Abstract
Tinnitus is the perception of a sound that has no external source. Sound stimuli can suppress spontaneous firing in auditory neurons long after stimulus offset. It is unknown how changes in sound stimulus parameters affect this forward suppression. Using in vivo extracellular recording in awake mice, we found that about 40 % of spontaneously active inferior colliculus (IC) neurons exhibited forward suppression of spontaneous activity after sound offset. The duration of this suppression increased with sound duration and lasted about 40 s following a 30-s stimulus offset. Pure tones presented at the neuron’s characteristic frequency (CF) were more effective in triggering suppression compared to non-CF or wideband noise stimuli. In contrast, non-CF stimuli often induced forward facilitation. About one third of IC neurons exhibited shorter suppression durations with each subsequent sound presentation. These characteristics of forward suppression are similar to the psychoacoustic properties of residual inhibition of tinnitus: a phenomenon of brief (about 30 s) suppression of tinnitus observed in tinnitus patients after sound presentations. Because elevated spontaneous firing in central auditory neurons has been linked to tinnitus, forward suppression of this firing with sound might be an underlying mechanism of residual inhibition.
Keywords: residual inhibition, inferior colliculus, mice, acoustic trauma
Introduction
Tinnitus is defined as the perception of sound when no external auditory stimulus is present. Over the course of a few decades, clinical tinnitus research has identified two major approaches for alleviation of tinnitus. One direction combines direct and indirect electrical brain stimulation including vagus nerve stimulation (see Smit et al. 2015; Langguth and De Ridder 2013; Hays et al. 2013; Vanneste and De Ridder 2012; Shore 2011). The other major directions utilize cognitive behavioral therapy and biofeedback (see Jun and Park 2013) or habituation training to decrease tinnitus perception and tinnitus-induced reactions (Jastreboff 1990). An external sound often acts as a tinnitus masker and usually decreases the relative distress from tinnitus (Schleuning and Johnson 1997). It has also been found that sound stimuli can act as tinnitus suppressor even after a sound has been terminated, the phenomenon known as residual inhibition (RI). This effect was first described in experiments in which tinnitus patients matched the pitch and timber of their tinnitus to various musical instruments (Spalding 1903). However, it was not until much later that RI was first systematically investigated (Feldmann 1971).
About 80 % of patients with tinnitus report some degree of RI (discussed by Vernon and Meikle 2003; Roberts et al. 2006). The duration of RI varies considerably among individuals, ranging from several seconds to hours, scaling logarithmically with the duration of the preceding masking sound (Hazell and Wood 1981; Terry et al. 1983). The majority of patients, however, experience tinnitus suppression from only about 5 to 30 s (Roberts 2007; Roberts et al. 2008). Recent reports have identified some basic psychoacoustic properties of RI (see Vernon and Meikle 2003; Roberts et al. 2006). The depth (magnitude of tinnitus reduction) and duration of RI largely depend on the intensity, duration, and spectrum of the sound used to induce RI. A recent RI study found that repetitive induction of RI leads to a reduction in its duration and depth (Sedley et al. 2015). The mechanism of RI has been a subject of debate among researchers (discussed by Vernon and Meikle 2003; Roberts et al. 2006). However, animal research has led to a core belief that the tinnitus percept is related to elevated spontaneous firing in central auditory neurons (see Kaltenbach 2011; Auerbach et al. 2014; Eggermont 2016). Our hypothesis is that forward suppression of this firing with sound might be an underlying mechanism of residual inhibition. Increased knowledge about this mechanism may not only shed light on the cause of tinnitus but also may help to develop an effective tinnitus treatment.
Two recent studies conducted on bats and mice have shown that brief sounds can trigger a long-lasting suppression of spontaneous firing in inferior colliculus neurons after sound cessation (Voytenko and Galazyuk 2010, 2011). Much like RI, the duration of this suppression increased with sound duration and sound intensity, although the sounds were much shorter (1.5 s) than those used to induce RI in humans. Since elevated spontaneous firing or hyperactivity in central auditory neurons has been linked to tinnitus (discussed by Roberts et al. 2010; Eggermont and Roberts 2012; Eggermont and Roberts 2015), suppression of this hyperactivity with sound may be an underlying mechanism of RI. Therefore, in this study, we tested neurons in the central auditory system of the mouse with stimuli akin to those used to induce RI, to determine the relationship between the characteristics of sound-triggered suppression and the psychoacoustic properties of RI.
Here, we show that the basic characteristics of suppression are similar to the psychoacoustic properties of the RI in humans, suggesting that this forward suppression might be an underlying mechanism of RI. Surprisingly to us, our data show that both normal animals and animals with behavioral signs of tinnitus exhibited long-lasting suppression. Therefore, it seems likely that forward suppression is a normal sound processing phenomenon in the auditory system, which may have unique relevance for people with tinnitus because of their abnormal central auditory processing.
Materials and Methods
Subjects
Fifty-five adult male CBA/CaJ mice were used in this study. To avoid startle variability which is known to result from hormone fluctuations of the estrous cycle, female mice were not used in this study (Plappert et al. 2005; Ison and Allen 2007). The experimental group consisted of 10 mice which were sound exposed unilaterally with the intent to induce tinnitus (see tinnitus induction and tinnitus assessment procedures below). Forty-five unexposed mice served as the control group. Mice were obtained from Jackson Laboratories and were approximately 12 weeks old with a mean weight of 27.5 g at the beginning of testing. Mice were housed in pairs within a colony room with a 12-h light–dark cycle (8 a.m. to 8 p.m.) at 25 °C. All procedures used in this study were approved by the Institutional Animal Care and Use Committee at Northeast Ohio Medical University.
Extracellular Recording
Each mouse was anesthetized using isoflurane inhalation (1.5–2.0 %, isoflurane administered by a precision vaporizer) prior to surgery. A midline incision of the skin over the cranium was made. The tissue overlying the skull then was removed and a small metal rod was glued to the skull using glass ionomer cement (3M ESPE, Germany). Following surgery, animals were allowed to recover for 1–2 days in individual holding cages.
Two days after surgery, each mouse was trained to stay inside a small plastic tube, to be used as a holding device during recording sessions. The metal rod on the head of the mouse was secured to a small holder designed to restrain the head of the animal, while the ears were unobstructed for free-field acoustic stimulation. Recordings were made in the central nucleus of the inferior colliculus (IC) in awake mice inside a single-walled sound attenuating chamber (Industrial Acoustics Company, Inc). A small hole (~50 μm) penetrating the dura was drilled in the skull overlying the IC, through which a recording electrode was inserted into the IC. Extracellular single-unit recordings were made with quartz glass micropipettes (10–20 MΩ impedance, 2–3 μm tip) filled with 0.5 M sodium acetate. Peak voltage of spikes typically exceeded background noise by a factor of 5. Neuronal spiking was identified either through background discharges or in response to acoustic search stimuli. The electrode was positioned into the drilled hole by means of a precision (1 μm) digital micromanipulator (Sutter, MP-285) using a surgical microscope (Leica MZ9.5). The relative position of each electrode was monitored from the readouts of digital micrometers using a common reference point on the skull. Vertical advancement of the electrode was made by a precision piezoelectric microdrive (Model 660, KOPF Instr.) from outside the sound attenuating chamber. Recorded action potentials were amplified (Dagan 2400A preamplifier), monitored audiovisually on a digital oscilloscope (DL1640, YOKOGAWA), digitized, and then stored on a computer hard drive using EPC-10 digital interface and PULSE software from HEKA Elektronik at a bandwidth of 10 kHz. The experimental protocol for a single recording lasted 30 to 40 min. It included determining frequency response tuning, assessing spontaneous firing, and sound-evoked firing followed by the test to assess changes in the neuronal firing following long-lasting sound stimulus presentations (up to 30 s). A single recording epoch for each sound presentation lasted about 2 min in order to assess neuronal firing 10 s before, 30 s during, and 90 s after each sound presentation. Throughout the recording session (3 to 4 h), the animal was offered water periodically and monitored for signs of discomfort. After a recording session, the exposed skull was covered with sterile bone wax, and the animal was returned to its holding cage. Experiments were conducted every 2–3 days for a maximum of 2 weeks. No sedative drugs were used during recording sessions. If the animal showed any signs of discomfort, the recording session was terminated and the mouse was returned to its cage.
Electrophysiology Data Analysis
Each neuron’s characteristic frequency, the frequency to which a given neuron responded with the lowest threshold, was determined manually by presenting pure tone stimuli at a wide range of frequencies and intensities. Sound stimuli were presented 40 dB above the neuronal response threshold, a level shown previously to be sufficient to reliably trigger long-lasting forward suppression of spontaneous activity (Voytenko and Galazyuk 2010). The response threshold was defined as the minimum sound level required to evoke a 50 % response rate from stimulus presentations. To determine the duration of suppression of the spontaneous firing elicited by sound stimuli, the spontaneous firing rates 5 s before were compared to post-stimulus firing rates. Changes in firing rates were difficult to assess from peristimulus time histograms (PSTHs); thus, we measured these changes over a 1-s sliding window. The window of analysis was initially aligned with the 0-ms point on the time axis of the PSTH and was shifted by 1-s increments until the end of the recording trace. Each point on the histograms in Figures 1, 2, 4, 5, 6, 7, and 8 was aligned with the start time of the analysis window. The 5 s preceding a stimulus was used to compute the mean value for the spontaneous firing rate. Suppression or facilitation of spontaneous firing was defined as the time interval following stimulus presentation that the spike rate was two standard deviations below or above the spontaneous rate (95 % confidence limits) recorded before the sound stimulus.
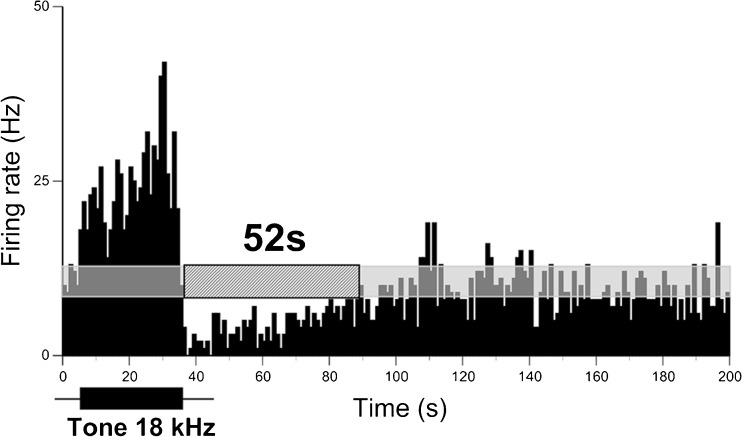
FIG. 1.
Long-lasting suppression of spontaneous firing in an IC neuron following a sound stimulus. PSTH of a single recording of an IC neuron in response to a pure tone (30-s duration) presented at the neuron’s CF (18 kHz) at 70 dB SPL or 40 dB above the neuron’s response threshold. Horizontal semitransparent bar represents an averaged level of spontaneous firing ±2 SD calculated based on spontaneous neuronal firing recorded during 5 s before the stimulus onset. The hashed bar indicates the duration of suppression (52 s, shown above). The sound stimulus is shown by a black horizontal bar below the histograms (same timescale as histogram). Bin size is 1 s.
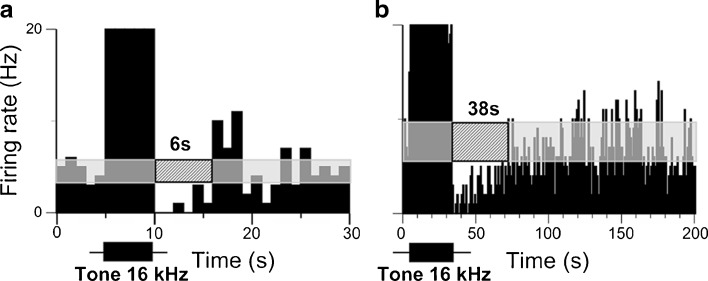
FIG. 2.
Suppression duration of spontaneous firing in an IC neuron increases with sound stimulus duration. PSTH of a single recording of an IC neuron in response to a pure tone of 5-s (a) and 30-s (b) duration presented at the neuron’s CF (16 kHz) at 65 dB SPL or 40 dB above the neuron’s response threshold. The duration of suppression was 6 s in a and 38 s in b. See legend of Figure 1 for other details.
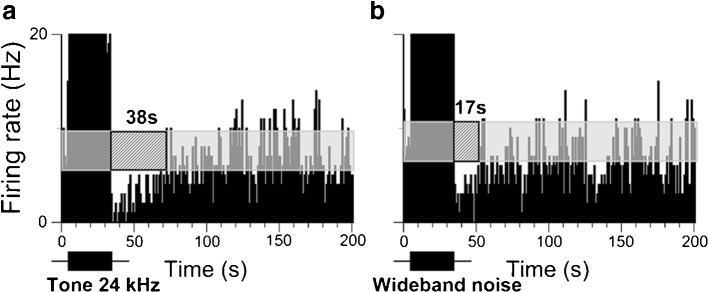
FIG. 4.
Duration of suppression in IC neurons in response to a pure tone at the neurons’ CF is longer than in response to a wideband noise. a PSTH of a single recording of an IC neuron to a 30-s pure tone at neuron’s CF (24 kHz) presented at 65 dB SPL or 40 dB above threshold. b PSTH of the same neuron in response to a wideband noise. To compensate for the power loss, the wideband noise was presented 10 dB louder (75 dB SPL). The duration of suppression was 38 s in a and 17 s in b. See legend of Figure 1 for other details.
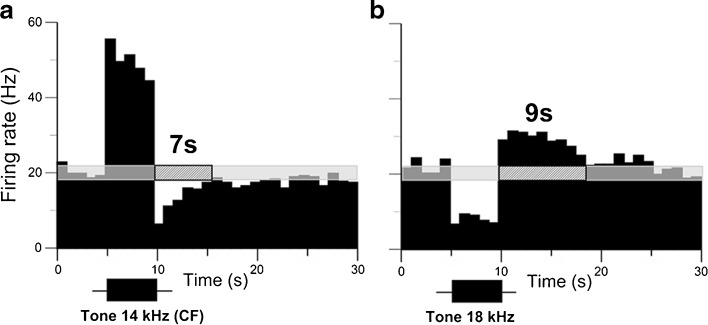
FIG. 5.
A small subset of IC neurons exhibited firing rate suppression in response to the neuron’s CF yet with facilitation to non-CFs. a PSTH of a single recording of an IC neuron in response to a 30-s pure tone at neuron’s CF (14 kHz) presented at 75 dB SPL or 40 dB above the neuron’s threshold. b PSTH of the same neuron in response to a non-CF (18 kHz). There was 7-s duration of suppression in a and 9 s of facilitation in b. See legend of Figure 1 for other details.
FIG. 6.
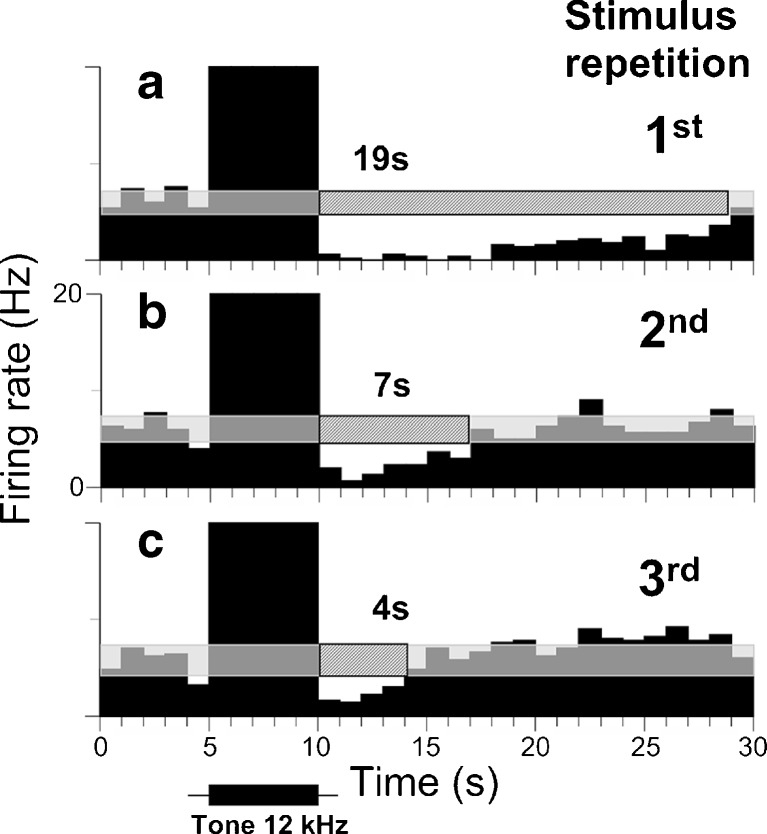
Duration of suppression decreased when a consecutive series of sound stimuli were presented. a PSTH of a single recording of an IC neuron to a 5-s pure tone at neuron’s CF (12 kHz) presented at 70 dB SPL or 40 dB above the neuron’s threshold. (b, c) PSTH of the same neuron in response to two more sound presentations. Note that duration of suppression decreased with each subsequent stimulus presentations (a 19 s, b 7 s, c 4 s). Bin size is 1 s. See legend of Figure 1 for other details.
FIG. 7.
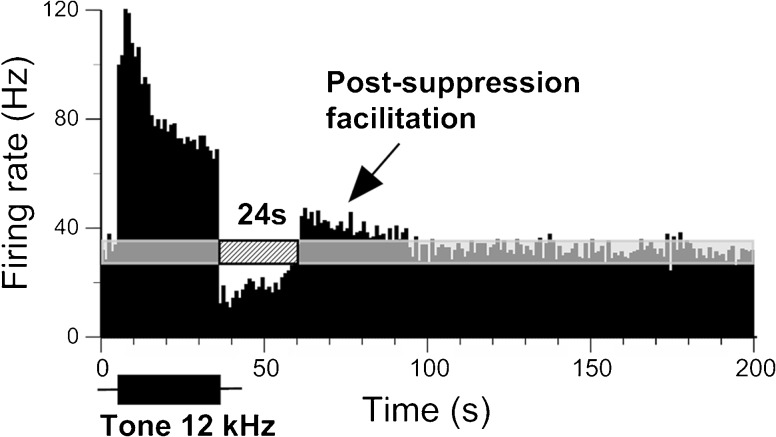
Facilitation of spontaneous firing in an IC neuron following sound-evoked suppression. PSTH of a single recording of an IC neuron in response to a 30-s pure tone presented at the neuron’s CF (12 kHz) at 60 dB SPL or 40 dB above the neuron’s response threshold. Black arrow indicates the facilitation. See legend of Figure 1 for other details.
FIG. 8.
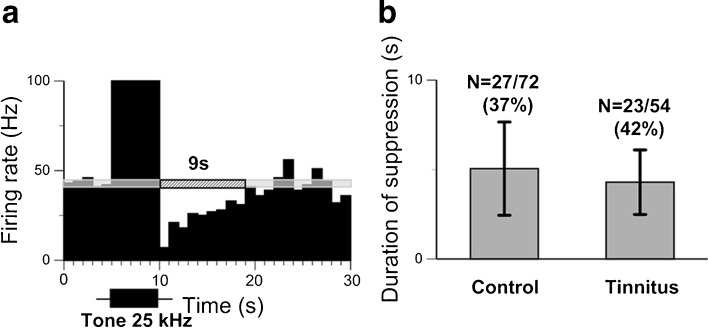
Suppression of spontaneous firing in IC neurons of tinnitus positive and naïve mice is similar. a PSTH of a single recording of an IC neuron in a tinnitus positive mouse to a 5-s pure tone presented at the neuron’s CF (25 kHz) at 70 dB SPL or 40 dB above the neuron’s response threshold. b Comparison of the duration of suppression to 5-s sound stimuli presented at neurons’ CF in IC neurons of the control and tinnitus positive mice. See legend of Figure 1 for other details.
Acoustic Trauma
Mice were anesthetized with an intramuscular injection of a ketamine/xylazine mixture (100/10 mg/kg). An additional injection (50 % of the initial dose) was given 30 min after the initial injection. Mice were unilaterally exposed to a 116 dB SPL one octave narrowband noise centered at 12.5 kHz (~8–17 kHz). This noise was generated using a waveform generator (Tektronix AFG 3021B), amplified (QSC RMX 2450), and played through a speaker (Fostex T925A Horn Tweeter). The loudspeaker was calibrated with a 0.25-in. microphone (Brüel and Kjaer 4135) connected to a measuring amplifier (Brüel and Kjaer 2525) and found to be ±4 dB between 10 and 60 kHz. During exposure, the speaker was located ~10 cm from the animal’s ear. During exposure, the left external ear canal was obstructed with a cotton plug followed by a Kwik-Sil silicone elastomer plug (World Precision Instruments). A cotton plug served as a blocker to prevent sticky silicone from damaging the tympanic membrane.
Gap Detection Test for Assessment of Tinnitus
Mice were assessed for chronic tinnitus 3 months after exposure, the time necessary for chronic tinnitus to be developed in mice (Longenecker and Galazyuk 2011). The ability of mice to detect a gap of silence preceding the startle stimulus was determined using commercial hardware/software equipment from Kinder Scientific, Inc. Mice were placed in a small restrainer situated on a plate with a pressure sensor. Any animal locomotor activity was detected by the sensor which measured its amplitude and stored data on the computer hard drive. Kinder Scientific software was used to generate a sequence of stimulus trials including a startle stimulus presented alone (STARTLE) and a startle stimulus paired with a gap (GAP+STARTLE) embedded into continuous background noise. The background consisted of narrowband (1/3 octave) noise centered at six different frequencies (10, 12.5, 16, 20, 25, and 31.5 kHz). This background noise level was constant (60 dB SPL) throughout the session. The startle stimulus was white noise presented at 110 dB SPL and lasted 20 ms. The gap of silence was 20 ms long and was presented 100 ms before (onset to onset) the startle stimulus.
For the gap detection test, parameters of our stimulus paradigm were set to levels which are typical for assuring a robust ∼30 % reduction in startle response amplitude caused by a preceding gap of silence in an otherwise continuous background sound (Ison et al. 2002; Turner et al. 2006; Longenecker and Galazyuk 2011).
The testing session started with an acclimation period lasting 3 min during which animals were exposed to a continuous 60 dB SPL wideband noise. Immediately afterwards, animals received 10 STARTLE-only trials in order to habituate their startle responses to a steady state level. For each of six background frequencies, we presented five STARTLE-only trials and five GAP+STARTLE trials. The STARTLE and GAP+STARTLE trials were pseudo-randomized. The inter-trial intervals were also pseudo-randomized between 7 and 15 s. After we completed testing all six background frequencies, the entire session was repeated one more time. Thus, during this testing for each background frequency, the total of 10 GAP+STARTLE trials and 10 STARTLE-only trials were presented. Prepulse inhibition of the acoustic startle reflex was used to assess animals’ hearing after sound exposure. The prepulse in this test was presented at the same sound level as the continuous background (60 dB SPL) used for the gap detection test. It served as a control to make sure that sound exposure did not compromise animals’ hearing, to ensure that the 60 dB SPL background presented during gap detection tests was still detectable. If no significant changes in the prepulse inhibition were found before and after exposure, the animal was considered to have satisfactory hearing performance.
Tinnitus Data Analysis
All waveforms collected during testing sessions were analyzed offline using a recently developed automatic method of startle waveform identification via a template matching paradigm (Grimsley et al. 2015). This approach allowed us to separate actual startle responses from a random animal background activity after capturing startle responses with high-speed video and a piezoelectric startle plate. We also used a mathematical approach to normalize startle response magnitudes of individual animals to their body mass (Grimsley et al. 2015). This mathematical conversion has two benefits: first, the procedure normalizes for mass, allowing legitimate comparisons between animals of different mass and inter-animal comparisons over time with differing masses, and second, it converts the forces sensed by the piezoelectric startle plate into a more readily understandable unit of distance jumped: the center of mass displacement.
For each background frequency, a total of 10 GAP+STARTLE trials and 10 STARTLE-only trials were presented. To calculate the GAP+STARTLE/STARTLE ratio, we calculated the mean for all STARTLE values. They changed little within one session. Then we divided each of 10 GAP+STARTLE values for a given background frequency by the startle mean value. These 10 ratio values at a given frequency were used to calculate mean and SD values. A one-way analysis of variance (ANOVA) was used to test for differences within a subject. The criterion for the presence of behavioral evidence of tinnitus was a significant reduction in gap-induced prepulse inhibition at one or several background frequencies compared to the preexposure values. During our data analysis, we found empirically that the 95 % confidence interval is an optimal must-reach criterion to demonstrate changes in gap or prepulse detection performance induced by sound exposure.
Results
Long-Lasting Suppression of Firing Activity in IC Neurons
Extracellular responses to long-lasting sound stimuli (5- or 30-s duration) were recorded from 201 IC neurons in 42 awake mice. The majority of neurons (87 %, 175/201) exhibited spontaneous activity with firing rates ranging from 0.2 to 36 spikes per second (sp/s). Since the focus of this study was to examine the effects of sound stimuli on spontaneous activity, we excluded neurons which did not exhibit spontaneous activity (13 %, 26/201) from our data analysis. More than one third of spontaneously active neurons (39.5 %, 69/175) exhibited suppression of their firing following sound stimulus termination. The remaining spontaneously active neurons (106/175) either showed suppression only during sound presentation (37/106) or no suppression at all (69/106). An extracellular response trace of a representative IC neuron exhibiting extended suppression is shown in Figure 1. This neuron had a spontaneous firing rate of 10.9 sp/s before a stimulus was presented. During the stimulus presentation, its firing rate was increased to 28 sp/s. After the stimulus, neuronal firing was suppressed for about 52 s and then returned to the pre-stimulus level.
Forward suppression in auditory neurons has been shown to increase with sound level (Voytenko and Galazyuk 2010). Therefore, for all recorded neurons in this study, we fixed the level at 40 dB above neuronal response threshold. The suppression was also sensitive to other sound stimulus parameters. Changes of stimulus spectrum or how the stimulus was presented could alter suppression or even reverse it into facilitation. Each of these stimulus-dependent effects is demonstrated below.
Effect of Sound Duration on Suppression Duration
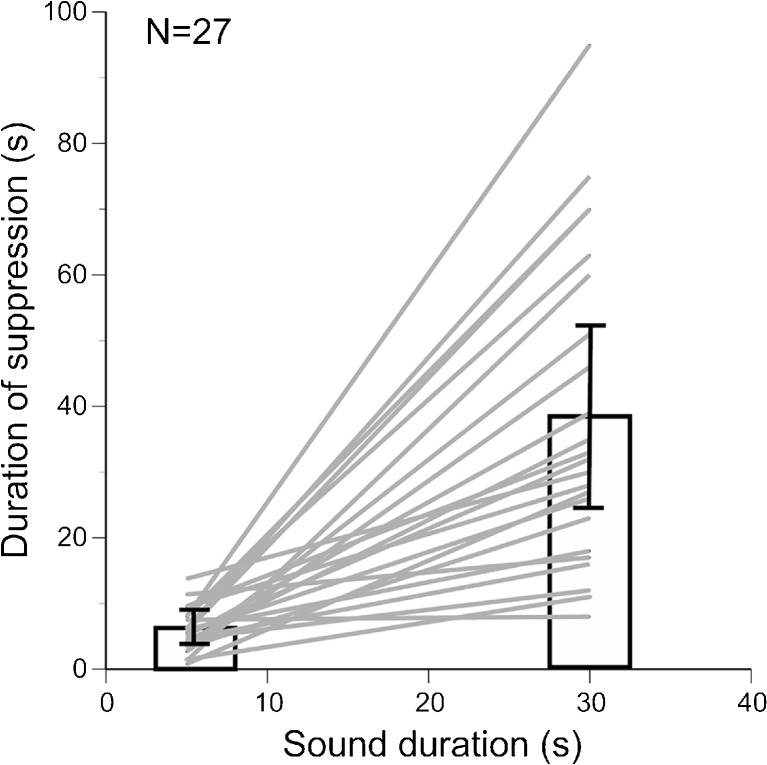
In agreement with our previous findings in bats (Voytenko and Galazyuk 2010, 2011), increasing the duration of the sound stimulus prolonged suppression of spontaneous firing in IC neurons in mice. A representative neuron in Figure 2 showed suppression lasting about 6 s in response to a 5-s pure tone presented at the neuron’s characteristic frequency (CF), the frequency at which a given neuron responds to the smallest sound intensity. When the sound duration was increased to 30 s, this neuron exhibited suppression for ~38 s. The population data on the IC neurons (27 units) tested with both 5- and 30-s sound durations showed that the average duration of the suppression was roughly correlated with the sound duration, 5.8 and 37.7 s, respectively (Fig. 3). However, a few neurons demonstrated suppressions which exceeded the stimulus duration two or even three times.
FIG. 3.
Duration of the suppression correlates with sound duration (r = 0.71, p < 0.0001). Suppression in 27 IC neurons was determined in response to both the 5-s and 30-s sound duration.
Effect of Stimulus Spectrum on Suppression Duration
IC neurons showed responses to both pure tones and wideband noises. We investigated whether the suppression in IC neurons was dependent on the spectral characteristics of the sound stimulus. Pure tones presented at the neuron’s CF were more effective in triggering a sustained suppression than noise stimuli (Fig. 4). The average duration of suppression from 12 IC neurons presented with both a 30-s pure tone at the neuron’s CF and a wideband noise ranged from 19 to 63 s (mean 39.7 ± 18.6) and from 9 to 31 (mean 19.4 ± 10.5), respectively. A two-sample t-test showed a significant difference between suppression from pure tones and wideband noise (t = 3.29, df = 22, p = 0.0017).
Effect of Non-Characteristic Sound Frequencies on the Post-Stimulus Firing
The difference in the duration of suppression between pure tones at the neurons’ CF and wideband noise suggests that the sound frequency might be critical to determine which changes in neuronal firing occur after sound presentation. To test this hypothesis, we studied the responses of 23 IC neurons to their CF and non-CFs. About half of these neurons (11/23) showed suppression to both types of stimuli but with longer suppression durations to the neurons’ CF. The average duration of suppression from 11 IC neurons to a 5-s pure tone presented at the neuron’s CF and a non-CF ranged from 1.48 to 13.8 s (mean 7.06 ± 3.38) and from 0.8 to 5.64 (mean 2.86 ± 1.38), respectively. A two-sample t-test indicated a significant difference in suppression between CF and non-CF (t = 3.8156, df = 20, p = 0.0005). A third of these neurons (7/23), however, exhibited long-lasting suppression to the CF; in contrast, these same neurons showed firing rate facilitation to non-CFs. A representative neuron exhibiting this type of response is shown in Figure 5. Typical for a majority of the IC neurons, the representative neuron exhibited sustained firing during the stimulus followed by a long-lasting suppression in response to its CF. In contrast, in response to a non-CF, the firing rate was suppressed during the stimulus but was facilitated after the stimulus (Fig. 5). The remaining 5 out of 23 neurons showed suppression to the CFs and no changes in firing in response to non-CFs.
Effect of Multiple Stimulus Presentations on the Suppression
We observed an interesting phenomenon resulting from sound stimuli presented consecutively. About one third of IC neurons exhibiting suppression (25/69 or 36 %) showed shortened suppression durations with each subsequent sound presentation. A representative neuron in Figure 6 showed suppression lasting 19 s to the first stimulus presentation (Fig. 6a), but when the same sound was presented again with a short delay, the duration of suppression decreased to 7 s (Fig. 6b). A subsequent sound presentation made this suppression even shorter, about 4 s (Fig. 6c). This phenomenon was not evident when inter-stimulus intervals were extended to several minutes.
Post-Suppression Facilitation
About 40 % (28/69) of IC neurons exhibiting suppression showed an increased firing rate compared with spontaneous firing immediately following the end of suppression. The duration of this post-suppression rebound varied among neurons, ranging from 5 to 42 s when a 30-s sound stimulus was presented. A representative neuron in Figure 7 elicited a 24-s suppression in response to a 30-s pure tone presented at the neuron’s CF. By the end of this suppression, the firing rate was significantly increased for about 33 s relative to the pre-stimulus level.
Suppression of Spontaneous Firing in Mice with Behavioral Evidence of Tinnitus
The data presented above were collected in normal, unexposed animals. To determine whether animals with tinnitus also exhibit long-lasting suppression, we studied a group of mice with behavioral evidence of tinnitus. Ten animals were exposed unilaterally for 1 hour to a 116 dB SPL one octave narrowband noise centered at 12.5 kHz, a common method in the field of tinnitus research used to induce tinnitus (see Galazyuk and Hebert 2015). Three months following exposure, behavioral testing identified evidence of tinnitus in 4 out of 10 exposed animals, a typical outcome for the given sound exposure. In agreement with numerous previous reports, the average spontaneous firing rate of IC neurons in mice with tinnitus was much higher compared to controls (Salvi et al. 2000; Kaltenbach and Afman 2000; Brozoski et al. 2002; Mulders and Robertson 2009; Dong et al. 2010; Longenecker and Galazyuk 2011). The firing rates of IC neurons in tinnitus positive mice ranged from 0.8 to 98 sp/s, with a mean of 27.71 sp/s, which was about five times higher than that of non-exposed animals (p < 0.00001). Similar to controls, about half of IC neurons (23/54 or 42 %) in the mice with behavioral evidence of tinnitus also showed long-lasting suppression (Fig. 8a). The duration of this suppression in tinnitus positive animals did not significantly differ from control animals (t = 1.18, df = 48, p = 0.24) (Fig. 8b).
Discussion
We found that nearly half of spontaneously active IC neurons, including data from both control and tinnitus animals, exhibited forward suppression of spontaneous activity after sound offset. The duration of this suppression increased with sound duration and lasted about 40 s following a 30-s stimulus offset. There are a number of striking similarities between the basic parameters of this forward suppression and RI. Since elevated spontaneous firing (hyperactivity) in auditory neurons has been linked to tinnitus (discussed by Roberts et al. 2010; Eggermont and Roberts 2012; Galazyuk et al. 2012), suppression of this hyperactivity with sound may explain a temporary relief from tinnitus during RI. To the best of our knowledge, our study presents the first evidence of a possible link between a neural mechanism of sound processing in auditory neurons and the behavioral phenomenon of RI. Below, we discuss the similarities between the RI and suppression individually as well as the possible significance of this suppression as a general sound processing phenomenon.
Similarities Between RI and Suppression
Duration of RI and Suppression Increases with Sound Duration
Although non-linear, the duration of RI has been shown to increase with sound duration (Feldmann 1971; Terry et al. 1983; Tyler et al. 1984). Audiologists typically test tinnitus patients for RI by using 30-s or 1-min sounds. In response to these stimuli, the majority of patients report RI lasting about 1 min on average (Roberts 2007). Similarly, the duration of the suppression in the auditory neurons in the present study also increased with sound duration and lasted on average 40 s in response to a 30-s sound stimulus (Figs. 2 and 3).
Effect of Stimulus Spectrum on Suppression Duration
Current literature concerning RI outlines some disagreement among studies on the differential effectiveness of pure tones vs noise for induction of RI. However, a majority of studies report that pure tones are more effective at inducing RI compared to wideband or even narrowband noise stimuli (Vernon and Meikle 1981; Olsen et al. 1996; Sockalingam et al. 2007). Both the depth and duration of RI are increased when the trigger sound matches the frequency range of the patient’s tinnitus (Roberts et al. 2006; Roberts 2007; Roberts et al. 2008). We observe a similar trend: pure tones were more likely to trigger longer suppression of neuronal firing in auditory neurons compared to wideband noise (Fig. 4).
An unusual phenomenon was observed in 30 % of auditory neurons when their responses were tested to CF and non-CFs. These neurons showed a typical suppression in response to neurons’ CF yet exhibited long-lasting facilitation to non-CFs. The forward suppression observed after CF sound is mediated by activation of the group I metabotropic glutamate receptors (Voytenko and Galazyuk 2011). The mechanism of facilitation observed after a non-CF stimulus is puzzling and requires further investigation. If this sound-evoked facilitation occurs in tinnitus patients, it would temporarily cause an increase of hyperactivity in the central auditory neurons, which in turn can increase loudness of tinnitus. A long-lasting increase in tinnitus loudness has been reported by some tinnitus patients during RI induction (Vernon and Schleuning 1978; Terry et al. 1983; Tyler et al. 1984; Lipman and Lipman 2007; Roberts et al. 2008, 2015). Our data strongly suggests that this unusual phenomenon might occur as a result of a mismatch between the frequencies of the sound stimulus and a patients’ tinnitus, especially in the case of tonal tinnitus. We hypothesize that if, for instance, a patient with 6-kHz tinnitus was presented a 10-kHz sound to induce RI, many hyperactive neurons having a CF of 6 kHz (tinnitus frequency) would be stimulated with a non-CF (10 kHz). Based on our results, some auditory neurons in the tinnitus frequency region would show firing rate facilitation instead of suppression which may be perceived as a temporary increase in tinnitus loudness. Future RI research is necessary to test this hypothesis.
The Effect of Multiple Stimulus Presentations on the Suppression
We demonstrated that some IC neurons exhibited a reduction in the duration and “depth” of the suppression of spontaneous firing rates when stimuli were presented with relatively short inter-stimulus intervals (Fig. 6). Interestingly, this effect resulting from repeated RI inductions has not been often reported in human RI studies. Therefore, it is possible that this phenomenon has not been widely observed and described. However, a recent intracranial mapping study on a single human patient did corroborate with our results (Sedley et al. 2015). When RI was induced repeatedly with relatively short inter-stimulus intervals, the efficacy of RI induction was largely reduced.
Post-Suppression Facilitation
The ~40 % of auditory neurons in our study that exhibited suppression also showed a momentary increase in the firing rate immediately following cessation of suppression (Fig. 7). Further research is needed to determine whether post RI facilitation is also a typical phenomenon during RI induction in humans.
What Does Neural Suppression Tell Us About the Mechanisms of RI?
Cumulative findings from the present study and our previous research (Voytenko and Galazyuk 2010, 2011) suggest that suppression is likely a universal sound processing phenomenon observed across species and appears to be tinnitus independent. We found no differences in the main features of the suppression in either tinnitus positive or negative mice. Although it has not been studied systematically in relation to RI, stimulus-induced suppression has been observed at almost all levels of the central auditory system and in different mammalian systems (Smith 1977; Harris and Dallos 1979; Relkin and Turner 1988; Ebert and Ostwald 1995; Portfors and Roberts 2007; Galazyuk et al. 2005; Nelson et al. 2009; Wehr and Zador 2005) including the cochlea nerve (Young and Sachs 1979). Therefore, it is not clear whether the forward suppression observed in the IC is formed at these levels or inherited from lower levels. Our previous studies suggest that suppression, at least at the level of IC, is formed locally and mediated by group I metabotropic glutamate receptors (Voytenko and Galazyuk 2011). Intracellular recordings from awake animals further reveal that this effect is likely to be presynaptic (Voytenko and Galazyuk 2010).
Since this suppression has been reported for multiple animal species, it would be logical to expect that this phenomenon should also be present in humans. This notion leads to the dubious question of why tinnitus-free people do not perceive this forward suppression. In an attempt to answer this question, it is useful to consider the theory that the tinnitus percept results from hyperactive or highly spontaneously active neurons. Based on this theory, elevated spontaneous activity arises in the central auditory system in response to cochlear damage (discussed by Roberts et al. 2010; Eggermont and Roberts 2012; Galazyuk et al. 2012; Auerbach et al. 2014). Apparently, the brain perceives this hyperactivity as a phantom sound or tinnitus. When a patient with tinnitus experiences RI after a sound stimulus, this sound stimulus may be lowering the spontaneous rate of his/her neurons for a brief period of time. Alternatively, normal individuals would not experience forward suppression because their spontaneous activity levels in the auditory system would be low enough to remain below the threshold of sensation. For these individuals, suppression of this activity with an external sound would be unnoticeable. As tinnitus is considered to be tightly linked to elevated spontaneous activity and we have shown that the characteristics of RI and neural response suppression are closely matched, it is likely that suppression of elevated spontaneous activity explains the suppression of tinnitus seen in ~80 % of patients during RI.
In addition to changes in spontaneous firing, increases in synchronized firing among auditory neurons have also been observed at multiple levels of the central auditory system after a hearing insult (Eggermont 2000; Noreña and Eggermont 2003; Finlayson and Kaltenbach 2009). This synchrony may also be responsible for the phantom sound percept in tinnitus patients (Roberts et al. 2010; Eggermont and Tass 2015). Although it was not tested directly in this study, synchrony should be greatly reduced during forward suppression due to a reduction of overall spontaneous activity. Future studies should test this hypothesis directly.
Suppression and Sound Processing
The present and previous researches suggest that post-stimulus suppression of spontaneous firing is a typical sound processing phenomenon. Real-world acoustic signals, including human speech, rarely occur in isolation and usually comprise a sequence of sound elements. Therefore, if a sound sequence occurs, the first sound of this sequence will be likely to trigger forward suppression, whereas subsequent sounds may maintain the suppression. Consequently, all but the first sound in a sequence will be processed by auditory neurons among little or no spontaneous activity (Voytenko and Galazyuk 2010). When spontaneous firing is absent or greatly suppressed, the presence of a spike in response to a sound is a more reliable indicator of the presence of a sound stimulus. Hence, suppression of spontaneous firing may serve as a mechanism of enhancing a signal-to-noise ratio during signal processing. However, there is some indirect evidence suggesting that the signal-to-noise ratio might not be the only advantage of the suppression. It has been demonstrated that response selectivity of auditory neurons to sound level (Galazyuk et al. 2000), frequency (Smalling et al. 2001), and duration (Zhou and Jen 2006) can be greatly enhanced if sound stimuli for assessing such selectivity are presented with high repetition rates. If the stimulation rate is high, the stimuli are likely to be analyzed by auditory neurons within the time of suppression. Unfortunately, little is known about the mechanism responsible for the enhancement of response acuity in auditory neurons responding to high stimulus rates. Further investigations are needed to ascertain the neural mechanisms mediating the suppression of spontaneous firing and its biological significance.
Acknowledgments
We would like to acknowledge Dr. Larry Roberts, Dr. Arnaud Noreña, Dr. Merri Rosen, Inga Kristaponyte, and Greg Nelson for their comments on earlier versions of this manuscript. This research was supported by grant R01 DC011330 to A.V. Galazyuk and F31 DC013498-01A1 to R. J. Longenecker from the National Institute on Deafness and Other Communication Disorders of the US Public Health Service.
Abbreviations
- RI
Residual inhibition
- IC
Inferior colliculus
- AC
Auditory cortex
- CF
Characteristic frequency
- PSTH
Peristimulus time histogram
Compliance with Ethical Standards
All procedures used in this study were approved by the Institutional Animal Care and Use Committee at Northeast Ohio Medical University.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Auerbach BD, Rodrigues PV, Salvi RJ. Central gain control in tinnitus and hyperacusis. Front Neurol. 2014;5:206. doi: 10.3389/fneur.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Mulders WH, Rodger J, Woo S, Robertson D. Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. Eur J Neurosci. 2010;31:1616–1628. doi: 10.1111/j.1460-9568.2010.07183.x. [DOI] [PubMed] [Google Scholar]
- Ebert U, Ostwald J. GABA can improve acoustic contrast in the rat ventral cochlear nucleus. Exp Brain Res. 1995;104:310–322. doi: 10.1007/BF00242016. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Sound-induced synchronization of neural activity between and within three auditory cortical areas. J Neurophysiol. 2000;83:2708–2722. doi: 10.1152/jn.2000.83.5.2708. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ (2016) Acquired hearing loss and brain plasticity. Hear Res xx:1–15 [DOI] [PubMed]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus: understanding abnormal and normal auditory perception. Front Syst Neurosci. 2012;6:53. doi: 10.3389/fnsys.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. Tinnitus: animal models and findings in humans. Cell Tissue Res. 2015;361:311–336. doi: 10.1007/s00441-014-1992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, Tass PA. Maladaptive neural synchrony in tinnitus: origin and restoration. Front Neurol. 2015;6:29. doi: 10.3389/fneur.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H. Homolateral and contralateral masking of tinnitus by noise-bands and by pure tones. Audiology. 1971;10:138–144. doi: 10.3109/00206097109072551. [DOI] [PubMed] [Google Scholar]
- Finlayson PG, Kaltenbach JA. Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear Res. 2009;256:104–117. doi: 10.1016/j.heares.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazyuk AV, Hebert S. Gap-prepulse inhibition of the acoustic startle reflex (GPIAS) for tinnitus assessment: current status and future directions. Front Neurol. 2015;6:88. doi: 10.3389/fneur.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazyuk AV, Llano D, Feng AS. Temporal dynamics of acoustic stimuli enhance amplitude tuning of inferior colliculus neurons. J Neurophysiol. 2000;83:128–138. doi: 10.1152/jn.2000.83.1.128. [DOI] [PubMed] [Google Scholar]
- Galazyuk AV, Lin W, Llano D, Feng AS. Leading inhibition to neural oscillation is important for time-domain processing in the auditory midbrain. J Neurophysiol. 2005;94:314–326. doi: 10.1152/jn.00056.2005. [DOI] [PubMed] [Google Scholar]
- Galazyuk AV, Wenstrup JJ, Hamid MA. Tinnitus and underlying brain mechanisms. Curr Opin Otolaryngol Head Neck Surg. 2012;20:409–415. doi: 10.1097/MOO.0b013e3283577b81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley CA, Longenecker RJ, Rosen MJ, Young JW, Grimsley JM, Galazyuk AV. An improved approach to separating startle data from noise. J Neurosci Methods. 2015;253:206–217. doi: 10.1016/j.jneumeth.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DM, Dallos P. Forward masking of auditory nerve fiber responses. J Neurophysiol. 1979;42:1083–1107. doi: 10.1152/jn.1979.42.4.1083. [DOI] [PubMed] [Google Scholar]
- Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res. 2013;207:275–299. doi: 10.1016/B978-0-444-63327-9.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell JW, Wood S. Tinnitus masking-a significant contribution to tinnitus management. Br J Audiol. 1981;15:223–230. doi: 10.3109/03005368109081442. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD. Pre-but not post-menopausal female CBA/CaJ mice show less prepulse inhibition than male mice of the same age. Behav Brain Res. 2007;185:76–81. doi: 10.1016/j.bbr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Ison JR, Castro J, Allen P, Virag TM, Walton JP. The relative detectability for mice of gaps having different ramp durations at their onset and offset boundaries. J Acoust Soc Am. 2002;112:740–747. doi: 10.1121/1.1490352. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- Jun HJ, Park MK. Cognitive behavioral therapy for tinnitus: evidence and efficacy. Korean J Audiol. 2013;17:101–104. doi: 10.7874/kja.2013.17.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA. Tinnitus: Models and mechanism. Hear Res. 2011;276:52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE (2000) Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res 140:165–172. doi:10.1016/s0378-5955(99)00197-5 [DOI] [PubMed]
- Langguth B, De Ridder D. Tinnitus: therapeutic use of superficial brain stimulation. Handb Clin Neurol. 2013;116:441–467. doi: 10.1016/B978-0-444-53497-2.00036-X. [DOI] [PubMed] [Google Scholar]
- Lipman RI, Lipman SP. Phase-shift treatment for predominant tone tinnitus. Otolaryngol Head Neck Surg. 2007;136:763–768. doi: 10.1016/j.otohns.2006.10.046. [DOI] [PubMed] [Google Scholar]
- Longenecker RJ, Galazyuk AV. Development of tinnitus in CBA/CaJ mice following sound exposure. J Assoc Res Otolaryngol. 2011;12:647–658. doi: 10.1007/s10162-011-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience. 2009;164:733–746. doi: 10.1016/j.neuroscience.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Nelson PC, Smith ZM, Young ED. Wide-dynamic-range forward suppression in marmoset inferior colliculus neurons is generated centrally and accounts for perceptual masking. J Neurosci. 2009;29:2553–2562. doi: 10.1523/JNEUROSCI.5359-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreña AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res. 2003;183:137–153. doi: 10.1016/S0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Olsen SØ, Nielsen LH, Osterhammel PAA, Rasmussen AN, Ludvigsen C, Westermann S. Experiments with sweeping pure tones for the inhibition of tinnitus. J Audiological Medicine. 1996;5:27–37. [Google Scholar]
- Plappert CF, Rodenbücher AM, Pilz PK. Effects of sex and estrous cycle on modulation of the acoustic startle response in mice. Physiol Behav. 2005;84:585–594. doi: 10.1016/j.physbeh.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Roberts PD. Temporal and frequency characteristics of cartwheel cells in the dorsal cochlear nucleus of the awake mouse. J Neurophysiol. 2007;98:744–756. doi: 10.1152/jn.01356.2006. [DOI] [PubMed] [Google Scholar]
- Relkin EM, Turner CW. A reexamination of forward masking in the auditory nerve. J Acoust Soc Am. 1988;84:584–591. doi: 10.1121/1.396836. [DOI] [PubMed] [Google Scholar]
- Roberts LE. Residual inhibition. Prog Brain Res. 2007;166:487–495. doi: 10.1016/S0079-6123(07)66047-6. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Moffat G, Bosnyak DJ. Residual inhibition functions in relation to tinnitus spectra and auditory threshold shift. Acta Otolaryngol Suppl. 2006;556:27–33. doi: 10.1080/03655230600895358. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Moffat G, Baumann M, Ward LM, Bosnyak DJ. Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. J Assoc Res Otolaryngol. 2008;9:417–435. doi: 10.1007/s10162-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30:14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Bosnyak DJ, Bruce IC, Gander PE, Paul BT. Evidence for differential modulation of primary and nonprimary auditory cortex by forward masking in tinnitus. Hear Res. 2015;327:9–27. doi: 10.1016/j.heares.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/S0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Schleuning AJ, Johnson RM. Use of masking for tinnitus. Int Tinnitus J. 1997;3:25–29. [PubMed] [Google Scholar]
- Sedley W, Gander PE, Kumar S, Oya H, Kovach CK, Nourski KV, Kawasaki H, Howard MA, 3rd, Griffiths TD. Intracranial mapping of a cortical tinnitus system using residual inhibition. Curr Biol. 2015;25:1208–1214. doi: 10.1016/j.cub.2015.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE. Plasticity of somatosensory inputs to the cochlear nucleus—implications for tinnitus. Hear Res. 2011;281:38–46. doi: 10.1016/j.heares.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalling JM, Galazyuk AV, Feng AS. Stimulation rate influences frequency tuning characteristics of inferior colliculus neurons in the little brown bat, Myotis lucifugus. Neuroreport. 2001;12:3539–3542. doi: 10.1097/00001756-200111160-00033. [DOI] [PubMed] [Google Scholar]
- Smit JV, Janssen ML, Schulze H, Jahanshahi A, Van Overbeeke JJ, Temel Y, Stokroos RJ. Deep brain stimulation in tinnitus: current and future perspectives. Brain Res. 2015;1608:51–65. doi: 10.1016/j.brainres.2015.02.050. [DOI] [PubMed] [Google Scholar]
- Smith RL. Short-term adaptation in single auditory nerve fibers: some poststimulatory effects. J Neurophysiol. 1977;40:1098–1111. doi: 10.1152/jn.1977.40.5.1098. [DOI] [PubMed] [Google Scholar]
- Sockalingam R, Dunphy L, Nam K, Gulliver M. Effectiveness of frequency-matched masking and residual inhibition in tinnitus therapy: a preliminary study. Audiol Med. 2007;5:92–102. doi: 10.1080/16513860701362124. [DOI] [Google Scholar]
- Spalding JA. Tinnitus, with a plea for its more accurate musical notation. Archives of Otology. 1903;32:263–272. [Google Scholar]
- Terry AM, Jones DM, Davis BR, Slater R. Parametric studies of tinnitus masking and residual inhibition. Br J Audiol. 1983;17:245–256. doi: 10.3109/03005368309081485. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Tyler RS, Conrad-Armes D, Smith PA. Postmasking effects of sensorineural tinnitus: a preliminary investigation. J Speech Hear Res. 1984;27:466–474. doi: 10.1044/jshr.2703.466. [DOI] [PubMed] [Google Scholar]
- Vanneste S, De Ridder D. Noninvasive and invasive neuromodulation for the treatment of tinnitus: an overview. Neuromodulation. 2012;15:350–360. doi: 10.1111/j.1525-1403.2012.00447.x. [DOI] [PubMed] [Google Scholar]
- Vernon JA, Meikle MB. Tinnitus masking: unresolved problems. CIBA Found Symp. 1981;85:239–262. doi: 10.1002/9780470720677.ch14. [DOI] [PubMed] [Google Scholar]
- Vernon JA, Meikle MB. Tinnitus: clinical measurement. Otolaryngol Clin N Am. 2003;36:293–305. doi: 10.1016/S0030-6665(02)00162-7. [DOI] [PubMed] [Google Scholar]
- Vernon J, Schleuning A. Tinnitus: a new management. Laryngoscope. 1978;88:413–419. doi: 10.1288/00005537-197803000-00005. [DOI] [PubMed] [Google Scholar]
- Voytenko SV, Galazyuk AV. Suppression of spontaneous firing in inferior colliculus neurons during sound processing. Neuroscience. 2010;165:1490–1500. doi: 10.1016/j.neuroscience.2009.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytenko SV, Galazyuk AV. mGluRs modulate neuronal firing in the auditory midbrain. Neurosci Lett. 2011;492:145–149. doi: 10.1016/j.neulet.2011.01.075. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Young ED, Sachs MB. Representation of steady-state vowels in the temporal aspects of the discharge patterns of populations of auditory-nerve fibers. J Acoust Soc Am. 1979;66:1381–1403. doi: 10.1121/1.383532. [DOI] [PubMed] [Google Scholar]
- Zhou X, Jen PH. Duration selectivity of bat inferior collicular neurons improves with increasing pulse repetition rate. Chin J Physiol. 2006;49:46–55. [PubMed] [Google Scholar]