Abstract
Several drugs, including aminoglycosides and platinum-based chemotherapy agents, are well known for their ototoxic properties. However, FDA-approved drugs are not routinely tested for ototoxicity, so their potential to affect hearing often goes unrecognized. This issue is further compounded for natural products, where there is a lack of FDA oversight and the manufacturer is solely responsible for ensuring the safety of their products. Natural products such as herbal supplements are easily accessible and commonly used in the practice of traditional eastern and alternative medicine. Using the zebrafish lateral line, we screened a natural products library to identify potential ototoxins. We found that the flavonoids quercetin and kaempferol, both from the Gingko biloba plant, demonstrated significant ototoxicity, killing up to 30 % of lateral line hair cells. We then examined a third Ginkgo flavonoid, isorhamnetin, and found similar levels of ototoxicity. After flavonoid treatment, surviving hair cells demonstrated reduced uptake of the vital dye FM 1-43FX, suggesting that the health of the remaining hair cells was compromised. We then asked if these flavonoids enter hair cells through the mechanotransduction channel, which is the site of entry for many known ototoxins. High extracellular calcium or the quinoline derivative E6 berbamine significantly protected hair cells from flavonoid damage, implicating the transduction channel as a site of flavonoid uptake. Since known ototoxins activate cellular stress responses, we asked if reactive oxygen species were necessary for flavonoid ototoxicity. Co-treatment with the antioxidant D-methionine significantly protected hair cells from each flavonoid, suggesting that antioxidant therapy could prevent hair cell loss. How these products affect mammalian hair cells is still an open question and will be the target of future experiments. However, this research demonstrates the potential for ototoxic damage caused by unregulated herbal supplements and suggests that further supplement characterization is warranted.
Electronic supplementary material
The online version of this article (doi:10.1007/s10162-016-0604-6) contains supplementary material, which is available to authorized users.
Keywords: Zebrafish, Herbal supplements, Hearing loss, Oxidative stress
INTRODUCTIONDeafness afflicts more than 300 million people worldwide and most often results from irreversible damage to sensory hair cells (Schacht 1999; Yang et al. 2015). Hearing loss is commonly caused by acoustic over-exposure, aging, or genetic mutations, but chemical ototoxins such as aminoglycoside antibiotics or platinum-based chemotherapy agents are also significant causes of hearing impairment (Rizzi and Hirose 2007; Rybak and Ramkumar 2007; Yang et al. 2015). Seligmann et al. (1996) identified over 130 drugs and compounds that pose an ototoxic threat, including antimalarial and anti-inflammatory agents. However, ototoxicity is not part of the Food and Drug Administration (FDA) approval process, so the ototoxic potential of new drugs is generally unknown until patients report hearing difficulties. The issue of occult ototoxicity is further compounded for natural products, where there is a lack of FDA regulation and manufacturers are solely responsible for ensuring the safety of their products.
Natural products are easily accessible and commonly used in the practice of traditional eastern and alternative medicine. Most natural products are purchased over the counter and taken in pill form or used as teas, powders, tinctures, or dried plants (National Institutes of Health 2015). Over 30 % of US adults and 12 % of children use natural products, including diverse compounds such as fish oil, which can reduce inflammation associated with rheumatoid arthritis, and Echinacea purpurea, a medicinal plant purported to reduce severity of common cold symptoms (Calder 2015; Karsch-Völk et al. 2015; Nestel et al. 2015). While some research has demonstrated significant benefits of natural compound use, such as the link between Omega-3 fatty acids in fish oil and heart health, other studies find little evidence to support health claims made by manufacturers (Bent 2008; Nestel et al. 2015). Furthermore, negative side effects caused by natural compound use have yet to be thoroughly tested. Several natural compounds are under consideration as anticancer agents, and cancer patients often self-medicate with over-the-counter supplements (Dy et al. 2004; Du et al. 2010). A greater understanding of the ototoxic potential of natural compounds is important to ensure public safety of these unregulated products. Here, we use the zebrafish lateral line as a platform to identify novel ototoxins from a natural compound library.
The lateral line system in larval zebrafish provides an excellent model for rapid identification and characterization of potential ototoxins (Ou et al. 2010; Coffin et al. 2014). The lateral line contains clusters of sensory organs called neuromasts that each contain ∼10–20 hair cells and associated supporting cells. Lateral line hair cells are stimulated by water-borne vibrations near the animal and sub-serve a number of behaviors, including orientation to flow, prey detection, and predator avoidance (Coombs et al. 1989; Montgomery et al. 1997; Sampson et al. 2013). Hair cells in the lateral line are homologous to mammalian inner ear hair cells and respond similarly to known ototoxins (Harris et al. 2003; Coffin et al. 2004; Ton and Parng 2005, reviewed in Ou et al. 2010). The external location of these cells allows for easy delivery of putative ototoxins and rapid assessment of their effects (Ou et al. 2010; Coffin et al. 2014).
Prior studies have successfully used the lateral line system to identify new ototoxins. For example, Chiu et al. (2008) used a screening approach to examine the ototoxic effects of 1040 FDA-approved drugs, 21 of which appeared significantly ototoxic. Experiments with mouse utricular cultures demonstrated that these putative ototoxins damaged mammalian hair cells (Chiu et al. 2008). In a similar screen of a chemotherapy drug library, Hirose et al. (2011) identified nine ototoxic compounds; four suspected ototoxins based on case reports and five novel ototoxic anticancer drugs. Further testing revealed synergistic hair cell toxicity in five multidrug combinations that are used clinically, suggesting that characterizing ototoxins in isolation is insufficient for adequate patient protection (Hirose et al. 2011). Collectively, these studies demonstrate the utility of the larval zebrafish lateral line for ototoxin studies with large numbers of compounds, an untenable approach in traditional mammalian models.
The goal of our study was to identify putative ototoxic agents in a library of 502 diverse natural compounds. Of the nine ototoxins that emerged from our screen, we selected two, quercetin and kaempferol, as well as the related compound isorhamnetin, for further study, as all three compounds are found in the popular herbal supplement Ginkgo biloba. We then used pharmacological manipulations to understand how these ototoxins damage hair cells.
METHODS
Animals
All experiments used 5–6 days post-fertilization (dpf) zebrafish obtained from paired or group matings in the Coffin Lab fish facility at Washington State University Vancouver. We used fish from one of two genetic lines: *AB wild-type fish or Tg(pou4f3:gap43-GFP) (hereafter referred to as Brn3c/mGFP) transgenic fish, which express membrane-bound green fluorescent protein (GFP) in hair cells (Xiao et al. 2005; Namdaran et al. 2012). All experiments were conducted at 28 °C in defined E2 embryo medium (EM) containing 994 μM MgSO4, 150 μM KH2PO4, 42 μM Na2HPO4, 986 μM CaCl2, 503 μM KCl, 14.9 mM NaCl, and 714 μM NaHCO3, with the pH adjusted to 7.2 (Westerfield 2000). All experiments were approved by the Institutional Animal Care and Use Committee at Washington State University.
Ototoxicity Screen
We screened the Enzo Natural Products library (Enzo, Farmingdale, NY, USA) for compounds that cause hair cell loss in the zebrafish lateral line. This library contains 502 mostly plant-derived compounds with diverse chemical structures, including compounds commonly found in popular herbal remedies. *AB larvae were incubated in 3 μM Yo-Pro-1 (Life Technologies, Grand Island, NY, USA) for 30 min to label the hair cells. Fish were then placed in a 24-well plate (three to four fish per well) and incubated for 24 h in 4 μg/mL of one natural compound. Two wells per plate were treated with DMSO only (the vehicle for the compound library), while an additional two received 100 μM neomycin (Sigma-Aldrich, St. Louis, MO, USA) as a positive control. Neomycin was added 1 h prior to hair cell assessment. After incubation, fish were anesthetized in 0.001 % MS-222 (Argent Labs, Redmond, WA, USA) and visualized on a Leica M165FC fluorescent stereomicroscope. Fish were assessed holistically for hair cell damage on all head neuromasts and were scored on a scale of 0–4, where 0 represents a dead fish, 1 represents a live fish with no visible hair cell fluorescence, 4 represents a full complement of hair cells, and 2–3 denote intermediate levels of hair cell survival (modified from Chiu et al. 2008; Vlasits et al. 2012). Any compound that resulted in an average score of 1 or less, with minimal to no fish mortality, was then re-screened to verify ototoxic potential.
Dose-Response Analyses of Hair Cell Death
Out of the nine compounds that made it through the second round of screening, we selected two for dose-response analyses based on their consistent hair cell toxicity profile. We also tested a third natural compound that did not register as ototoxic in our initial screen but was found in herbal supplements in combination with the two screen hits selected for further study. We tested a range of concentrations of each potential ototoxin (0.5–50 μM), with the concentration range centered on the initial screen concentration (∼14 μM). Larvae (8–12 per treatment) were placed in custom fish transfer baskets, and then treated with a potential ototoxin for 24 h. Control fish were treated with vehicle only (DMSO or ethanol (EtOH), as appropriate for the target ototoxin). Fish were then rinsed in fresh EM and hair cells assessed using vital dye labeling in live, anesthetized larvae, or with cell counts in Brn3c/mGFP transgenic larvae.
For vital dye assessment, we used a DASPEI scoring protocol originally developed by Harris et al. 2003 (see also Coffin et al. 2009, 2013a). After natural compound treatment, fish were then incubated for 15 min in a 0.005 % solution of the mitochondrial dye 2-(4-(dimethylamino) styryl)-N-ethylpyridinium iodide (DASPEI, Life Technologies), which specifically labels lateral line hair cells using our treatment protocol. Fish were then rinsed twice in fresh EM, anesthetized with MS-222, and viewed on a Leica M165FC fluorescent stereomicroscope. We assessed 10 neuromasts per animal (IO1, IO2, IO3, IO4, M2, SO1, SO2, MI1, MI2, O2; see Raible and Kruse 2000), scoring each neuromast based on relative fluorescent intensity (0 = no fluorescent labeling, 1 = moderate labeling, or 2 = bright labeling) and summed the scores, resulting in a maximum score of 20 per fish (Harris et al. 2003). In larval zebrafish, neuromasts develop in stereotyped locations (Raible and Kruse 2000). Thus, if a neuromast was missing during assessment, it was most likely due to damage caused by the compound under scrutiny rather than a developmental anomaly. This method has proven highly reliable and reproducible in prior studies (Harris et al. 2003; Owens et al. 2009; Coffin et al. 2013a, b).
To further validate hair cell loss, we also performed direct hair cell counts in both Yo-Pro-1-labeled *AB larvae and in Brn3c/mGFP larvae. Following natural compound treatment, fish were anesthetized with 0.001 % MS-222, mounted on bridged coverslips, and viewed with a Leica DMRB compound fluorescent microscope. We counted hair cells within five head neuromasts per animal (IO1, IO2, IO3, M2, OP1) and summed the counts to arrive at one value per fish.
Assessment of Sub-Lethal Hair Cell Damage
We examined hair cell function using uptake of the vital dye FM 1-43, which is taken up by the mechanotransduction channel at the apical end of the hair bundle, thereby serving as a proxy for transduction channel function (Gale et al. 2001; Meyers et al. 2003). *AB fish were incubated in 50 μM of a natural compound for 24 h, and then rinsed 4× in fresh EM and incubated for 30 s in 3 μM FM 1-43FX (the fixable analog of FM 1-43, Life Technologies). Fish were rinsed 4× in fresh EM, euthanized with 0.01 % MS-222, and fixed in 4 % paraformaldehyde (PFA) overnight at 4 °C. Fish were then rinsed in phosphate-buffered saline (PBS, Life Technologies) and stored in 1:1 PBS/glycerol (Sigma). Fish were imaged on a Leica SP8 confocal with a 488-nm laser, ×20 objective, and ×5 digital zoom. Images were collected using a HyD detector with 590–680-nm detection; gain and laser power were constant for all experiments. Optical sections of the SO2 neuromast in each fish were collected (selected for ease of access) and compressed into a single maximum-point projection using the Leica LAS software. Fluorescence was quantified in ImageJ v. 1.48 (NIH, Bethesda, MD, USA) by masking the entire neuromast and calculating mean fluorescence for that neuromast, then subtracting mean background fluorescence to yield net mean fluorescence for each neuromast (Uribe et al. 2015). Fluorescence from treated neuromasts was then normalized to the average control (untreated neuromast) value for that imaging day in order to control for variations in fluorescence between sampling days.
Dye Labeling of Hair Cells
The preceding sections describe use of several different vital dyes, all of which provide complementary information about the relative health of hair cells in the lateral line. In the initial screen, the nuclear dye Yo-Pro-1 was used to identify putative ototoxins because fish could be labeled with Yo-Pro-1 prior to compound treatment, allowing for rapid assessment of compound-treated fish after the 24-h incubation period. Our screen assessment was therefore sensitive to sub-lethal damage to hair cells, particularly nuclear condensation, a hallmark of classical apoptosis, allowing us to identify compounds that caused nuclear changes without outright hair cell death (Santos et al. 2006; Owens et al. 2008). Yo-Pro-1 labeling was also used to quantify hair cells in our dose-response analyses, as the nuclear labeling allows for unambiguous identification of individual hair cell nuclei (Coffin et al. 2009). In contrast, hair cell-specific DASPEI is sensitive to changes in mitochondrial potential and relative quantification of DASPEI fluorescence on a neuromast-by-neuromast basis is a rapid means of assessing hair cell health, as reductions in DASPEI fluorescence are highly correlated with hair cell survival (Harris et al. 2003; Coffin et al. 2013a). The separate use of DASPEI and Yo-Pro-1 in our dose-response analyses allowed for independent validation of hair cell damage. The third dye, FM 1-43FX, enters hair cells via the mechanotransduction channel (Gale et al. 2001; Meyers et al. 2003). Therefore, when FM 1-43FX is administered to damaged hair cells, relative hair cell fluorescence is an indicator of transduction channel function, allowing us to further explore the condition of hair cells that were not directly killed by ototoxin treatment (Kawashima et al. 2011). In combination, the use of these vital dyes allows for a more complete assessment of hair cell status in response to a potentially toxic insult.
Natural Compound Uptake
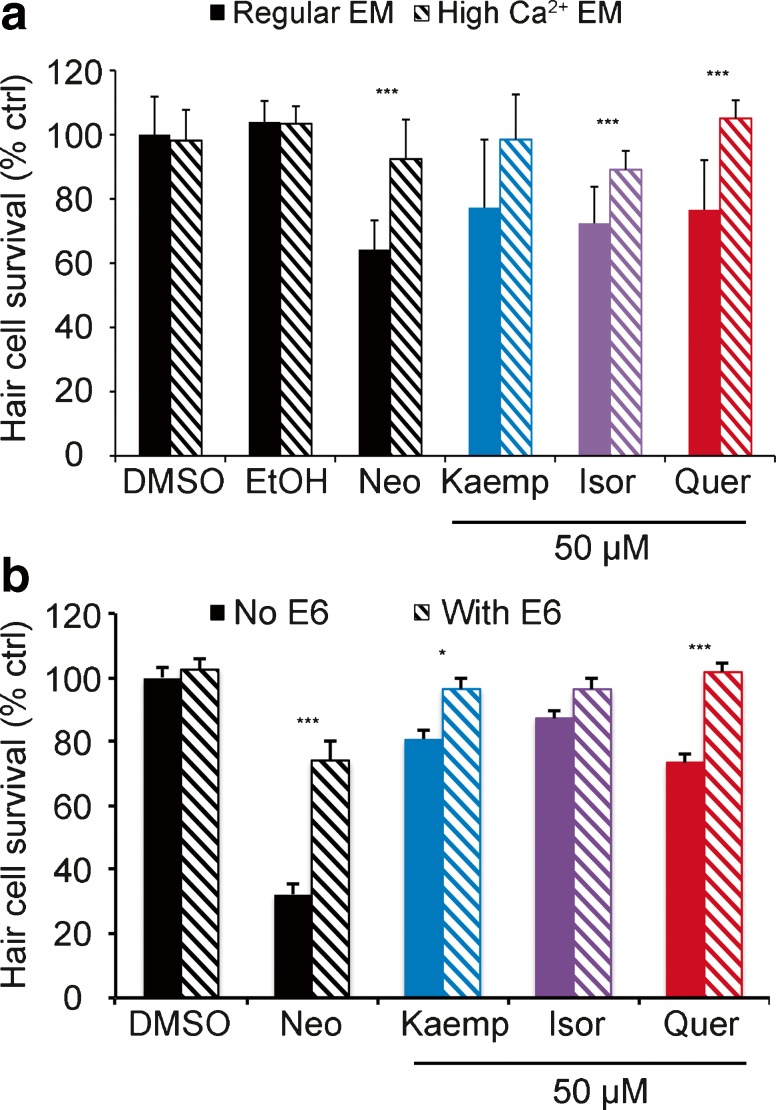
Two types of known ototoxins, aminoglycoside antibiotics and platinum-based chemotherapy agents, enter hair cells through mechanotransduction channels at the apical tip of the hair bundle (Marcotti et al. 2005; Alharazneh et al. 2011; Thomas et al. 2013). As a first step in understanding how ototoxic natural compounds damage hair cells, we used high-extracellular Ca2+ to block the transduction channel, which attenuates aminoglycoside ototoxicity in the lateral line by reducing ototoxin entry (Eatock 2000; Coffin et al. 2009). For high-calcium experiments, fish were treated for 24 h in a single natural compound in either our normal E2 EM (∼1 mM CaCl2) or in medium with 2.1 mM CaCl2 (Coffin et al. 2009). Controls were incubated in the same EM with an equal volume of the appropriate solvent, either DMSO or EtOH. Fish were then rinsed in E2 EM and hair cells were assessed with DASPEI. We also examined an additional mechanotransduction blocker, the quinoline derivative E6 berbamine. E6 attenuates aminoglycoside and FM 1-43 entry into hair cells, consistent with prior research on related quinoline-ring derivatives (Ou et al. 2009, 2012; Kruger et al. 2016). Furthermore, the parent compound berbamine attenuates mechanotransduction current in mouse cochlear hair cells, consistent with a permeant channel block (C. Kros, personal communication). Fish were incubated for 15–30 m in 500 nM E6, and then co-treated with E6 and 50 μM flavonoid for 24 h. Hair cells were assessed with DASPEI.
Cell Death Signaling
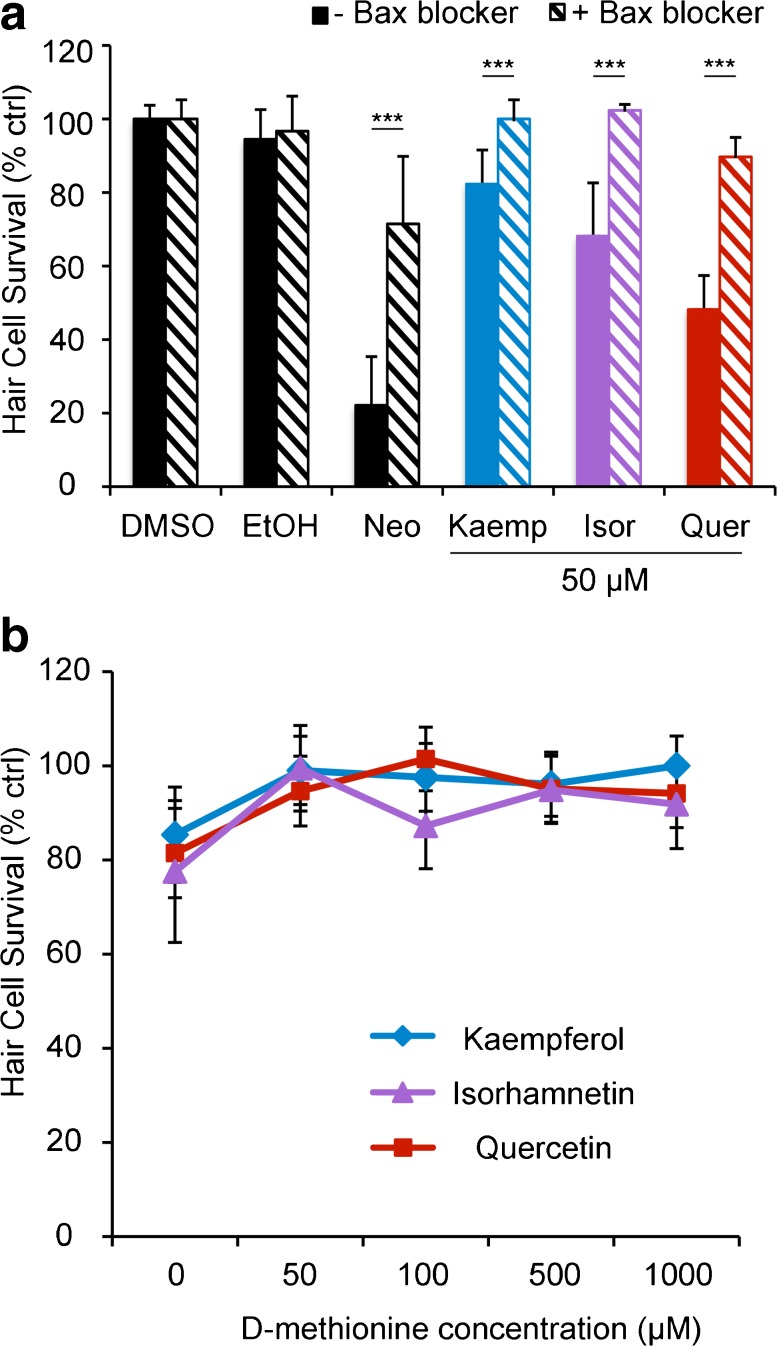
We used pharmacological manipulation of oxidative stress and intrinsic cell death pathways to examine the intracellular signaling events underlying natural compound-induced damage. Fish were treated with either the antioxidant N-acetyl-cysteine (NAC, 25–250 μM, Sigma) or D-methionine (D-Met, 50–1000 μM, Sigma), or the Bax channel blocker (±)-1-(3,6-dibromocarbazol-9-yl)-3-piperazin-1-yl-propan-2-ol, bis TFA (2.5 μM. EMD Millipore, Billerica, MA, USA; Bombrun et al. 2003; Coffin et al. 2013a). Fish were incubated for 15–30 min in one of these three cell signaling modulators, and then co-treated for 24 h in the cell signaling modulator and a natural compound (or equal volume of vehicle for controls). Hair cells were assessed with DASPEI or by direct counts in Brn3c/mGFP fish as described above. For Bax manipulation experiments, neomycin was used as a positive control (Coffin et al. 2013a).
Ototoxin Interactions
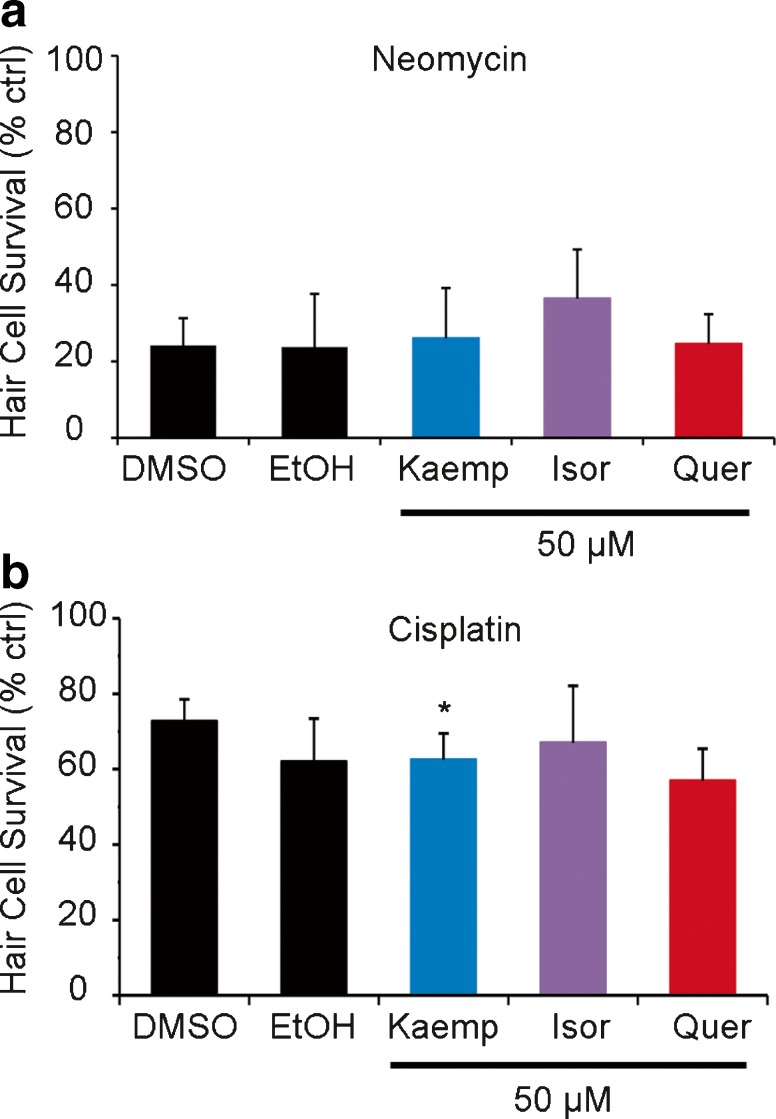
In order to determine how these newly discovered putative ototoxins interact with known hair cell toxins, we performed combinatorial experiments using the three potential ototoxins with either the aminoglycoside antibiotic neomycin or the chemotherapy agent cisplatin. For neomycin experiments, fish were incubated for 1 h in natural compound (or vehicle control), then co-treated for 30 min with natural compound and 100 μM neomycin, rinsed 4× in EM, and allowed to recover for 1 h (Coffin et al. 2009). Cisplatin experiments were conducted similarly except that fish were instead co-treated for 24 h in natural compound and 50 μM cisplatin (Washington State University veterinary pharmacy, Pullman, WA; Vlasits et al. 2012).
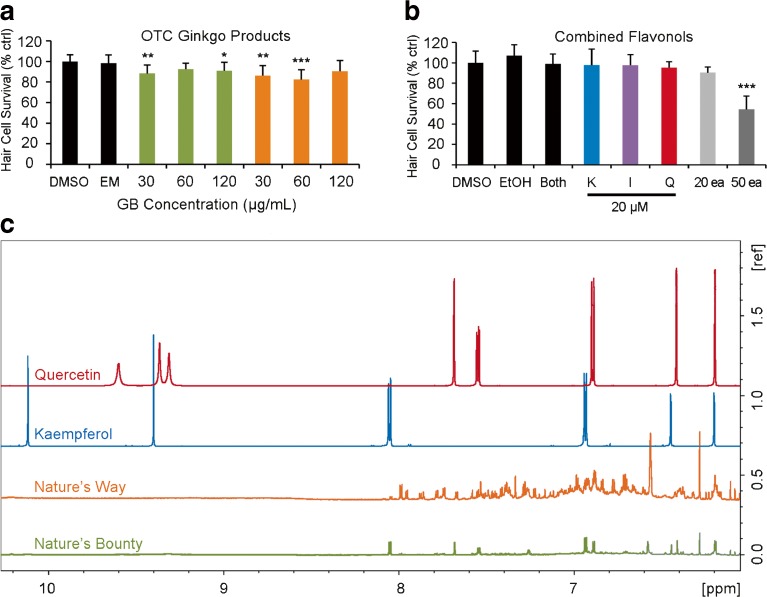
Finally, we examined the effect of natural compound combinations on hair cell survival. Fish were treated for 24 h with each natural compound singly or with all three natural compounds at variable concentrations. As all three putative ototoxins naturally co-occur in G. biloba, a popular herbal supplement, we also examined two over-the-counter G. biloba preparations for their potential to damage hair cells. Fish were treated for 24 h in a 20–120 mg/mL solution prepared from Nature’s Bounty or Nature’s Way (Amazon.com) or DMSO (vehicle control). Hair cells in all combinatorial experiments were assessed with DASPEI scoring or counts in Brn3c/mGFP fish. Chemical make-up of commercial G. biloba preparations was determined by 1H NMR spectroscopy (950 MHz) conducted at Research Triangle Institute (Research Triangle Park, North Carolina, USA).
Statistical Analysis
All data were analyzed by one- or two-way ANOVA or by pair wise comparisons using Bonferroni-corrected t tests, as appropriate, using Prism 6.0. Significance values are reported as p < 0.05. Unless specified, all data are presented as mean ± standard error.
RESULTS
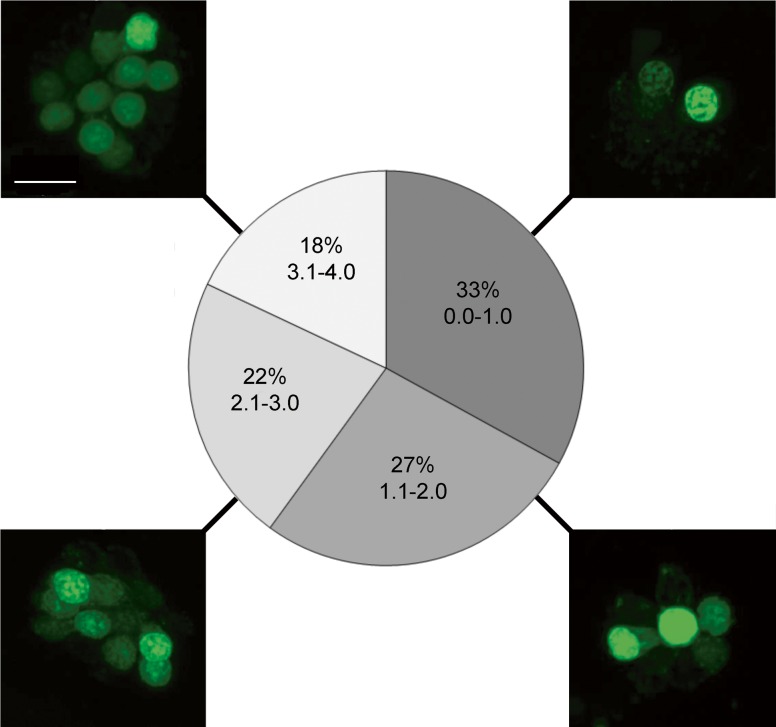
Using the zebrafish lateral line to assess ototoxicity, we screened the Enzo Screen-Well Natural Product Library for compounds that induce hair cell death. In the initial screen, 165 compounds had an average score of 1 or less out of a maximum score of 4, suggesting substantial hair cell loss or high fish mortality (Fig. 1). Upon re-screening, the 61 compounds that were not overtly toxic to the fish, we found 9 candidates that consistently induced moderate hair cell death: morin, andrographolide, sclerotiorin, cephaeline, taxifolin, wedelolactone, homobutein, quercetin, and kaempferol. Our final number dropped from 61 to 9 compounds because the screen produced several false positives, which is not unusual in a phenotypic screen (Chiu et al. 2008; Rennekamp and Peterson 2015). We selected only robust “hits” for further examination. Supplemental Table 1 lists all 61 potential hair cell toxins.
FIG. 1.
Ototoxicity screen results of the ENZO Natural Products Library. a Confocal images of a representative neuromast depicting each level of damage on a 1–4 scale, where 1 represents few to no hair cells, and 4 describes a fish with healthy neuromasts. Scale bar = 10 μm and applies to all images. b Percentage breakdown of the ototoxic potential of the 502 natural compounds. N ∼ 2300. Of the 165 compounds in the 0–1 category (darkest gray), 61 were not toxic to the fish and were re-screened in duplicate. A list of all 61 compounds is shown in Supplemental Table 1.
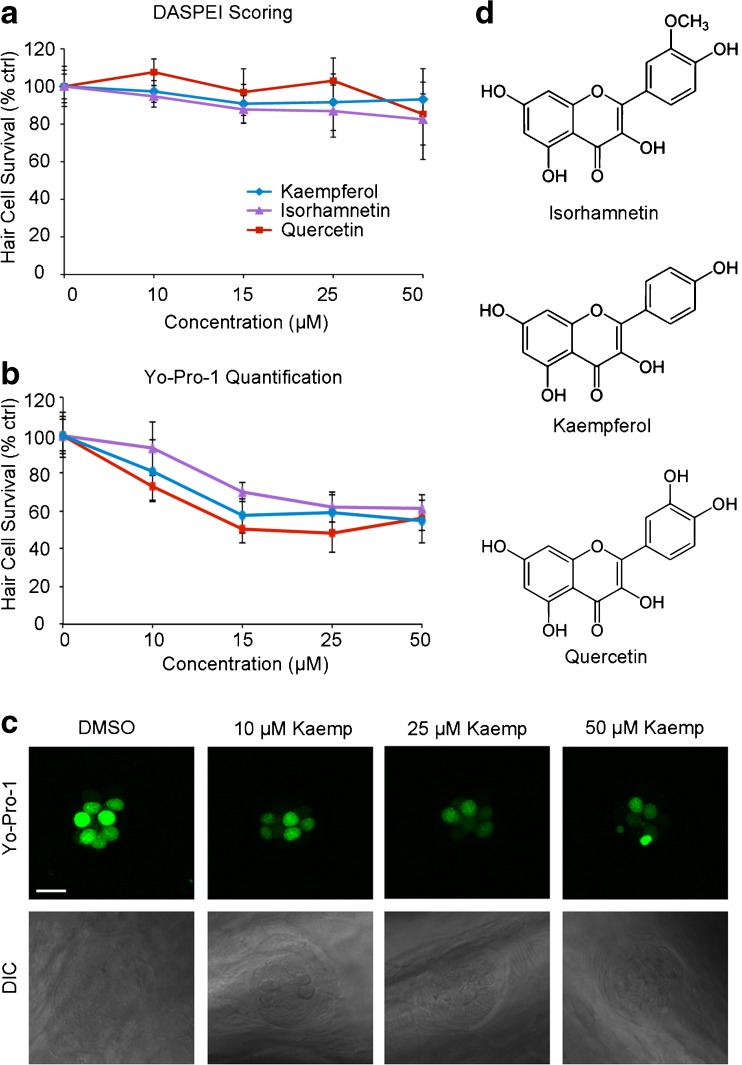
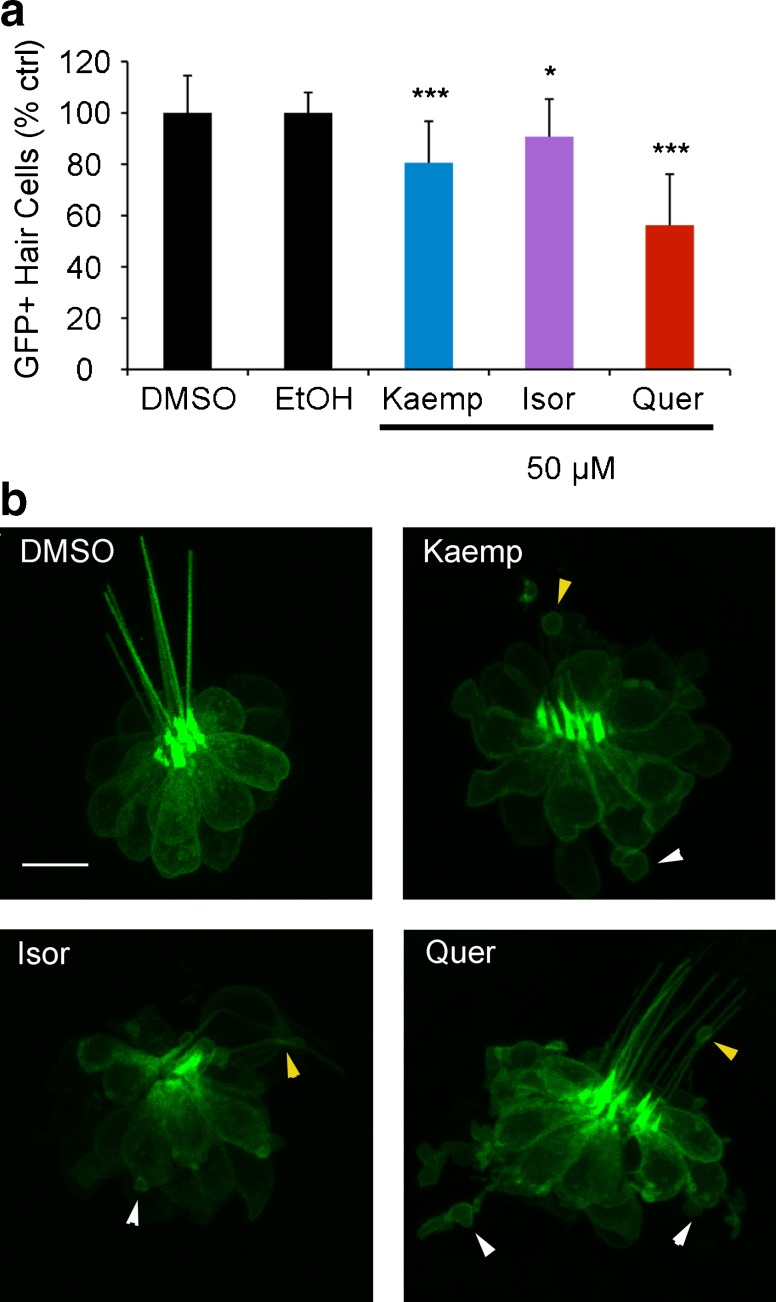
We chose to further evaluate quercetin (quer) and kaempferol (kaemp) because they are both flavonoid extracts from the G. biloba tree and consistently received a score of 1 to 2 in the re-screening process. We also included a third compound, isorhamnetin (isor), in our study because isor is the third most common Ginkgo flavonoid and shares a similar chemical structure (Fig. 2). Using DASPEI labeling, we found that 15 or 25 μM kaemp; 15, 25, and 50 μM isor; or 50 μM quer induced moderate hair cell damage (Fig. 2a). DASPEI fluorescence is dependent on mitochondrial membrane potential (Bereiter-Hahn 1976), and alterations in mitochondrial potential may not always accurately reflect hair cell death. Therefore, to validate the damage observed during DASPEI scoring, we counted hair cells in both Yo-Pro-1-labeled larvae and in transgenic Brn3c/mGFP zebrafish. Using Yo-Pro-1, we observed dose-dependent hair cell loss after 24-h treatment with each flavonoid (Fig. 2b). Similarly, we found a modest but statistically significant reduction in GFP+ hair cells following flavonoid exposure, with 50 μM of each compound causing significant hair cell loss (Fig. 3a). Remaining GFP+ hair cells showed significant signs of morphological damage, specifically membrane protrusions at both the apical and basal ends of the cell (Fig. 3b). These data demonstrate that Ginkgo flavonoids significantly damage lateral line hair cells.
FIG. 2.
Three plant flavonoids demonstrate moderate ototoxicity. These compounds were selected out of the nine compounds from the screen that moderately damaged hair cells. a DASPEI assessment of neuromast fluorescence shows that each compound modestly damages hair cells across concentrations (24-h exposure). One-way ANOVA; Isor: F4, 115 = 12.09, p < 0.0001, 15 μM*, 25 and 50 μM***; Kaemp: F4, 103 = 3.28, p = 0.014, 15 and 25 μM*; Quer: F4, 133 = 9.339, p < 0.001, 50 μM***. N = 8–31 fish per treatment. Asterisks denote significant post hoc comparisons vs. the appropriate vehicle control (*p < 0.05,**p < 0.01, ***p < 0.001). b Hair cell counts in Yo-Pro-1-labeled larvae confirm the ototoxic potential of each compound (24-h exposure). One-way ANOVA; Isor: F4, 45 = 50.85, p < 0.0001, 15, 25, and 50 μM all cause significant damage (Bonferroni-corrected post hoc test, p < 0.001). Kaemp: F4, 39 = 33.78, p < 0.0001, all concentrations significantly damage hair cells (Bonferroni-corrected post hoc test, p < 0.01). Quer: F4, 42 = 47.93, p < 0.0001, all concentrations significantly damage hair cells (Bonferroni-corrected post hoc test, p < 0.001). N = 8–11 per treatment. For both a and b, control fish were treated with DMSO (vehicle for Kaemp and Isor) or EtOH (vehicle for Quer), and data are normalized to the appropriate vehicle-only control for each flavonoid. All error bars are ±S.E.M. c Representative images of Yo-Pro-1-labeled neuromasts (top row) and DIC images of the same neuromasts (bottom row) from kaempferol-treated fish. Scale bar = 10 μm and applies to all panels. d Chemical structures of each flavonoid.
FIG. 3.
a Flavonoids cause modest hair cell loss in Brn3c/mGFP larvae. Fish were treated with 50 μM flavonoid or appropriate vehicle control and the number of GFP+ hair cells was quantified after 24 h. Asterisks denote significant comparisons (Bonferroni-corrected t tests, *p < 0.05, ***p < 0.001). N = 15–43 per treatment. b Flavonoids cause morphological damage to remaining hair cells. Confocal images (brightest-point projections) of a neuromast from a control fish treated with DMSO (the solvent for kaempferol and isorhamnetin, left panel) and a fish treated with each flavonoid. Ethanol is the solvent for quercetin, and similar to DMSO, has no effect on hair cell morphology or survival. All three compounds cause both apical and basal damage, as indicated by small pockets of cellular material traveling up the kinocillium (apical damage, yellow arrowheads) or flaking off the bottom of the neuromast (basal damage, white arrowheads). Scale bar = 10 μm and applies to all images.
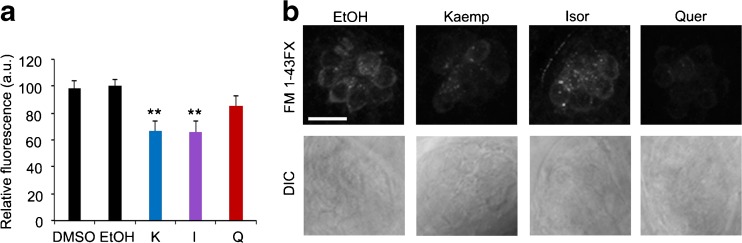
We further examined sub-lethal effects of flavonoid exposure using the fluorescent vital dye FM 1-43FX, which enters hair cells through the transduction channel and thereby serves as a proxy for transduction channel function (Gale et al. 2001; Meyers et al. 2003). Kaemp and isor significantly reduced FM 1-43FX intensity, while quer did not (Fig. 4). Collectively, these data suggest that G. biloba flavonoids induce modest hair cell loss, and that there is sub-lethal damage to the remaining cells including a possible reduction in hair cell function.
FIG. 4.
Flavonoids reduce FM 1-43FX uptake by hair cells. a Mean relative fluorescence (normalized to the appropriate vehicle-only control and quantified in arbitrary units) of all images taken for each flavonoid at 50 μM concentration (24-h exposure) demonstrates a significant reduction in fluorescent intensity (one-way ANOVA, F4, 86 = 4.331, p = 0.003). **p < 0.01. N = 20–24, bars are +S.E.M. b Representative images showing FM 1-43FX fluorescence in control (EtOH) and treated neuromasts. Top images show FM 1-43FX labeling, while bottom panels show DIC images for the same neuromast. The scale bar in the top left image is 10 μm and applies to all images. Brightness was equally increased by 60 % across all images, and the images were converted to grayscale to increase visibility for print; no changes were made to the images used for fluorescence quantification.
Cell Signaling Modulation Reduces Flavonoid-Induced Ototoxicity
We then asked if flavonoid-induced cell signaling mechanisms are similar to that of known ototoxins, specifically aminoglycoside antibiotics. Aminoglycosides enter hair cells via apically located mechanotransduction channels (Marcotti et al. 2005; Alharazneh et al. 2011; Vu et al. 2013). Calcium modulates the open probability of the transduction channel, and high extracellular calcium attenuates aminoglycoside entry and subsequent hair cell toxicity, likely by closing the channel (Eatock 2000; Coffin et al. 2009). We found that increasing the extracellular calcium concentration significantly protected hair cells from quer or isor toxicity, but not from kaemp (Fig. 5a). As expected, calcium conferred significant protection from aminoglycoside (neomycin) damage, consistent with prior reports (Coffin et al. 2009). As further validation of the calcium data, we examined the effect of E6 berbamine, a quinoline derivative that also attenuates aminoglycoside entry into hair cells, likely by blocking the transduction channel (Kruger et al. 2016; C. Kros, personal communication). E6 significantly protected hair cells from kaemp or quer damage (Fig. 5b). These data suggest that flavonoids may enter hair cells through the mechanotransduction channel, or in an alternate transduction-dependent manner, and subsequently activate intracellular signaling pathways.
FIG. 5.
Transduction channel blockers attenuate flavonoid damage. a High extracellular calcium protects hair cells from flavonoid damage. EM containing 2.1 mM Ca2+ (hashed bars) attenuated hair cell damage relative to normal EM (1 mM Ca2+, solid bars). No protection was observed against kaemp (p = 0.07). b E6 berbamine also reduced damage due to quer or kaemp. No protection was observed against isor in this experiment (p = 0.19). Only the DMSO control is shown in this experiment but the data are also representative of EtOH-only controls. In both experiments, neomycin was used as a positive control. Significant pairwise comparisons were determined with Bonferroni-corrected t tests and denoted with asterisks (*p < 0.05, ***p < 0.001). N = 7–16 per treatment, bars are +S.E.M.
We then examined potential downstream signaling molecules, again comparing signaling events between flavonoids and aminoglycoside antibiotics. In the lateral line, neomycin damage activates mitochondrial-associated death pathways and relies on the activity of Bax, a member of the Bcl2 protein family (Wei et al. 2001; Coffin et al. 2013a). We exposed hair cells to a Bax inhibitor for 1 h before co-treating the cells with a flavonoid for 24 h and found that Bax inhibition significantly protected hair cells from all three flavonoids, as well as from neomycin (the positive control).
Aminoglycoside exposure is also associated with an increase in reactive oxygen species (ROS), and antioxidants confer some protection from aminoglycoside damage (e.g., Evans and Halliwell 1999; Sergi et al. 2004). We found that the antioxidant D-methionine significantly attenuated flavonoid-induced hair cell damage in a dose-dependent manner, and similar results were observed with N-acetyl-cysteine, a second antioxidant (Fig. 6b and data not shown). These data suggest that our flavonoids likely increase production of ROS in hair cells.
FIG. 6.
Cell signaling modulators attenuate flavonoid ototoxicity. a A Bax channel blocker reduced flavonoid-induced hair cell death. Fish were pre-treated with 2.5 μM Bax blocker, then co-treated with Bax blocker and 50 μM flavonoid for 24 h. Neomycin was used as a positive control. Significance values (determined by Bonferroni-corrected t tests) are denoted by asterisks (***p < 0.001). b Hair cells exposed to D-methionine were significantly less damaged than were those without the antioxidant treatment. D-met significantly protects hair cells from all three flavonoids (one-way ANOVA, isor: F 4, 55 = 7.89, p < 0.0001, significant protection is seen with 50, 500, and 1000 μM; kaemp: F 4, 55 = 7.14, p = 0.0001, significant protection at all concentrations; quer: F 4, 55 = 10.84, p < 0.0001, significant protection at all concentrations). N = 12 and bars are +S.E.M.
Flavonoid Interactions with Known Ototoxins
Given that aminoglycosides and flavonoids appear to damage hair cells using similar intracellular mechanisms, we next asked how the two interact at the level of hair cell damage. None of these flavonoids significantly modulated neomycin-induced hair cell loss (Fig. 7a). We also examined the interaction of flavonoids with cisplatin, an ototoxic chemotherapy agent, as some flavonoids (e.g., quercetin) have been suggested as anticancer drugs in multi-drug chemotherapy regimens (Chen and Chen 2013; Zhang et al. 2015). Flavonoid treatment did not substantially alter cisplatin-induced hair cell damage. Overall, these data suggest that G. biloba flavonoids do not influence ototoxicity of neomycin or cisplatin.
FIG. 7.
Flavonoids do not substantially modulate damage from known ototoxins. a No flavonoid significantly influenced the way hair cells respond to neomycin. b All groups were exposed to cisplatin with the indicated flavonol or vehicle control (DMSO for kaemp and isor, EtOH for quer). Kaemp significantly increased cisplatin-induced ototoxicity (Bonferroni-corrected t test, p = 0.02). N = 10 (for both a and b) and bars are +S.E.M.
Over-The-Counter Ginkgo Formulas Do Not Substantially Damage Hair Cells
Finally, we asked if over-the-counter (OTC) G. biloba formulas show ototoxic properties. We examined two popular G. biloba preparations, Nature’s Way and Nature’s Bounty. Our results indicate that the 30 and 120 mg/mL Nature’s Bounty and the 30 and 60 mg/mL of Nature’s Way formulas are all significantly ototoxic (Fig. 8a). However, these effects are quite modest and may not be biologically meaningful. We also combined the pure flavonoid extracts to simulate an OTC formula and found that only the maximum amount (50 μM each) of combined flavonoids caused significant damage (Fig. 8b). Given that OTC natural compounds are not regulated and ingredients are therefore not standardized, we then asked if these OTC G. biloba preparations contained our chosen flavonoids. NMR spectroscopy demonstrates that the Nature’s Bounty formula contains signatures of quercetin and kaempferol, while these chemical signatures could not be confirmed in the Nature’s Way preparation because of overlapping constituents at higher levels (Fig. 8c). The NMR peaks downfield from 9 ppm represent hydroxyl groups present in the flavonoids as demonstrated by the spectra of standards. These NMR peaks are not clearly visible (with the same intensities) in the OTCs perhaps due to the chemical heterogeneities in the Ginkgo supplements or due to any reactions of these hydroxyl groups during the process of manufacturing.
FIG. 8.
a Some over-the-counter (OTC) formulas of Ginkgo biloba have ototoxic potential. Certain concentrations were significantly ototoxic (one-way ANOVA, Nature’s Bounty (green): F 3, 40 = 4.824, p = 0.006; Nature’s Way (orange): F 3, 39 = 7.013, p < 0.001). b The combination of purified flavonoids was significantly ototoxic (one-way ANOVA, F 7, 68 = 17.49, p < 0.0001). Fish were treated with a combined solution of 20 μM flavonoid (20 μM kaemp, isor, and quer) or a combined solution containing 50 μM of each flavonoid. Pairwise comparisons show that significant hair cell loss was only seen with 50 μM of each flavonoid combined in one treatment. “Both” indicates a combination of DMSO and EtOH as the combined vehicle control. a, b Significance values are denoted by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). N = 10 fish per group and all bars are +S.E.M. c 1H NMR spectra (950 MHz) of both OTC formulas of G. biloba as compared to quercetin or kaempferol standard compounds. Nature’s Bounty appears to contain both flavonoids, while the flavonoid composition of the Nature’s Way formulation could not be confirmed due to the overlap of NMR signals of other constituents that were present at higher levels. N = 32 experiments for each sample.
DISCUSSION
The purpose of this investigation was to identify potentially ototoxic compounds from a broad range of natural products that are not FDA-regulated nor tested for ototoxicity. Of the 502 compounds initially assessed, 33 % appeared toxic to zebrafish hair cells, or to the fish as a whole. Our initial screening protocol relied on overall fluorescent intensity of hair cells across the fish, which is a common but cursory means for assessing hair cell survival (e.g., Owens et al. 2008; Chiu et al. 2008; Vlasits et al. 2012). Screens are a powerful method for identifying compounds that modulate a desired phenotype but prone to false positives, as the goal is to maximize the chances of observing the phenotype of interest (Chiu et al. 2008; Rennekamp and Peterson 2015). We re-screened all initial “hits” in duplicate to validate our findings, and then used additional methods to quantify damage caused by kaempferol and quercetin. These compounds are rich in many food sources, including raspberries and onions, several medicinal herbs used in traditional Chinese medicine, and G. biloba, a popular herb touted for its purported memory-boosting effects and tinnitus relief (Donnapee et al. 2014; Fu et al. 2015; Heinonen and Gaus 2015). We also examined a third Ginkgo flavonoid, isorhamnetin, which did not meet our initial criteria for re-testing (original score of 2) but has a similar chemical structure to quer and kaemp. We found that each flavonoid was modestly ototoxic and that greater hair cell damage was observed following combined treatment with all three flavonoids. In contrast, OTC formulations of G. biloba did not show substantial ototoxicity. OTC formulations may contain much lower concentrations of flavonoids than what we tested individually (24 % by weight, Drieu 1986). Given the lack of regulation, it is difficult to determine the exact chemical distribution in many OTC preparations. Our 1H NMR data did not conclusively demonstrate flavonoid presence in the Nature’s Way formula, suggesting that these flavonoids may not be present in all OTC Ginkgo compounds. It was difficult to confirm the presence of quer or kaemp in Nature’s Way due to other constituents present in the same region, complicating the spectrum. Some herbal preparations contain additional botanicals or synthetic bioactives that are not reported on the label, complicating “medicinal” use of these compounds (e.g., Jiang et al. 2011; Campbell et al. 2013; Heinonen and Gaus 2015). A recent DNA barcoding study demonstrated that 9 of 40 OTC Ginkgo preparations marketed in the USA did not contain Ginkgo DNA (Little 2014). However, our data clearly demonstrate that purified Ginkgo flavonoids, and possibly complete Ginkgo extracts, have ototoxic potential.
We observed moderate hair cell damage following 24-h treatment with each flavonoid. These flavonoids caused a modest decrease in DASPEI fluorescence and a reduction in the number of Yo-Pro-1-labeled nuclei, suggesting a loss of mitochondrial membrane potential and decrease in hair cell viability. These data are corroborated by our observation of morphological damage, including flaking of the cell body and kinocilial blebbing, both signs of programmed cell death (Dinh et al. 2015). We also noted a significant reduction in FM 1-43FX uptake in kaemp- or isor-treated hair cells, suggesting reduced transduction channel function or potential hair bundle damage (Gale et al. 2001; Meyers et al. 2003). While each flavonoid exhibited a unique damage profile, our collective data demonstrate that these flavonoids reduce hair cell viability.
We then compared flavonoid-induced hair cell damage to damage induced by well-studied ototoxins, specifically aminoglycoside antibiotics. Aminoglycosides enter hair cells via the mechanotransduction channel, and inhibiting drug entry is sufficient to confer protection (Ricci and Fettiplace 1998; Marcotti et al. 2005; Wang and Steyger 2009; Alharazneh et al. 2011). We found that high extracellular calcium, which attenuates aminoglycoside entry into hair cells via modulation of transduction channel opening, was sufficient to reduce flavonoid ototoxicity. Similar protection was observed with the quinoline derivative E6 berbamine, another transduction channel modulator that also protects hair cells from aminoglycoside damage (Kruger et al. 2016). These data suggest that flavonoids may also enter hair cells via the transduction channel and go on to affect intracellular processes. The transduction channel is a non-specific cation channel that allows passage of several large molecules, including the aminoglycoside neomycin (614 g/mol) and the vital dye FM 1-43FX (611 g/mol). With molecular weights from 286 to 316 g/mol, Ginkgo flavonoids are considerably smaller than are other molecules that pass through the transduction channel. However, we cannot exclude alternative explanations, particularly for our calcium data; it is possible that flavonoids damage hair cells in a calcium-dependent manner that is independent of the transduction channel (Esterberg et al. 2013, 2014).
We then asked if aminoglycosides and flavonoids activate similar intracellular signaling mechanisms. Bcl-2 protein family members modulate hair cell death from a variety of toxins in rodent models, and Bax inhibition prevents neomycin ototoxicity in the zebrafish lateral line (Cunningham et al. 2004; Vicente-Torres and Schacht 2006; Yamashita et al. 2008; Pfannenstiel et al. 2009; Coffin et al. 2013a). Pharmacological inhibition of Bax conferred substantial protection from each flavonoid, demonstrating a shared mechanism of action with neomycin-induced damage. These data are also consistent with prior studies demonstrating flavonoid-induced Bax up-regulation in cancer cells (Duo et al. 2012; Dai et al. 2015; Zhang et al. 2015). In flavonoid-treated cancer cells, Bax expression is linked to increased ROS generation, and quer and kaemp increase ROS in multiple cell lines (Sahu and Gray 1994; Metodiewa et al. 1999; Zhang et al. 2015). Several lines of evidence demonstrate increased ROS generation in aminoglycoside-damaged hair cells, and antioxidants confer a protective benefit in multiple animal models of aminoglycoside ototoxicity (Schacht 1999; Lee et al. 2004; Coffin et al. 2013b). The antioxidants D-methionine and NAC significantly prevented flavonoid-induced hair cell damage. Collectively, these data suggest that flavonoids and aminoglycosides may activate similar cell death signaling pathways, and that flavonoid damage may be consistent across cell types. Additional research is warranted to determine the full suite of signaling pathways in flavonoid-damaged hair cells, including experiments using ROS indicators to further understand the timing and magnitude of oxidative stress.
G. biloba and its major constituents have diverse effects on cells. Discovery of the pro-oxidant properties of Ginkgo flavonoids prompted research on the antineoplastic potential of these compounds (Chirumbolo 2013). For example, quer may exert pro-apoptotic effects on mammalian tumor cells by a variety of mechanisms, including inhibition of cell survival pathways and modulation of the tumor-suppressor p53 (Kim and Lee 2007; Siegelin et al. 2009; Vargas et al. 2011). Ginkgo flavonoids have also been proposed as part of multi-drug chemotherapy regimens (Teng et al. 2006; Luo et al. 2010; Chen and Chen 2013). For example, Luo et al. (2010) demonstrated that kaemp sensitizes ovarian cancer cells to cisplatin. We therefore examined the combined ototoxic potential of cisplatin and our Ginkgo flavonoids and found little to no synergistic effect on lateral line hair cells. These findings conflict with a study by Lee et al. (2015), who concluded that our highest concentration of quer, 50 μM, reduced cisplatin-induced hair cell damage in zebrafish embryos. The Lee et al. study used 4 h of quer and cisplatin co-treatment, quite different from the 24-h treatment used here, and also used a cisplatin dose 20 times higher than that in our study. We think that our prolonged, low-dose treatment more closely mimics clinical scenarios, but additional work is needed to resolve these differences.
Conflicting reports pepper the robust literature on Ginkgo flavonoids. Some studies report that the antioxidant properties of quer or isor protect cardiomyocytes from a variety of stressors, while a recent study demonstrates adverse effects of quer on mouse cardiomyocyte function, likely by increasing oxidative stress (Sun et al. 2013; Ruiz et al. 2015; Li et al. 2016). Similarly, recent work by Sagit et al. (2015) and Yang et al. (2011) found that quer attenuated gentamicin ototoxicity in rats and guinea pigs, while Miman et al. (2002) showed that Ginkgo enhanced amikacin ototoxicity. By contrast, we did not find significant modulation of neomycin damage with any of our flavonoids. Whether this discrepancy is due to a difference in animal model or ototoxin is an open question, as different aminoglycosides can activate different hair cell death mechanisms (Owens et al. 2009; Coffin et al. 2013a, b). Neither the Sagit et al. or Yang et al. studies report ototoxicity of Ginkgo flavonoids alone. However, these studies primarily rely on changes in ABR thresholds, which may not be sensitive to modest hair cell damage. Confounding reports of potential ototoxicity or otoprotection have been demonstrated for both statin drugs and estrogens, suggesting that hair cells may show diverse responses to many chemical modulators (Borghi et al. 2002; Hultcrantz et al. 2006; Chiu et al. 2008).
Our findings confront the notion that natural compounds are risk averse by virtue of their organic nature. Many of these natural compounds go on to form the basis of drugs used in clinical treatments, some in combination with other possible ototoxins. Our study demonstrates flavonoid ototoxicity in the zebrafish lateral line; whether these compounds damage mammalian hair cells remains an open question. Our flavonoid concentrations are likely higher than are serum concentrations in human patients. One clinical study examined serum metabolites in adults treated with quercetin supplements and found metabolite concentrations up to 2 μM. Our zebrafish model in some ways resembles an in vitro system, and in vitro studies in cancer cells use flavonoid concentrations in line with our experiments (Cialdella-Kam et al. 2012). How these concentrations translate to human studies is not fully characterized. We could not find published case reports of an association between G. biloba or its constituents and hearing loss, but given the modest hair cell damage we observed, it is unlikely that Ginkgo consumption would be linked with hearing loss based on observational studies. Clinical trials with Ginkgo preparations focus largely on dementia (Gautheir and Schlaefke 2014; Solfrizzi and Panza 2015), and these studies often occur in elderly populations where mild hearing loss would likely be attributed to age-related causes. One clinical trial (NCT01139281) examines Ginkgo as a potential otoprotectant from cisplatin-induced hearing loss, but trial results are not yet available.
The zebrafish lateral line is an attractive model for ototoxicity research because lateral line hair cells respond similarly to known ototoxins, and this system was previously used to identify occult ototoxins from multiple drug libraries—ototoxins that also showed toxicity in mammalian systems (Chiu et al. 2008; Hirose et al. 2011). We therefore think it likely that these flavonoids could induce damage in mammalian hair cells if the compound reaches the inner ear in sufficient quantities. Orally administered Ginkgo leads to detectable flavonoid concentrations in the brain, demonstrating that the blood-brain barrier is permeable to Ginkgo constituents (Rangel-Ordóñez et al. 2010). Occasional oral administration of G. biloba would likely cause little ototoxicity, as plasma concentrations are generally low in rat studies (176–341 ng/mL after a single administration; Rangel-Ordóñez et al. 2010). However, chemotherapy studies have proposed intravenous administration of G. biloba or isolated flavonoids, which would greatly increase circulating flavonoid levels (Hauns et al. 2001; Jones et al. 2004). Future studies are needed to determine if Ginkgo flavonoids induce mild toxicity to mammalian inner ear hair cells, and if the ototoxic potential outweighs the potential benefit of G. biloba for other conditions, such as to prevent cognitive deficits in dementia patients (Herrschaft et al. 2012; von Gunten et al. 2015).
Electronic supplementary material
(XLSX 13 kb)
Acknowledgements
This research was supported by the National Institutes of Health award R15DC013900 and Washington State University Vancouver funds to A.B.C. We thank Matthew Kruger, Travis Long, and Heather Wiedenhoft for experimental assistance and Chris Riso and Alex Young for fish husbandry expertise. We also thank two anonymous reviewers for the comments that significantly improved the manuscript.
Footnotes
Sarah Neveux and Nicole K. Smith contributed equally to this work.
Contributor Information
Sarah Neveux, Email: sarahneveux@gmail.com.
Nicole K. Smith, Email: nickikalani@gmail.com
Anna Roche, Email: annasroche@gmail.com.
Bruce E. Blough, Email: beb@rti.org
Wimal Pathmasiri, Email: wpathmasiri@rti.org.
Allison B. Coffin, Phone: +1-360-546-9748, Email: Allison.coffin@wsu.edu
References
- Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, Ricci AJ. Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One. 2011;6(7):e22347. doi: 10.1371/journal.pone.0022347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent S. Herbal medicine in the United States: review of efficacy, safety, and regulation. J of Gen Intern Med. 2008;23:854–859. doi: 10.1007/s11606-008-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter-Hahn J. Dimethylaminostyrylmethylpyridiniumiodine (DASPMI) as a fluorescent probe for mitochondria in situ. Biochim Biophys Acta. 1976;423:1–14. doi: 10.1016/0005-2728(76)90096-7. [DOI] [PubMed] [Google Scholar]
- Bombrun A, Gerber P, Casi G, Terradillos O, Antonsson B, Halazy S. 3,6-dibromocarbazole piperazine derivatives of 2-propanol as first inhibitors of cytochrome c release via Bax channel modulation. J of Medicinal Chem. 2003;46:4365–4368. doi: 10.1021/jm034107j. [DOI] [PubMed] [Google Scholar]
- Borghi C, Modugno G, Pirodda A. Possible role of HMG-CoA reductase inhibitors for the treatment of sudden sensorineural hearing loss (SSHL) Med Hypotheses. 2002;58:399–402. doi: 10.1054/mehy.2001.1535. [DOI] [PubMed] [Google Scholar]
- Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1854:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Campbell N, Clark JP, Stecher VJ, Thomas JW, Callanan AC, Donnelly BF, Goldstein I, Kaminetsky JC. Adulteration of purported herbal and natural sexual performance enhancement dietary supplements with synthetic phosphodiesterase type 5 inhibitors. J Sex Med. 2013;10:1842–1849. doi: 10.1111/jsm.12172. [DOI] [PubMed] [Google Scholar]
- Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirumbolo S. Quercetin and cancer prevention and therapy. Integr Cancer Ther. 2013;12(2):97–102. doi: 10.1177/1534735412448215. [DOI] [PubMed] [Google Scholar]
- Chiu LL, Cunningham LL, Raible DW, Rubel EW, Ou HC. Using the zebrafish lateral line to screen for ototoxicity. J Assoc Res Otolaryngol. 2008;9:178–190. doi: 10.1007/s10162-008-0118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdella-Kam L, Nieman DC, Sha W, Meaney MP, Knab AM, Shanely RA. Dose-response to 3 months of quercetin-containing supplements on metabolite and quercetin conjugate profile in adults. Br J Nutr. 2012;109(11):1923–1933. doi: 10.1017/S0007114512003972. [DOI] [PubMed] [Google Scholar]
- Coffin AB, Brignull H, Raible DW, Rubel EW. Hearing loss, protection, and regeneration in the larval zebrafish lateral line. In: Coombs S, Bleckmann H, Fay RR, Popper AN, editors. The lateral line system. New York: Springer; 2014. pp. 313–347. [Google Scholar]
- Coffin AB, Reinhart K, Owens K, Raible D, Rubel E. Extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Hear Res. 2009;253:42–51. doi: 10.1016/j.heares.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Rubel EW, Raible DW. Bax, Bcl2, and p53 differentially regulate neomycin- and gentamicin-induced hair cell death in the zebrafish lateral line. J Assoc Res Otolaryngol. 2013;14:645–659. doi: 10.1007/s10162-013-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Williamson KL, Mamiya A, Raible DW, Rubel EW. Profiling drug induced cell death pathways in the zebrafish lateral line. Apoptosis. 2013;18:393–408. doi: 10.1007/s10495-013-0816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Kelley M, Manley GA, Popper AN. Evolution of sensory hair cells. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the vertebrate auditory system. New York: Springer; 2004. pp. 55–94. [Google Scholar]
- Coombs S, Görner P, Münz H. The mechanosensory lateral line. New York: Springer; 1989. [Google Scholar]
- Cunningham LL, Matsui JI, Warchol ME, Rubel EW. Overexpression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. J of Neurobio. 2004;60:89–100. doi: 10.1002/neu.20006. [DOI] [PubMed] [Google Scholar]
- Dai W, Gao Q, Qiu J, Yuan J, Wu G, Shen G. Quercetin induces apoptosis and enhances 5-FU therapeutic efficacy in hepatocellular carcinoma. Tumour Biol. 2015;1:1–7. doi: 10.1007/s13277-015-4501-0. [DOI] [PubMed] [Google Scholar]
- Dinh C, Goncalves S, Bas E, Water T, Zine A. Molecular regulation of auditory hair cell death and approaches to protect sensory receptor cells and/or stimulate repair following acoustic trauma. Front in Cell Neuro. 2015;9:96. doi: 10.3389/fncel.2015.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnapee S, Li J, Yang X, Ge AH, Donkor PO, Gao XM, Chang YX. Cuscuta chinensis Lam.: a systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. J Ethnopharmacol. 2014;157:292–308. doi: 10.1016/j.jep.2014.09.032. [DOI] [PubMed] [Google Scholar]
- Drieu K. Preparation and definition of Ginkgo biloba extract. Presse Med. 1986;15:1455–1457. [PubMed] [Google Scholar]
- Du G, Lin H, Yang Y, Zhang S, Wu X, Wang M, Han G. Dietary quercetin combining intratumoral doxorubicin injection synergistically induces rejection of established breast cancer in mice. Intl Immunopharmacol. 2010;10:819–826. doi: 10.1016/j.intimp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Duo J, Ying GG, Wang GW, Zhang L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol Med Reports. 2012;5:1453–1456. doi: 10.3892/mmr.2012.845. [DOI] [PubMed] [Google Scholar]
- Dy GK, Bekele L, Hanson LJ, Furth A, Mandrekar S, Sloan JA, Adjei AA. Complementary and alternative medicine use by patients enrolled onto phase I clinical trials. J of Clin Oncol. 2004;22:4810–4815. doi: 10.1200/JCO.2004.03.121. [DOI] [PubMed] [Google Scholar]
- Eatock RA. Adaptation in hair cells. Ann Rev Neuro. 2000;23:285–314. doi: 10.1146/annurev.neuro.23.1.285. [DOI] [PubMed] [Google Scholar]
- Esterberg R, Hailey DW, Coffin AB, Raible DW, Rubel EW. Disruption of intracellular calcium regulation is integral to aminoglycoside-induced hair cell death. J Neurosci. 2013;33(17):7513–7525. doi: 10.1523/JNEUROSCI.4559-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg R, Hailey DW, Rubel EW, Raible DW. ER-mitochondrial calcium flow underlies vulnerability of mechanosensory hair cells to damage. J Neurosci. 2014;32(29):9703–9719. doi: 10.1523/JNEUROSCI.0281-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P, Halliwell B. Free radicals and hearing: cause, consequence, and criteria. Annals NY Acad of Sci. 1999;884:19–40. doi: 10.1111/j.1749-6632.1999.tb08633.x. [DOI] [PubMed] [Google Scholar]
- Fu R, Zhang Y, Peng T, Guo Y, Chen F. Phenolic composition and effects on allergic contact dermatitis of phenolic extracts Sapium sebiferum (L.) Roxb. leaves. J Ethnopharmacol. 2015;162:176–180. doi: 10.1016/j.jep.2014.12.072. [DOI] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautheir S, Schlaefke S. Efficacy and tolerability of Ginkgo biloba extract EGb 761 in dementia: a systematic review and meta-analysis of randomized placebo-controlled trials. Clin Interv Aging. 2014;9:2065–2077. doi: 10.2147/CIA.S72728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2003;4:219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauns B, Häring B, Köhler S, Mross K, Unger C. Phase II study of combined 5-fluorouracil/Ginkgo biloba extract (GBE 761 ONC) therapy in 5-fluorouracil pretreated patients with advanced colorectal cancer. Phytother Res. 2001;15(1):34–38. doi: 10.1002/1099-1573(200102)15:1<34::AID-PTR755>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Heinonen T, Gaus W. Cross matching observations on toxicological and clinical data for the assessment of tolerability and safety of Ginkgo biloba leaf extract. Toxicology. 2015;327:95–115. doi: 10.1016/j.tox.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Herrschaft H, Nacu A, Likhachev S, Sholomov I, Hoerr R, Schlaefke S. Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: a randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J Psychiatr Res. 2012;46:716–723. doi: 10.1016/j.jpsychires.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Hirose Y, Simon JA, Ou HC. Hair cell toxicity in anti-cancer drugs: evaluating an anti-cancer drug library for independent and synergistic toxic effects on hair cells using the zebrafish lateral line. J Assoc Res Otolaryngol. 2011;12:719–728. doi: 10.1007/s10162-011-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultcrantz M, Simonoska R, Stenberg A. Estrogen and hearing: a summary of recent investigations. Acta Oaot-laryngol. 2006;126:10–14. doi: 10.1080/00016480510038617. [DOI] [PubMed] [Google Scholar]
- Jiang B, Ma C, Motley T, Kronenberg F, Kennelly EJ. Phytochemical fingerprinting to thwart black cohosh adulteration: a 15 Actaea species analysis. Phytochem Anal. 2011;22:339–351. doi: 10.1002/pca.1285. [DOI] [PubMed] [Google Scholar]
- Jones DJ, Lamb JH, Verschoyle RD, Howells LM, Butterworth M, Lim CK, Ferry D, Farmer PB, Gescher AJ. Characterization of metabolites of the putative cancer chemopreventive agent quercetin and their effect on cyclo-oxygenase activity. Br J Cancer. 2004;91(6):121301219. doi: 10.1038/sj.bjc.6602091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsch-Völk M, Barrett B, Linde K. Echinacea for preventing and treating the common cold. JAMA. 2015;313:618–619. doi: 10.1001/jama.2014.17145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Géléoc GSG, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, Griffith AJ. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest. 2011;121:4796–4809. doi: 10.1172/JCI60405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Lee YJ. TRAIL apoptosis is enhanced by quercetin rhough Aky dephosphorylation. J Cell Biochem. 2007;100(4):998–1009. doi: 10.1002/jcb.21098. [DOI] [PubMed] [Google Scholar]
- Kruger M, Boney R, Ordoobadi AJ, Sommers TF, Trapani JG, Coffin AB. Natural bizbenzoquinoline derivatives protect zebrafish lateral line sensory hair cells from aminoglycoside toxicity. Frontiers Cell Neurosci. 2016 doi: 10.3389/fncel.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Nakagawa T, Kim TS, Iguchi F, Endo T, Kita T, Ito J. Signaling pathway for apoptosis of vestibular hair cells of mice due to aminoglycosides. Acta Otolaryngol. 2004;124:69–74. doi: 10.1080/03655230310016799. [DOI] [PubMed] [Google Scholar]
- Lee SK, Oh KH, Chung AY, Park HC, Lee SH, Kwon SY, Choi J. Protective role of quercetin against cisplatin-induced hair cell damage in zebrafish embryos. Human & Experi Toxicol. 2015;34:1043–1052. doi: 10.1177/0960327114567766. [DOI] [PubMed] [Google Scholar]
- Li C, Wang T, Zhang C, Xuan J, Su C, Wang Y. Quercetin attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Gene. 2016;577(2):275–280. doi: 10.1016/j.gene.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Little DP. Authentication of Ginkgo biloba herbal dietary supplements using DNA barcoding. Genome. 2014;57:513–516. doi: 10.1139/gen-2014-0130. [DOI] [PubMed] [Google Scholar]
- Luo H, Daddysman MK, Rankin GO, Jiang B-H, Chen YC. Kaempferol enhances cisplatin’s effect on ovarian cancer cells through promoting apoptosis caused by down regulation of cMyc. Cancer Cell Intl. 2010;10:16. doi: 10.1186/1475-2867-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Netten S, Kros C. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol. 2005;567:505–521. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metodiewa D, Jaiswal AK, Cenas N, Dickancaité E, Segura-Aguilar J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radical Bioand Med. 1999;26:107–116. doi: 10.1016/S0891-5849(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miman MC, Ozturn O, Iraz M, Erdem T, Olmez E. Amikacin ototoxicity enhanced by Ginkgo biloba extract (EGb 761) Hear Res. 2002;169:121–129. doi: 10.1016/S0378-5955(02)00385-4. [DOI] [PubMed] [Google Scholar]
- Montgomery JC, Baker CF, Carton AG. The lateral line can mediate rheotaxis in fish. Nature. 1997;390:960–963. doi: 10.1038/40135. [DOI] [Google Scholar]
- Namdaran P, Reinhart KE, Owens KN, Raible DW, Rubel EW. Identification of modulators of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2012;32:3516–3528. doi: 10.1523/JNEUROSCI.3905-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Health (2015) Medline Plus: Herbal Medicine. Retrieved from: http://www.nlm.nih.gov/medlineplus/herbalmedicine.html
- Nestel P, Clifton P, Colquhoun D, Noakes M, Mori TA, Sullivan D, Thomas B. Indications for Omega-3 long chain polyunsaturated fatty acid in the prevention and treatment of cardiovascular disease. Heart Lung Circ. 2015;24:769–779. doi: 10.1016/j.hlc.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Owens KN, Coffin AB, Hong LS, Bennett KOC, Rubel EW, Raible DW. Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct cell death pathways. Hear Res. 2009;253:32–41. doi: 10.1016/j.heares.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Santos F, Roberts B, Linbo T, Coffin AB, Knisely AJ, Simon JA, Rubel EW, Rabile DW. Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet. 2008;4(2):e1000020. doi: 10.1371/journal.pgen.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Cunningham LL, Francis SP, Brandon CS, Simon JA, Raible DW, Rubel EW. Identification of FDA-approved drugs and bioactives that protect hair cells in the zebrafish (Danio rerio) lateral line and mouse (Mus musculus) utricle. J Assoc Res Otolaryngol. 2009;10(2):191–203. doi: 10.1007/s10162-009-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Keating S, Wu P, Simon JA, Raible DW, Rubel EW. Quinoline ring derivatives protect against aminoglycoside-induced hair cell death in the zebrafish lateral line. J Assoc Res Otolaryngol. 2012;13(6):759–770. doi: 10.1007/s10162-012-0353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Santos F, Raible DW, Simon JA, Rubel EW. Drug screening for hearing loss: using the zebrafish lateral line to screen for drugs that prevent and cause hearing loss. Drug Discov Today. 2010;15:265–271. doi: 10.1016/j.drudis.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannenstiel SC, Praetorius M, Plinkert PK, Brough DE, Staecker H. Bcl-2 gene therapy prevents aminoglycoside—induced degeneration of auditory and vestibular hair cells. Audiol Neurootol. 2009;14:254–266. doi: 10.1159/000192953. [DOI] [PubMed] [Google Scholar]
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J of Comp Neurol. 2000;421:189–198. doi: 10.1002/(SICI)1096-9861(20000529)421:2<189::AID-CNE5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Rangel-Ordóñez L, Nöldner M, Schubert-Zsilavecz M, Wurglics M. Plasma levels and distribution of flavonoids in rat brain after single and repeated doses of standardized Ginkgo biloba extract EGb 761®. Planta Med. 2010;76:1683–1690. doi: 10.1055/s-0030-1249962. [DOI] [PubMed] [Google Scholar]
- Rennekamp AJ, Peterson RT. 15 years of zebrafish chemical screening. Curr Opin Chem Biol. 2015;24:58–70. doi: 10.1016/j.cbpa.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Fettiplace R. Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J Physiol. 1998;506:159–173. doi: 10.1111/j.1469-7793.1998.159bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi MD, Hirose K. Aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 2007;15:352–357. doi: 10.1097/MOO.0b013e3282ef772d. [DOI] [PubMed] [Google Scholar]
- Ruiz LM, Salazar C, Jensen E, Ruiz PA, Tiznado W, Quintanilla RA, Barreto M, Elorza AA. Quercetin affects erythropoiesis and heart mitochondrial function in mice. Oxidative Med Cell Longev. 2015;2015:836301. doi: 10.1155/2015/836301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72:931–935. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- Sagit M, Korkmaz F, Gürgen S, Gundogdu R, Akcadag A, Ozcan I. Quercetine attenuates the gentamicin-induced ototoxicity in a rat model. Intl J Ped Otorhinolaryngol. 2015;79:2109–2114. doi: 10.1016/j.ijporl.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Sahu SC, Gray GC. Kaempferol-induced nuclear DNA damage and lipid peroxidation. Cancer Lett. 1994;85:159–164. doi: 10.1016/0304-3835(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Sampson JA, Duston J, Croll RP. Superficial neuromasts facilitate non-visual feeding by larval striped bass (Morone saxatilis) J Exp Biol. 2013;216:3522–3530. doi: 10.1242/jeb.087395. [DOI] [PubMed] [Google Scholar]
- Santos F, MacDonald G, Rubel EW, Raible DW. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio) Hear Res. 2006;213:25–33. doi: 10.1016/j.heares.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Schacht J. Antioxidant therapy attenuates aminoglycoside-induced hearing loss. Annals NY Aca of Sci. 1999;884:125–130. [PubMed] [Google Scholar]
- Seligmann H, Podoshin L, Ben-David J, Fradis M, Goldsher M. Drug-induced tinnitus and other hearing disorders. Drug Saf. 1996;14:198–212. doi: 10.2165/00002018-199614030-00006. [DOI] [PubMed] [Google Scholar]
- Sergi B, Fetoni AR, Ferraresi A, Troiani D, Azzena GB, Paludetti G, Maurizi M. The role of antioxidants in protection from ototoxic drugs. Acta Otolaryngol. 2004;124:42–45. doi: 10.1080/03655230410017111. [DOI] [PubMed] [Google Scholar]
- Siegelin MD, Reuss DE, Habel A, Rami A, von Deilmling A. Quercetin promotes degradation of surviving and thereby enhances death-receptor-mediated apoptosis in glioma cells. Neuro-Oncology. 2009;11(2):122–131. doi: 10.1215/15228517-2008-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfrizzi V, Panza F. Plant-based nutraceutical interventions against cognitive impairment and dementia: meta-analytic evidence of efficacy of a standardized Ginkgo biloba extract. J Alzheimers Dis. 2015;43(2):605–611. doi: 10.3233/JAD-141887. [DOI] [PubMed] [Google Scholar]
- Sun J, Sun G, Meng X, Wang H, Luo Y, Qin M, Ma B, Wang M, Cai D, Guo P, Sun X. Isorhamnetin protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. PLoS One. 2013;8(5):e64526. doi: 10.1371/journal.pone.0064526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng BS, Lu YH, Wang ZT, Tao XY, Wei DZ. In vitro anti-tumor activity of isorhamnetin isolated from Hippophae rhamnoides L. against BEL-7402 cells. Pharmacol Res. 2006;54:186–194. doi: 10.1016/j.phrs.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Hailey DW, Stawicki TM, Wu P, Coffin AB, Rubel EW, Raible DW, Simon JA, Ou HC. Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. J Neurosci. 2013;33:4405–4414. doi: 10.1523/JNEUROSCI.3940-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton C, Parng C. The use of zebrafish for assessing ototoxic and otoprotective agents. Hear Res. 2005;208:79–88. doi: 10.1016/j.heares.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Uribe PM, Kawas LH, Harding JW, Coffin AB. Hepatocyte growth factor mimetic protects lateral line hair cells from aminoglycoside exposure. Front Cell Neuro. 2015;9:3. doi: 10.3389/fncel.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas AJ, Sittadjody S, Thangasamy T, Mendoza EE, Limesand KH, Burd R. Exploiting tyrosinase expression and activity in melanocytic tumors: quercetin and the central role of p53. Integr Cancer Ther. 2011;10(4):328–340. doi: 10.1177/1534735410391661. [DOI] [PubMed] [Google Scholar]
- Vicente-Torres MA, Schacht J. A BAD link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. J Neurosci Res. 2006;83:1564–1572. doi: 10.1002/jnr.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasits AL, Simon JA, Raible DW, Rubel EW, Owens KN. Screen of FDA-approved drug library reveals compounds that protect hair cells from aminoglycosides and cisplatin. Hear Res. 2012;294:153–165. doi: 10.1016/j.heares.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gunten A, Schlaefke S, Überla K. Efficacy of Ginkgo biloba extract EGb 761® in dementia with behavioural and psychological symptoms: a systematic review. World J Biol Psychiatry. 2015;27:1–12. doi: 10.3109/15622975.2015.1066513. [DOI] [PubMed] [Google Scholar]
- Vu AA, Nadaraja GS, Huth ME, Luk L, Kim J, Chai R, Ricci AJ, Cheng AG. Integrity and regeneration of mechanotransduction machinery regulate aminoglycoside entry and sensory cell death. PLoS One. 2013;8:e54794. doi: 10.1371/journal.pone.0054794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Steyger PS. Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells. J Assoc Res Otolaryngol. 2009;10:205–219. doi: 10.1007/s10162-009-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong W-X, Cheng EH-Y, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio) Eugene: University of Oregon; 2000. [Google Scholar]
- Xiao T, Roeser T, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 2005;132:2955–2967. doi: 10.1242/dev.01861. [DOI] [PubMed] [Google Scholar]
- Yang CH, Schrepfer T, Schacht J. Age-related hearing impairment and the triad of acquired hearing loss. Front Cell Neurosci. 2015;9:276. doi: 10.3389/fncel.2015.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita D, Minami SB, Kanzaki S, Ogawa K, Miller JM. Bcl-2 genes regulate noise-induced hearing loss. J Neurosci Res. 2008;86:920–928. doi: 10.1002/jnr.21533. [DOI] [PubMed] [Google Scholar]
- Yang TH, Young YH, Liu SH. EGb 761 (Ginkgo biloba) protects cochlear hair cells against ototoxicity induced by gentamicin via reducing reactive oxygen species and nitric oxide-related apoptosis. J Nutr Biochem. 2011;22:886–894. doi: 10.1016/j.jnutbio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Cheng G, Qiu H, Zhu L, Ren Z, Zha W, Liu L. The p53-inducible gene 3 involved in flavonoid-induced cytotoxicity through the reactive oxygen species-mediated mitochondrial apoptotic pathway in human hepatoma cells. Food and Function. 2015;6:1518–1525. doi: 10.1039/C5FO00142K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 13 kb)