Abstract
Intratympanic gentamicin therapy is widely used clinically to treat the debilitating symptoms of Ménière’s disease. Cochleotoxicity is an undesirable potential side effect of the treatment and the risk of hearing loss increases proportionately with gentamicin concentration in the cochlea. It has recently been shown that gentamicin is readily absorbed through the oval window in guinea pigs. The present study uses quantitative functional measures of vestibular and cochlea function to investigate the efficacy of treating the vestibule by applying a small volume of gentamicin onto the stapes footplate in guinea pigs. Vestibular and cochlea function were assessed by recording short latency vestibular evoked potentials in response to linear head acceleration and changes in hearing threshold, respectively, 1 and 2 weeks following treatment. Histopathology was analyzed in the crista ampullaris of the posterior semi-circular canal and utricular macula in the vestibule, and in the basal and second turns of the cochlea. In animals receiving gentamicin on the stapes footplate, vestibular responses were significantly suppressed by 72.7 % 2 weeks after treatment with no significant loss of hearing. This suggests that the vestibule can be treated directly by applying gentamicin onto the stapes footplate.
Keywords: Gentamicin, Aminoglycoside, Vestibulotoxicity, Ototoxicity, Oval window, Stapes, Stapediovestibular joint, Annular ligament, Intratympanic, Pharmacokinetics
Introduction
It is well documented that the debilitating symptoms of vertigo experienced by Ménière’s disease (MD) sufferers can be reduced with intratympanic administration of aminoglycoside antibiotics. Direct injection with a small-gauge needle through an anesthetized eardrum is the preferred delivery method since the procedure can be performed quickly and inexpensively in a physician’s office. Furthermore, there is no evidence that use of a tympanostomy tube, microcatheter, or other delivery device provides benefit over intratympanic administration (Carey 2004). When injected into the middle ear, aminoglycoside is absorbed into inner ear fluid, gaining access to the sensory cells in both the vestibule and cochlea. Aminoglycosides are toxic to inner ear sensory cells. As a result, part of the vestibular system is destroyed, which in turn suppresses vertigo symptoms. However, since aminoglycosides are also cochleotoxic, patients can experience the undesirable side effect of permanent unilateral hearing loss. Gentamicin has emerged as the preferred aminoglycoside used in MD treatment because it is substantially less cochleotoxic than other aminoglycosides such as streptomycin (Lange 1989).
The degree of hearing loss associated with the treatment is directly related to the gentamicin concentration in the cochlea (Plontke et al. 2002; Salt et al. 2008). The dosages, timing, and method of gentamicin therapy used previously, as well as the endpoints selected to curtail therapy, differ greatly (Chia et al. 2004; Salt et al. 2008; McCall et al. 2010). Present gentamicin dosage protocols are empirically based, developed over many years during numerous clinical studies in which a sufficient dosage to adequately suppress vestibular symptoms or vestibular sensitivity was balanced against the degree of hearing loss caused by the treatment. The large variation in dosage protocols in previous clinical studies makes a comparison of patient outcomes difficult. However, the reported risk of additional hearing loss associated with intratympanic gentamicin treatment is approximately 21 %, with a range of 0–37 % (Diamond et al. 2003; Carey 2004).
It has been widely accepted that gentamicin applied intratympanically enters the round window membrane (RWM) (Smith and Myers 1979; Goycoolea 2001; Becvarovski et al. 2002) then spreads locally from scala tympani (ST) to scala vestibuli (SV)/vestibule through the interstitial spaces of the spiral ligament (Plontke et al. 2002). The preferential effects on vestibular function, rather than hearing, were assumed to be due to a greater gentamicin sensitivity of vestibular hair cells than cochlear hair cells (Aran et al. 1995; Nakashima et al. 2000). However, in a previous study (King et al. 2013), we found that the oval window (OW) was permeable to gentamicin in guinea pigs suggesting an alternative route for gentamicin to enter the vestibule. We observed that shifts in hearing threshold (8–32 kHz) and morphological changes in vestibular hair cells in the utricle were more influenced by gentamicin entry through the OW than the RWM.
The focus of the present study is to investigate the efficacy of treating the vestibule directly by applying a small volume of low concentration gentamicin solution onto the stapes footplate only. Changes in hearing threshold and cellular morphology were analyzed to assess cochleotoxicity.
To assess vestibular function quantitatively, short latency vestibular evoked potentials (VsEP) were recorded in response to linear head acceleration pulses, pre-treatment and 1 and 2 weeks after treatment.
Materials and Methods
Animal Preparation
The study was approved by the Royal Victorian Eye and Ear Hospital (Melbourne, Australia) Animal Ethics Committee (Ethics Approval 13/287AB & 13/276AB). Fourteen tri-color adult guinea pigs of either sex, weighing between 622 and 740 g, were used in the study and randomly assigned to a treatment group. The animals were anesthetized with Ketamine (Troy Laboratories Pty Ltd., Australia; 60 mg/kg) and Xylazil-20 (Troy Laboratories Pty Ltd., Australia; 4 mg/kg) administered intramuscularly. Anesthesia was monitored during the experiment using pedal and ocular reflexes and supplemented as necessary with 67 % of the initial dose. One milliliter of Lignocaine-20 (Troy Laboratories Pty Ltd., Australia) was administered subcutaneously to the surgical sites prior to making the incisions. Temgesic (Reckitt Benckiser; 0.05 mg/kg) and Baytril (Bayer AG; 20 mg/kg) were administered subcutaneously following surgery for analgesia and infection control, respectively.
Surgical Procedure
A 3-cm surgical incision was made along the midline of the cranium to expose the cranial bone. After the periosteum was removed with a scalpel blade, a stainless steel bolt was cemented upside down to the skull near bregma using dental cement (Paladur, Heraeus Kulzer). The wound was sutured closed around the bolt and the bolt remained in place for the duration of the 2-week experiment.
Using a dorsolateral posterior-auricular surgical approach, the facial nerve canal was exposed. In the same ear, the bulla was opened using a scalpel blade to expose the round window niche. A small section of bony shelf overlying the stapes footplate was removed with a 0.3 mm 90 ° pic (Kaisers) to expose the stapes footplate.
A wire recording electrode (175 μm diameter 90/10 Platinum/Iridium (Pt/Ir) Teflon-coated wire with the end exposed) was inserted 6 mm into the facial nerve canal and cemented to nearby bone to hold the electrode in place for the duration of the experiment. An electrical connector was connected to the electrode during recordings. At the first curvature, the facial nerve is separated from the vestibulocochlear nerve (VIIIth cranial nerve) by a thin bony partition, enabling potentials to be recorded within close proximity to the nerve without mechanically damaging it (Bohmer 1995; Bohmer et al. 1995; Oei et al. 2001; Kingma and Wit 2010; Bremer et al. 2012; Chihara et al. 2013).
Vestibular Evoked Potentials
All physiological recordings were taken in a sound-treated electrically shielded room with the contralateral ear occluded with an ear mold compound (Otoform, Dreve Germany) to attenuate hearing. Animals were held in position using a custom-made bite bar and nose clamp during the recordings.
To accelerate the head, a B71 audiometric bone-conductor (Radioear Corp., USA) was attached to the bolt cemented to the skull. To monitor head acceleration, a tri-axial accelerometer (Dimension Engineering, USA) was mounted onto a metal platform that was connected to the bolt. After connection to the animal, but before each set of VsEP recordings, the system was calibrated to produce graded vertical head accelerations of 1–8 g for each animal. The bone-conductor was controlled by computer-generated stimuli using a data acquisition system (National Instruments USB-6366 X series DAQ, Igor Pro, Wavemetrics). The stimuli consisted of 1 ms pulses of alternating polarity to minimize electromagnetic induced artifact. Measurements were averaged over 100 repetitions, delivered at a rate of 20 Hz, sampled at a rate of 100 kHz, with 2 repeats. Forward noise-masking (80 dB SPL) was presented to the ipsilateral ear via a speaker 0.1 m from the pinna to suppress acoustic responses to the vibration stimuli.
VsEP responses were recorded differentially (ISO-80 Bio-Amplifier, World Precision Instruments) from the Pt/Ir wire electrode in the facial nerve canal using a connector clipped to the recording electrode, and a 250 μm diameter Pt/Ir reference electrode placed in neck musculature. A subcutaneous ground electrode was placed further caudally in the thigh.
Vestibular function was assessed by peak-to-peak amplitude measurements of the first N1-P1 wave of the VsEP waveform. Initial baseline recordings were taken prior to treatment (T = 0). Recordings were taken again 1 week (T = 1) and 2 weeks (T = 2) after treatment. For each animal, the N1-P1 amplitudes in the T = 1, 2 week recordings were individually normalized to the initial pre-treatment T = 0 recording to assess changes in vestibular function. Post-mortem VsEP recordings were taken in some animals to ensure artifact suppression techniques were adequate.
Acoustically Evoked Potentials
Computer-generated acoustic stimuli (5 ms tone pips with 1 ms rise/fall times at frequencies 2, 8, 16, 24, and 32 kHz) were delivered free-field from a loudspeaker (4″ Vifa XT25TG30–04) placed 0.1 m from the ipsilateral pinna. Stimulus intensity was attenuated in 5 dB steps between 80 and 10 dB SPL. Acoustically evoked potentials (AEP) were recorded using the same methods as the VsEP recordings and auditory function was assessed by changes in hearing threshold.
Gentamicin Delivery
Following the initial physiological recordings, 1 μL of 5 mg/ml solution of gentamicin sulfate (G3632, Sigma Aldrich Australia) in phosphate buffered saline (PBS) (n = 5) or 1 μL of PBS (n = 5) was administered onto the stapes footplate. The solution was delivered using a 5 μL Hamilton syringe fitted with a fine custom-made cannula, connected to a syringe driver (Micro4™ Microsyringe Pump, World Precision Instruments, USA). Delivery was visually confirmed to not contact the RWM. The wound was sutured closed with the animal left in position for 30 min and allowed to recover.
Animals were carefully monitored during recovery for signs of jaw function impediment or distress caused by the wire electrode located in the facial nerve canal. One and 2 weeks after treatment, animals were anesthetized for AEP and VsEP recordings. Following the final two-week AEP and VsEP recordings, animals were euthanized with an intraperitoneal injection of 0.5 mg/kg Lethabarb (Virbac Pty Ltd., Australia), perfused and the inner ears removed for histological analysis.
Histology
Animals were perfused by an intracardiac injection of heparinsed isotonic saline followed by 4 % paraformaldehyde (PFA) solution (Sigma Aldrich, Australia). Both inner ears were removed and fixed in 4 % PFA overnight. All subsequent histological processing and analysis was done blinded to the treatment group. Cochleae were cut with a bone saw in a parallel plane to the RWM and decalcified in 10 % (w/v) ethylenediaminetetraacetic acid (Sigma Aldrich, Australia) for up to 6 weeks. Specimens were cryoprotected in graded sucrose solutions (10–30 %), embedded in O.C.T (Tissue-tek) and frozen at −80 °C. Sections of 10 μm thickness were taken every 60 μm, mounted on a glass slide, stained with Harris Hematoxylin solution (Sigma Adrich, Australia) and Eosin Y solution (Sigma Aldrich, Australia), and cover slipped. Digital images of the vestibular end organs and cochlea regions of interest were taken using a 20× objective (Axio Imager 2 upright microscope, Carl Zeiss) and Axio Vision software (version 4.2.8, Carl Zeiss) for histological analysis.
Histopathology
The same morphological criteria described in a previous study (King et al. 2013) were used to identify and quantify vestibular hair cells and supporting cells. Briefly, type I hair cells (HCs) were identified by flask-shaped cell bodies surrounded by an afferent nerve calyx, a spherical nucleus with heterogeneous chromatin, a stereocilia bundle, and a cuticular plate (Lindeman 1969; Merchant 1999). These were distinguished from type II HCs, which were identified by a cylindrical shape, an ovoid nucleus with homogenous chromatin, superficial spatial location in the sensory epithelium, stereocilia bundle, cuticular plate, and the absence of a nerve calyx surrounding the cell body. Type I HCs were manually counted in three consecutive mid-sections of utriculi macula, and the crista ampullaris in the posterior semi-circular canal. In cases where the morphology appeared abnormal, if a spherical nucleus was present with evidence of a nerve calyx surrounding the nucleus, it was counted as a type I HC regardless of whether a stereociliary bundle was present or not.
Inner hair cells (IHCs) and outer hair cells (OHCs) in the lower and upper regions of the basal turn of the cochlea were counted. IHC and OHC were identified by the presence of a cell nucleus and were counted in three consecutive mid-modiolar sections, each separated by 60 μm. All cell counts were averaged across the three consecutive sections. To eliminate observer bias, all histological sections were assessed in a blinded manner using consistent morphological criteria.
Statistical Analysis
Change in N1-P1 VsEP amplitude, normalized percentage of N1-P1 amplitude remaining, and change in hearing threshold across treatment groups were individually subjected to one-way ANOVA analysis at each time point. Cell counts in each region of interest across groups were subjected to one-way ANOVA analysis. Effect sizes and pairwise multiple comparisons were evaluated post hoc using the Holm-Sidak test. When homogeneity as tested by the Shapiro-Wilk method failed, Kruskal-Wallis ANOVA on ranks was performed. Differences were considered statistically significant when p < 0.05. Statistical calculations were performed using Sigmaplot V 13.0 (Systat Software Inc.).
Results
Short Latency Vestibular Evoked Potentials
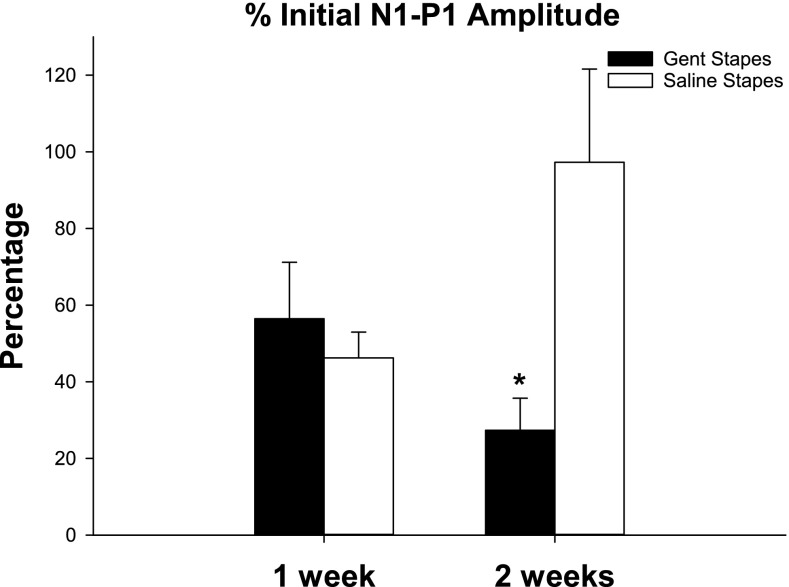
Typical examples of VsEP recordings from each group are shown in Figure 1 and the average change in VsEP amplitude over time for each group is shown in Figure 2. For each animal, the N1-P1 amplitude at T = 1, 2 weeks was compared to its pre-treatment N1-P1 amplitude. There was no significant difference in N1-P1 amplitude between the groups before the treatment (T = 0). N1-P1 amplitude decreased in both groups 1 week after treatment, suggesting that the surgical procedure itself affected vestibular function that had not resolved by that time. The difference between the gentamicin treated group (56.46 %, SEM 14.73 of pre-treatment N1-P1 amplitude, n = 5) and control group (46.24 %, SEM 6.73 of pre-treatment N1-P1 amplitude, n = 5) at T = 1 week was not significant. At 2 weeks after treatment, vestibular function improved in the control group (97.27 %, SEM 8.38 of pre-treatment N1-P1 amplitude, n = 5), but continued to decline in the gentamicin treated group (27.34 %, SEM 24.31 of pre-treatment N1-P1 amplitude, n = 5), suggesting the losses were due to gentamicin-induced vestibulotoxicity. The difference between the groups was significant at T = 2 weeks (p = 0.026, F 1,8 = 7.388, df 1, ANOVA).
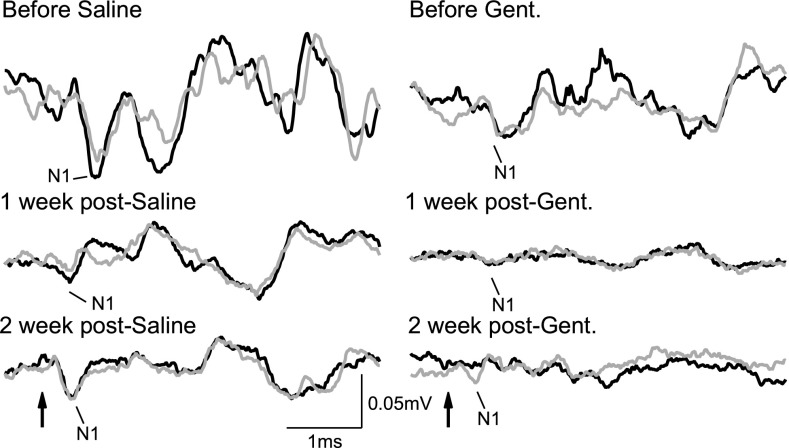
Fig. 1.
Typical vestibular evoked potentials (VsEP) recorded with an electrode inserted into the facial nerve canal in guinea pigs from each treatment group at baseline (before saline/gentamicin) and T = 1, 2 weeks after treatment. Simultaneous acoustic masking with 80 dB SPL noise was delivered to the ipsilateral ear. Arrow indicates onset of 8 g head acceleration. Each trace is composed of two repeated recordings that are represented as black and gray traces.
Fig. 2.
Change in short latency vestibular evoked potentials (VsEP) following gentamicin (T = 1 week, n = 5; T = 2 weeks, n = 5) or saline (T = 1 week, n = 4; T = 2 weeks, n = 5) application on the stapes footplate, expressed as a percentage of initial (T = 0) N1-P1 amplitude (individually normalized). Vestibular function decreased in both groups at T = 1 week. At T = 2 weeks, function improved in the control group but continued to decline in the gentamicin-treated group. The difference between the treatment groups at T = 2 weeks was stat. sig. (indicated by asterisk). Bars indicate SEM.
Acoustically Evoked Potentials
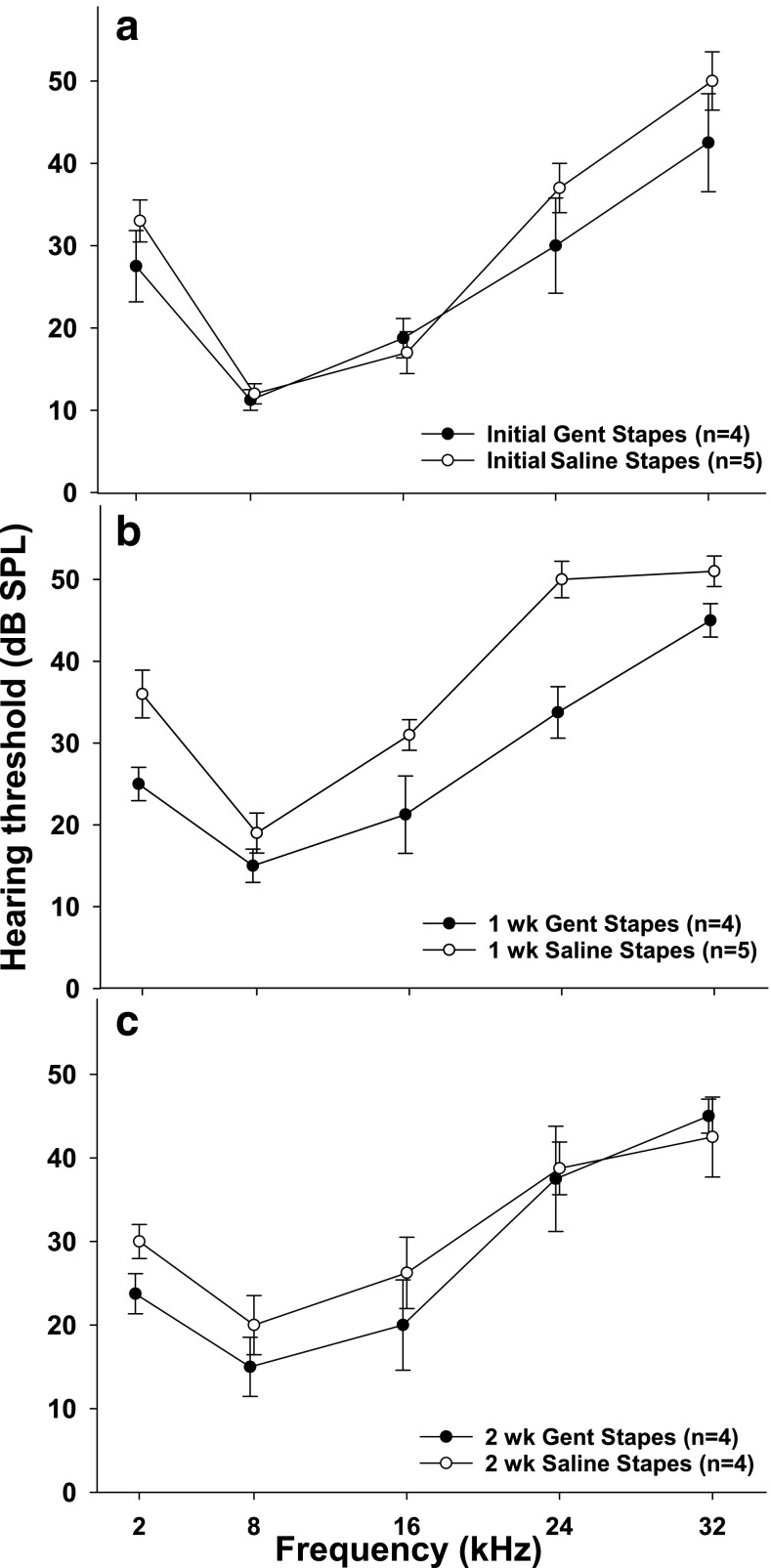
There were no significant differences in AEP threshold between the gentamicin treated animals and saline controls in the initial baseline (T = 0) recordings or T = 2 week recordings; however, the differences at T = 1 week were statistically significant at 2 kHz (p = 0.022, F 1,8 = 8.556, df 1, ANOVA) and 24 kHz (p = 0.003, F 1,8 = 18.778, df 1, ANOVA) (Fig. 3). The differences between groups (T = 1 week) at 8 kHz (p = 0.266, F 1,8 = 1.464, df 1, ANOVA), 16 kHz (p = 0.075, F 1,8 = 4.365, df 1, ANOVA), and 32 kHz (p = 0.068, F 1,8 = 4.667, df 1, ANOVA) were not statistically significant. There were no significant differences measured over time (T = 0, 1, 2 weeks) within the gentamicin group. The statistically significant increase in AEP thresholds observed in the control group at T = 1 week may have been related to the surgical procedure itself which had not resolved by that time. Importantly, the elevated hearing threshold was temporary and had recovered by T = 2 weeks.
Fig. 3.
Acoustically evoked potential (AEP) thresholds at five stimulus frequencies measured a before treatment (T = 0), b 1 week, and c 2 weeks following gentamicin or saline administration on the stapes footplate. Bars indicate SEM. There were no significant differences in threshold observed between the groups at any time point, or within the gentamicin group over time.
Vestibular Morphology
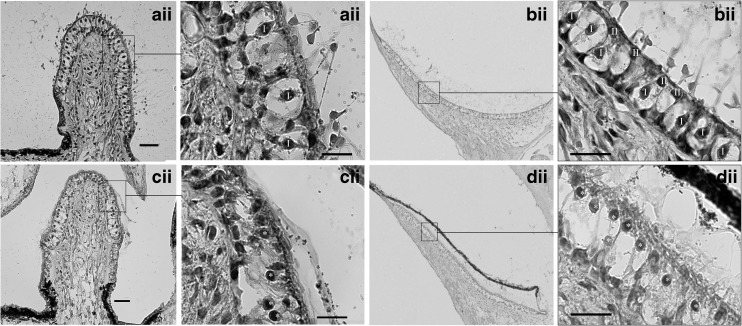
Typical examples of vestibular morphology in hematoxylin and eosin stained sections from the crista ampullaris and utricle from each treatment group are shown in Figure 4. During the analysis, it was observed that several animals had abnormal type 1 hair cells where the nerve calyx was distorted, the spatial localization of the nucleus was closer to the cuticular plate (making the distinction between type I and type II difficult at times), the afferent nerve branch was thin or discontinuous, and there was reduced or absent stereociliary bundles (see Fig. 4Cii–Dii). In these cases, a type I HC was recorded when a spherical nucleus was present within an evident nerve calyx (distorted or not) regardless of whether if appeared normal or not or a stereociliary bundle was present or not. After unblinding of the histological analysis, it was evident that only gentamicin treated animals exhibiting abnormal morphology (see Fig. 4c–d); however, it is worth noting that not all animals treated with gentamicin exhibited abnormal morphology.
Fig. 4.
Typical examples of gentamicin-induced morphological changes in vestibular hair cells in the crista ampullaris of the posterior semi-circular canal (a, c) and utricle (b, d) in hematoxylin and eosin stained sections, 2 weeks following treatment. Low power magnification: 20× objective (Ai–Di). High power magnification: 100× objective (Aii–Dii). All scale bars = 20 μm. Ai–Bi indicate crista ampullaris and utricle respectively following saline administration to the stapes footplate. The majority of type 1 hair cells (marked I) exhibited normal morphological appearance characterized by flask-shaped cell bodies surrounded by a nerve calyx (chalice) and stereocilia bundle. Ci–Di indicate crista ampullaris and utricle respectively following gentamicin administration on the stapes footplate. Extensive damage was caused to type 1 hair cells characterized by calyceal distortion, nuclei migration toward the cuticular plate and reduced stereocilia bundles. Aii–Dii indicate higher magnifications of the outlined areas shown in Ai–Di, respectively.
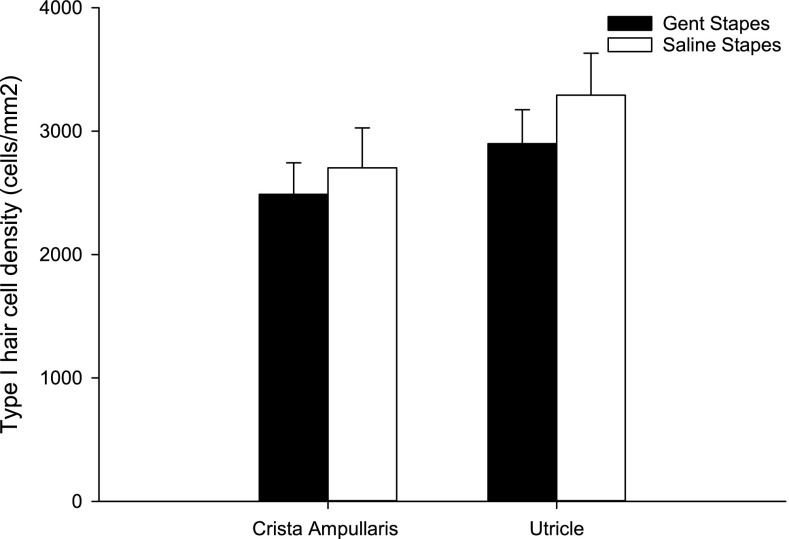
Type I HCs were counted in three consecutive mid-sections of crista ampullaris in the posterior semi-circular canal and utricle for both treatment groups as shown in Figure 5. The counts were individually normalized to area and averaged. The density of type I HCs in both the crista ampullaris (gentamicin on stapes, n = 4; controls, n = 4) and utricle (gentamicin on stapes, n = 5; controls, n = 5) were lower in the group receiving gentamicin on the stapes footplate compared to the control group 2 weeks after treatment; however, this did not reach statistical significance.
Fig. 5.
Type 1 hair cell counts (individually normalized to area and expressed as cell density) in the crista ampullaris (CA) of the posterior semi-circular canal and utricle (U) following gentamicin (CA n = 4, U n = 5) or saline (CA n = 4, U n = 5) administration on the stapes footplate. Hair cell density decreased in both regions in the group receiving gentamicin on the stapes compared to controls; however, this did not reach statistical significance. Bars indicate SEM.
Cochlear Morphology
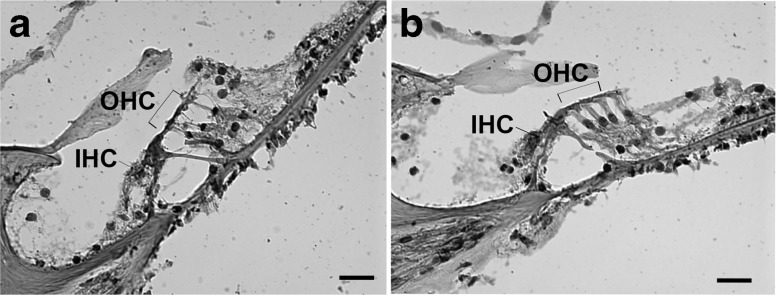
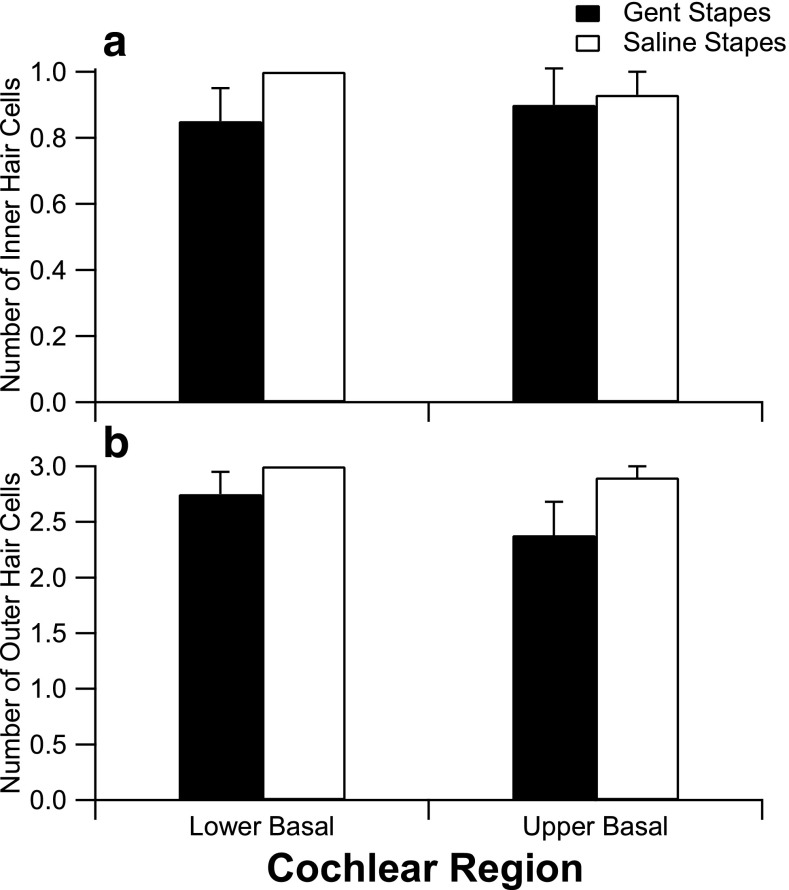
Typical examples of cellular morphology from each treatment group are shown in Figure 6. Inner and outer hair cells were counted in the lower and upper region of the basal turn in three consecutive mid-modiolar sections for each treatment group (T = 2 weeks following treatment) as shown in Figure 7. The number of IHCs and OHCs present in the basal turn was lower in the group that received gentamicin on the stapes footplate; however, this did not reach statistical significance.
Fig. 6.
Typical examples of hair cell morphology in the basal turn of the cochlea 2 weeks following treatment shown in hematoxylin and eosin stained sections. 40× magnification, scale bars = 20 μm. a Gentamicin applied to the stapes footplate. b Saline applied to the stapes footplate.
Fig. 7.
Effects of gentamicin on cochlear hair cells in the basal turn. a Number of inner hair cells present 2 weeks following treatment (gentamicin on stapes: lower basal (n = 4), upper basal (n = 3); saline on stapes: lower basal (n = 4), upper basal (n = 4)). b Number of outer hair cells (gentamicin on stapes: lower basal (n = 4), upper basal (n = 3); saline on stapes: lower basal (n = 4), upper basal (n = 4)). Hair cell counts were averaged over three consecutive sections, each spaced 60 μm apart. The number of surviving inner and outer hair cells present in the lower and upper basal turn was lower in the group that received gentamicin on the stapes footplate; however, the differences were not statistically significant. Error bars indicate SEM.
Discussion
Two weeks after a small volume (1 μL) of gentamicin solution (5 mg/mL) was applied directly onto the stapes footplate in guinea pigs; there was a significant loss of 72.66 % of N1-P1 VsEP amplitude in response to an 8 g vertical head acceleration without significant shifts in hearing threshold. This is the first study using quantitative functional measures to show that vestibular function can be suppressed by applying gentamicin exclusively onto the OW in guinea pigs.
Despite a dramatic reduction in vestibular function following gentamicin treatment, there was no significant reduction in the number of type I HCs. However, abnormal morphology of putative type I HCs was observed in animals that had been treated with gentamicin, but not saline treated control animals. The most parsimonious explanation therefore is that these abnormalities were caused by gentamicin toxicity. Furthermore, since there is evidence of stereocilia reduction, calyceal distortion, and thinner/absent afferent nerve branches in these gentamicin treated animals, it is likely that many of these putative type I HCs were non-functional. This would explain why the vestibular HC counts do not reflect the reduction in VsEP amplitude observed.
Initial baseline recordings were far noisier than both sets of recordings after treatment in all animals. Presumably, the noise drops after treatment from a reduction in cochlear microphonics and/or spontaneous VIIIth nerve activity and stabilization of the electrode within the nerve. Importantly, the 2-week post treatment recording exhibit a similar level of background noise, with the saline treated animals exhibiting a robust VsEP, while the VsEP in the gentamicin-treated animals was small or absent.
There was a temporary elevation in AEP threshold and reduction in N1-P1 amplitude in the saline treated controls at 1-week post treatment. However, both hearing and vestibular function returned to baseline 2 weeks after treatment, suggesting that the surgical procedure itself may have affected both hearing and vestibular function in these animals. Temporary threshold shifts observed after acoustic over exposure exhibit a similar time course whereby full recovery can take 2 weeks to occur (Kujawa and Liberman 2009).
Cochleotoxicity is an undesirable potential side effect of intratympanic gentamicin therapy and the risk of hearing loss increases proportionately with gentamicin dosage (concentration, volume, and administration time) (Plontke et al. 2002; Salt et al. 2008). When a large volume of gentamicin is injected through the tympanic membrane, it is in contact with and absorbed by both the RWM and OW. Attempts have been made to deliver gentamicin more specifically to the vestibule by surgically occluding the RWM with connective tissue before intratympanic injection; however, a significant number of patients (27 %) still experienced hearing loss (Quaranta et al. 1999). Applying a small volume of gentamicin solution directly onto the OW reduces the likelihood of gentamicin absorption through the RWM compared with conventional intratympanic delivery. This may in turn lower the gentamicin concentration in the cochlea, resulting in improved hearing outcomes compared to intratympanic delivery.
In the current study, we also optimized our targeting of the OW by removal of a small section of the bony shelf obstructing the stapes footplate and careful positioning of the animal to locate the stapes footplate physically below the RWM. Using a micromanipulator, the fine cannula was carefully advanced into the space where the section of bony shelf was removed, advanced past the side of the cochlea and positioned just above the stapes footplate (below the RWM). However, improved targeting of the OW does not eliminate the risk of hearing loss altogether. Following OW absorption, passive diffusion occurs from vestibule/scala vestibuli perilymph to scala tympani through semi-permeable tissues. In previous studies (King et al. 2013), we observed statistically significant hearing losses in the groups that received high dosages of gentamicin (3 μL of 337 mg/mL, 2 μL of 40 mg/mL, respectively) on the OW compared to the groups receiving the same dosage on the RWM. This suggests that either the rate of drug elimination is higher from scala tympani in the cochlea following RWM entry or that gentamicin is absorbed more readily through the OW than the RWM. By reducing the gentamicin concentration in the current study to 2 μL of 5 mg/mL, we were able to significantly suppress vestibular function by 72.66 % without significantly increasing AEP thresholds.
There are anatomical differences in the stapediovestibular joint between species; however, this is not expected to be a major factor influencing drug permeability of the OW. For instance, the guinea pig stapediovestibular joint comprises hyaline cartilage on the articulating surfaces of the stapes footplate rim and OW frame, a fluidic articular cavity, and epithelial membranes overlying the structure (middle ear mucosa) (Tanaka and Motomura 1981). In humans, the stapes is attached to the perimeter of the OW by an annular ligament (Merchant and Nadol 2010). Without tight junctions present, substances would be expected to readily pass through this ligament. This has been demonstrated in magnetic resonance imaging (MRI) studies where gadolinium readily penetrated the OW in guinea pigs (King et al. 2011), rats, and humans (Zou et al. 2005).
Other factors may influence OW absorption such as the presence of endolymphatic hydrops. A recent MRI study in humans showed the distribution of an MRI contrast agent was compromised in two patients with endolymphatic hydrops (Shi et al. 2014), from which it was concluded that OW absorption was reduced by the presence of endolymphatic hydrops. Vestibular hydrops can cause the otoliths to lie against the stapes footplate and there is often fibrosis connecting the stapes to the membranous labyrinth in MD sufferers (Schuknecht 1993; Wackym et al. 1994; Nadol 1977), which may impede drug entry through the OW. Additionally, if MD involves a chronic immune pathology, as is a commonly held theory, it is plausible that the permeability of the blood-labyrinth barrier, and clearance of molecules from the ear to blood may be higher in MD ears (Hirose et al. 2014; Floc’h et al. 2014). It is not presently clear if clearance rates are abnormal in MD ears, but it should be taken into consideration, along with pharmacokinetics, when delivering drugs to the OW or RWM for the treatment of MD.
Acknowledgements
This study was funded by the Garnett Passe & Rodney Williams Memorial Foundation. This authors wish to thank Mr. Rodney Millard, Mr. Mark Harrison, and Dr. Mohit Shivdasani from the Bionics Institute and Professor Ian Curthoys from the University of Sydney for their input into setting up the VsEP recording equipment at the Bionics Institute; Professor Alec Salt from Washington University School of Medicine, St Louis, USA, for technical advice; Ms. Shefin George from the Bionics Institute for her assistance with perfusions; and Miss Prudence Neilson from University of Melbourne for preparing histology slides. The time invested by A/Prof Fallon, Prof Shepherd, Dr. Brown were supported by research grants from the NHMRC (GNT1081478) and the Garnett Passe and Rodney Williams Memorial Foundation.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- Aran JM, Chappert C, Dulon D, Erre JP, Aurousseau C. Uptake of amikacin by hair cells of the guinea pig cochlea and vestibule and ototoxicity: comparison with gentamicin. Hear Res. 1995;82:179–183. doi: 10.1016/0378-5955(94)00175-P. [DOI] [PubMed] [Google Scholar]
- Becvarovski Z, Bojrab DI, Michaelides EM, et al. Round window gentamicin absorption: an in vivo human model. Laryngoscope. 2002;112(9):1610–1613. doi: 10.1097/00005537-200209000-00015. [DOI] [PubMed] [Google Scholar]
- Bohmer A. Short latency vestibular evoked responses to linear acceleration stimuli in small mammals: masking effects and experimental applications. Acta Otolaryngol Suppl. 1995;520(Pt1):120–123. doi: 10.3109/00016489509125206. [DOI] [PubMed] [Google Scholar]
- Bohmer A, Hoffman LF, Honrubia V. Characterization of vestibular potentials evoked by linear acceleration pulses in the chinchilla. Am J Otol. 1995;16:498–504. [PubMed] [Google Scholar]
- Bremer GH, de Groot JC, Versnal H, Klis SF. Combined administration of kanalycin and furosemide does not result in loss of vestibular function in Guinea pigs. Audiol Neurootol. 2012;17:25–38. doi: 10.1159/000327256. [DOI] [PubMed] [Google Scholar]
- Carey J. Intratympanic gentamicin for the treatment of Meniere’s disease and other forms of peripheral vertigo. Otolaryngol Clin N Am. 2004;37:1075–1090. doi: 10.1016/j.otc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Chia SH, Garnst AC, Anderson JP, Harris JP. Intratympanic gentamicin therapy for Mèniére’s disease: a meta analysis. Otol Neurotol. 2004;25:544–552. doi: 10.1097/00129492-200407000-00023. [DOI] [PubMed] [Google Scholar]
- Chihara Y, Wang V, Brown DJ. Evidence for the utricular origin of the vestibular short-latency-evoked potential (VsEP) to bone-conducted vibration in guinea pig. Exp Brain Res. 2013;229(2):157–170. doi: 10.1007/s00221-013-3602-5. [DOI] [PubMed] [Google Scholar]
- Diamond C, O’Connell DA, Hornig JD, et al. Systematic review of intratympanic gentamicin in Meniere’s disease. J Otolaryngol. 2003;32(6):351–361. doi: 10.2310/7070.2003.13863. [DOI] [PubMed] [Google Scholar]
- Floc’h JL, et al. Markers of cochlear inflammation using MRI. J Magn Reson Imaging. 2014;39(1):150–161. doi: 10.1002/jmri.24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goycoolea MV. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol. 2001;121(4):437–447. doi: 10.1080/000164801300366552. [DOI] [PubMed] [Google Scholar]
- Hirose K, et al. Systemic lipopolysaccharide compromises the blood-labyrinth barrier and increases entry of serum fluorescein into the perilymph. J Assoc Res Otolaryngol. 2014;15(5):707–719. doi: 10.1007/s10162-014-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King EB, Salt AN, Eastwood HT, O’Leary SJ. Direct entry of gadolinium into the vestibule following intratympanic applications in Guinea pigs and the influence of cochlear implantation. JARO. 2011;12(6):741–751. doi: 10.1007/s10162-011-0280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King EB, Salt AN, Kel GE, Eastwood HT, O’Leary SJ. Gentamicin administration on the stapes footplate causes greater hearing loss and vestibulotoxicity than round window administration in guinea pigs. Hear Res. 2013;304:159–166. doi: 10.1016/j.heares.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma CM, Wit HP. The effect of changes in perilymphatic K+ on the vestibular evoked potential in the guinea pig. Eur Arch Otorhinolaryngol. 2010;267:1679–1684. doi: 10.1007/s00405-010-1298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neuroscience. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange G. Gentamicin and other ototoxic antibiotics for the transtympanic treatment of Menière’s disease. Arch Otorhinolaryngol. 1989;246(5):269–270. doi: 10.1007/BF00463571. [DOI] [PubMed] [Google Scholar]
- Lindeman H (1969) "Regional differences in structure of the vestibular sensory regions." The Journal of Laryngology & Otology 83.01: 1-17 [DOI] [PubMed]
- McCall AA, Leary Swan EE, Borenstein JT, Sewell WF, Kujawa SG, McKenna MJ. Drug delivery for treatment of inner ear disease: current state of knowledge. Ear & Hearing. 2010;31:156–165. doi: 10.1097/AUD.0b013e3181c351f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SN. A method for quantitative assessment of vestibular otopathology. Laryngoscope. 1999;109:1560–1569. doi: 10.1097/00005537-199910000-00004. [DOI] [PubMed] [Google Scholar]
- Merchant SN, Nadol JB (2010) Schuknecht’s Pathology of the Ear, People’s Medical Publishing House USA, 3rd Edition
- Nadol JB. Positive Hennebert’s sign in Ménierè’s disease. Arch Otolaryngol. 1977;103:524–530. doi: 10.1001/archotol.1977.00780260054005. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Teranishi M, Hibi T, Kobayashi M, Umemura M. Vestibular and cochlear toxicity of aminoglycosides: a review. Acta Otolaryngol. 2000;120:904–911. doi: 10.1080/00016480050218627. [DOI] [PubMed] [Google Scholar]
- Oei MLYM, Segenhout JM, Wit HP, Albers FW. The vestibular evoked response to linear, alternating, acceleration pulses without acoustic masking as a parameter of vestibular function. Acta Otolaryngol. 2001;121:62–67. doi: 10.1080/000164801300006290. [DOI] [PubMed] [Google Scholar]
- Plontke SKR, Wood AWW, Salt AN. Analysis of gentamicin kinetics in fluids of the inner ear with round window administration. Otol Neurotol. 2002;23:67–974. doi: 10.1097/00129492-200211000-00026. [DOI] [PubMed] [Google Scholar]
- Quaranta A, Aloisi A, De Benedittis G, Scaringi A. Intratympanic therapy for Ménière’s disease. High-concentration gentamicin with round-window protection. Ann N Y Acad Sci. 1999;884:410–424. doi: 10.1111/j.1749-6632.1999.tb08658.x. [DOI] [PubMed] [Google Scholar]
- Salt AN, Gill RM, Plontke SK. Dependence of hearing changes on the dose of intratympanically applied gentamicin: a meta-analysis using mathematical simulations of clinical drug delivery protocols. Laryngoscope. 2008;118:1793–1800. doi: 10.1097/MLG.0b013e31817d01cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuknecht HF. Pathology of the ear. 2. Philadelphia/Baltimore: Lea & Febiger; 1993. [Google Scholar]
- Shi H, Li Y, Yin S, Zou J. The predominant vestibular uptake of gadolinium through the oval window pathway is compromised by endolymphatic hydrops in Ménière’s disease. Otol Neurotol. 2014;35(2):315–322. doi: 10.1097/MAO.0000000000000196. [DOI] [PubMed] [Google Scholar]
- Smith BM, Myers MG. The penetration of gentamicin and neomycinin to perilymph across the round window membrane. Otolaryngol Head Neck Surg. 1979;87(6):888–891. doi: 10.1177/019459987908700625. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Motomura S. Permeability of the labyrinthine window in guinea pigs. Arch Otorhinolarygnol. 1981;233:67–73. doi: 10.1007/BF00464276. [DOI] [PubMed] [Google Scholar]
- Wackym PA, Schuknecht HF, Ward PH, Linthicum FH, Kerner MM, Aframian D, et al. Blinded control study of endolymphatic duct and sac fibrosis in Meniere’s disease. In: Filipo R, Barbara M, et al., editors. Meniere’s disease: perspectives in the 90s. Amsterdam/New York: Kugler Publishing; 1994. pp. 209–215. [Google Scholar]
- Zou J, Pyykkö I, Bjelke B, Dastidar P, Toppila E. Communication between the perilymphatic scalae and spiral ligament visualized by in vivo MRI. Audiol Neurotol. 2005;10:145–152. doi: 10.1159/000084024. [DOI] [PubMed] [Google Scholar]