Abstract
We describe a multiplex PCR assay to identify and discriminate between isolates of Campylobacter coli, Campylobacter jejuni, Campylobacter lari, and Campylobacter upsaliensis. The C. jejuni isolate F38011 lpxA gene, encoding a UDP-N-acetylglucosamine acyltransferase, was identified by sequence analysis of an expression plasmid that restored wild-type lipopolysaccharide levels in Escherichia coli strain SM105 [lpxA(Ts)]. With oligonucleotide primers developed to the C. jejuni lpxA gene, nearly full-length lpxA amplicons were amplified from an additional 11 isolates of C. jejuni, 20 isolates of C. coli, 16 isolates of C. lari, and five isolates of C. upsaliensis. The nucleotide sequence of each amplicon was determined, and sequence alignment revealed a high level of species discrimination. Oligonucleotide primers were constructed to exploit species differences, and a multiplex PCR assay was developed to positively identify isolates of C. coli, C. jejuni, C. lari, and C. upsaliensis. We characterized an additional set of 41 thermotolerant isolates by partial nucleotide sequence analysis to further demonstrate the uniqueness of each species-specific region. The multiplex PCR assay was validated with 105 genetically defined isolates of C. coli, C. jejuni, C. lari, and C. upsaliensis, 34 strains representing 12 additional Campylobacter species, and 24 strains representing 19 non-Campylobacter species. Application of the multiplex PCR method to whole-cell lysates obtained from 108 clinical and environmental thermotolerant Campylobacter isolates resulted in 100% correlation with biochemical typing methods.
Campylobacteriosis, gastroenteritis caused by the thermotolerant Campylobacter spp. (Campylobacter coli, Campylobacter jejuni, Campylobacter lari, and Campylobacter upsaliensis), is the most commonly reported gastrointestinal bacterial disease in developed countries (44). C. jejuni is most often implicated as the causative agent of campylobacteriosis, followed by C. coli (46), C. upsaliensis (23), and C. lari (35). While C. lari and C. upsaliensis cause far fewer cases of disease, both species have been recognized as emerging human pathogens (23, 46, 51). The true incidence of disease caused by non-C. jejuni Campylobacter spp. is difficult to estimate. This is, in part, due to difficulties in distinguishing between Campylobacter species based solely on phenotypic properties commonly used in clinical laboratories (30). In order to better understand the epidemiology of Campylobacter spp. and facilitate its control, genetic methods that distinguish between members of this species need to be developed.
Housekeeping genes encode proteins that carry out essential functions in a cell. As such, housekeeping genes make excellent targets for species-specific probes for several reasons. Notably, the products encoded by these genes are functionally constrained; thus, diversity present in the coding region can be used for phylogenetic analysis (37). The gene lpxA encodes the enzyme LpxA, a UDP-N-acetylglucosamine acyltransferase that catalyzes the first step of lipid A biosynthesis and subsequently lipopolysaccharide biosynthesis (38). Mutations in lpxA result in a loss of viability in gram-negative bacteria (38), with the exception of Neisseria meningitidis (36). The substrate for LpxA, an (R)-3-hydroxy fatty acid, is also used by the enzymatic product of the fabZ gene (28). The FabZ protein is involved in the dehydration of β-hydroxylacyl-acyl carrier protein moieties to the corresponding trans-2-acyl-acyl carrier protein product, thereby initiating fatty acid biosynthesis (15, 28). The genes fabZ and lpxA are commonly associated in tandem in gram-negative bacteria, which is also the case for the sequenced C. jejuni strain NCTC 11168 (15, 28, 33, 39, 42).
The ability to distinguish between Campylobacter species is important in the identification of Campylobacter sources and transmission routes (12, 50). Oligodeoxynucleotide primers and PCR assays that distinguish between one or more combinations of the thermotolerant Campylobacter species have been described (1, 4, 5, 9, 11, 13, 16, 21, 48). The advantages of PCR-based assays are that they can be performed quickly and relatively cheaply (29). Unfortunately, many of the assays currently available for Campylobacter spp. suffer from a lack of sensitivity or specificity (14, 32). Other assays require a combination of PCR amplification, coupled with restriction endonuclease digestion of the PCR amplicon by two to four enzymes, to specifically differentiate the thermotolerant Campylobacter spp. (11, 16).
Recently, species-specific microarrays have been described for the rapid identification of Campylobacter spp. (19, 49). These methods have great potential for epidemiological studies, but given the cost of the technology and the lack of microarray facilities in most clinical laboratories, they are currently not practical for routine application. We have developed a multiplex PCR assay, based on nucleotide sequence differences in the lpxA gene, to distinguish between C. coli, C. jejuni, C. lari, and C. upsaliensis. This multiplex PCR assay has been shown to be rapid, sensitive, specific, and robust.
(A portion of this work was presented at the 2002 Conference for Research Workers in Animal Diseases, St. Louis, Mo.)
MATERIALS AND METHODS
Reference strains and culture conditions.
The reference strains and isolates supplied from individual culture collections used in this study are listed in Table 1. Thermotolerant Campylobacter spp. (C. coli, C. jejuni, C. lari, and C. upsaliensis) were cultured on Mueller-Hinton medium (Remel, Lenexa, Kans.) supplemented with 5% sheep blood (Invitrogen, Carlsbad, Calif.) and 1.5% agar (Difco, Franklin Lakes, N.J.) at 43°C in a microaerobic atmosphere (5% O2, 10% CO2, 10% H2, 75% N2).
TABLE 1.
Reference bacterial isolates used, and their nucleotide sequence accession numbers, and amplicon sizes in the lpxA multiplex PCR assay
| Species | Isolate no. | Isolate sourcea | Amplicon size (bp) | Accession no.b |
|---|---|---|---|---|
| Campylobacter coli | M275 | Human; Arizona | 391 | AY531509 |
| RM1051 | Human; Canada | 391 | AY531503; identical to M275 | |
| RM1166 | Chicken | 391 | AY531502; identical to M275 | |
| RM1505 | 391 | AY531501; identical to M275 | ||
| RM1530 | Sheep; USA | 391 | AY531500; identical to M275 | |
| RM1531 | Marmoset | 391 | AY531499; identical to M275 | |
| RM1533 | Human; USA | 391 | AY531497; identical to M275 | |
| RM2225 | Chicken; USA | 391 | AY531508; identical to M275 | |
| RM2228 | Chicken; USA | 391 | AY531507; identical to M275 | |
| RM1532 | Human; USA | 391 | AY531498 | |
| RM1857 | Human | 391 | AY531496 | |
| WA31 | Water; NZ | 391 | AY598971; identical to RM1857 | |
| KLC5104 | Human; NZ | 391 | AY598972; identical to RM1857 | |
| RM1858 | Human | 391 | AY531495 | |
| RM1865 | Human | 391 | AY531494 | |
| RM1878; ATCC 43474 | Human | 391 | AY531493 | |
| RM1896 | Swine; USA | 391 | AY531492 | |
| RM1897 | Swine; USA | 391 | AY531491 | |
| RM3232 | Swine | 391 | AY531504 | |
| RM3230c | Swine; Australia | 391 | AY531506; identical to RM3232 | |
| RM3231c | Swine; Australia | 391 | AY531505; identical to RM3232 | |
| WA27 | Water; NZ | 391 | AY531510 | |
| KLC4366 | Human; NZ | 391 | NS | |
| KLC5039 | Human; NZ | 391 | NS | |
| CC39.4 | Swine; Missouri | 391 | NS | |
| T1631 | Human; Arizona | 391 | NS | |
| T2144 | Human; Arizona | 391 | NS | |
| T2232 | Human; Arizona | 391 | NS | |
| Campylobacter jejuni | F38011 | Human; Arizona | 331 | AY531520 |
| RM1221 | Chicken; USA | 331 | AY531521; identical to F38011 | |
| RM3672 | Canada Geese; California | 331 | AY531512; identical to F38011 | |
| RM3673 | Canada Geese; California | 331 | AY531511; identical to F38011 | |
| S2B | Chicken; USA | 331 | AY598974; identical to F38011 | |
| FZ917T | Human; NZ | 331 | AY598976; identical to F38011 | |
| NCTC 11168 | Human; UK | 331 | AL111168 | |
| ANR0493 | Human; NZ | 331 | AY531523; identical to NCTC11168 | |
| YG108S | Human; NZ | 331 | AY598977; identical to NCTC11168 | |
| YG936T | Human; NZ | 331 | AY598978; identical to NCTC11168 | |
| RM3664 | Canada geese; California | 331 | AY531519 | |
| RM3665 | Canada geese; California | 331 | AY531518; identical to RM3664 | |
| RM3666 | Canada geese; California | 331 | AY531517; identical to RM3664 | |
| RM3667 | Canada geese; California | 331 | AY531516; identical to RM3664 | |
| ESR 081 | Human; NZ | 331 | AY598979; identical to RM3664 | |
| RM3668 | Canada geese; California | 331 | AY531515 | |
| RM3669 | Canada geese; California | 331 | AY531514; identical to RM3668 | |
| RM3670 | Canada geese; California | 331 | AY531513; identical to RM3668 | |
| KLC2851 | Human; NZ | 331 | AY531522 | |
| M129 | Human; Arizona | 331 | AY598973 | |
| 81116 | 331 | AY598975 | ||
| KLC4271 | Human; NZ | 331 | AY598980 | |
| ZJ638R | Human; NZ | 331 | AY598981 | |
| 81-176 | Human; Minnesota | 331 | NS | |
| Campylobacter lari | RM1890 | Human | 233 | AY531476 |
| RM2810 | 233 | AY531488; identical to RM1890 | ||
| RM2821 | Human; Canada | 233 | AY531483; identical to RM1890 | |
| RM2100 | Human; USA | 233 | AY531474 | |
| RM2099 | Human | 233 | AY531475; identical to RM2100 | |
| RM2808 | 233 | AY531490; identical to RM2100 | ||
| RM2809 | 233 | AY531489; identical to RM2100 | ||
| RM2817 | Horse; Sweden | 233 | AY531487; identical to RM2100 | |
| RM2820 | Seagull | 233 | AY531484; identical to RM2100 | |
| RM2826 | Human; Belgium | 233 | AY531478; identical to RM2100 | |
| DRI879 | Human; NZ | 233 | AY598982; identical to RM2100 | |
| RM2819 | Seagull; Canada | 233 | AY531485 | |
| RM2822 | Human | 233 | AY531482 | |
| RM2823 | Human; Canada | 233 | AY531481 | |
| EC007 | Water; NZ | 233 | AY598983; identical to RM2823 | |
| EC009 | Water; NZ | 233 | AY598984 | |
| RM2824 | River water; UK | —d | AY531480 | |
| RM2825 | Human; Canada | — | AY531479 | |
| RM3659 | River water; UK | — | AY531477 | |
| NZRM2622 | 233 | NS | ||
| Campylobacter upsaliensis | ATCC 43954 | Canine; Sweden | 206 | AY598985 |
| RM1488 | Human; Canada | 206 | AY531471 | |
| ATCC 49815 | ||||
| RM2094 | Human; USA | 206 | AY531469; identical to RM1488 | |
| C1137 | Canine; Washington | 206 | AY598988; identical to RM1488 | |
| RM3937 | Human; California | 206 | Identical to RM1488 | |
| RM3939 | Human; California | 206 | AY598996; identical to RM1488 | |
| RM3940 | Human, California | 206 | Identical to RM1488 | |
| RM3941 | Human; California | 206 | Identical to RM1488 | |
| RM3943 | Human; California | 206 | Identical to RM1488 | |
| RM3945 | Human; California | 206 | Identical to RM1488 | |
| RM3946 | Canine; California | 206 | Identical to RM1488 | |
| RM3947 | Canine; California | 206 | Identical to RM1488 | |
| RM3949 | Canine; California | 206 | Identical to RM1488 | |
| RM3950 | Canine; California | 206 | Identical to RM1488 | |
| RM2089 | Sheep | 206 | AY531472 | |
| RM2093 | Human; USA | 206 | AY531470 | |
| RM3195 | Human; South Africa | 206 | AY531473 | |
| RM3777 | Human; South Africa | 206 | AY598986 | |
| C1138 | Canine; Washington | 206 | AY598989 | |
| RM2092 | Human | 206 | AY598987 | |
| RM3779 | Human; South Africa | 206 | AY598990 | |
| RM3778 | Human; South Africa | 206 | Identical to RM3779 | |
| RM3781 | Human; South Africa | 206 | Identical to RM3779 | |
| RM3786 | Human; South Africa | 206 | Identical to RM3779 | |
| RM3780 | Human; South Africa | 206 | AY598991 | |
| RM3776 | Human; South Africa | 206 | Identical to RM3780 | |
| RM3784 | Human; South Africa | 206 | Identical to RM3780 | |
| RM3783 | Human; South Africa | 206 | AY598992 | |
| RM3785 | Human; South Africa | 206 | AY598993 | |
| RM3942 | Human; California | 206 | AY598995 | |
| RM3944 | Human; California | 206 | AY598994 | |
| RM3948 | Canine; California | 206 | Identical to RM3944 | |
| RM3782 | Human; South Africa | 206 | NS |
NZ, New Zealand; UK, United Kingdom.
NS, not sequenced.
These isolates were formerly classified as Campylobacter hyoilei.
—, no band was amplified during the PCR assay.
Campylobacter concisus ATCC 33237, ATCC 43643, ATCC 51561, and ATCC 51562; Campylobacter curvus ATCC 35224, ATCC 33237, and RM3269; Campylobacter fetus subsp. fetus NCTC 10842, RM2086, RM2087, and RM2088; Campylobacter fetus subsp. venerealis NCTC 10354, RM3224. RM3225, ATCC 19438, ATCC 33561, and ATCC 33226; Campylobacter gracilis ATCC 33226; Campylobacter helveticus ATCC 51209 and ATCC 51210; Campylobacter hyointestinalis ATCC 35217, RM1558, RM1559, RM1561, RM2101, and RM3773; Campylobacter lanienae NCTC 13003 and NCTC 13004; Campylobacter mucosalis ATCC 43264 and ATCC 43265; Campylobacter rectus ATCC 33238 and RM3276; Campylobacter showae ATCC 51146; and Campylobacter sputorum ATCC 33491 and ATCC 51146 were cultured on triple sugar iron medium at 37°C under anaerobic conditions as described previously (31). Bacteroides urealyticus NZRM 2009 was cultured on nonselective sheep blood agar at 37°C under anaerobic conditions. E. coli strains SM105 and SM101 were cultured aerobically at 30°C or 43°C on Luria-Bertani (LB) medium (Difco, Franklin Lakes, N.J.) or LB medium supplemented with 1.5% agar.
All other bacterial species (Arcobacter cryaerophilus ATCC 43158 and ATCC 49615; Arcobacter butzleri ATCC 49616, RM1588, and RM1591; Arcobacter nitrofigilis ATCC 33309; Arcobacter skirrowii CCUG10374, ATCC 51400, and LMG10234; Bacillus cereus NCTC 8035; Bacillus subtilis NCTC 3610; Enterobacter aerogenes NCTC 10006; Enterobacter faecalis NCTC 775; Escherichia coli ATCC 25922; Helicobacter pylori NZRM2925; Klebsiella pneumoniae NCTC 9633; Listeria innocua NCTC 11288; Listeria monocytogenes NCTC 7073; Morganella morganii NCTC 235; Proteus vulgaris ATCC 13315; Pseudomonas aeruginosa NCTC 10662; Salmonella enterica serovar Menston NCTC 7836; and Shigella flexneri NCTC 5) were cultured overnight in brain heart infusion broth (Difco) at 35°C. Ampicillin was added at a concentration of 50 μg ml−1 as required to maintain expression plasmids.
Nonreference clinical and environmental Campylobacter isolates.
Additional Campylobacter organisms, designated nonreference isolates, were obtained from clinical (n = 16) and environmental (n = 92) sources in South Canterbury, New Zealand (2, 51). The majority of these isolates were obtained from surface water (n = 34), with seven isolates obtained from beef feces, four isolates from beef offal, seven isolates from whole chicken carcasses, 10 isolates from dairy cow feces, five isolates from dog feces, four isolates from duck feces, one isolate from rabbit feces, 13 isolates from sheep feces, and seven isolates from sheep offal.
Phenotypic characterization.
Clinical and environmental isolates were examined for darting motility and Gram stained for identification to the genus level. To tentatively establish species identification, conventional methods available for sodium hippurate hydrolysis and nalidixic acid sensitivity were used (24).
Genomic DNA extractions.
Validation of the lpxA multiplex PCR was conducted with purified genomic DNA for reference strains. Bacterial growth from a single agar plate was harvested with 4 ml of sterile phosphate-buffered saline and a sterilized glass rod. A 1-ml aliquot of cellular growth was transferred to a 1.5-ml microcentrifuge tube, and cells were collected by centrifugation in a benchtop centrifuge at 5,800 × g, 4°C, for 5 min. Genomic DNA was extracted with the method of Pospiech and Neumann (34). DNA was quantified with a Hitachi U2000 spectrophotometer. Extracted genomic DNA was stored at −20°C in sterile distilled water supplemented with a final RNase A concentration of 20 μg ml−1.
Campylobacter genomic DNA from clinical and environmental isolates was extracted by heat lysis of bacterial cell cultures (21). For these experiments, bacteria were harvested after 48 h of growth from culture medium and suspended in 2 ml of sterile distilled water with a sterile cotton applicator stick. A 1-ml aliquot of the cell suspension was transferred to a 1.5-ml microcentrifuge tube, and the cell mixture was boiled in a water bath for 10 min. Cell debris was pelleted by centrifugation at 10,300 × g, 4°C, for 10 min. The supernatant was transferred to a new 1.5-ml tube and subsequently used as a template for PCR amplification.
Cloning of the C. jejuni lpxA gene.
An F38011 plasmid expression library (20) was used to transform E. coli strain SM105 [lpxA(Ts)] to ampicillin resistance (Apr). Ampicillin-resistant transformants were patch streaked onto two plates of LB plus agar and ampicillin and incubated at either 30 or 43°C. Plasmids were recovered from transformants capable of growth at the nonpermissive temperature (43°C). The insert from one plasmid, pCI1, was subsequently sequenced and characterized further (17).
PCR.
The oligodeoxynucleotide sequencing primers used were obtained from Sigma Genosys (The Woodlands, Tex.). The lpxA nucleotide sequence of C. coli isolate M275 was determined with primers lpxAF9625 and lpxAR0025 (Table 2). Primers 0301 and 0304 were used to amplify a nearly full-length copy of lpxA and the upstream flanking region (793 bp) from isolates of C. coli, C. jejuni, C. lari, and C. upsaliensis. The primer pair lpxAF0301 and lpxARKK2m was used to generate a 521-bp PCR amplicon from additional isolates of C. coli, C. jejuni, C. lari, and C. upsaliensis. Forward primers complementary to the lpxA nucleotide sequence of C. coli (lpxAC. coli), C. jejuni (lpxAC. jejuni), C. lari (lpxAC. lari), and C. upsaliensis (lpxAC. upsaliensis) were designed with the program Oligo (version 6, MBI Inc, Cascade, Colo.). In combination with the primer lpxARKK2m, amplicons of 391, 331, 233, and 206 bp, respectively, were generated.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| Forward primers | |
| IpxAF9625 | TGC GTC CTG GAG ATA GGC |
| 0301 | CTT AAA GCN ATG ATA GTR GAY AAR |
| IpxAC. coli | AGA CAA ATA AGA GAG AAT CAG |
| IpxAC. jejuni | ACA ACT TGG TGA CGA TGT TGT A |
| lpxAC. lari | TRC CAA ATG TTA AAA TAG GCG A |
| lpxAC. upsaliensis | AAG TCG TAT ATT TTC YTA CGC TTG TGT G |
| Reverse primers | |
| lpxAR0025 | TAG GCA TTA TTT TTA CCC CTA TAG ACA G |
| 0304 | ACA GGR ATT CCR CGY TTT GTY TC |
| lpxARKK2m | CAA TCA TGD GCD ATA TGA SAA TAH GCC AT |
For PCR assays, 10 pmol of forward primer μl−1 and 10 pmol of reverse primer μl−1 were mixed in a 25-μl reaction volume containing 200 μM each deoxynucleoside triphosphate (Invitrogen, Carlsbad, Calif.), 1× reaction buffer without MgCl2 (Qiagen, Valencia, Calif.), an additional 2 mM MgCl2 (Qiagen, Valencia, Calif.), and 1.25 units of Taq DNA polymerase (Qiagen). Genomic DNA, 20 to 50 ng, or the equivalent amount of DNA from a whole-cell lysate was then added, and the reaction mixtures were placed into an MJ Research (Waltham, Mass.) thermocycler. The cycling conditions used were 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min, with a final extension time of 5 min (35 cycles total). For multiplex PCR assays, 10 pmol of each forward primer μl−1 was added to the reaction mixture with 30 pmol of the lpxARKK2m reverse primer μl−1. Each reaction mixture was examined by agarose gel electrophoresis through 3% agarose, and bands were visualized with UV light after staining with ethidium bromide. Images were captured on a Kodak Electrophoresis and Documentation Analysis System 120 (Fisher Scientific, Pittsburgh, Pa.).
Nucleotide sequence analysis.
The DNA sequence of the full-length lpxA gene from C. coli isolate M275, C. jejuni isolate F38011, and the remaining lpxA PCR amplicons were determined either with an ABI Prism 377 or with an ABI Prism 3100 genetic analyzer (Applied Biosystems, Foster City, Calif.). For nucleotide sequence reactions, dye terminator technology was used, and nucleotide sequence from both strands of the DNA template was determined. Nonincorporated nucleotides and oligonucleotide primers were removed from PCR mixtures with the DyeEx purification kit (Qiagen, Valencia, Calif.). Twofold redundancy of each PCR amplicon was achieved by determining the nucleotide sequence of both strands. The nucleotide sequence of the lpxA gene from C. jejuni isolate F38011 and C. coli isolate M275 were determined in two independent experiments, generating fourfold redundancy for these isolates. Accession numbers for the lpxA genes are listed in Table 1.
Phylogenetic analysis of the lpxA nucleotide sequence.
Nucleotide sequence data were aligned with the program Clustal W (45). Phylogenetic and molecular evolutionary analyses were conducted with MEGA version 2.1 (22). Phylogenetic trees were constructed with the unweighted pair group method with arithmetic mean analysis (40). Evolutionary distances were calculated with the method of Jukes and Cantor (18). Bootstrap analysis (10) was performed with 1,000 replicates.
RESULTS
Cloning and sequencing of the C. jejuni F38011 lpxA gene.
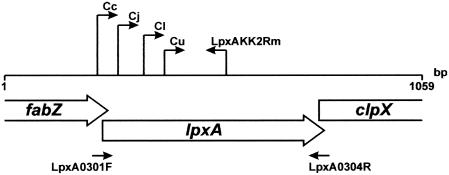
The lpxA gene from C. jejuni isolate F38011 was identified based on its ability to restore lipid A biosynthesis in E. coli strain SM105, which contains a temperature-sensitive mutation in lpxA, at 43°C (data not shown) (17). In total, 1,059 bp of lpxA and flanking DNA sequence was determined. The complete nucleotide sequence of the lpxA gene from C. jejuni F38011 was determined to be 789 bp, with an overall G+C content of 34.2 mol%. The nucleotide sequence of C. jejuni F38011 exhibits 98.9% identity with the lpxA gene of C. jejuni NCTC 11168 (33). The ATG start codon of C. jejuni F38011 lpxA overlaps the putative TAA stop codon of the upstream gene fabZ (Fig. 1). A partial open reading frame with amino acid similarity to that of the translated product of the gene clpX was located downstream (33).
FIG. 1.
Arrangement of the lpxA locus within the Campylobacter jejuni F38011 genome. The lpxA gene, flanked at the 5′ end by fabZ and at the 3′ end by clpX (direction of transcription shown by the arrows), is shown. The numbers indicate the nucleotide sequence obtained from the F38011-derived expression plasmid pCI1. Also indicated (solid arrowheads underneath the gene designations) are the relative positions of the PCR and sequencing primers lpxA0301F and lpxA0304R. The relative position of the species-specific primer positions are indicated as follows: Cc, C. coli; Cj, C. jejuni; Cl, C. lari; and Cu, C. upsaliensis, with LpxAKK2Rm representing the universal lpxA reverse primer.
Identification of Campylobacter species-specific lpxA sequences.
PCR primers flanking the C. jejuni lpxA gene (9625 and 0025) were used to amplify the lpxA region from C. coli. Comparison of the F38011 lpxA gene and flanking region sequences to the same region of the C. coli isolate M275 genome revealed 137 changes (12.9%, data not shown), including an 11-bp intergenic spacer separating fabZ from lpxA. The overall sequence divergence between these two species suggested that the lpxA locus might be useful in distinguishing C. jejuni from C. coli as well as from other thermotolerant Campylobacter spp. To determine if the fabZ-lpxA sequences could be used to distinguish Campylobacter isolates from one another, additional amplicons generated from phenotypically and genetically defined C. coli, C. jejuni, C. lari, and C. upsaliensis isolates were sequenced. Amplicons, generated with primers lpxAF0301 and lpxAR0304 were obtained from an additional 20 isolates of C. coli, 12 isolates of C. jejuni, 16 isolates of C. lari, and five isolates of C. upsaliensis (Table 1).
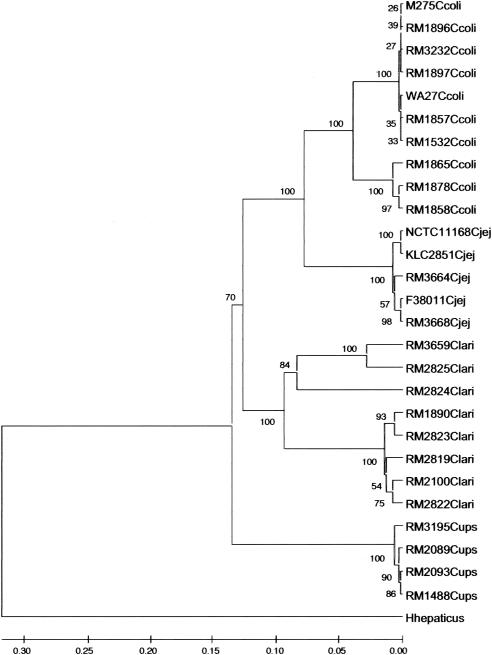
Phylogenetic analysis of the lpxA sequences from the 55 thermotolerant Campylobacter isolates clearly separated each species into distinct clades (Fig. 2). Intraspecies variation was also observed. Comparison of the C. coli isolates revealed two discrete clusters that possessed 96% identity. The larger of the two clusters consisted of seven highly similar lpxA alleles derived from 17 isolates of different geographies and animal hosts. Over the 757-bp sequence, only 5 bp were variable (0.66% difference). Of interest was that two isolates formerly identified as Campylobacter hyoilei (RM3230 and RM3231) were located in this cluster and were indistinguishable from C. coli isolate RM3232. The smaller C. coli branch was defined by 48 unique changes over the 757-bp sequence (6.34%). The three isolates constituting the smaller cluster, RM1858, RM1865, and RM1878, were clinical isolates and were over 98% identical (12 variable bp over 757 bp). The lpxA gene sequences from the C. jejuni isolates were more closely related to those of C. coli (less than 7% unique differences) than either C. lari or C. upsaliensis. The C. jejuni isolates were more homogeneous than the isolates of C. coli (greater than 98% identity), and five alleles were represented in these 13 isolates.
FIG. 2.
Phylogenetic analysis of the lpxA gene of 53 isolates of thermotolerant Campylobacter spp. The lpxA gene from isolates of C. coli, C. jejuni, C. lari, and C. upsaliensis was amplified from genomic DNA by PCR with primers lpxAF0301 and lpxAR0304. Amplicons were sequenced, and the primer regions were removed to facilitate the alignment. In total, 746 bp of C. jejuni, and C. lari nucleotide sequence, 754 bp of C. upsaliensis nucleotide sequence, and 757 bp of C. coli nucleotide sequence were aligned. The lpxA gene of Helicobacter hepaticus (ATCC 51499) was used to root the tree. The scale at the bottom of the figure is a measure of genetic identity. Numbers at the branches indicate the percent bootstrap support for each node. Note that the following isolates were not included in the figure for clarity; the lpxA sequences from these isolates were represented as follows. C. coli RM3232 also represents isolates RM3230 and RM3231 and M275 also represents isolates RM1051, RM1166, RM1505, RM1530, RM1531, RM1533, RM2225, and RM2228. C. jejuni RM3664 also represents isolates RM3665, RM3666, and RM3667; NCTC 11168 also represents ANR0493; RM3668 also represents RM3669 and RM3670; and F38011 also represents isolates RM1221, RM3672, and RM3673. C. lari RM2100 also represents RM2099, RM2808, RM2809, RM2817, RM2820, and RM2826 and RM1890 also represents RM2810 and RM2821. C. upsaliensis: RM1488 also represents RM2094.
The clades containing the C. lari and C. upsaliensis lpxA sequences were distinct from the C. coli and C. jejuni clades (88% and 86.5% nucleotide identity, respectively). Four of the five isolates of C. upsaliensis analyzed contained unique lpxA alleles. Too few samples were present to conclusively demonstrate geographic clustering within these samples. Similar to the situation within C. coli, analysis of the C. lari lpxA sequences revealed a heterogeneous collection of isolates composed of more than one distinctive cluster. The main cluster of 13 isolates had 30 variable nucleotides over 746 bp (4.0% variability) that resulted in five lpxA alleles. The second, smaller branch consisted of two isolates (RM2825 and RM3659) that differed at 38 of 746 bp (5.1%) and thus represented two distinct alleles of C. lari lpxA. One of these isolates, RM3659, was urease positive. These two isolates differed from the major C. lari group at 90 of 746 positions (12.1%). A third branch consisting of the RM2824 lpxA sequence was also present. This isolate had the most distinct lpxA allele of all the C. lari isolates sequenced, differing at 108 of 746 positions (14.5%) from the major C. lari group.
A more detailed comparison of the lpxA nucleotide sequences from the 55 thermotolerant isolates discussed above revealed several regions that could be used to develop species-specific probes (Fig. 3A to D). A unique feature of the C. coli lpxA sequences was an 11-bp intergenic spacer region separating fabZ from lpxA. C. upsaliensis also contained a similar intergenic spacer region of 8 bp, but this sequence differed in composition from that of C. coli. A sequence located 41 bp downstream from the lpxA start codon was highly conserved between C. jejuni isolates but diverged (8 or 9 of 22 bp within C. coli, 9 or 11 of 22 bp within C. lari, and 12 of 22 bp within C. upsaliensis) in other thermotolerant isolates. Similarly, a 20-bp region 141 bp downstream from the putative lpxA start site was strongly conserved among the majority of C. lari isolates (typified by isolate RM1890) but differed in 7 to 10 of 20 bp from isolates of C. upsaliensis, 6 to 8 of 20 bp from C. coli, and 6 to 9 of 20 bp between isolates of C. jejuni. The three isolates representing the lesser-represented branches of C. lari (RM2825, RM3659, and RM2824) were also divergent within this region. The nucleotide region representing C. upsaliensis differed from C. coli and C. jejuni in 9 to 11 positions and C. lari in 11 to 14 positions over the 27-bp region.
FIG. 3.
Nucleotide sequence alignments of lpxA alleles from C. jejuni, C. coli, C. lari, and C. upsaliensis isolates. The nucleotide sequences shown are composites of all of the sequences of the isolates of each species sequenced with the exception of C. coli and C. lari. The two major clusters of C. coli, designated 1 and 2, are individually displayed, with the species-specific primer shown in bold and underlined below the target species. C. coli 1 represent the C. coli isolates including M275, while C. coli 2 represents isolates such as RM1865. The three major clusters of C. lari are designated C. lari 1, 2 and 3 and represent isolates including RM1890, RM2825, and RM2824, respectively. An s indicates the species-specific oligonucleotide sequence. The numbering above each panel refers to the nucleotide sequencerelative to the C. jejuni lpxA start site (indicated as +1 in panel A). Note that the putative start codons for C. coli (GTG) and C. upsaliensis (TTG) differ from the ATG start codon of C. jejuni and C. lari. A dash (—) indicates identical nucleotides, and a star (*) indicates the presence of a gap. Divergent bases are indicated with the following nomenclature: R, A or G; Y, C or T; M, A or C; S, C or G; W, A or T; H, A or C or T; and D, A or T or G. Panel A, C. coli species-specific primer shown in bold and underlined; panel B, C. jejuni species-specific primer shown in bold and underlined; panel C, C. lari species-specific primer shown in bold and underlined; panel D, C. upsaliensis species-specific primer shown in bold and underlined; panel E; universal lpxA reverse primer LpxAKK2Rm is shown in bold and underlined.
We also sequenced a variable number of nucleotides (ranging from 176 to 661) from an additional two C. coli, nine C. jejuni, three C. lari, and 27 C. upsaliensis isolates from New Zealand, South Africa, and the United States to support our initial sequencing results (Table 1). These sequences revealed identity in the regions selected for the species-specific primers. However, an additional four C. jejuni, two C. upsaliensis, and one C. lari lpxA allele were uncovered. In total, the nucleotide sequences of 10 C. coli, 9 C. jejuni, 9 C. lari, and 14 C. upsaliensis lpxA alleles have been deposited in GenBank.
Generation of a multiplex PCR assay.
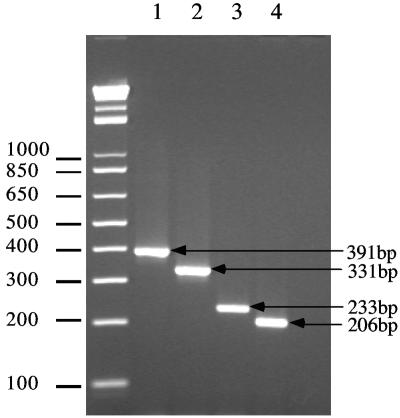
Based on the sequence data above, we designed species-specific primers to the unique regions of C. coli, C. jejuni, C. lari, and C. upsaliensis. The species-specific primers were tested against purified chromosomal DNA isolated from reference strains of the thermotolerant Campylobacter, nonthermotolerant Campylobacter, and non-Campylobacter isolates (Table 1 and Materials and Methods). The PCR assay showed almost 100% specificity (the ability of the primer to amplify the targeted species) and 97% sensitivity (the ability of the species-specific primer to amplify all members within the species). All of the C. coli, C. jejuni, and C. upsaliensis isolates reacted with their respective specific primer set to generate a PCR amplicon (Fig. 4, Table 1). Only 3 of the 20 C. lari isolates failed to generate a lari-specific PCR product; these nonreacting C. lari isolates have been shown to be genetically diverse from the main group of isolates (8) (Fig. 3C). Within each of the thermotolerant species, an amplicon was only generated with the specific-species primer set, and no cross-reactivity was observed with any of the species-specific primer sets to any other bacterial species tested with the exception of Campylobacter helveticus. The C. upsaliensis-specific primer set generated a weaker but detectable band in both isolates of C. helveticus (data not shown).
FIG. 4.
Multiplex PCR analysis of Campylobacter coli, Campylobacter jejuni, Campylobacter lari, and Campylobacter upsaliensis. Species-specific PCR amplicons were resolved after electrophoresis through a 3% agarose gel. Lane 1, C. coli (391 bp); lane 2, C. jejuni (331 bp); lane 3, C. lari (233 bp); and lane 4, C. upsaliensis (206 bp). A DNA ladder (in base pairs) is shown on the left-hand edge of the gel.
Whole-cell lysates made from environmental and clinical Campylobacter isolates were also tested by the multiplex PCR assay; 77 isolates from all sources except dog and rabbit feces were identified as C. jejuni, and 20 isolates from all sources except beef offal, whole chicken carcasses, and dog and duck feces were identified as C. coli. All five of the C. upsaliensis isolates identified were from dog feces, whereas four of the six isolates identified as C. lari were from surface water. The remaining two isolates were from clinical samples. The species identification for all isolates correlated with their species designations as determined previously (2, 51). These results indicate that a highly accurate species-specific multiplex PCR assay for the rapid identification of thermotolerant species of Campylobacter has been achieved. The PCR worked equally well in our hands with purified genomic DNA and whole-cell lysate material from field isolates.
DISCUSSION
The thermotolerant Campylobacter species C. coli, C. jejuni, C. lari, and C. upsaliensis continue to be a major concern with regard to human gastroenteritis. Epidemiological investigations have uncovered numerous point sources for these organisms, but in spite of this, there has been no apparent reduction in reported incidences. Molecular assays, such as the multiplex PCR assay developed in this study, should further expand our knowledge of Campylobacter epidemiology by allowing multiple species identification and detection from numerous potential point sources. The lpxA sequences that we obtained represent isolates from a range of habitats and geographies, mainly from the United States and New Zealand. Further genotypic data collected by pulsed-field gel electrophoresis support the groupings obtained with the lpxA sequence data (data not shown). The multiplex PCR assay described in this report was able to identify C. coli, C. jejuni, C. lari, and C. upsaliensis strains isolated from both human and environmental sources with either purified genomic DNA or DNA obtained after heat lysis.
The multiplex PCR primers used in this study were based on nucleotide sequences generated from a relatively large sample of thermotolerant isolates. Nucleotide sequence analysis of the lpxA gene from C. coli, C. jejuni, C. lari, and C. upsaliensis strains was readily able to discriminate these bacterial isolates into individual species. Within the lpxA sequence, regions of divergence and conservation were located to generate a robust, specific and sensitive PCR assay. Nucleotide sequence analysis also revealed a limited number of lpxA alleles within each species. In C. coli, an 11-nucleotide intergenic spacer separates the genes fabZ and lpxA. This spacer is completely absent from C. jejuni and C. lari isolates, and a modified version is present in C. upsaliensis. There are an additional 111 nucleotide changes over the length of the C. coli and C. jejuni lpxA genes (14%). Comparatively, the genes encoding heptosyl transferase I, an enzyme necessary for lipopolysaccharide core biosynthesis, and Campylobacter adhesion to fibronectin protein (CadF), have 19.8% and 12.6% nucleotide differences, respectively, between C. coli and C. jejuni (20, 21). Similarly, unique regions were located in the lpxA sequences of C. lari and C. upsaliensis, while overall the sequences were distinct from that of C. jejuni.
Recently, the ability of DNA sequences to distinguish between strains of C. jejuni and other organisms (6, 7, 43) has been reported. Given the variability in the lpxA nucleotide sequence observed within the thermotolerant species in this study, future nucleotide sequence analysis of lpxA alleles may also be a useful component in strain discrimination. Unlike the flaA gene, which has been analyzed in a similar manner (26), the lpxA gene is less likely to be influenced by recombination and is capable of discriminating between the closely related species C. coli and C. jejuni. While nucleotide sequence data have been used in the design of many of the existing species-specific Campylobacter primer sets, there are only a few examples in which the extent of sequence variability among isolates within or across species has been determined (26, 27, 48). Analysis of an increased number of isolates from a single species in the primer target region should facilitate the development of more accurate and precise primer sets.
An unanticipated finding of our study was the apparent heterogeneity that exists at a genetic level between two C. coli clusters. Others have argued that bacterial strains that are less than 97% identical at the 16S ribosomal DNA gene level will not give a DNA reassociation of greater than 60%, defining them as possibly new species (41). Clearly caution must be used in making this interpretation based solely on a single genetic marker such as the lpxA DNA sequence (47). We are currently in the process of extending our genetic and phenotypic analyses of C. coli in order to support our initial findings with the lpxA allele.
Taxonomically, urease-positive thermophilic Campylobacter spp. have been classified as C. lari (3). Several investigators have found that isolates within the C. lari taxon are very heterogeneous (8, 25). With multilocus enzyme electrophoresis, Matsuda et al. (25) showed that urease-positive thermophilic campylobacters are genetically hypervariable and form a cluster separate from the main C. lari cluster, while Duim et al. (8) used fragment length polymorphism, whole-cell protein profiles, and limited DNA-DNA hybridization analyses to reach a similar conclusion. In fact, Duim et al. suggest that there may be as many as five genogroups.
While our single-gene data do not permit as extensive a phylogenetic analysis as multilocus enzyme electrophoresis or fragment length polymorphism, it may be possible, with the lpxA sequence, to rapidly identify members of each genogroup. It is therefore of interest to note that with the eight overlapping strains used in this study and that of Duim et al. (8), identical classification results were obtained (i.e., RM2817, RM2819, RM2821, RM2822, RM2823, and RM2826 are all group 1, RM2825 is a group III isolate, and RM2824 is a group IV isolate). We are in the process of validating this initial observation.
In summary, we have described a multiplex PCR method that rapidly identifies thermotolerant campylobacters as C. coli, C. jejuni, C. lari, and C. upsaliensis. We are currently expanding the Campylobacter taxa distinguished by the lpxA gene as well as investigating the relationship among isolates of C. coli and C. lari as revealed by their lpxA nucleotide sequences.
Acknowledgments
We thank Paula Scholes (Institute for Environmental Science and Research, Limited, Christchurch Science Centre, Christchurch, New Zealand) for expert technical help.
This work was supported, in part, by a grant from the USDA-CSREES National Integrated Food Safety Initiative (Proposal No. 2002-03842) as well as grants from the Brian Mason Science and Technical Trust (New Zealand) and the University of Canterbury (New Zealand) research fund.
REFERENCES
- 1.Al Rashid, S. T., I. Dakuna, H. Louie, D. Ng, P. Vandamme, W. Johnson, and V. L. Chan. 2000. Identification of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, Arcobacter butzleri, and A. butzleri-like species based on the glyA gene. J. Clin. Microbiol. 38:1488-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, M., A. Ball, M. Devane, N. Garrett, B. Gilpin, A. Hudson, J. Klena, C. Nicol, M. Savill, P. Scholes, and D. Williams. 2002. Potential transmission routes of Campylobacter from environment to humans. A report to the New Zealand Ministry of Health. Christchurch Science Center of the Institute of Environmental Science and Research, Ltd., Christchurch, New Zealand.
- 3.Bolton, F. J., A. V. Holt, and D. Hutchinson, N. 1985. Urease-positive thermophilic campylobacters. Lancet i:1217-1218. [DOI] [PubMed] [Google Scholar]
- 4.Chuma, T., S. Hashimoto, and K. Okamoto. 2000. Detection of thermophilic Campylobacter from sparrows by multiplex PCR: the role of sparrows as a source of contamination of broilers with Campylobacter. J. Vet. Med. Sci. 62:1291-1295. [DOI] [PubMed] [Google Scholar]
- 5.Cloak, O. M., and P. M. Fratamico. 2002. A multiplex polymerase chain reaction for the differentiation of Campylobacter jejuni and Campylobacter coli from a swine processing facility and characterization of isolates by pulsed-field gel electrophoresis and antibiotic resistance profiles. J. Food Prot. 65:266-273. [DOI] [PubMed] [Google Scholar]
- 6.Dingle, K. E., F. M. Colles, D. R. A. Wareing, R. Ure, A. J. Fox, F. E. Bolton, H. J. Bootsma, R. J. L. Willems, R. Urwin, and M. C. J. Maiden. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 39:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dingle, K. E., N. van den Braak, F. M. Colles, L. J. Price, D. L. Woodward, F. G. Rodges, H. P. Endtz, A. van Belkum, and M. C. J. Maiden. 2001. Sequence typing confirms that Campylobacter jejuni strains associated with Guillain-Barré and Miller-Fisher syndromes are of diverse genetic lineage, serotype and flagella type. J. Clin. Microbiol. 39:3346-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duim, B., J. A. Wagenaar, J. R. Dijkstra, J. Goris, H. P. Endtz, and P. A. R. Vandamme. 2004. Identification of distinct Campylobacter lari genogroups by amplified fragment length polymorphism and protein electric profiles. Appl. Environ. Microbiol. 70:18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyers, M., S. Chapelle, G. Van Camp, H. Goossens, and D. De Wachter. 1993. Discrimination among thermophilic Campylobacter species by polymerase chain reaction amplification of 23S rRNA gene fragments. J. Clin. Microbiol. 31:3340-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 11.Fermér, C., and E. O. Engvall. 1999. Specific PCR identification and differentiation of the thermophilic campylobacters Campylobacter jejuni, C. coli, C. lari, and C. upsaliensis. J. Clin. Microbiol. 37:3370-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frost, J. A. 2001. Current epidemiological issues in human campylobacteriosis. J. Appl. Microbiol. 90:85S-95S. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez, I., K. A. Grant, P. T. Richardson, S. F. Park, and M. D. Collins. 1997. Specific identification of the enteropathogens Campylobacter jejuni and Campylobacter coli by using a PCR test based on the ceuE gene encoding a putative virulence determinant. J. Clin. Microbiol. 35:759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorkiewicz, G., G. Feierl, C. Schober, F. Dieber, J. Köfer, R. Zechner, and E. L. Zechner. 2003. Species-specific identification of campylobacters by partial 16S rRNA gene sequencing. J. Clin. Microbiol. 41:2537-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath, R. J., and C. O. Rock. 1996. Roles of the FabA and FabZ beta-hydroxyacyl-acyl carrier protein dehydratases in Escherichia coli fatty acid biosynthesis. J. Biol. Chem. 271:27795-27801. [DOI] [PubMed] [Google Scholar]
- 16.Hurtado, A., and R. J. Owen. 1997. A molecular scheme based on 23S rRNA gene polymorphisms for rapid identification of Campylobacter and Arcobacter species. J. Clin. Microbiol. 35:2401-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibbitt, J. C. 1997. Cloning and characterisation of the lpxA gene in Campylobacter jejuni. M.Sc. University of Canterbury, Christchurch, New Zealand.
- 18.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, New York, N.Y.
- 19.Keramas, G., D. D. Bang, M. Lund, M. Madsen, S. E. Rasmussen, H. Bunkenborg, P. Telleman, and C. B. V. Christensen. 2003. Development of a sensitive DNA microarray suitable for rapid detection of Campylobacter spp. Mol. Cell. Probes 17:187-196. [DOI] [PubMed] [Google Scholar]
- 20.Klena, J. D., S. A. Gray, and M. E. Konkel. 1998. Cloning, sequencing, and characterization of the lipopolysaccharide biosynthetic enzyme heptosyltransferase I gene (waaC) from Campylobacter jejuni and Campylobacter coli. Gene 222:177-185. [DOI] [PubMed] [Google Scholar]
- 21.Konkel, M. E., S. A. Gray, B. J. Kim, S. G. Garvis, and J. Yoon. 1999. Identification of the enteropathogens Campylobacter jejuni and Campylobacter coli based on the cadF virulence gene and its product. J. Clin. Microbiol. 37:510-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 23.Labarca, J. A., J. Sturgeon, L. Borenstein, N. Salem, S. M. Harvey, E. Lehnkering, R. Reporter, and L. Mascola. 2002. Campylobacter upsaliensis: another pathogen for consideration in the United States. Clin. Infect. Dis. 34:e59-60. [DOI] [PubMed] [Google Scholar]
- 24.Lior, H. 1984. New, extended biotyping scheme for Campylobacter jejuni, Campylobacter coli, and “Campylobacter laridis.” J. Clin. Microbiol. 20:636-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuda, M., A. Kaneko, T. Stanley, B. C. Millar, M. Miyajima, P. G. Murphy, and J. E. Moore. 2003. Characterization of urease-positive thermophilic campylobacter subspecies by multilocus enzyme electrophoresis. Appl. Environ. Microbiol. 69:3308-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meinersmann, R. J., L. O. Helsel, P. I. Fields, and K. L. Hiett. 1997. Discrimination of Campylobacter jejuni isolates by fla gene sequencing. J. Clin. Microbiol. 35:2810-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metherell, L. A., J. M. J. Logan, and J. Stanley. 1999. PCR-enzyme-linked immunosorbent assay for detection and identification of Campylobacter species: application to isolates and stool samples. J. Clin. Microbiol. 37:433-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohan, S., T. M. Kelly, S. S. Eveland, C. R. H. Raetz, and M. S. Anderson. 1994. An Escherichia coli gene (fabZ) encoding (3R)-hydroxymyristoyl acyl carrier protein dehydrase. Relation to fabA and suppression of mutations in lipid A biosynthesis. J. Biol. Chem. 269:32896-32903. [PubMed] [Google Scholar]
- 29.Olive, D. M., and P. Bean. 1999. Principles and applications of methods for DNA-based typing of microbial organisms. J. Clin. Microbiol. 37:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.On, S. L. W. 1996. Identification methods for campylobacters, helicobacters, and related organisms. Clin. Microbiol. Rev. 9:405-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.On, S. L. W., H. I. Atabay, and J. E. L. Corry. 1999. Clonality of Campylobacter sputorum bv. paraureolyticus determined by macrorestriction profiling and biotyping, and evidence for long-term persistent infection in cattle. Epidemiol. Infect. 122:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.On, S. L. W., and P. J. Jordan. 2003. Evaluation of 11 PCR assays for species-level identification of Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 41:330-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quall, M. A. Rajandrean, K. M. Rutherford, A. H. M. Van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 34.Pospiech, A., and B. Neumann. 1995. A versatile quick prep of genomic DNA from Gram-positive bacteria. Trends Genet. 11:217-218. [DOI] [PubMed] [Google Scholar]
- 35.Prasad, K. N., A. K. Dixit, and A. Ayyagari. 2001. Campylobacter species associated with diarrhoea in patients from a tertiary care centre of north India. Indian J. Med. Res. 114:12-17. [PubMed] [Google Scholar]
- 36.Pridmore, A. C., and Others. 2001. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via toll-like receptor (TLR) 2 but not via TLR/MD2. J. Infect. Dis. 183:89-96. [DOI] [PubMed] [Google Scholar]
- 37.Priest, F., G., and B. Austin. 1993. Modern bacterial taxonomy, 2nd ed. Chapman and Hall, New York, N,Y.
- 38.Raetz, C. R. H., and W. Dowhan. 1990. Biosynthesis and function of phospholipids in Escherichia coli. J. Biol. Chem. 265:1235-1238. [PubMed] [Google Scholar]
- 39.Servos, S., S. Khan, and D. Maskell. 1996. Cloning and expression of genes encoding lipid A biosynthesis from Haemophilus influenza type b. Gene 175:137-141. [DOI] [PubMed] [Google Scholar]
- 40.Sneath, P. H. A., and R. R. Sokal. 1973. Numerical taxonomy, p. 230-234. W. H. Freeman and Company, San Francisco, Calif.
- 41.Stackebrandt, E., and B. M. Goebel. 1994. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 42.Steeghs, L., M. Berns, J. ten Hove, A. de Jong, P. Roholl, L. van Alphen, J. Tommassen, and P. van der Ley. 2002. Expression of foreign LpxA acyltransferases in Neisseria meningitidis results in modified lipid A with reduced toxicity and retained adjuvant activity. Cell. Microbiol. 4:599-611. [DOI] [PubMed] [Google Scholar]
- 43.Suerbaum, S., M. Lohrengel, A. Sonnevend, F. Ruberg, and M. Kist. 2001. Allelic diversity and recombination in Campylobacter jejuni. J. Bacteriol. 183:2553-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tauxe, R. V. 1992. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 9-19. In I. Nachamkin, M. J. Blaser, and L. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology Press, Washington, D.C.
- 45.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandamme, P. 2000. Taxonomy of the family Campylobacteraceae, p. 3-27. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter. American Society for Microbiology Press, Washington, D.C.
- 47.Vandamme, P., B. Pot, M. Gillis, P. De Vos, K. Kersters, and J. Swings. 1996. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol. Rev. 60:407-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Doorn, L.-J., B. A. J. Giesendorf, R. Bax, B. A. M. van der Zeijst, P. VanDamme, and W. G. V. Quint. 1997. Molecular discrimination between Campylobacter jejuni, Campylobacter coli, Campylobacter lari and Campylobacter upsaliensis by polymerase chain reaction based on a novel putative GTPase gene. Mol. Cell. Probes 11:177-185. [DOI] [PubMed] [Google Scholar]
- 49.Volokhov, D., V. Chizhikov, K. Chumakov, and A. Rasooly. 2003. Microarray-based identification of thermophilic Campylobacter jejuni, C. coli, C. lari, and C. upsaliensis. J. Clin. Microbiol. 41:4071-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wassenaar, T. M., and D. G. Newell. 2000. Genotyping of Campylobacter spp. Appl. Environ. Microbiol. 66:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Werno, A., J. D. Klena, G. M. Shaw, and D. R. Murdoch. 2002. Fatal case of Campylobacter lari prosthetic joint infection and bacteremia in an immunocompetent patient. J. Clin. Microbiol. 40:1053-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]