Abstract
Introduction
The association between age at injury (AAI) and long-term cognitive outcome of traumatic brain injuries (TBI) is debatable.
Methods
Eligible participants with a history of TBI from Alzheimer's Disease Neuroimaging Initiative were divided into a childhood TBI (cTBI) group (the AAI ≤ 21 years old) and an adult TBI (aTBI) group (the AAI > 21 years old).
Results
The cTBI group has a higher Everyday Cognition total score than the aTBI group. All perceived cognitive functions are worse for the cTBI group than for the aTBI group except memory. By contrast, the cTBI group has higher assessment scores on either the Boston Naming Test or Rey Auditory Verbal Learning Test than the aTBI group.
Discussion
The AAI is associated with the long-term cognitive outcomes in older adults with a history of TBI.
Keywords: Alzheimer's disease, Executive function, Dementia, TBI
1. Introduction
Age at injury (AAI) has been shown to influence cognitive outcome in patients with traumatic brain injuries (TBI) [1]. However, the association between AAI and the long-term cognitive outcome of TBI is debatable. On one hand, the AAI was not associated with the cognitive outcome measured within the first year after TBI for children who sustained TBI before 6 years of age [2]. The AAI was also not an effective predictor for the long-term cognitive outcome in patients with a history of severe TBI when they were evaluated at an average of 14 years after TBI [3]. On the other hand, pediatric TBI patients with a younger AAI (<8 years old) were associated with a worse cognitive outcome when they were tested at least 6 years after TBI [4]. Moreover, a younger AAI was reported to be associated with a better long-term cognitive outcome of TBI in a study with a follow-up duration of 30 years [1]. Although the AAI has been studied with regard to its effects on cognitive performance in young patients with TBI [4], [5], [6], [7], no study has been done to compare the long-term cognitive outcome between patients sustained with childhood TBI (cTBI) and adult TBI (aTBI). In this report, the AAI was investigated for its relationship with the long-term cognitive outcome of TBI by analyzing the cognitive performance of elderly participants enrolled in the Alzheimer's Disease Neuroimaging Initiative (ADNI). The findings have important implications for making prognosis and therapeutic plans for patients with a history of TBI.
2. Methods
2.1. ADNI
Data used in the preparation of this report were obtained from the ADNI database (adni.loni.usc.edu). ADNI is the result of efforts by many coinvestigators from a broad range of academic institutions and private corporations. Participants have been recruited from over 50 sites across the United States and Canada. To date, ADNI has recruited over 1800 adults, aged 55 to 90 years, to participate in the research, consisting of cognitively normal older individuals, people with early or late mild cognitive impairment, and people with early Alzheimer's disease (AD). Further information can be found at http://www.adni-info.org/ and in previous reports [8], [9], [10], [11], [12], [13].
2.2. AAI
ADNI participants with a TBI history were selected by searching keywords from the medical history database as previously described [14]. For multiple traumatic brain injuries with the same severity (mild vs. moderate or severe), the date for the first injury was used to determine the AAI. When one TBI was more severe than the other ones, the date for the most severe TBI was used to derive the AAI. Based on the AAI, all participants with a history of TBI were divided into a cTBI group (the AAI ≤ 21 years old) and an aTBI group (the AAI > 21 years old).
2.3. Cognitive assessments
All the participants had completed a battery of neuropsychological tests including Everyday Cognition (ECog) ratings, Boston Naming Test (BNT), and Rey Auditory Verbal Learning Test (RAVLT). The subjective ECog ratings are used to assess the participants' perceptions about their capability to perform normal everyday tasks, in comparison to activity levels 10 years prior, on a five-point scale (1 = no change or actually performs better than 10 years ago; 2 = occasionally performs the task worse but not all of the time; 3 = consistently performs the task a little worse than 10 years ago; 4 = performs the task much worse than 10 years ago; 5 = participant/caregiver does not know) [15]. The ECog ratings cover multiple cognitive domains, including language, memory, visual spatial ability, and executive function, including planning, organization, and divided attention. The BNT is a language function test sensitive to both aphasia and object recognition deficit with a maximum score of 30 points. The RAVLT is a test for episodic memory to recall a list of words immediately after presentation and recall and recognize the words after a 30-minute delay interval [16].
2.4. Statistic analysis and figures
Two-way analysis of covariance was used to compare ECog ratings and cognitive performance on the BNT and RAVLT between the cTBI and aTBI groups, using AAI and baseline diagnosis as independent variables. Baseline age, gender, and education were controlled as potential confounding factors. A multivariate analysis of covariance (MANCOVA) model was used to compare ECog performance in different domains between the cTBI and aTBI groups. Results are shown in the form of mean ± standard error, and P < .05 is considered as significant for all statistical analyses with SPSS (version 23.0; IBM Corp., Armonk, NY, USA). Figures were created using Microsoft Excel or SigmaPlot (version 10.0).
3. Results
3.1. The AAI shows a bimodal distribution
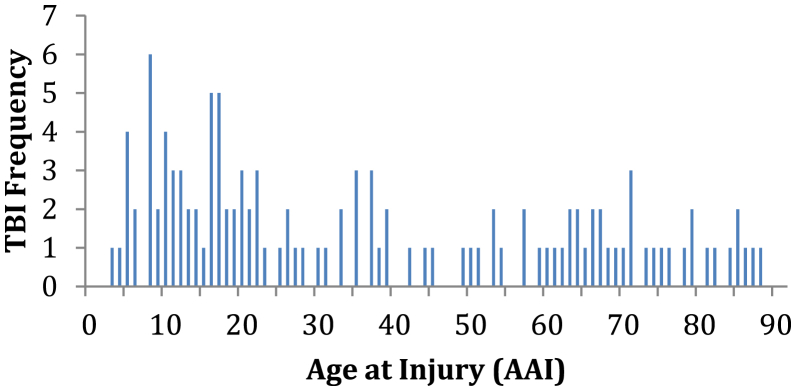
In this study, the average lag time was 39.21 ± 23.07 years (n = 119), which refers to the delay between the sustaining time of TBI and the cognitive assessment time. The AAI of all participants with TBI showed a typical bimodal distribution. The first peak appeared in the range of 5 to 40 years old; the other peak was in the range of 60 to 90 years old (see Fig. 1).
Fig. 1.
The AAI showed a bimodal distribution. Abbreviations: AAI, age at injury; TBI, traumatic brain injuries.
3.2. AAI is associated with ECog ratings
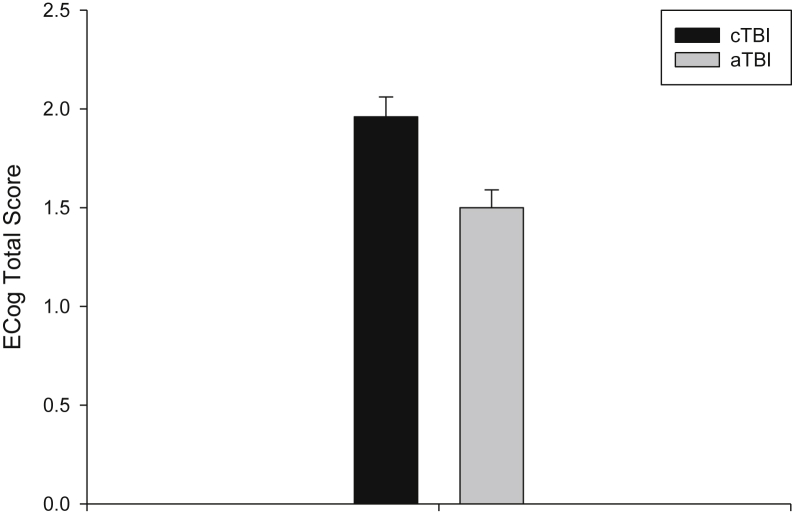
The cTBI group had an ECog total score of 1.96 ± 0.10 (95% confidence interval [CI]: 1.76–2.16, n = 31). This is higher than the same measure for the aTBI group of 1.50 ± 0.09 (95% CI: 1.32–1.69, n = 33, P = .002; Fig. 2). Furthermore, AAI interacts with baseline diagnosis for affecting the ECog total score (P = .012), such that the effects of AAI were seen in participants with a baseline diagnosis of cognitive impairments, especially those with AD, but not with cognitively normal participants. Subsequent MANCOVA showed that the cTBI group has significantly higher ECog assessment scores than the aTBI group for all ECog domains except memory (P = .105; Table 1).
Fig. 2.
The AAI affected the ECog total score. The cTBI group had a higher ECog total score than the aTBI group. Abbreviations: aTBI, adult TBI; AAI, age at injury; cTBI, childhood TBI; ECog, everyday cognition.
Table 1.
The cTBI group had more complaints on the ECog than the aTBI group in all domains
| ECog domain | cTBI | 95% CI | aTBI | 95% CI | P |
|---|---|---|---|---|---|
| Executive function | 1.90 ± 0.11 (n = 31) | 1.68–2.12 | 1.38 ± 0.10 (n = 32) | 1.17–1.58 | .001 |
| Organization | 1.91 ± 0.12 (n = 31) | 1.68–2.15 | 1.45 ± 0.12 (n = 32) | 1.22–1.68 | .009 |
| Planning function | 1.74 ± 0.11 (n = 31) | 1.51–1.97 | 1.13 ± 0.11 (n = 32) | 0.91–1.36 | .001 |
| Language | 2.05 ± 0.13 (n = 31) | 1.79–2.32 | 1.49 ± 0.13 (n = 32) | 1.23–1.75 | .005 |
| Divided attention | 2.07 ± 0.15 (n = 31) | 1.78–2.36 | 1.53 ± 0.14 (n = 32) | 1.25–1.82 | .013 |
| Visual spatial ability | 1.66 ± 0.09 (n = 31) | 1.48–1.84 | 1.24 ± 0.09 (n = 32) | 1.06–1.42 | .003 |
| Memory | 2.23 ± 0.14 (n = 31) | 1.95–2.50 | 1.90 ± 0.13 (n = 32) | 1.64–2.17 | .105 |
Abbreviations: cTBI, childhood TBI; ECog, everyday cognition; aTBI, adult TBI; CI, confidence interval.
3.3. AAI is associated with performance on BNT and RAVLT
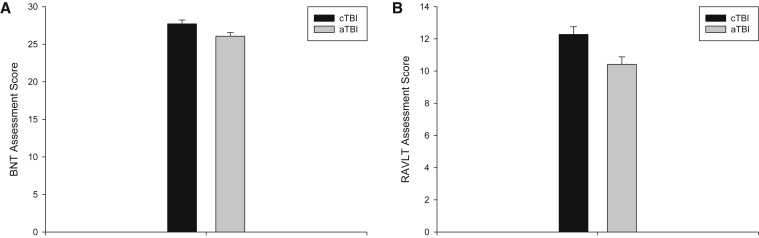
The analyses showed that the cTBI and aTBI groups have significantly different performance on the BNT or RAVLT delayed recognition (Fig. 3). The BNT total score is 27.72 ± 0.50 (95% CI: 26.72–28.72, n = 47) for the cTBI group, which is higher than the same measure for the aTBI group of 26.07 ± 0.48 (95% CI: 25.11–27.03, n = 64, P = .025). Similarly, the cTBI group has a RAVLT total score of 12.28 ± 0.48 (95% CI: 11.32–13.23, n = 47), which is higher than the same measure for the aTBI group of 10.42 ± 0.46 (95% CI: 9.51–11.34, n = 64) (P = .009).
Fig. 3.
The AAI had effects on language and memory functions. The cTBI group had a significantly better performance than the aTBI group on two cognition function tests, the BNT (panel A) and the RAVLT delayed recognition (panel B). Abbreviations: aTBI, adult TBI; AAI, age at injury; BNT, Boston Naming Test; cTBI, childhood TBI; RAVLT, Rey Auditory Verbal Learning Test; TBI, traumatic brain injuries.
4. Discussion
This retrospective study investigated the association between AAI and the long-term cognitive outcome in elderly participants with a TBI history. The participants with a TBI history were divided into cTBI and aTBI groups based on the sustaining age of TBI. The cognitive performance on ECog ratings, BNT and RAVLT, was compared between the two groups of participants.
The mean lag time was more than 39 years in the present study. This is the longest follow-up duration for studying the long-term cognitive outcome of TBI in the literature. The bimodal distribution of AAI was expected because the youngest and the oldest members of a population are always at the greatest risk for sustaining TBI [17].
The cTBI group has higher ECog scores than the aTBI group in all domains, although the difference in the memory domain does not reach significance (Table 1). In general, a higher ECog item assessment score represents more subjective complaints. However, the ECog rating differences seen in this report are probably not clinically significant due to the small differences between the aTBI and cTBI groups. The absolute ECog rating values are below 2 for both cTBI and aTBI groups (2 = occasionally performs the task worse but not all of the time), suggesting minimal subjective complaints in these groups.
However, the cTBI group has a significantly better performance on both the BNT and RAVLT recognition scores than the aTBI group. The results suggest that immature brains are more resilient for maintaining the episodic memory/recognition and language functions than mature brains. The findings are consistent with those from a previous report that adult TBI patients with a relatively younger AAI (55–64 years old) were suggested to be more resilient to dementia development than those with an older AAI (65–74 years old) [18]. However, the convergence of subjective ratings (more complaints in cTBI) and objective performance (better performance in cTBI) is interesting and should be further investigated.
This study has several limitations. Preinjury social economic status (SES) has been shown to be associated with the long-term cognitive outcome of TBI [3]. Preinjury ability was also identified as a significant predictor of postinjury cognitive status in those patients with cTBI [19], [20]. However, neither preinjury SES nor preinjury ability was controlled as potential confounding factors in the present study due to unavailability of information. In addition, the history of TBI was based on self-report information from either participants or informants, which may bring some inaccuracy. Although injury severity has been reported to be associated with the long-term cognitive outcome of TBI [3], [21], [22], [23], the role of TBI severity was not investigated in this study because of the self-reported medical history. Finally, participants with a history of TBI were divided into the cTBI and aTBI groups using an arbitrary age of 21 years old. Because a significantly higher rate of cognitive dysfunction is only seen in patients with TBI sustaining after age 11 years than in the general population [24], it might be more reasonable to group patients by brain maturation phases in future studies [25].
In summary, the findings from this study showed that age at injury is associated with the long-term cognitive outcome of individuals with a history of TBI. People with TBI occurring before age 22 years had better cognitive performance in language and episodic memory/recognition than those with aTBI, despite an increased level of self-perceived cognitive decline in all cognitive domains on the ECog assessments.
Research in context.
-
1.
Systematic review: The association of age at injury (AAI) with the long-term cognitive outcome of traumatic brain injuries (TBI) is debatable. The authors reviewed the effects of AAI of TBI on the long-term cognitive outcomes using PubMed as the main literature source. The relevant citations are appropriately cited.
-
2.
Interpretation: AAI was demonstrated to be associated with the long-term cognitive outcome of individuals with a history of TBI. People with childhood TBI showed better cognitive performance in language and episodic memory/recognition than those with adult TBI.
-
3.
Future directions: Studies with large samples and a prospective design are needed to replicate the current findings. The present study can be deepened by investigating the role of the following factors: (1) TBI severity; (2) preinjury economic status and ability; (3) a logical classification of TBI based on the brain maturation process.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). Analyses in the present report were supported by NIA R01 AG19771 (to A.J.S.), NIA K01 AG049050 (S.L.R.), and funding from Indiana Alzheimer Disease Center (P30 AG10133). The authors recognize the contributions of Dr. Ulla Connor, Esen Gokpinar-Shelton, and Matthew Hume from the International Center for Intercultural Communication at IU School of Liberal Arts for their editorial support.
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Himanen L., Portin R., Isoniemi H., Helenius H., Kurki T., Tenovuo O. Longitudinal cognitive changes in traumatic brain injury: a 30-year follow-up study. Neurology. 2006;66:187–192. doi: 10.1212/01.wnl.0000194264.60150.d3. [DOI] [PubMed] [Google Scholar]
- 2.Prasad M.R., Ewing-Cobbs L., Swank P.R., Kramer L. Predictors of outcome following traumatic brain injury in young children. Pediatr Neurosurg. 2002;36:64–74. doi: 10.1159/000048355. [DOI] [PubMed] [Google Scholar]
- 3.Hoofien D., Vakil E., Gilboa A., Donovick P.J., Barak O. Comparison of the predictive power of socio-economic variables, severity of injury and age on long-term outcome of traumatic brain injury: sample-specific variables versus factors as predictors. Brain Inj. 2002;16:9–27. doi: 10.1080/02699050110088227. [DOI] [PubMed] [Google Scholar]
- 4.Verger K., Junqué C., Jurado M.A., Tresserras P., Bartumeus F., Nogués P. Age effects on long-term neuropsychological outcome in paediatric traumatic brain injury. Brain Inj. 2000;14:495–503. doi: 10.1080/026990500120411. [DOI] [PubMed] [Google Scholar]
- 5.Anderson V., Spencer-Smith M., Leventer R., Coleman L., Anderson P., Williams J. Childhood brain insult: can age at insult help us predict outcome? Brain. 2009;132:45–56. doi: 10.1093/brain/awn293. [DOI] [PubMed] [Google Scholar]
- 6.Donders J., Warschausky S. Neurobehavioral outcomes after early versus late childhood traumatic brain injury. J Head Trauma Rehabil. 2007;22:296–302. doi: 10.1097/01.HTR.0000290974.01872.82. [DOI] [PubMed] [Google Scholar]
- 7.Levin H.S., Culhane K.A., Mendelsohn D., Lilly M.A., Bruce D., Fletcher J.M. Cognition in relation to magnetic resonance imaging in head-injured children and adolescents. Arch Neurol. 1993;50:897–905. doi: 10.1001/archneur.1993.00540090008004. [DOI] [PubMed] [Google Scholar]
- 8.Jack C.R., Jr., Bernstein M.A., Borowski B.J., Gunter J.L., Fox N.C., Thompson P.M. Update on the magnetic resonance imaging core of the Alzheimer's disease neuroimaging initiative. Alzheimers Dement. 2010;6:212–220. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagust W.J., Bandy D., Chen K., Foster N.L., Landau S.M., Mathis C.A. The Alzheimer's Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement. 2010;6:221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen R.C., Aisen P.S., Beckett L.A., Donohue M.C., Gamst A.C., Harvey D.J. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saykin A.J., Shen L., Foroud T.M., Potkin S.G., Swaminathan S., Kim S. Alzheimer's Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimers Dement. 2010;6:265–273. doi: 10.1016/j.jalz.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trojanowski J.Q., Vandeerstichele H., Korecka M., Clark C.M., Aisen P.S., Petersen R.C. Update on the biomarker core of the Alzheimer's Disease Neuroimaging Initiative subjects. Alzheimers Dement. 2010;6:230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiner M.W., Aisen P.S., Jack C.R., Jr., Jagust W.J., Trojanowski J.Q., Shaw L. The Alzheimer's disease neuroimaging initiative: progress report and future plans. Alzheimers Dement. 2010;6:202–211. doi: 10.1016/j.jalz.2010.03.007. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W., Risacher S.L., McAllister T.W., Saykin A.J. Traumatic brain injury and age at onset of cognitive impairment in older adults. J Neurol. 2016;263:1280–1285. doi: 10.1007/s00415-016-8093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farias S.T., Mungas D., Reed B.R., Cahn-Weiner D., Jagust W., Baynes K. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rey A. Presses Universitaires de France; Paris: 1964. L'examen clinique en psychologie. [Google Scholar]
- 17.McCrea M.A. Oxford University Press; USA: 2008. Mild traumatic brain injury and postconcussion syndrome: the new evidence base for diagnosis and treatment. [Google Scholar]
- 18.Gardner R.C., Burke J.F., Nettiksimmons J., Kaup A., Barnes D.E., Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71:1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson V., Catroppa C. Recovery of executive skills following paediatric traumatic brain injury (TBI): a 2 year follow-up. Brain Inj. 2005;19:459–470. doi: 10.1080/02699050400004823. [DOI] [PubMed] [Google Scholar]
- 20.Anderson V., Le Brocque R., Iselin G., Eren S., Dob R., Davern T.J. Adaptive ability, behavior and quality of life pre and posttraumatic brain injury in childhood. Disabil Rehabil. 2012;34:1639–1647. doi: 10.3109/09638288.2012.656789. [DOI] [PubMed] [Google Scholar]
- 21.Anderson V., Catroppa C., Morse S., Haritou F., Rosenfeld J. Recovery of intellectual ability following traumatic brain injury in childhood: impact of injury severity and age at injury. Pediatr Neurosurg. 2000;32:282–290. doi: 10.1159/000028956. [DOI] [PubMed] [Google Scholar]
- 22.Aaro Jonsson C., Catroppa C., Godfrey C., Smedler A.C., Anderson V. Individual profiles of predictors and their relations to 10 years outcome after childhood traumatic brain injury. Brain Inj. 2013;27:831–838. doi: 10.3109/02699052.2013.775493. [DOI] [PubMed] [Google Scholar]
- 23.Johnson V.E., Stewart W. Traumatic brain injury: age at injury influences dementia risk after TBI. Nat Rev Neurol. 2015;11:128–130. doi: 10.1038/nrneurol.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teasdale T.W., Engberg A.W. Cognitive dysfunction in young men following head injury in childhood and adolescence: a population study. J Neurol Neurosurg Psychiatry. 2003;74:933–936. doi: 10.1136/jnnp.74.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson S.B., Blum R.W., Giedd J.N. Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J Adolesc Health. 2009;45:216–221. doi: 10.1016/j.jadohealth.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]