Highlights
-
•
CareHPV tests were used to compare screen-and-treat and colposcopy management.
-
•
Screen-and-treat strategy with HPV testing was found to be very cost-effective.
-
•
CAPE has screened > 25,000 women in the Paracentral region.
-
•
Over 70% of screen-positive women received recommended treatment within six months.
-
•
CAPE is an example of public-private partnership resulting in paradigm change.
Keywords: HPV, Cervical cancer, Screening, Implementation, Screen-and-treat
1. Cervical cancer landscape in El Salvador
Cervical cancer is the leading cause of cancer death among women in El Salvador (GLOBOCAN, 2013). At most, 47% of women are screened appropriately, (Murillo et al., 2008) and 40% of women with an abnormal diagnosis do not receive adequate follow-up (Agurto et al., 2006). Screening for high-risk human papilloma virus (HPV) is a relatively new strategy for detection. Following a positive HPV test, the World Health Organization (WHO) has endorsed both colposcopy referral and “screen-and-treat” as management strategies (Guidelines, 2011). Screen-and-treat programs with alternatives to colposcopy benefit low-resource countries such as El Salvador, as these are less costly and result in a higher proportion of women receiving treatment (Goldie et al., 2005). Since a low-cost HPV screening is a relatively new technology, the most effective strategy for implementation of an HPV testing-based screening program has not yet been established.
Here we describe a large-scale screening and treatment intervention in El Salvador as a partnership between the Ministry of Health (MINSAL) and Basic Health International (BHI), a non-governmental organization. The participation of multiple entities proved crucial in the success of the program. We detail the implementation process and collaboration among stakeholders as a blueprint for similar efforts in other low-resource settings.
2. Initial planning for cervical cancer prevention in El Salvador (CAPE)
At the International Papillomavirus Conference in July 2010, the biotechnology company Qiagen launched Qiagencares, a program dedicated to improving access to screening methods for infectious diseases in developing countries, with a particular focus on eliminating cervical cancer through public-private partnerships. One of the Qiagencares initiatives was the donation of 1 million low-cost careHPV tests (Qiagen Inc., Gaithersburg, MD), a tool developed in partnership with PATH and the Bill and Melinda Gates Foundation, to low-income countries over a five-year period (Solomon et al., 2002). El Salvador immediately expressed interest in implementing an HPV-testing program in partnership with BHI to target rural women between the ages of 30 and 49.
In November 2010, BHI organized stakeholder meetings that included representatives from MINSAL, the Pan American Health Organization (PAHO), the U.S. National Cancer Institute (NCI) at the National Institutes of Health (NIH), and professional organizations for demographics and reproductive health in El Salvador. The goal was to identify best practices to integrate HPV-testing into the existing cervical cancer control program. One strategy discussed was immediate cryotherapy treatment of all eligible HPV-positive women. Previous evidence suggests that this method results in lower prevalence of high-grade cervical cancer precursors (Denny et al., 2010). Consensus was reached to initiate a pilot implementation comparing this strategy with the conventional colposcopy management.
Representatives of Qiagen, BHI, and MINSAL met in El Salvador in January, 2011 to agree on the guidelines for submitting a proposal for the HPV tests donation. It was established that it was important to evaluate feasibility of screen-and-treat algorithms through follow-up rates and cost-effectiveness analyses. The team collaborated with epidemiologist, clinicians, and heath economists in order to evaluate these goals. In March 2011, BHI and MINSAL representatives visited a project sponsored by PATH in rural areas of Nicaragua to observe implementation of HPV testing (Jeronimo et al., 2014).
The proposal for the initial phase of the 2–5 year program was submitted to Qiagen in August 2011 and the donation was received a year later. A memorandum of understanding was signed between MINSAL and BHI where the latter committed to provide start-up financial support and serve as a technical advisor. This public-private partnership launched in October 2012 through the Cervical Cancer Prevention in El Salvador (CAPE) program (Fig. 1).
Fig. 1.
CAPE summary.
Phases 1 and 2 consisted of colposcopy and screen-and-treat management strategies, while all women were assigned to screen-and treat in Phase 3.
The participation of Qiagen was limited to the donation of tests and machines and training for lab technicians. The company had no part in implementation, data collection or analyses and no financial or personal relationship with the authors. Analyses were conducted retrospectively on de-identified data and all procedures were approved by the national ethics review board of El Salvador.
3. CAPE Phase 1
The initial donation consisted of 2000 HPV tests for provider collection and an additional 2000 tests for self-collection on the same women in four primary health units. Two machines were donated to process the tests. The health units that were chosen were places where BHI had a working relationship with local MINSAL personnel after previously conducting screening projects that involved visual inspection with acetic acid (VIA) (Cremer et al., 2010, Cremer et al., 2011).
CAPE personnel were MINSAL health providers who underwent training tailored to their role. Physicians and nurses received updates on the natural history of cervical cancer and tutorials on sample collection. Health promoters, lab technicians and support staff attended various educational sessions depending on their experience. All tests were processed in labs run by MINSAL.
The primary goal of Phase 1 was to identify the most successful screening plan as indicated by completion of six-month follow-up. The targeted 2000 women were assigned by MINSAL to one of two management strategies based on place of residence with the goal of placing equal numbers of women in each group. Demographic variables of participants in Phases 1 and 2 are provided on Table 1 (Cremer et al., 2016a, Cremer et al., 2016b). Women were visited by health promoters at home and invited to participate. Women then attended the local clinic for HPV screening. Those who tested HPV-positive received either, 1) colposcopy with or without biopsy as indicated, and care according to pathology results and local guidelines, or 2) visual assessment triage (VAT) and immediate treatment for cryotherapy-eligible women (“screen-and-treat”). BHI provided additional funding to contact eligible women after 6 months to maximize follow-up visits.
Table 1.
Demographic characteristics of women in CAPE Phases 1 and 2.
| Phase 1a |
Phase 2b |
|||
|---|---|---|---|---|
| Colposcopy management (n = 1000) | Screen-and-treat (n = 1000) | Colposcopy management (n = 3963) | Screen-and-treat (n = 4087) | |
| Mean age | 38.5 | 38.3 | 37.8 | 38.3 |
| Education | ||||
| None/element | 65.4% | 68.6% | 73.9% | 74.4% |
| Middle | 22.9% | 17.2% | 29.1% | 25.6% |
| Other | 11.7% | 14.3% | n/a | n/a |
| Sexual debut (age) | ||||
| < 16 | 19.6% | 17.4% | 24.4% | 26.4% |
| 16–19 | 50.8% | 52% | 38.1% | 38.8% |
| 20 + | 29.6% | 30.6% | 37.5% | 34.8% |
| Residence | ||||
| Rural | 95.8% | 89.6% | 80% | 84.9% |
| Urban | 4.2% | 10.4% | 20% | 15.1% |
| Last screened | ||||
| Never or > 3 yrs | 26% | 43.7% | 35.9% | 42.3% |
| < 3 yrs ago | 74% | 54.5% | 64.1% | 57.6% |
Concordance of HPV test results between self and provider samples was 98%. The screen-and-treat group was significantly more likely to complete treatment within the recommended time frame compared to women in the colposcopy cohort (98.3% vs. 68.8%, p < .001) (Cremer et al., 2016a). While Phase 1 established the feasibility of achieving high coverage and compliance with recommended care, outcomes were only observed for one round of screening.
To project long-term health and economic outcomes and inform policy-makers, a mathematical model of HPV infection and cervical carcinogenesis was constructed using epidemiologic data from El Salvador and empirical data from Phase 1. The evaluation by health economists concluded that a screen-and-treat strategy was more cost-effective than referral to colposcopy (Campos et al., 2015). Thus, MINSAL decided to scale up the initiative to investigate the optimal algorithm in a larger population. The scale-up aimed to reach over 60% of the target population in the original four health units.
4. CAPE Phase 2
In Phase 2 (October, 2013–July, 2014), CAPE expanded to include 8000 women across eight health units. Qiagen donated 16,000 tests for both provider- and self-sampling and two more careHPV machines. During Phase 1, two labs had processed approximately 4000 samples. In Phase 2, MINSAL made further investments including hiring information system personnel to begin work on a national registry and adding a dedicated staff person to process HPV samples. The rate of agreement between self-sampled and provider-sampled HPV tests was high (93.7%). Outcomes were similar to those of Phase 1 with women in the screen and treat model significantly more likely to complete treatment than their counterparts (88.4% vs. 44.2%, p < .001) (Cremer et al., 2016b).
5. CAPE Phase 3
Given previous results, MINSAL determined to proceed with only the screen-and-treat strategy beginning in May 2015. The goal was to provide an organized system to cover 20,000 women per year, a rate that would reach 80% of the target population within five years. All samples were collected by providers since product labeling does not indicate self-collection. Health promoters learned to identify under-screened women and paperwork was reduced from multiple forms to a single one. Preliminary numbers indicate that scale-up has been effective to date in reaching the target population with high follow-up rates for those in need of treatment (forthcoming).
6. New guidelines
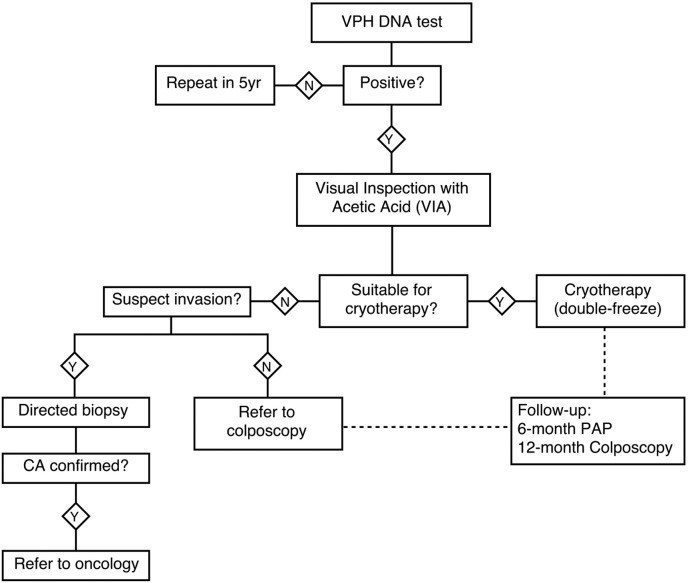
In March 2014, PAHO worked with MINSAL to host a policy dialog to update the Salvadorian national guidelines for cervical cancer screening and treatment. > 115 key stakeholders from medical associations—general physicians, gynecology, pathology, cytotechnicians—and members of the National Alliance for Cervical Cancer participated in the discussion. The centerpiece for discussion was the new PAHO/WHO guideline which promotes the use of all available screening tests (conventional cytology, liquid based cytology, visual inspection with acetic acid (VIA), and HPV DNA testing), and endorses treatment of precancerous lesions with cryotherapy, LEEP or cold knife conization, and provides various alternatives for service delivery. The most relevant recommendation from WHO was that immediate treatment for women who test HPV positive is the preferred approach because it is most effective and can reach the most women.
It took several months for all stakeholders to accept the new evidence that favored screen and treat over cytology management. Key thought leaders within MINSAL recognized barriers in the existing cytology-based screening program despite years of investment and helped make the case for a screen-and-treat program (Maza et al., 2016). Eventually, a decision was reached to update the national cervical cancer guideline to include the model exemplified by CAPE for women aged 30 to 59 (Fig. 2) (Lineamientos, 2015).
Fig. 2.
MINSAL's new guidelines.
In 2015, El Salvador's Ministry of Health published its new HPV-test cervical cancer screening guidelines for women aged 30 to 59.
7. Political will
The successful completion of Phase 1 demonstrated that HPV testing is feasible in low-resource settings. Continuing evidence-based education to Salvadorian providers, government officials and other stakeholders further demonstrated the advantages of HPV screening. The cost-effectiveness of this model was crucial in cementing support for the continuation of CAPE. Before Phase 3 began, MINSAL announced that cancer control is among its new priorities and ultimately made HPV testing a central part of its new guideline. This outcome was only possible through the continued engagement of MINSAL with public, private, national and international thought leaders. The collaboration among different stakeholders was essential in mobilizing and maintaining political will to support the program.
8. Financial support and collaborations
Multiple organizations provided support to CAPE. Costs were shared by BHI and MINSAL with BHI receiving major funding from the Einhorn Family Charitable Trust, the Union for International Cancer Control (UICC) in Phases 1 and 2, and by PATH and the Einhorn Family Charitable Trust in Phase 3.
Rotary International sponsored advocacy campaigns; MD Anderson Cancer Center, NCI/NIH, Global Coalition for Cervical Cancer (GC3), and the University of Southern California provided technical assistance and continuing medical education; and the American Society for Colposcopy and Cervical Pathology conducted clinical trainings. PAHO, the International Agency for Research on Cancer (IARC), and Center for Disease Control and Prevention (CDC) were essential in disseminating the new WHO guidelines to key stakeholders.
9. Opportunities and challenges
The most successful outcome of CAPE to date has been that in Phases 1 and 2, over 10,000 women have been screened with an overall estimate of 12% HPV positivity. > 70% of HPV positive women have received proper follow-up within six months, a significant improvement in screening coverage and treatment rates (Murillo et al., 2008, Maza et al., 2016). Incoming data suggests that Phase 3 will be equally encouraging. MINSAL continues to work on information systems to follow and monitor HPV positive women, a key element for the long-term success of the program. MINSAL has also announced plans to expand services for countrywide HPV testing and treatment as resources permit. Thus, an organized screening program system has replaced the opportunistic approach that had been historically used.
Significant challenges in the implementation of CAPE include the advocacy needed to counter health providers' resistance to changing the traditional paradigm and the logistics of introducing a new medical product into the country. On two occasions, HPV test shipments were lost due to lack of proper temperature control by local couriers. Special customs permissions were needed to release testing materials and MINSAL and BHI worked with Qiagen to fix or replace malfunctioning components and ensure reagents were properly transported and stored.
Finally, it is essential that procurement of HPV tests is included in the national budget if El Salvador is to successfully implement a national screening plan. Discussions of this issue involved multiple meetings between BHI and the Minister and the Vice Minister of Health. In 2017 MINSAL will procure tests to continue the regional implementation with the hope of phased national introduction of the program in the coming years. Although budgetary constraints remain a significant hurdle in the scale-up of cervical cancer control efforts, public-private partnerships can overcome obstacles to make meaningful changes at the national level.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
The authors gratefully acknowledge the support of the Einhorn Family Charitable Trust and PATH. This work has been supported by a grant received from UICC under the UICC Cervical Cancer Initiative.
Contributor Information
Philip E. Castle, Email: bhisubmissions@gmail.com.
Miriam Cremer, Email: mcremer@basichealth.org.
References
- Agurto I., Sandoval J., De La Rosa M., Guardado M. Improving cervical cancer prevention in a developing country. Int. J. Qual. Health Care. 2006;18(2):81–86. doi: 10.1093/intqhc/mzi100. [DOI] [PubMed] [Google Scholar]
- Campos N.G., Maza M., Alfaro K., Gage J.C., Castle P.E., Felix J.C. The comparative and cost-effectiveness of HPV-based cervical cancer screening algorithms in El Salvador. Int. J. Cancer. 2015;137(4):893–902. doi: 10.1002/ijc.29438. [DOI] [PubMed] [Google Scholar]
- Cremer M., Bullard K., Maza M., Peralta E., Moore E., Garcia L. Cytology versus visual inspection with acetic acid among women treated previously with cryotherapy in a low-resource setting. International journal of gynaecology and obstetrics. 2010;111(3):249–252. doi: 10.1016/j.ijgo.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Cremer M., Conlisk E., Maza M., Bullard K., Peralta E., Siedhoff M. Adequacy of visual inspection with acetic acid in women of advancing age. International journal of gynaecology and obstetrics. 2011;113(1):68–71. doi: 10.1016/j.ijgo.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Cremer M.L., Maza M., Alfaro K.M., Kim J.J., Ditzian L.R., Villalta S. Introducing a high-risk HPV DNA test into a public sector screening program in El Salvador. J. Low Genit. Tract. Dis. 2016;20(2):145–150. doi: 10.1097/LGT.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer M.L., Maza M., Alfaro K., Morales Velado M., Felix J., Castle P., Kim J., Gage J. Scale-up of an human papillomavirus testing implementation program in El Salvador. J. Low Genit Tract Dis. 2016;21(1):26–32. doi: 10.1097/LGT.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny L., Kuhn L., Hu C.-C., Tsai W.-Y., Wright T.C. Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial. J. Natl. Cancer Inst. 2010;102(20):1557–1567. doi: 10.1093/jnci/djq342. [DOI] [PubMed] [Google Scholar]
- GLOBOCAN . International Agency for Research on Cancer; 2013. 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]http://globocan.iarc.fr Available from: [Google Scholar]
- Goldie S.J., Gaffikin L., Goldhaber-Fiebert J.D., Gordillo-Tobar A., Levin C., Mahé C. Cost-effectiveness of cervical-cancer screening in five developing countries. N. Engl. J. Med. 2005;353(20):2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- Guidelines W.H.O. World Health Organization; Geneva: 2011. Use of Cryotherapy for Cervical Intraepithelial Neoplasia. [Internet]http://www.ncbi.nlm.nih.gov/books/NBK138476/ Available from: [PubMed] [Google Scholar]
- Jeronimo J., Bansil P., Lim J., Peck R., Paul P., Amador J.J. A multicountry evaluation of care HPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer. Int. J. Gynecol. Cancer. 2014;24(3):576–585. doi: 10.1097/IGC.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministerio de Salud, San Salvador; El Salvador: March 2015. Lineamientos técnicos para la prevención y control del cáncer cérvico uterino y de mama.http://asp.salud.gob.sv/regulacion/pdf/lineamientos/lineamientos_prevencion_cancer_cervico_uterino_y_de_mama_v3.pdf Available at. [Google Scholar]
- Maza M., Matesanz S., Alfaro K., Alonzo T.A., Masch R., Calderon S. Adherence to recommended follow-up care after high-grade cytology in El Salvador. Int J Healthcare. 2016;2(2):31. [Google Scholar]
- Murillo R., Almonte M., Pereira A., Ferrer E., Gamboa O.A., Jeronimo J. Cervical cancer screening programs in Latin America and the Caribbean. Vaccine. 2008;26(Suppl. 11):L37–L48. doi: 10.1016/j.vaccine.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Solomon D., Davey D., Kurman R. The 2001 Bethesda system: terminology for reporting results of cervical cytology. JAMA. 2002;287(16):2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]