Abstract
Introduction
To prospectively determine the diagnostic value of cerebrospinal fluid (CSF) levels total-tau (tau) to amyloid-β1–42 ratio (Aβ1–42) ratio (tau/Aβ1–42 ratio), phosphorylated-tau (p-tau) to tau ratio (p-tau/tau ratio), neurofilament light chain (NfL) and YKL40 in the late-onset frontal lobe syndrome, in particular for the differential diagnosis of behavioral variant frontotemporal dementia (bvFTD) versus primary psychiatric disorders (PSY).
Method
We included patients with a multidisciplinary 2-year-follow-up diagnosis of probable/definite bvFTD (n = 22) or PSY (n = 25), who underwent a detailed neuropsychiatric clinical examination, neuropsychological test battery, and magnetic resonance imaging at baseline. In all cases, CSF was collected through lumbar puncture at baseline. We compared CSF biomarker levels between the two groups and measured the diagnostic accuracy for probable/definite bvFTD, using the follow-up diagnosis as the reference standard.
Results
The best discriminators between probable/definite bvFTD and PSY were the levels of CSF NfL (area under the curve [AUC] 0.93, P < .001, 95% confidence interval [CI] 0.85–1.00), p-tau/tau ratio (AUC 0.87, P < .001, 95% CI 0.77–0.97), and YKL40 (AUC 0.82, P = .001, 95% CI 0.68–0.97). The combination of these three biomarkers had a sensitivity of 91% (95% CI 66%–100%) at a specificity of 83% (95% CI 65%–95%) with an AUC of 0.94 (P < .001, 95% CI 0.87–1.00) for bvFTD. CSF tau/Aβ1–42 ratio was less accurate in differentiating between bvFTD and PSY.
Discussion
We found a good diagnostic accuracy for higher levels of CSF NfL and YKL40 and reduced p-tau/tau ratio in distinguishing bvFTD from PSY. We advocate the use of these CSF biomarkers as potential additional tools to neuroimaging in the diagnosis of bvFTD versus PSY.
Keywords: Frontotemporal dementia, Psychiatric disorders, Cerebrospinal fluid, Biomarker, Neurofilament, p/t ratio, YKL40
1. Introduction
Clinical differentiation between behavioral variant frontotemporal dementia (bvFTD) from primary psychiatric disorders such as major depressive disorder, bipolar disorder (BD), or schizophrenia (SZ) forms a great challenge due to symptomatic overlap [1], [2]. These disorders share clinical features that are generally characterized by changes in the regulation of social, interpersonal, and personal conduct and cognitive impairment. For example, the neuropsychiatric feature apathy is frequently present in both bvFTD and primary psychiatric disorders [3]. Although bvFTD and primary psychiatric disorders can often be discriminated through imaging techniques, these investigations have a relatively low sensitivity (magnetic resonance imaging [MRI]) or specificity ([18F]-fluorodeoxyglucose–positron emission tomography ([18F]FDG-PET)) [4]. Therefore, ancillary biomarkers that are able to distinguish between bvFTD and primary psychiatric disorders at a higher accuracy are needed. Apart from patient management perspectives, this is becoming even more important in light of future treatment development in both bvFTD and primary psychiatric disorders.
Cerebrospinal fluid (CSF) biomarkers are of great potential because they are considered to reflect pathological processes taking place in the brain. CSF biomarkers have proven to be of great diagnostic value in the diagnosis of Alzheimer's disease (AD), whereby levels of CSF total-tau (CSF tau) and phosphorylated-tau (CSF p-tau181) are increased, and levels of CSF amyloid-β1–42 (CSF Aβ1–42) are decreased reflecting amyloid plaque load and severity of neurodegeneration, respectively [5].
For bvFTD, several studies investigated the utility of CSF biomarkers. However, in contrast to AD, the pathology of the clinical phenotype bvFTD is heterogeneous and consists of either TAR-DNA binding protein 43 (TDP43) inclusions or tau pathology in most cases, whereas a smaller proportion (<10%) has fused in sarcoma protein pathology [6]. Because most CSF studies in FTD rely on clinically diagnosed cases, various results have been found regarding the conventional biomarkers (CSF tau, p-tau181, and Aβ1–42). More recently, however, a decreased phosphorylated-tau to total-tau ratio (p-tau/tau ratio), increased CSF neurofilament light chain (NfL), and YKL40 have been identified as markers of underlying TDP43 pathology in FTD [7], [8], [9], [10], whereas these markers have also been found to be accurate for subjects with underlying tau pathology and correlated with survival [9]. In addition, NfL is a marker for axonal dysfunction or degeneration and YKL40 (chitinase-3 like-1, cartilage glycoprotein-39) is a glycoprotein that is produced by activated microglia and reflects inflammatory processes [11], [12].
Although primary psychiatric disorders are considered to lack neuropathological changes, several CSF biomarkers have been investigated in psychiatric illnesses based on the persistence of cognitive impairment in euthymic or remitted patients [13] or on morphological abnormalities of the brain [14]. For example, in patients with BD, levels of CSF NfL and YKL40 were found to be higher than in healthy controls [15], [16], whereas levels of CSF Aβ1–42, tau and p-tau181 were found to be similar compared with healthy controls [17]. In SZ, CSF Aβ1–42 was found to be significantly lower with equal levels of tau and p-tau181 compared to healthy controls [18]. Thus, although there is some evidence of axonal dysfunction in primary psychiatric disorders, it is unknown to what extent levels of CSF biomarkers are elevated or decreased compared to bvFTD patients.
Therefore, in the present study, we aim to prospectively determine the diagnostic value of CSF levels of total-tau to amyloid-β1–42 ratio (tau/Aβ1–42 ratio), p-tau/tau ratio, NfL, and YKL40 in a cohort presenting with a late-onset frontal lobe syndrome, in particular for the differential diagnosis of bvFTD versus primary psychiatric disorders. By selecting bvFTD and a heterogeneous group of primary psychiatric disorders, this study resembles a daily clinical practice where different primary psychiatric disorders can overlap with bvFTD.
2. Methods
2.1. Participants
All subjects were participants of the late-onset frontal lobe (LOF) study, a multicenter prospective study conducted between April 2011 and June 2015 [19]. In the LOF study, 137 patients who presented with behavioral changes consisting of apathy, disinhibition, and/or compulsive/stereotypical behavior between 45 and 75 years of age were included. Patients were included in the study when they had a score ≥11 on the Frontal Behavioural Inventory [20] or a score ≥10 on the Stereotypy Rating Inventory [21]. All patients received full neurological and psychiatric examination at baseline and at 2-year follow-up. Cognitive screening tests included the Mini–Mental State Examination [22], the frontal assessment battery [23], and a neuropsychological battery concerned the domains of attention/concentration, memory, language, visuospatial, executive, and social functioning. Psychiatric evaluation included an interview by a psychiatrist, the Montgomery Aberg Depression Rating Scale [24] for depressive symptoms, the positive and negative symptom scale for psychotic symptoms [25], and the MINI-PLUS Diagnostic interview [26] to assess primary psychiatric disorders. A consensus diagnosis between the neurologist and the psychiatrist was made at baseline based on the clinical information and additional investigations, including results of conventional CSF biomarkers (CSF tau, p-tau181, and Aβ1–42; ratio was not used), MRI, and [18F]FDG-PET. After 2 years of follow-up, the neuropsychiatric examination, neuropsychological tests, and the MRI of the brain were repeated, followed by establishment of the final multidisciplinary diagnosis. Diagnoses were based on the consensus guidelines for various types of dementia including the frontotemporal dementia consensus criteria, and the psychiatric diagnoses were based on current psychiatric criteria [27], [28], [29], [30], [31], [32].
From the original LOF cohort of 137 cases included at baseline, a total of 21 patients were excluded at follow-up. Three patients were excluded from the final analysis with a 2-year follow-up diagnosis of possible bvFTD, whereas three patients died without postmortem verification or a clear clinical diagnosis. Fifteen patients were lost to follow-up.
For the present study, we included participants with a final multidisciplinary diagnosis at 2-year follow-up who had undergone a diagnostic lumbar puncture at baseline with either a primary psychiatric diagnosis (n = 25) or a probable/definite bvFTD (n = 22) diagnosis. Subsequently, the diagnostic accuracies of CSF biomarkers (tau/Aβ1–42 ratio, p-tau/tau ratio, NfL, and YKL40) at baseline were calculated, using the follow-up diagnosis as reference standard. Participants in the psychiatric group had a clinical diagnosis of major/minor depression (n = 11), bipolar disorder (n = 4), autism spectrum (n = 1), anxiety disorder (n = 1), obsessive–compulsive disorder (n = 1), and personality disorder (n = 7). In the bvFTD group, we included patients with a final multidisciplinary diagnosis of probable bvFTD (n = 19) or definite bvFTD (n = 3). We excluded patients with CSF AD profile or lower CSF Aβ1–42 levels according to previously published cutoff values; <550 pg/mL for CSF Aβ1–42, >375 pg/mL for CSF tau, and >52 pg/mL CSF p-tau181 [5]. Furthermore, for the present study, we excluded patients with other types of dementia (n = 30), other neurologic disorders (n = 8), subjective cognitive decline (n = 5), and other diagnoses (n = 9), using the follow-up diagnosis as the reference standard. The local institutional ethical review board approved this study, and a written informed consent was obtained from all participants.
2.2. CSF biomarkers analyses
CSF was obtained with a lumbar puncture (LP), after informed consent had been signed. The LP was performed according to a standard medical procedure in the lateral position (L3-L4, L4-L5, or L5-S1 intervertebral space) by a 25-gauge needle and syringe. CSF was collected in polypropylene tubes and centrifuged within an hour. CSF samples were centrifuged for 10 minutes at 4°C. The supernatant was stored in 0.5-mL aliquots at −20°C. CSF levels of NfL were measured by using ELISAs (Uman) according to the manufacturers instructions as described before [33]. YKL40 CSF levels were measured by using human YKL40 (MicroVue YKL40 EIA kit, Quidel, CA, USA). Performance of the assays was evaluated using CSF pools as internal controls. The coefficient of variation (CV) was calculated for each sample in duplicate as the standard deviation divided by the mean. Intraassay and interassay CVs were calculated for NfL (1.3% and 6.1%) and YKL40 (2.8% and 9.5%). Laboratory analysis of levels of CSF tau, p-tau181, and Aβ1–42 concentrations was performed using sandwich ELISAs (Fujirebio/Innogenetics, Belgium) on a routine basis.
2.3. Statistical analysis
Data analysis was performed using IBM SPSS statistics version 22.0 (IBM SPSS Statistics, Armonk, NY, USA) for Mac. Clinical and demographic baseline characteristics were compared between groups using independent Student t-tests for normally distributed continuous data. Assumptions for normality were checked and if not normally distributed after log-transformation, a Mann-Whitney test was used. For categorical data, chi-square tests were used. The comparison of levels CSF tau, CSF p-tau181, CSF Aβ1–42, CSF tau/Aβ1–42, CSF p-tau/tau ratio, CSF NfL, and CSF YKL40 between probable/definite bvFTD and primary psychiatric disorders were made using nonparametric tests (Mann–Whitney U test). Receiver operating characteristic (ROC) curves were plotted for CSF tau/Aβ1–42, CSF p-tau/tau ratio, CSF NfL, and CSF YKL40, and we calculated the area under the curves (AUCs) with 95% confidence interval (CI). We calculated the best cutoff value using the Youden index for CSF markers with AUC >0.80 [34]. Furthermore, a binary logistic regression was used to combine the best CSF markers (AUC >0.80; CSF p-tau/tau ratio, CSF NfL, and CSF YKL40) in one model (ENTER). The linearity of the associations was studied before the logistic regression for continuous data of the CSF biomarkers. Potential multicollinearity was investigated for the multivariable model using the variance inflation factor (VIF) for each of the independent variables in the multivariable model using linear regression analyses and variables (VIF < 3). The model was plotted in a ROC curve, and we calculated AUC with 95% CI. A P-value of <.05 was considered statistically significant.
3. Results
3.1. Clinical and demographical characteristics
The final cohort consisted of 22 patients with probable/definite bvFTD, of whom 19 patients had probable bvFTD and 3 definite bvFTD. Definite bvFTD included a histopathologically proven tauopathy and two cases carrying a C9orf72 hexanucleotide repeat expansion. In the primary psychiatric group, a total of 25 patients were included. The clinical and demographical characteristics of patients with probable/definite bvFTD and primary psychiatric disorders are presented in Table 1. There were no significant differences in clinical and demographical characteristics between the two groups.
Table 1.
Clinical characteristics and levels of CSF biomarkers
| Characteristics | Probable/definite bvFTD (n = 22) | Primary psychiatric disorders (n = 25) | P value |
|---|---|---|---|
| Age, years | 62.9 (6.3) | 60.6 (7.00) | .25 |
| Gender, % male | 59 | 84 | .06 |
| Education, verhage | 4.42 (1.32) | 4.35 (1.46) | .57 |
| MMSE | 26.2 (2.8) | 25.6 (2.9) | .49 |
| FAB | 14.1 (4.2) | 14.8 (2.8) | .52 |
| CSF Aβ1–42, pg/mL∗ | 1103 (607–1460) | 1057 (632–1472) | .29 |
| CSF tau, pg/mL∗ | 379 (182–993) | 278 (152–648) | .02 |
| CSF p-tau181, pg/mL∗ | 40 (16–76) | 47 (16–112) | .24 |
| CSF p-tau/tau ratio∗ | 0.11 (0.05–0.2) | 0.17 (0.09–0.22) | <.001 |
| CSF tau/Aβ1–42 ratio∗ | 0.34 (0.19–0.76) | 0.27 (0.15–0.55) | .006 |
| CSF NfL, pg/mL∗,† | 5630 (821–26,599) | 759 (369–1542) | <.001 |
| CSF YKL40, ng/mL∗,† | 399 (224–712) | 254 (150–394) | .001 |
Abbreviations: bvFTD, behavioral variant frontotemporal dementia; Verhage, education score comparable with the International Standard Classification of Education (UNESCO, Paris, 1976); MMSE, Mini–Mental State Examination; FAB, frontal assessment battery; CSF, cerebrospinal fluid; Aβ1–42, amyloid-β1–42; Tau, total-tau; p-tau181, phosphorylated-tau; NfL, neurofilament light chain.
NOTE. Data are mean (standard deviation) unless otherwise stated.
Mean, minimum–maximum.
CSF NfL missing eight bvFTD cases and four psychiatric cases, CSF YKL40 seven bvFTD cases and four psychiatric cases.
3.2. Levels of CSF biomarkers
Mean CSF levels for both groups are shown in Table 1. We found significant higher CSF tau levels in bvFTD compared to primary psychiatric disorders. CSF p-tau181 levels, however, were similar between the bvFTD group and the psychiatric group. CSF Aβ1–42 levels were not significantly different.
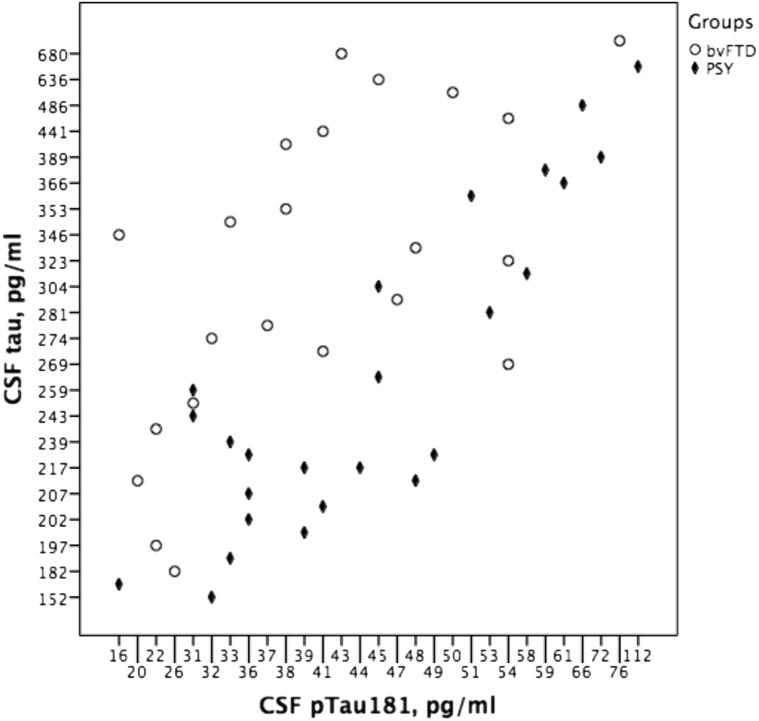
Furthermore, the mean CSF p-tau/tau ratio was significantly lower in the bvFTD group than in patients with a primary psychiatric disorder (Fig. 1). Similarly, the CSF tau/Aβ1–42 ratio was higher in the bvFTD group compared to the primary psychiatric group.
Fig. 1.
CSF tau relative to CSF p-tau181 levels per diagnostic group. Abbreviations: bvFTD, behavioral variant frontotemporal dementia; CSF, cerebrospinal fluid; p-tau181, phosphorylated-tau; Psy, primary psychiatric disorders.
Mean CSF NfL levels were >7 fold higher in the probable/definite bvFTD group compared to the primary psychiatric disorders. The CSF YKL40 levels were 1.4 fold higher in the probable/definite bvFTD group compared to those in the primary psychiatric disorders. Of note, although the levels of NfL and YKL40 in the group of psychiatric disorders were lower than in bvFTD, they were higher than levels described in control subjects, which are typically in the range of 254–365 pg/mL for CSF NfL [12], [15], [35] and around 200 ng/mL for CSF YKL40 [8], [36].
3.3. Diagnostic accuracy for CSF markers
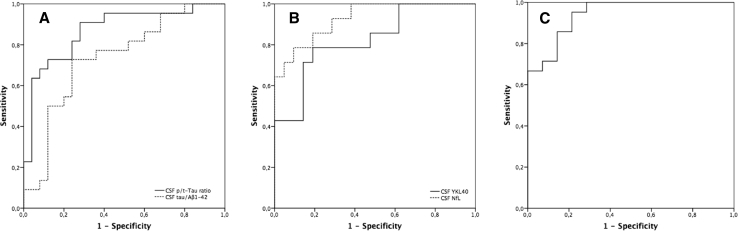
ROC curves of CSF biomarkers are shown in Fig. 2. The highest AUC was reached for CSF NfL (AUC 0.93, P < .001, 95% CI 0.85–1.00) followed by the CSF p-tau/tau ratio (AUC 0.87, P < .001, 95% CI 0.77–0.97). For CSF YKL40, the AUC for probable/definite bvFTD was relatively good (AUC 0.82, P = .001, 95% CI 0.68–0.97). The diagnostic accuracy for CSF tau/Aβ1–42 ratio was moderate with an AUC of 0.74 (P = .006, 95% CI 0.54–0.84).
Fig. 2.
ROC curves of CSF biomarkers for probable/definite bvFTD versus primary psychiatric disorders. (A) CSF p-/t-tau ratio (AUC 0.87, P < .001, 95% CI 0.77–0.97), CSF tau/Aβ1–42 (AUC 0.74, P = .006, 95% CI 0.54–0.84); (B) CSF NfL (AUC 0.93, P < .001, 95% CI 0.85–1.00), CSF YKL40 (AUC 0.82, P = .001, 95% CI 0.68–0.97); (C) combination of CSF NfL, YKL40, and p-/t-tau ratio (AUC of 0.94, P < .001, 95% CI 0.87–1.00). Abbreviations: AUC, area under the curve; bvFTD, behavioral variant frontotemporal dementia; CI, confidence interval; CSF, cerebrospinal fluid; ROC, receiver operating characteristic.
For CSF NfL, CSF p-tau/tau ratio, and CSF YKL40, we calculated the optimal cutoff value to discriminate probable/definite bvFTD from psychiatric disorders. The optimal cutoff for CSF NfL for probable/definite bvFTD versus primary psychiatric disorders was >1063 pg/mL with a sensitivity of 79% at specificity of 91%. For the CSF p-tau/tau ratio, we found a sensitivity of 91% at a specificity of 72% at a cutoff <0.15 for probable/definite bvFTD. CSF YKL40 had a sensitivity of 67% with a specificity of 81% at a cutoff >324 ng/mL.
By using a logistic regression model, the combination of CSF NfL, p-tau/tau ratio, and YKL40 explained 73.8% (chi-square test 27.6, df 3, P < .001) of the variance. This model had a sensitivity of 91% (95% CI 66%–100%) at a specificity of 83% (95% CI 65%–95%). The ROC curve, shown in Fig. 2, had an AUC of 0.94 (P < .001, 95% CI 0.87–1.00).
4. Discussion
We found that the best discriminators between probable/definite bvFTD and primary psychiatric disorders were the CSF NfL levels and the p-tau/tau ratio. Likewise, we showed that the levels of CSF YKL40 were increased in probable/definite bvFTD and had a good diagnostic accuracy for probable/definite bvFTD. The combination of these three CSF biomarkers showed the highest diagnostic accuracy in differentiating probable/definite bvFTD from primary psychiatric disorders in patients with behavioral changes. The value of tau/Aβ1–42 ratio in distinguishing between these disorders was less accurate, although there was a higher ratio in the group of probable/definite bvFTD.
The most important clinically relevant finding was that the combination of CSF NfL, p-tau/tau ratio, and YKL40 has proven to be a good diagnostic tool to discriminate between probable/definite bvFTD and primary psychiatric disorders. Combining these CSF biomarkers showed a sensitivity of 91% with a specificity of 83% for probable/definite bvFTD. This results in a similar or even higher diagnostic accuracy for bvFTD than frontotemporal changes on neuroimaging [4], [37], [38]. Thus, we advocate the use of CSF, in particular, these three biomarkers, in the diagnostic process of patients with changes in the regulation of social, interpersonal, and personal conduct and cognitive impairment.
Levels of CSF NfL were significantly increased in probable/definite bvFTD and had a good diagnostic accuracy with a sensitivity of 79% at a specificity of 91% for probable/definite bvFTD. This is in line with previous findings of increased CSF NfL levels in TDP43 and tau-pathology FTLD cases in comparison with AD and subjective memory complaints (SMCs) [9], [36]. Higher CSF NfL levels were also found in a more heterogeneous FTD group in a large clinically established dementia cohort including healthy controls [35]. Moreover, it also reflects the disease severity in specific FTD cases, indicating the clinical relevance of this CSF biomarker [39]. The intermediate CSF NfL levels in our probable/definite bvFTD cohort is probable due to NfL being a marker for axonal dysfunction or degeneration [40], which is a hallmark of neurodegenerative disorders. In contrast, no sustainable evidence exists for axonal loss in the pathophysiology of primary psychiatric disorders. Nevertheless, we found somewhat elevated CSF NfL levels in our heterogeneous group of primary psychiatric disorders compared to previous described levels in healthy controls [12], [15], [35]. This finding is in line with one study where levels of CSF NfL were higher in BD compared to healthy controls [15], suggesting some axonal loss or dysfunction in specific primary psychiatric disorders presenting with behavioral changes.
The ratio of p-tau/tau appeared a good diagnostic test for distinguishing between probable/definite bvFTD and primary psychiatric disorders with a sensitivity of 91% and a specificity of 72%. In recent publications, the diagnostic value of CSF p-tau/tau ratio for differentiation between FTLD-Tau and FTLD-TDP pathology was proven to be accurate [7], [41]. In general, our findings in clinical bvFTD are comparable with Pijnenburg et al. [9], who found similar reduced CSF p-tau/tau values in proven TDP-FTLD and tau-FTLD pathology compared to SMCs. The most likely explanation of the reduced CSF p-tau/tau ratio in our heterogeneous group of bvFTD is that it reflects neuronal degeneration in an active disease leading to increased total CSF tau levels, with relative stable levels of CSF p-tau181. It appears that hyperphosphorylated-tau, in contrast to what is the case in AD, is not a specific marker for bvFTD [5].
Moreover, various results have been found for CSF tau levels in clinically diagnosed FTD cases. Although some studies reported normal CSF tau levels, others reported moderately increased CSF tau levels [42], [43]. One group reported decreased CSF tau levels in clinical and definite bvFTD [44]. Others, however, have not reproduced this finding. From these studies, it appears that the diagnostic value of CSF tau for bvFTD is relatively low [45], [46]. Nearly all these previous studies compared FTD with SMC or healthy controls, but never with primary psychiatric patients, where the differential diagnosis is clinically most challenging [43], [46].
The increased marker YKL40 in bvFTD compared to primary psychiatric disorders is a novel finding. YKL40 is a glycoprotein that is produced by activated microglia and reflects inflammatory processes that may contribute to the neural or synaptic dysfunction in neurodegenerative disorders and also in some primary psychiatric disorders [11], [12]. Moreover, YKL40 is found to be elevated in AD patients and other neurodegenerative disorders [47], [48], [49]. However, the sensitivity and disease specificity of this marker is still uncertain because other studies found no increased levels CSF YKL40 in neurodegenerative disorders [50], [51], [52]. A possible explanation of our increased levels of YKL40 for bvFTD in contrast to other studies could be the careful adherence to the bvFTD diagnostic criteria providing less heterogeneity in diagnostic groups than in some other studies. Another explanation might be that neuroinflammatory activity in bvFTD is stage dependent because we mostly included early-stage cases. For primary psychiatric disorders, higher levels of CSF YKL40 have been described in bipolar patients and found to be associated with the severity of cognitive impairment [12]. Although a neuroinflammatory response might not be specific for neurodegenerative disorders, higher levels of YKL40 in our group of probable/definite bvFTD may reflect the presence of more neuroinflammation in bvFTD than in primary psychiatric disorders. Still, our result of increased YKL40 in psychiatric patients in comparison to previous described levels in controls [8] supports the hypothesis that neuroinflammation plays a role in specific psychiatric diseases too [53].
Another finding of our study was the higher tau/Aβ1–42 ratio in bvFTD compared to primary psychiatric disorders. As described in several studies, decreased or equal CSF Aβ1–42 values are found in FTD cases compared to healthy controls [43], [54]. We found essentially identical values for CSF Aβ1–42 in bvFTD and primary psychiatric disorders. As a consequence, the higher CSF tau/Aβ1–42 is probably due to the higher CSF tau levels in the bvFTD group.
Few CSF biomarker studies have been performed in primary psychiatric disorders and mainly investigated the conventional markers such as Aβ1–42, tau, and p-tau181. For example, decreased CSF Aβ1–42 levels have been described in SZ and depressive patients compared to healthy elderly controls, while compared to AD, higher levels of CSF Aβ1–42 were found [18], [55]. In a different study, patients with SZ had normal levels of CSF tau and CSF p-tau181 compared to age-matched healthy controls [18]. In comparison with these studies, we were unable to demonstrate significant lower Aβ1–42 for the psychiatric group compared to bvFTD. A possible explanation for their findings is that lower CSF Aβ1–42 levels in these psychiatric patients are a result of a prodromal stage of neurodegeneration or a secondary underlying neurodegenerative disorder. Overall, primary psychiatric disorders are unlikely to be associated with AD-related pathology.
Limitations of this study should be acknowledged to interpret the findings. One limitation is the small number of cases with a definite FTD diagnosis, based on autopsy and genetic testing. We relied on the clinical consensus diagnosis at 2-year follow-up. Although we applied this 2-year follow-up, we are unable to completely rule out a concomitant or underlying neurodegenerative disease for the patients in the primary psychiatric group with higher CSF tau, YKL40, and NfL. The absence of clinical decline or changes on neuroimaging in 2 years makes a neurodegenerative cause of the frontal symptoms less likely and slow progressive cases of bvFTD that mimic psychiatric disorders were identified by testing of C9orf72 repeat expansion in all subjects. On the other hand, our cohort represents the heterogeneous group of bvFTD and a wide range of primary psychiatric diagnoses in a daily clinical practice. However, this heterogeneity may have influenced the results on CSF markers, as these disorders may be associated with different biological processes. Furthermore, with a small sample size, caution must be applied, as the findings are less generalizable and so future studies must validate our findings.
In conclusion, we found a good diagnostic accuracy for the CSF biomarkers NfL, YKL40, and p-tau/tau ratio in distinguishing probable/definite bvFTD from primary psychiatric disorders in patients presenting with behavioral changes. In general, this study strengthens the idea that the use of CSF biomarkers as a potential additional tool to neuroimaging in distinguishing bvFTD from primary psychiatric disorders has diagnostic relevance and may be incorporated in future diagnostic guidelines for bvFTD.
Research in Context.
-
1.
Systematic review: We conducted multiple Medline searches to initially identify all cerebrospinal fluid (CSF) studies for behavioral variant frontotemporal dementia (bvFTD) and primary psychiatric disorders. In addition, we searched the reference list of identified articles.
-
2.
Interpretation: In this study, we clearly demonstrate a good diagnostic accuracy for higher levels of CSF neurofilament light chain (NfL) and YKL40 and reduced p/t-tau ratio in distinguishing bvFTD from primary psychiatric disorders. In general, this study strengthens the idea that the use of CSF biomarkers as a potential additional tool to neuroimaging in distinguishing bvFTD from primary psychiatric disorders has diagnostic relevance.
-
3.
Future directions: Future studies should further investigate the relationship between clinical symptoms and CSF levels and how these CSF markers change over time. Finally, CSF biomarkers might monitor progression in bvFTD and be incorporated in future diagnostic guidelines for bvFTD.
References
- 1.Woolley J.D., Khan B.K., Murthy N.K., Miller B.L., Rankin K.P. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease. J Clin Psychiatry. 2011;72:126–133. doi: 10.4088/JCP.10m06382oli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanata S.C., Miller B.L. The behavioural variant frontotemporal dementia (bvFTD) syndrome in psychiatry. J Neurol Neurosurg Psychiatry. 2016;87:501–511. doi: 10.1136/jnnp-2015-310697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijverberg E.G.B., Dols A., Krudop W.A., Peters A., Kerssens C.J., van Berckel B.N. Diagnostic accuracy of the frontotemporal dementia consensus criteria in the late-onset frontal lobe syndrome. Dement Geriatr Cogn Disord. 2016;41:210–219. doi: 10.1159/000444849. [DOI] [PubMed] [Google Scholar]
- 4.Vijverberg E.G., Wattjes M.P., Dols A., Krudop W.A., Möller C., Peters A. Diagnostic accuracy of MRI and additional [18F]FDG-PET for behavioral variant frontotemporal dementia in patients with late onset behavioral changes. J Alzheimers Dis. 2016;53:1287–1297. doi: 10.3233/JAD-160285. [DOI] [PubMed] [Google Scholar]
- 5.Duits F.H., Teunissen C.E., Bouwman F.H., Visser P.J., Mattsson N., Zetterberg H. The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement. 2014;10:713–723.e2. doi: 10.1016/j.jalz.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Cairns N.J., Bigio E.H., Mackenzie I.R., Neumann M., Lee V.M., Hatanpaa K.J. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borroni B., Benussi A., Archetti S., Galimberti D., Parnetti L., Nacmias B. Csf p-tau 181/tau ratio as biomarker for TDP pathology in frontotemporal dementia. Amyotroph Lateral Scler Frontotemporal Degeneration. 2015;16:86–91. doi: 10.3109/21678421.2014.971812. [DOI] [PubMed] [Google Scholar]
- 8.Teunissen C.E., Elias N., Koel-Simmelink M.J., Durieux-Lu S., Malekzadeh A., Pham T.V. Novel diagnostic cerebrospinal fluid biomarkers for pathologic subtypes of frontotemporal dementia identified by proteomics. Alzheimer's Demen Diagn Assess Dis Monit. 2016;2:86–94. doi: 10.1016/j.dadm.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pijnenburg Y.A.L., Verwey N.A., van der Flier W.M., Scheltens P., Teunissen C.E. Discriminative and prognostic potential of cerebrospinal fluid phosphoTau/tau ratio and neurofilaments for frontotemporal dementia subtypes. Alzheimer's & Dementia: Diagnosis. Assess Dis Monit. 2015;1:505–512. doi: 10.1016/j.dadm.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wald M.L., Santillo A.F., Passant U., Zetterberg H., Rosengren L., Nilsson C. Cerebrospinal fluid neurofilament light chain protein levels in subtypes of frontotemporal dementia. BMC Neurol. 2013;13:54. doi: 10.1186/1471-2377-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellwig K., Kvartsberg H., Portelius E., Andreasson U., Oberstein T.J., Lewczuk P. Neurogranin and YKL-40: independent markers of synaptic degeneration and neuroinflammation in Alzheimer's disease. Alzheimer's Res Ther. 2015;7:74. doi: 10.1186/s13195-015-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolstad S., Jakobsson J., Sellgren C., Ekman C.J., Blennow K., Zetterberg H. Cognitive performance and cerebrospinal fluid biomarkers of neurodegeneration: a study of patients with bipolar disorder and healthy controls. PLoS ONE. 2015;10:e0127100. doi: 10.1371/journal.pone.0127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meesters P.D., Schouws S., Stek M., de Haan L., Smit J., Eikelenboom P. Cognitive impairment in late life schizophrenia and bipolar I disorder. Int J Geriatr Psychiatry. 2013;28:82–90. doi: 10.1002/gps.3793. [DOI] [PubMed] [Google Scholar]
- 14.Kempton M.J., Geddes J.R. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsson J., Bjerke M., Ekman C.-J., Sellgren C., Johansson A.G., Zetterberg H. Elevated concentrations of neurofilament light chain in the cerebrospinal fluid of bipolar disorder patients. Neuropsychopharmacology. 2014;39:2349–2356. doi: 10.1038/npp.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolstad S., Jakobsson J., Sellgren C., Isgren A., Ekman C.-J., Bjerke M. CSF neuroinflammatory biomarkers in bipolar disorder are associated with cognitive impairment. Eur Neuropsychopharmacol. 2015;25:1091–1098. doi: 10.1016/j.euroneuro.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Forlenza O.V., Aprahamian I., Radanovic M., Talib L.L., Camargo M.Z., Stella F. Cognitive impairment in late-life bipolar disorder is not associated with Alzheimer's disease pathological signature in the cerebrospinal fluid. Bipolar Disord. 2016;18:63–70. doi: 10.1111/bdi.12360. [DOI] [PubMed] [Google Scholar]
- 18.Frisoni G.B., Prestia A., Geroldi C., Adorni A., Ghidoni R., Amicucci G. Alzheimer's CSF markers in older schizophrenia patients. Int J Geriatr Psychiatry. 2010;26:640–648. doi: 10.1002/gps.2575. [DOI] [PubMed] [Google Scholar]
- 19.Krudop W.A., Kerssens C.J., Dols A., Prins N.D., Möller C., Schouws S. Building a new paradigm for the early recognition of behavioral variant frontotemporal dementia: Late Onset Frontal Lobe Syndrome study. Am J Geriatr Psychiatry. 2014;22:735–740. doi: 10.1016/j.jagp.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Kertesz A., Nadkarni N. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc. 2000;6:460–468. doi: 10.1017/s1355617700644041. [DOI] [PubMed] [Google Scholar]
- 21.Shigenobu K., Ikeda M., Fukuhara R., Maki N., Hokoishi K., Nebu A. The Stereotypy Rating Inventory for frontotemporal lobar degeneration. Psychiatry Res. 2002;110:175–187. doi: 10.1016/s0165-1781(02)00094-x. [DOI] [PubMed] [Google Scholar]
- 22.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Dubois B., Slachevsky A., Litvan I., Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. The Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 25.Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 26.Sheehan D.V., Lecrubier Y., Sheehan K.H. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–23. [PubMed] [Google Scholar]
- 27.Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Demen. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roman G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 30.McKeith I.G., Dickson D.W., Lowe J., Emre M., O'Brien J.T., Feldman H. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association . American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders, 4th edition Text Revision (DSM-IV-TR) [Google Scholar]
- 32.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koel-Simmelink M.J., Vennegoor A., Killestein J., Blankenstein M.A., Norgren N., Korth C. The impact of pre-analytical variables on the stability of neurofilament proteins in CSF, determined by a novel validated SinglePlex Luminex assay and ELISA. J Immunological Methods. 2014;402:43–49. doi: 10.1016/j.jim.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Skillbäck T., Farahmand B., Bartlett J.W., Rosén C., Mattsson N., Nägga K. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83:1945–1953. doi: 10.1212/WNL.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 36.Olsson B., Lautner R., Andreasson U., Öhrfelt A., Portelius E., Bjerke M. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. The Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 37.Mendez M.F., Shapira J.S., McMurtray A., Licht E., Miller B.L. Accuracy of the clinical evaluation for frontotemporal dementia. Arch Neurol. 2007;64:830–835. doi: 10.1001/archneur.64.6.830. [DOI] [PubMed] [Google Scholar]
- 38.Pijnenburg Y.A., Mulder J.L., Van Swieten J.C., Uitdehaag B.M.J., Stevens M., Scheltens P. Diagnostic accuracy of consensus diagnostic criteria for frontotemporal dementia in a memory clinic population. Dement Geriatr Cogn Disord. 2008;25:157–164. doi: 10.1159/000112852. [DOI] [PubMed] [Google Scholar]
- 39.Scherling C.S., Hall T., Berisha F., Klepac K., Karydas A., Coppola G. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann Neurol. 2014;75:116–126. doi: 10.1002/ana.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bacioglu M., Maia L.F., Preische O., Schelle J., Apel A., Kaeser S.A. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron. 2016;91:56–66. doi: 10.1016/j.neuron.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Hu W.T., Watts K., Grossman M., Glass J., Lah J.J., Hales C. Reduced CSF p-Tau181 to Tau ratio is a biomarker for FTLD-TDP. Neurology. 2013;81:1945–1952. doi: 10.1212/01.wnl.0000436625.63650.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoonenboom N.S., Reesink F.E., Verwey N.A., Kester M.I., Teunissen C.E., van de Ven P.M. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78:47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- 43.Verwey N.A., Kester M.I., van der Flier W.M., Veerhuis R., Berkhof H., Twaalfhoven H. Additional value of CSF amyloid-beta 40 levels in the differentiation between FTLD and control subjects. J Alzheimers Dis. 2010;20:445–452. doi: 10.3233/JAD-2010-1392. [DOI] [PubMed] [Google Scholar]
- 44.Grossman M., Farmer J., Leight S., Work M., Moore P., Van Deerlin V. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer's disease. Ann Neurol. 2005;57:721–729. doi: 10.1002/ana.20477. [DOI] [PubMed] [Google Scholar]
- 45.Irwin D.J., Trojanowski J.Q., Grossman M. Cerebrospinal fluid biomarkers for differentiation of frontotemporal lobar degeneration from Alzheimer's disease. Front Aging Neurosci. 2013;5:6. doi: 10.3389/fnagi.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pijnenburg Y.A., Schoonenboom N.S., Rosso S.M., Mulder C., Van Kamp G.J., van Swieten J.C. CSF tau and A 42 are not useful in the diagnosis of frontotemporal lobar degeneration. Neurology. 2004;62:1649. doi: 10.1212/01.wnl.0000123014.03499.a7. [DOI] [PubMed] [Google Scholar]
- 47.Rosén C., Andersson C.H., Andreasson U., Molinuevo J.L., Bjerke M., Rami L. Increased levels of chitotriosidase and YKL-40 in cerebrospinal fluid from patients with Alzheimer's disease. Dement Geriatr Cogn Disord Extra. 2014;4:297–304. doi: 10.1159/000362164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsson B., Hertze J., Lautner R., Zetterberg H., Nägga K., Höglund K. Microglial markers are elevated in the prodromal phase of Alzheimer's disease and vascular dementia. J Alzheimers Dis. 2013;33:45–53. doi: 10.3233/JAD-2012-120787. [DOI] [PubMed] [Google Scholar]
- 49.Craig-Schapiro R., Perrin R.J., Roe C.M., Xiong C., Carter D., Cairns N.J. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vinther-Jensen T., Budtz-Jørgensen E., Simonsen A.H., Nielsen J.E., Hjermind L.E. YKL-40 in cerebrospinal fluid in Huntington's disease—a role in pathology or a nonspecific response to inflammation? Parkinsonism Relat Disord. 2014;20:1301–1303. doi: 10.1016/j.parkreldis.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Mattsson N., Tabatabaei S., Johansson P., Hansson O., Andreasson U., Månsson J.-E. Cerebrospinal fluid microglial markers in Alzheimer's disease: elevated chitotriosidase activity but lack of diagnostic utility. Neuromol Med. 2011;13:151–159. doi: 10.1007/s12017-011-8147-9. [DOI] [PubMed] [Google Scholar]
- 52.Janelidze S., Hertze J., Zetterberg H., Landqvist Waldö M., Santillo A., Blennow K. Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer's disease. Ann Clin Transl Neurol. 2015;3:12–20. doi: 10.1002/acn3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao B., Passos I.C., Mwangi B., Bauer I.E., Zunta-Soares G.B., Kapczinski F. Hippocampal volume and verbal memory performance in late-stage bipolar disorder. J Psychiatr Res. 2016;73:102–107. doi: 10.1016/j.jpsychires.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pijnenburg Y.A., Schoonenboom S.N., Mehta P.D., Mehta S.P., Mulder C., Veerhuis R. Decreased cerebrospinal fluid amyloid beta (1-40) levels in frontotemporal lobar degeneration. Journal of Neurology. Neurosurg Psychiatry. 2006;78:735–737. doi: 10.1136/jnnp.2006.105064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nascimento K.K., Silva K.P., Malloy-Diniz L.F., Butters M.A., Diniz B.S. Plasma and cerebrospinal fluid amyloid-β levels in late-life depression: a systematic review and meta-analysis. J Psychiatr Res. 2015;69:35–41. doi: 10.1016/j.jpsychires.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]