Abstract
Eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome, is a rare systemic disease situated between primary small vessel vasculitides associated with antineutrophil cytoplasmic antibodies (ANCAs) and hypereosinophilic syndromes (HES). Here, we present a case of EGPA in a 38-year-old male, with a previous diagnosis of asthma, who presented with fever, migratory lung infiltrates and systemic eosinophilia that was refractory to previous courses of antibiotics. This case highlights the importance of the primary care physician understanding the differential diagnosis of pulmonary eosinophilic syndromes.
Keywords: Eosinophilic granulomatosis with polyangiitis, Vasculitis, Hypereosinophilic syndromes, Eosinophilia
1. Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome (CSS) [1], was first described in 1951 by Churg and Strauss as a rare disease characterized by disseminated necrotizing vasculitis with extravascular granulomas occurring exclusively among patients with asthma and tissue eosinophilia [2], [3], [4]. EGPA is a disease situated between primary systemic vasculitides [1] and hypereosinophilic disorders [5], [6]. Within this dual categorization, EGPA is classified among small-vessel vasculitides associated with antineutrophil cytoplasmic antibodies (ANCAs) and hypereosinophilic syndromes (HESs) [5], which are syndromes with accompanying hypereosinophilia [6]. Both vessel inflammation and eosinophilic proliferation have been proposed to contribute to organ damage, but the clinical presentations are heterogeneous, and the respective roles of vasculitis and hypereosinophilia in the disease process are not well understood [3].
Here, we present a case of EGPA in a 38-year-old male, with a previous diagnosis of asthma, who presented with fever, migratory lung infiltrates and systemic eosinophilia that was refractory to previous courses of antibiotics.
2. Case report
A 38-year-old male, non-smoker, with a previous diagnosis of asthma from childhood, presented with fever, productive cough with haemoptoic sputum and upper airway respiratory symptoms. He reported that his asthma had worsened in previous years and was refractory to medications. With an exception of budesonide/formoterol (400/12 μg twice daily in the last 6 months) and prednisone (10 mg once daily in the last 2 months), he denied using other medications. One month prior to admission, he had intermittent fever and a worsening of the cough, which became more intense and productive and was associated with dyspnoea. He had received multiple antibiotic courses (azithromycin for 10 days, levofloxacin for 10 days and amoxicillin-clavulanate for 14 days) without success.
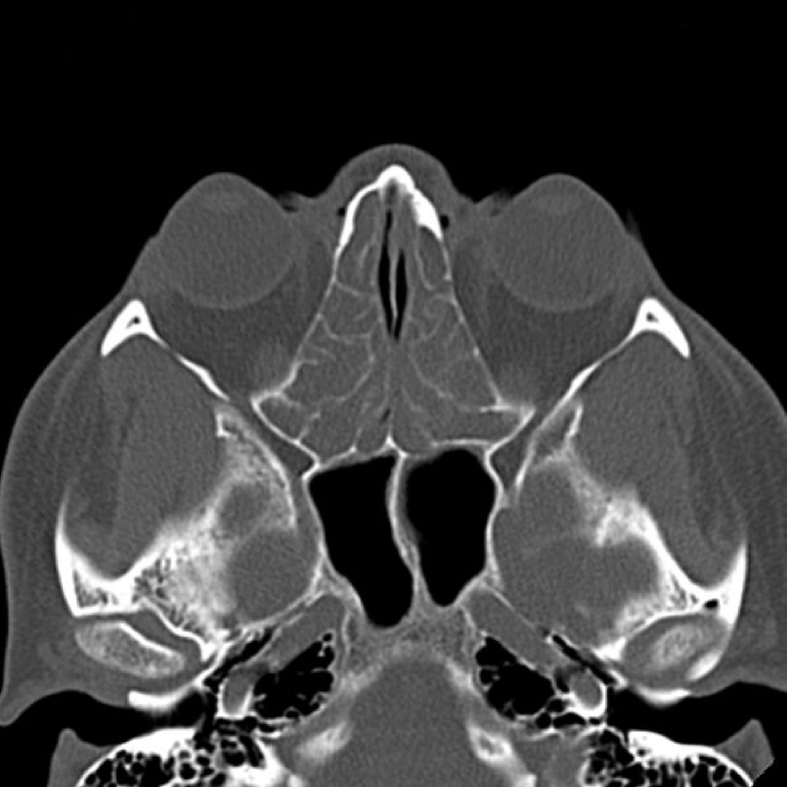
A chest computed tomography (CT) showed diffuse ground-glass opacification (GGO) (Fig. 1), and a paranasal sinuses CT revealed opacification of the frontal and ethmoidal sinuses (Fig. 2). Pulmonary function tests indicated a severe obstructive pattern and no post-bronchodilator response (forced vital capacity (FVC) = 59% predicted; forced expiratory volume in 1 s (FEV1) = 35% predicted; FEV1/FVC = 50%; total lung capacity (TLC) = 89% predicted; residual volume (RV) = 177% predicted; RV/TLC = 191% predicted; diffusing capacity for carbon monoxide (DLco) = 69% predicted). Laboratory examinations demonstrated leucocytosis (14400/mm3) with marked eosinophilia (3168 mm3/22%) and normal renal function, and a urine dipstick test revealed urinary occult blood (2+) without erythrocyte dysmorphism, elevated immunoglobulin (Ig)E and a reactive p-ANCA (myeloperoxidase) on two separate occasions. Stool microscopy did not identify any ova, cysts or parasites, and serum antibody tests for the parasites Fasciola hepatica, Strongyloides spp., Trichinella spp., Taenia solium, Schistosoma mansoni and Toxocara canis were negative. Antigen-specific IgE antibody test to Aspergillus fumigatus was also negative. Despite the negative results of the serologies, it was opted for the administration of albendazole 400 mg once daily for three days, as antiparasitic prophylaxis. An open lung biopsy was performed and demonstrated intense perivascular eosinophilic inflammatory infiltrate (Fig. 3, Fig. 4), confirming the diagnosis of EGPA [4], [7], [8] (Table 1). Posteriorly, a transthoracic echocardiogram and electroneuromyography for complementary investigation were performed and produced normal results.
Fig. 1.
Chest computed tomography showing diffuse ground-glass opacities.
Fig. 2.
Paranasal sinuses computed tomography showing opacification of ethmoidal sinuses.
Fig. 3.
A diffuse chronic inflammatory infiltrate with a marked presence of eosinophils (star) is found near the bronchioles.
Fig. 4.
Arterial vessels present thickening of the wall (arrow) due to muscular hypertrophy and fibrosis of the intima, in a plexiform arrangement that appears to present several vascular lights. Note the eosinophil infiltrate near the bronchiole (star).
Table 1.
Distinct classification criteria for eosinophilic granulomatosis with polyangiitis.
The patient received prednisone 40 mg once daily (0.5 mg/kg/day) for four months leading to a resolution of respiratory and systemic symptoms, lungs infiltrates (Fig. 5) and eosinophilia. Currently, he is tapering out prednisone and started azathioprine for glucocorticoid sparing.
Fig. 5.
Chest computed tomography after induction therapy with glucocorticoids showing improvement of ground-glass opacities.
3. Discussion
Vasculitic disorders are characterized by blood vessel inflammation, which can result in many symptoms due to ischaemia/infarction, caused by occlusion or reduction of blood flow, or haemorrhage, caused by a rupture of committed vessels [9]. ANCA-associated vasculitis (AAV) includes EGPA, microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA). Our patient had EGPA, which is the rarest form of AAV [10]. EGPA is approximately 2–10 times less common than the other forms of AAV, with a prevalence and annual incidence estimated at 2–22 and 0.5–3.7 per million, respectively [11]. The mean age at diagnosis is 48 years, although it may occur at all ages without a clear sex predominance [12]. ANCAs, usually p-ANCA/myeloperoxidase ANCA, may be found in only 30–40% of EGPA patients [10].
EGPA is increasingly considered a syndromic condition of several clinically or pathogenically distinct subgroups. Clinical manifestations in EGPA tend to divide patients into two subsets of the disease, with a predominance of vasculitic or eosinophilic manifestations, and ANCA can differentiate between these two subsets [1], [3]. This subclassification contrasts ANCA-positive and ANCA-negative EGPA. The first group is significantly more likely to have disease manifestations associated with small-vessel vasculitis, including necrotizing glomerulonephritis, mononeuritis and purpura, whereas the latter group is significantly more likely to have cardiac and lung involvement [13], [14]. Although the literature indicates that lung infiltrates are more related to negative ANCA, this patient had a pulmonary involvement with ANCA-positive EGPA.
EGPA has traditionally been classified as occurring in three phases [3], [15]. The prodromal phase can start from months up to years before the other phases and can last for a long time. EGPA is characterized by upper respiratory symptoms, asthma and general symptoms, such as arthralgia, myalgia, malaise, fever and weight loss [3]. The eosinophilic phase is characterized by peripheral eosinophilia and organ involvement, including lung, cardiac and gastrointestinal involvement. Migratory infiltrates in lung imaging is another hallmark of EGPA and one of the diagnostic criteria of the American College of Rheumatology. Chest CT scanning is a more sensitive method for evaluating the infiltrates [3]. The vasculitic phase presents with constitutional symptoms, such as fatigue, fever and weight loss. Paradoxically, an apparent improvement of asthma symptoms can also occur. Peripheral neuropathy can occur as multiplex mononeuritis or sensorimotor peripheral neuropathy. Renal manifestations can range from isolated urinary abnormalities to rapidly progressive glomerulonephritis. The most common presentation is pauci-immune focal and segmental necrotizing glomerulonephritis, with or without crescents, which usually involve less than 50% of the glomeruli [3]. Skin lesions are also a prominent feature of the vasculitic phase and occur most commonly as palpable purpura and nodules [3]. In our patient, a persistent microscopic haematuria was observed in urine analyses, without erythrocyte dysmorphism. Despite these findings, renal function remained normal during the follow-up evaluation.
Asthma is a chronic inflammatory airway disease that is characterized by a specific inflammatory cell response resulting in a swollen, oedematous, hyper-reactive airway. It is the main manifestation during the prodromal phase of the disease. It is present in 96–100% of EGPA patients [16]. Asthma symptoms generally precede the onset of the systemic disease by several years and generally become corticodependent [7], [17]. Unlike our patient, the adult-onset of asthma is the most common presentation [7]. Other upper respiratory symptoms are present in a majority of patients (47–93%). These symptoms include allergic rhinitis, nasal polyps, recurrent or chronic sinusitis [16]. In this case report, the patient had an asthma diagnosis since childhood, but with a recent worsening of the symptoms, cough and dyspnoea, which was followed by upper airway symptoms, haemoptoic sputum and constitutional symptoms, representing the prodromal phase of EGPA. CT imaging of the paranasal sinus confirmed chronic sinusitis, which evolved into the frontal and ethmoidal sinuses in this patient's case.
Eosinophilia is one of the hallmarks of the development of this syndrome into AAV. It can be found in peripheral, sputum, bronchoalveolar lavage and tissue eosinophilia. It has been associated with several diseases that affect the small and large airways, as with many other lung diseases. Indeed, marked eosinophilia observed during the course of lung disease is not a common event, and thus, when it occurs, it generally indicates a specific diagnosis [18]. A peripheral blood eosinophilia >10% is one of the clinical criteria for EGPA diagnosis. In this report, the patient's blood exams demonstrated leucocytosis with marked eosinophilia (22%), which meets an additional criterion of the American College of Rheumatology.
Cardiac involvement is the major cause of death and poor prognosis in EGPA, occurring in as many as 27–47% of EGPA cases [11]. Clinical manifestations include congestive heart failure, myocarditis, pericarditis, valvular heart abnormalities myocardial ischaemia and arrhythmia [11], [19]. It is more commonly found in ANCA-negative patients and directly correlates with blood eosinophilia [11]. In this report, the patient had no clinical manifestations of heart disease and no suggestive findings in transthoracic echocardiogram and electrocardiogram.
Extravascular eosinophil can be evaluated by performing a tissue biopsy. Histopathological analysis of the lungs has revealed that EGPA can present with an eosinophil-rich granulomatous inflammation of the airways in addition to small- and medium-vessel vasculitis [9], [20], [21]. EGPA was originally described as a pathological triad consisting of eosinophilic infiltration, necrotizing vasculitis and extravascular granuloma formation. The early phase of the disease is characterized by extravascular tissue infiltration by eosinophils of any organ. In the vasculitis phase, signs of inflammation are observed in small to medium vessel walls. Vasculitis is characterized by fibrinoid necrosis and eosinophilic vessel wall inflammation [3]. ANCA-positive patients more frequently exhibit vasculitis in histological specimens [9]. As the last investigative step of this patient, an open lung biopsy was performed, and specimen analysis showed perivascular eosinophilic infiltration, which is characteristic of the vasculitic phase, with eosinophilic infiltration to tissues, resulting in inflammation in small and medium vessels. However, it is important to remark that many eosinophilic inflammatory infiltrates are not specific to EGPA and have been reported in other vasculitis, mainly in GPA [1]. Migration of eosinophils into the lung is a multistep process, and undergoes a precise mechanism depending on the location of the tissue eosinophilia [22].
An important differential diagnosis in our case was HES. HES shares many features with EGPA, such as peripheral hypereosinophilia and tissue eosinophilia with organ dysfunction or damage. Differentiating between these two conditions has therapeutic and prognosis repercussions. For this reason, it has been proposed that patients who present with asthma, an eosinophil count above 1500/mm3 and systemic manifestations but no vasculitis in histology specimens or ANCA, may be considered to suffer from HES [6], [23].
Neurologic involvement is observed in up to 60–70% of patients [10], [18]. Patients may present with multiplex mononeuritis or a mixed sensorial and motor peripheral neuropathy. The central nervous system is involved in 25% of cases with neurological involvement [3]. Although the patient did not present any neurological symptoms, an electroneuromyography was performed to complement the evaluation. This examination showed a normal pattern.
EGPA has some classical imaging findings. The most common lung radiographic manifestations are transient, bilateral, non-segmental areas of consolidation without a predilection of any specific lung segment or zone [7], [24]. GGO areas or consolidation in either a patchy or mainly peripheral distribution are the most common observations on high-resolution CT. [24], [25]. Other abnormalities include small centrilobular nodules, and less common observations are larger nodules and interlobular septal thickening. Pleural effusion, a rare manifestation, has been described in a few patients [25]. The patient's chest CT showed patchy GGO, the most common finding of EGPA in his examination, and a few small centrilobular nodules.
Pulmonary function tests (spirometry, body plethysmography and DLco test) were performed and revealed a severe obstructive pattern with no bronchodilator response. Airway obstruction is the most common pattern found on spirometry in EGPA. Many patients may have an irreversible airflow obstruction, which was observed in our patient [26]. Interestingly, the evaluation of DLco is important in cases of pulmonary involvement of EGPA, in which alveolar haemorrhage can occur slightly or as a life-threating manifestation, thereby increasing the DLco value [27]. Another cause that may increase the DLco value in EGPA is asthma [28]. However, our patient had a reduced DLco that, in EGPA, has been associated with severe pulmonary infiltrates or pulmonary vascular abnormalities [29], [30]. Thus, we believe that pulmonary microangiopathy with endothelial cell damage induced by vasculitis in pulmonary blood vessels may justify, at least in part, the decrease in DLco observed in our patient [29]. Corroborating with this finding, it is worth noting that our patient had ANCA-positive, which is associated with small-vessels vasculitis and more significantly decreased DLco [13], [14], [30].
Because EGPA is a rare disease, there is a lack of high quality clinical trials. Thus, expert recommendations for the treatment of EGPA are commonly made by an analogy to clinical trial data derived for other forms of AAV. Our patient had an excellent response to glucocorticoids (0.5–1.5 mg/kg per day), which are the mainstay of EGPA treatment and dramatically improved the prognosis [31]. Posteriorly, he converted to maintenance therapy with azathioprine, a less toxic immunosuppressive drug, in combination with a tapering dose of glucocorticoids [19], [32]. He had no markers of poor prognosis according to the “five factor score” (FFS) [33] (Table 2), which predicts survival, and did not require additional immunosuppression. In patients with FFS ≥2 or severe organ dysfunction (e.g., heart and central nervous system), intravenous cyclophosphamide plus intravenous high dose glucocorticoids (methylprednisolone 1000 mg for three days) have been proposed [34], [35], [36]. In refractory disease, the use of rituximab (anti-CD20 monoclonal antibody) [37], [38], [39], [40] and mepolizumab (anti-IL-5 monoclonal antibody) has been reported with success [41], [42], [43].
Table 2.
Prognostic criteria predicting survival in eosinophilic granulomatosis with polyangiitis.
| Revised 2011 five-factor score [17] |
|---|
|
|
|
|
|
Conflict of interest
The authors have no potential conflicts of interest to disclose.
References
- 1.Jennette J.C., Falk R.J., Bacon P.A., Basu N., Cid M.C., Ferrario F. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013 Jan;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Churg J., Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am. J. Pathol. 1951 Mar-Apr;27(2):277–301. [PMC free article] [PubMed] [Google Scholar]
- 3.Greco A., Rizzo M.I., De Virgilio A., Gallo A., Fusconi M., Ruoppolo G. Churg-Strauss syndrom. Autoimmun. Rev. 2015 Apr;14(4):341–348. doi: 10.1016/j.autrev.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Masi A.T., Hunder G.G., Lie J.T., Michel B.A., Bloch D.A., Arend W.P. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990 Aug;33(8):1094–1100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 5.Simon H.U., Rothenberg M.E., Bochner B.S., Weller P.F., Wardlaw A.J., Wechsler M.E. Refining the definition of hypereosinophilic syndrome. J. Allergy Clin. Immunol. 2010 Jul;126(1):45–49. doi: 10.1016/j.jaci.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valent P., Klion A.D., Horny H.P., Roufosse F., Gotlib J., Weller P.F. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J. Allergy Clin. Immunol. 2012 Sep;130(3):607–612. doi: 10.1016/j.jaci.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanham J.G., Elkon K.B., Pusey C.D., Hughes G.R. Systemic vasculitis with asthma and Eosinophilia: a clinical approach to the Churg-strauss syndrome. Med. Baltim. 1984 Mar;vol. 63(2):65–81. doi: 10.1097/00005792-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Watts R., Lane S., Hanslik T., Hauser T., Hellmich B., Koldingsnes W. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyartetritis nodosa for epidemiology studies. Ann. Rheum. Dis. 2007 Feb;66(2):222–227. doi: 10.1136/ard.2006.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A., Dogra S., Sharma K. Granulomatous vasculitis. Dermatol. Clin. 2015 Jul;33(3):475–487. doi: 10.1016/j.det.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Moosig F. Eosinophilic granulomatosis with polyangiitis: future therapies. Presse Med. 2013 Apr;42(4 Pt 2):510–512. doi: 10.1016/j.lpm.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Mahra A., Moosig F., Neumann T., Szczeklik W., Taillé C., Vaglio A. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): evolutions in classification, etiopathogenesis, assessment and management. Curr. Opin. Rheumatol. 2014 Jan;26(1):16–23. doi: 10.1097/BOR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 12.Gibelin A., Maldini C., Mahr A. Epidemiology and etiology of wegener granulomatosis, microscopic polyangiitis, churg-strauss syndrome and goodpasture syndrome: vasculitides with frequent lung involvement. Semin. Respir. Crit. Care Med. 2011 Jun;32(3):264–273. doi: 10.1055/s-0031-1279824. [DOI] [PubMed] [Google Scholar]
- 13.Sablé-Fourtassou R., Cohen P., Mahr A., Pagnoux C., Mouthon L., Jayne D. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann. Intern. Med. 2005 Nov;143(9):632–638. doi: 10.7326/0003-4819-143-9-200511010-00006. [DOI] [PubMed] [Google Scholar]
- 14.Sinico R.A., Di Toma L., Maggiore U., Bottero P., Radice A., Tosoni C. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum. 2005 Sep;52(9):2926–2935. doi: 10.1002/art.21250. [DOI] [PubMed] [Google Scholar]
- 15.Mouthon L., Dunogue B., Guillevin L. Diagnosis and classification of eosinophilic granulomatosis with polyangiitis (formely name Churg-Strauss syndrome) J. Autoimmun. 2014 Feb-Mar;48–49:99–103. doi: 10.1016/j.jaut.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Baldini C., Talarico R., Della Rossa A., Bombardieri R. Clinical manifestations and treatment of Churg–Strauss syndrome. Rheum. Dis. Clin. North Am. 2010 Aug;36(3):527–543. doi: 10.1016/j.rdc.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Abril A., Calamia K.T., Cohen M.D. The Churg Strauss syndrome (allergic granulomatous angiitis): review and update. Semin. Arthritis Rheum. 2003 Oct;33(2):106–114. doi: 10.1016/s0049-0172(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 18.Woolnough K., Wardlaw A. Eosinophilia in pulmonary disorders. Immunol. Allergy Clin. N. Am. 2015 Aug;35(3):477492. doi: 10.1016/j.iac.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Sinico R.A., Bottero P. Churg-Strauss angiitis. Best. Pract. Res. Clin. Rheumatol. 2009 Jun;23(3):355–366. doi: 10.1016/j.berh.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Churg A. Recent advances in the diagnosis of Churg-Strauss syndrome. Mod. Pathol. 2001 Dec;14(12):1284–1293. doi: 10.1038/modpathol.3880475. [DOI] [PubMed] [Google Scholar]
- 21.Katzenstein A.L. Diagnostic features and differential diagnosis of Churg-Strauss syndrome in the lung: a review. Am. J. Clin. Pathol. 2000 Nov;114(5):767–772. doi: 10.1309/F3FW-J8EB-X913-G1RJ. [DOI] [PubMed] [Google Scholar]
- 22.Wardlaw A.J. Molecular basis for selective eosinophil trafficking in asthma: a multistep paradigm. J. Allergy Clin. Immunol. 1999 Nov;104(5):917–926. doi: 10.1016/s0091-6749(99)70069-2. [DOI] [PubMed] [Google Scholar]
- 23.Gotlib J. World Health Organization-defined eosinophilic disorders: 2014 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2014 Mar;89(3):325–337. doi: 10.1002/ajh.23664. [DOI] [PubMed] [Google Scholar]
- 24.Silva C.I., Müller N.L., Fujimoto K., Johkoh T., Ajzen S.A., Churg A. Churg-Strauss syndrome: high resolution CT and pathologic findings. J. Thorac. Imaging. 2005 May;20(2):74–80. doi: 10.1097/01.rti.0000155268.00125.de. [DOI] [PubMed] [Google Scholar]
- 25.Worthy S.A., Müller N.L., Hansell D.M., Flower C.D. Churg-Strauss syndrome: the spectrum of pulmonary CT findings in 17 patients. Am. J. Roentgenol. 1998 Feb;170(2):297–300. doi: 10.2214/ajr.170.2.9456932. [DOI] [PubMed] [Google Scholar]
- 26.Cottin V., Khouatra C., Dubost R., Glérant J.C., Cordier J.F. Persistent airflow obstruction in asthma of patients with Churg-Strauss syndrome and long-term follow-up. Allergy. 2009 Apr;64(4):589–595. doi: 10.1111/j.1398-9995.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 27.Kostianovsky A., Hauser T., Pagnoux C., Cohen P., Daugas E., Mouthon L., Miossec P. Pulmonary haemorrhage complicating Wegener's granulomatosis and microscopic polyarteritis. Br. Med. J. 1985 Jun;290(6484):1775–1778. doi: 10.1136/bmj.290.6484.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saydain G., Beck K.C., Decker P.A., Cowl C.T., Scanlon P.D. Clinical significance of elevated diffusing capacity. Chest. 2004 Feb;125(2):446–452. doi: 10.1378/chest.125.2.446. [DOI] [PubMed] [Google Scholar]
- 29.Utsugi M., Ishizuka T., Hisada T., Sato K., Ishizuka T., Dobashi K. Hypoxemia with high alveolar-arterial oxygen gradient but no lung involvement in a patient with Churg-Strauss syndrome: case report. Int. J. Immunopathol. Pharmacol. 2008 Jan-Mar;21(1):251–253. doi: 10.1177/039463200802100131. [DOI] [PubMed] [Google Scholar]
- 30.Li J., Zhang L., Zhao W., Zhu M., Xue Y., Dai H. Clinical analysis of 43 patients with eosinophilic granulomatosis with polyangiitis. Zhonghua Yi Xue Za Zhi. 2016 Mar;96(10):787–791. doi: 10.3760/cma.j.issn.0376-2491.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Mukhtyar C., Guillevin L., Cid M.C., Dasgupta B., de Groot K., Gross W. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann. Rheum. Dis. 2009 Mar;68(3):310–317. doi: 10.1136/ard.2008.088096. [DOI] [PubMed] [Google Scholar]
- 32.Pagnoux C., Guilpain P., Guillevin L. Churg-Strauss syndrome. Curr. Opin. Rheumatol. 2007 Jan;19(1):25–32. doi: 10.1097/BOR.0b013e3280119854. [DOI] [PubMed] [Google Scholar]
- 33.Guillevin L., Pagnoux C., Seror R., Mahr A., Mouthon L., Le Toumelin P. The five-factor score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Med. Baltim. 2011 Jan;vol. 90(1):19–27. doi: 10.1097/MD.0b013e318205a4c6. [DOI] [PubMed] [Google Scholar]
- 34.Cohen P., Pagnoux C., Mahr A., Arène J.P., Mouthon L., Le Guern V. Churg-Strauss syndrome with poor-prognosis factors: a prospective multicenter trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in forty-eight patients. Arthritis Rheum. 2007 May;57(4):686–693. doi: 10.1002/art.22679. [DOI] [PubMed] [Google Scholar]
- 35.Moosig F., Bremer J.P., Hellmich B., Holle J.U., Holl-Ulrich K., Laudien M. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg-Strauss, EGPA): monocentric experiences in 150 patients. Ann. Rheum. Dis. 2013 Jun;72(6):1011–1017. doi: 10.1136/annrheumdis-2012-201531. [DOI] [PubMed] [Google Scholar]
- 36.Samson M., Puéchal X., Devilliers H., Ribi C., Cohen P., Stern M. Long-term outcomes of 118 patients with eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) enrolled in two prospective trials. J. Autoimmun. 2013 Jun;43:60–69. doi: 10.1016/j.jaut.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Muñoz S.A., Gandino I.J., Orden A.O., Allievi A. Rituximab in the treatment of eosinophilic granulomatosis with polyangiitis. Reumatol. Clin. 2015 May-Jun;11(3):165–169. doi: 10.1016/j.reuma.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Mohammad A.J., Hot A., Arndt F., Moosig F., Guerry M.J., Amudala N. Rituximab for the treatment of eosinophilic granulomatosis with polyangiitis (Churg-Strauss) Ann. Rheum. Dis. 2016 Feb;75(2):396–401. doi: 10.1136/annrheumdis-2014-206095. [DOI] [PubMed] [Google Scholar]
- 39.Fanouriakis A., Kougkas N., Vassilopoulos D., Fragouli E., Repa A., Sidiropoulos P. Rituximab for eosinophilic granulomatosis with polyangiitis with severe vasculitic neuropathy: case report and review of current clinical evidence. Semin. Arthritis Rheum. 2015 Aug;45(1):60–66. doi: 10.1016/j.semarthrit.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Pagnoux C., Groh M. Optimal therapy and prospects for new medicines in eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) Expert Rev. Clin. Immunol. 2016 Oct;12(10):1059–1067. doi: 10.1080/1744666X.2016.1191352. [DOI] [PubMed] [Google Scholar]
- 41.Moosig F., Gross W.L., Herrmann K., Bremer J.P., Hellmich B. Targeting interleukin-5 in refractory and relapsing Churg-Strauss syndrome. Ann. Intern. Med. 2011 Sep;115(5):341–343. doi: 10.7326/0003-4819-155-5-201109060-00026. [DOI] [PubMed] [Google Scholar]
- 42.Kim S., Marigowda G., Oren E., Israel E., Wechsler M.E. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J. Allergy Clin. Immunol. 2010 Jun;125(6):1336–1343. doi: 10.1016/j.jaci.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 43.Varrichi G., Bagnasco D., Borrielo F., Heffler E., Canonica G.W. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr. Opin. Allergy Clin. Immunol. 2016 Apr;16(2):186–200. doi: 10.1097/ACI.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]