Abstract
In this study we tested whether legumes can improve the growth and N and S nutrition of rapeseed in an intercropping system and compared the effect of mixtures on legume N-fixation and soil N-resources. Rapeseed was cultivated in low N conditions in monocrops using one (R) or two plants (RR) per pot and in mixtures with lupine, clover or vetch.
The R monocrop was the most relevant control, intraspecific competition inducing a significant growth delay resulting in a significantly lower leaf number, in RR monocrop compared to R and the three mixtures considered. Plant biomass, and the N and S contents of rapeseed grown in mixtures were the same than those measured in R monocrop. Compared to the monocrop, the proportion of N derived from the atmosphere was increased by 34, 140 and 290% in lupine, clover and vetch, respectively when intercropped with rapeseed. In mixture with clover and lupine, the soil N pool at harvest was higher than in other treatments, while N export by crop was constant. Legumes suffered from competition for soil S resulting in a decrease of 40% in their S content compared to the monocrop. Compared to rapeseeds grown in R monocrop and in mixture with lupine and vetch, rapeseed mixed with clover showed significantly higher SPAD values in old leaves.
In our conditions, mixing legumes with rapeseed is relevant to reduce N fertilization and improve nutrition and growth of rapeseed.
Keywords: Agriculture, Plant biology
1. Introduction
Excessive use of nitrogen fertilizers in intensive agricultural systems has affected the balance of the global nitrogen (N) cycle, resulting in negative environmental impacts. In most intensive monocrops, the inefficient use of nitrogen fertilizers can lead to N losses by denitrification and leaching into the environment. For example, nitrate leaching can be responsible for the eutrophication of waters while the nitrous oxide produced by denitrification plays an important role in ozone depletion, and both of these adversely affect climate and human health (Galloway et al., 2003). Thus, new cultural practices must be developed in order to decrease N inputs and improve the agro-environmental balance of this crop.
In this context, intercropping through the process of biological N fixation (BNF) by legumes (Fabaceae) offers an environmentally sustainable source of N and can partly substitute or replace external N inputs (Garg and Geetanjali, 2007; Peoples et al., 2009). Intercropping is defined as the growth of two or more crops in proximity in the same field during a growing season to promote interaction between them (Willey, 1979). Previous studies have demonstrated that growing legumes with a cereal crop has a positive impact on yield, yield stability and the grain N content of the cereal (Hauggaard-Nielsen et al., 2001a; Jensen, 1996a). Indeed, it has been frequently observed that cereal-legume mixtures offer improvements in the use of N resources (Vandermeer, 1989; Loreau and Hector, 2001), mainly due to the fact that species do not compete for the same resource niche (located in either the soil or as dinitrogen N2) at the same time and in the same space (Hauggaard-Nielsen et al., 2001a; Bedoussac and Justes, 2010a). For example, in cereal-pea mixtures, a cereal with a high N requirement is more competitive for soil mineral nitrogen due a deeper and faster growing root system and the N nutrition of the legume depends mainly on symbiotic fixation of dinitrogen (N2) (Jensen, 1996a; Corre-Hellou et al., 2007; Bedoussac and Justes, 2010b). Many studies using different 15N-labelling methods have demonstrated that legumes can deposit a significant amount of N into the soil, which can be transferred from the legume to the non-fixing neighbouring plants (for review: Fustec et al., 2010; Chalk et al., 2014). It is also well documented that intercropping of legumes and cereals increases the efficiency of BNF compared to a monocrop (Jensen, 1996b; Xiao et al., 2004). Moreover, the percentage of legume N derived from the biological fixation of N2 (%BNF) is higher in low N input than in high N input systems (Jensen, 1996a; Corre-Hellou et al., 2007). Owing to this optimized use of resources, legume-cereal intercropping may sustain the yield of both crops under low N inputs (Hauggaard-Nielsen et al., 2001b, 2008; Andersen et al., 2005; Naudin et al., 2010; Pelzer et al., 2012). For example, Pelzer et al. (2012) have shown that pea–wheat intercrops allow maintenance of wheat grain production and require less than half of the nitrogen fertilizer compared to wheat grown alone.
Rapeseed (Brassica napus L.) is an important agricultural crop that requires a large amount of N and sulfur (S) inputs to maintain yield and the quality of harvest products (Colnenne et al., 1998; Dubousset et al., 2010; D’Hooghe et al., 2014). Although rapeseed is considered a nitrophilic plant, it is characterized by a low N use efficiency with only half the N derived from fertilizers recovered in the harvested seeds (Schjoerring et al., 1995). These N (150–250 kg ha−1: Rathke et al., 2006) and S (30–50 kg ha−1: Pedersen et al., 1998) fertilizations are crucial and are performed at the beginning of spring at the bolting stage of rapeseed to promote efficient growth, pod filling and yield. Some field experiments conducted on two mustard species (Brassica campestris Var. Toria and Sinapis alba L. cv. Gisilba) mixed with legumes have not shown any benefit to mustard yield compared with mustard as a sole crop (Waterer et al., 1994; Banik et al., 2000). However, Jamont et al. (2013) have shown that intercropping faba bean (Vicia faba L. spp. minor cv. Divine) with rapeseed under low N-conditions has a positive effect on dry weight and N contents in rapeseed mainly due to the niche complementarity between the both species in sharing soil N resources. Cortés-Mora et al. (2010) have also shown that the yield and N contents of some Brassica species were significantly greater in legume-supported intercrops than when monocropped. In addition, these authors have detected N transfer from the legume to the Brassica species at the early stages of growth. Accordingly, mixing legumes with rapeseed seems to be relevant (especially at the bolting stage of rapeseed) to reduce the amount of N fertilizer or to improve rapeseed nutrition in low N conditions. However, although recent results demonstrate benefits of legumes on Brassica yield, Brassica-legume mixtures still remain sparsely documented and require more knowledge of their practical application.
In this study, three legume species (lupine, clover and vetch) were used to examine the suitability of legumes in mixtures with rapeseed in low N conditions. The first aim of this study was to evaluate whether combining rapeseed with legumes can improve its growth and mineral nutrition (especially N and S) compared to two types of rapeseed monocrops that considered either one or two plants per pot (R and RR) to determine their relevance as controls. The second aim was to compare the effect of mixtures on the N2 fixation capacity of legumes (%Ndfa) and on soil N resources.
2. Materials and methods
2.1. Plant growth conditions and experimental design
Seeds of rapeseed (Brassica napus var. Boheme) and three legumes that differ in their growth habit: upright lupine (Lupinus albus var. Orus), ground-covering clover (Trifolium incarnatum var. Cegalo) and climbing vetch (Vicia sativa var. Nacre), were germinated in a greenhouse on perlite over demineralized water for 1 week in the dark, followed by 2 weeks in the light. After first leaf emergence, seedlings were planted in pots (50 cm height and 14 cm diameter) filled with 12 kg of sand-soil mixture - to generate low N conditions and limit flush of N mineralization - (v/v: 2/1) with the following parameters: sand: quartz BB 0.8–1.4 mm diameter (SIBELCO, Paris, France) and soil: pH 6.1, clay 36.7%, silt 41.3%, total N 0.32% and total S 0.1%. The pots were watered exclusively with deionized water and brought back to initial weight every day to maintain initial soil humidity, which was fixed at 25%. Plants were grown with a thermoperiod of 20/17 °C day/night and a photoperiod of 16 h. Natural light was supplemented with high pressure sodium lamps (Philips, MASTER Green Power T400W) supplying an average photosynthetically active radiation of 350 μmol photons m−2 s−1 at canopy height. Seven plant combinations were established: two types of rapeseed monocrops (Gibson et al., 1999) of either one (R) or two rapeseed plants (RR) per pot; lupine (L), clover (C) and vetch (V) as monocrops; and rapeseed-Lupine (R-Lupine), rapeseed-Clover (R-Clover) and rapeseed-Vetch (R-Vetch) in mixture systems. Legume monocrops consisted of two plants per pot and mixture systems consisted of one rapeseed plant and one legume per pot.
2.2. Growth of rhizobium strains and plant inoculation conditions
To enhance the BNF of legumes in controlled growth conditions (see below), the unsterilized soil (to preserve native soil biota, especially microorganisms involved in N and S mineralization processes) was inoculated with specific strains of bacteria of each host legume (Mazurier, 1989; Laguerre et al., 1992, 1994; Table 1). The different strains were provided from the core collection of UMR1347 Agroécologie, Institut National de la Recherche Agronomique, Dijon, France and were conserved at –80 °C in Bergersen's medium (Bergersen, 1961). Before plant inoculation, each bacterial strain was grown on 100 ml of Bergersen's medium modified by the addition of 0.2 g.l−l of yeast extract and adjusted to pH 6.8 under sterilized conditions. This culture medium was incubated for 48 h at 28 °C under low orbital agitation (200 rpm). Bacterial cultures were suspended in 100 ml of sterile deionized water and vortexed to obtain homogeneous inoculum suspensions. Each inoculum suspension was applied to the soil of each host legume (5 ml per pot) at the time of seedling transplantation in the greenhouse.
Table 1.
Bacterial species and strains of the host legumes.
| Strains | Species | Host legumes | References |
|---|---|---|---|
| T354 (MSDJ1056) | Rhizobium leguminosarum bv. trifolii | Trifolium incarnatum var. Cegalo | Mazurier, 1989 |
| P221 (MSDJ0469) | Rhizobium leguminosarum bv. viciae | Vicia sativa var. Nacre | Laguerre et al., 1992 |
| LL13 (MSDJ718) | Bradyrhizobium sp. | Lupinus albus var. Orus | Laguerre et al., 1994 |
2.3. Plant harvest
All plants were harvested after three months of growth. The shoots were separated from the roots. For each rapeseed plant, leaves were separated based on their time of emergence (defined as the leaf rank number) and the leaf rank number incremented from the oldest to the younger leaves. According to Ruiz-Espinoza et al. (2010), a SPAD-502 chlorophyll meter (Minolta, Tokyo, Japan) was used as a relevant non-destructive method to estimate leaf chlorophyll contents of rapeseed. In the monocrops, the roots of the two plants were pooled in the same sample. In the mixtures, the roots of each plant (rapeseed and legume) were separated. Root samples were carefully washed with deionized water. In each pot, aliquots of soil from which roots were carefully removed (using a magnifying glass) were collected. Each sample (plant organs and soil) was weighed and oven dried (60 °C) for DW determination and ground to fine powder before total N, 15N and total S analysis.
2.4. Total N, δ15 and total S analysis
Aliquots of 5 or 20 mg of DW of each plant organ or soil sample were placed into tin capsules for isotopic analysis, respectively. The total N and S contents and the isotopic ratio 15N/14N were determined by analysing samples with a continuous flow Isotope-Ratio Mass Spectrometer (IRMS) (Horizon, NU Instruments, Wrexham, United Kingdom) linked to a C/N/S analyser (EA3000, Euro Vector, Milan, Italy). The total N or S amount (Ntot or Stot) in each organ was calculated as:
Ntotal (or Stotal) = %N (or %S) x DW/100
The δ15N was calculated as:
δ15N = (Rsample – Rstandard)/Rstandard x 1000
Where Rsample and Rstandard are the isotopic ratios 15N/14N of the sample and standard (atmospheric dinitrogen gas, 0.3663 Atom % 15N), respectively.
2.5. Determination of the atmospheric dinitrogen (N2) fixation capacity of each legume species (β value)
To determine the N2 fixation capacity of each legume species, specific plant culture conditions were used. The β parameter is defined as the δ15N value of legumes grown in sand with a nutrient solution free of nitrogen and is a prerequisite for the calculation of the proportion of a legume's %Ndfa, as previously described by Shearer and Kohl (1986). Seedlings of legumes (lupine, clover and vetch) were planted in pots (2 L) perforated at the base (free-draining) and filled with 2.5 kg of sand. Legumes were inoculated with specific rhizobium strains (Table 1). Each pot was watered for 40 days with 0.25 L day−1 of N-free nutrient solution containing: K2SO4 1 mM, KH2PO4 0.4 mM, K2HPO4 0.15 mM, CaCl2 3 mM, MgSO4 0.5 mM, EDTA 2NaFe 0.2 mM, H3BO3 14 μM, MnSO4 5 μM, ZnSO4 3 μM, CuSO4 0.7 μM, Na2MoO4 0.7 μM, CoCl2 0.1 μM. After 40 days, plants were harvested, weighed and oven dried (60 °C) for DW determination and ground to fine powder before IRMS analysis to determine the δ15N corresponding to the β. Three replicates (three independent pots) of each legume were considered for this experiment.
2.6. Determination of the %Ndfa by legume species grown in monocrops or in mixtures with rapeseed
Legumes can take up two N sources: soil N (δ15N > 0) and atmospheric dinitrogen with δ15N = 0. Thus, an increase of dinitrogen fixation lead to a decrease of δ15N in legumes.
%Ndfa was determined from plants grown in pots filled with a sand-soil mixture (w/w: 2/1) as previously described, using the natural abundance δ15N method according to the following formula given by Shearer and Kohl (1986):
%Ndfa = (δ15Nnon-fixingplant - δ15Nlegume)/(δ15N non-fixingplant – β) x 100
Where:
δ15Nnon-fixingplant is the δ15N value of rapeseed monocrops (R and RR) grown in a sand-soil mixture,
δ15Nlegume is the δ15N value of legumes grown in monocrops or in mixtures with rapeseed in a sand-soil mixture.
2.7. Data and statistical analysis
The experiment was performed with five replicates except for the Ndfa determination, which was performed with three replicates. The resulting variations in data are expressed as the means ± S.E for n = 5 (or n = 3 for %Ndfa determination). As our main purpose was to compare individual intercrop systems to controls (not to check for general significant trends between 5 crops), we performed Student's t tests between each intercrop and control after verifying compliance of normality.
3. Results
3.1. In mixture, the growth of rapeseed is unaffected
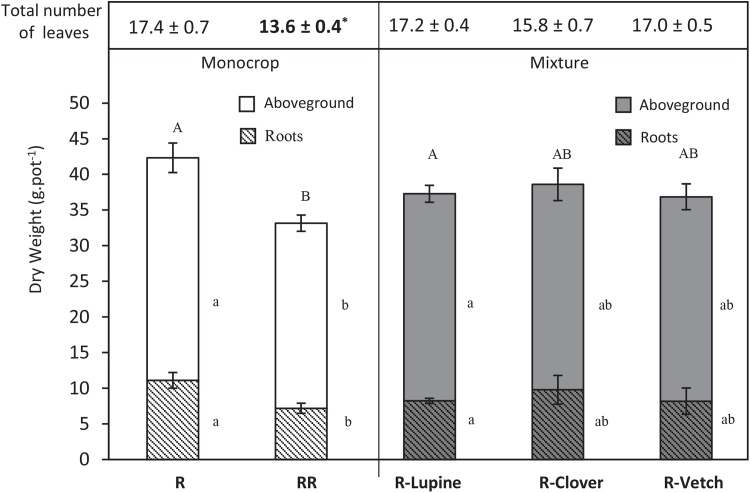
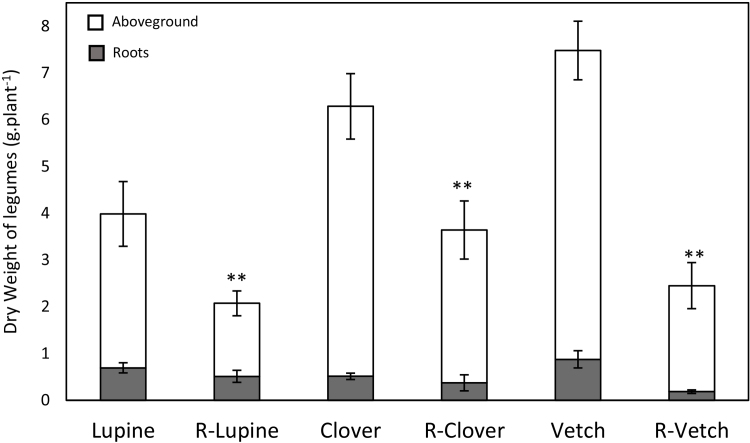
In monocrops, the total dry weight (DW) of rapeseed grown alone (R) was significantly higher than the total DW of two rapeseed plants grown together (RR; Fig. 1). The same trend was noticed for both aboveground and root DW. In the RR monocrop, the total leaf number of rapeseed was significantly lower than the R monocrop or rapeseed grown in mixtures (R-Lupine, R-Clover and R-Vetch), where the values were all similar. These results suggest a delay in the growth of rapeseed in the RR monocrop compared to the R monocrop and mixtures. Among all mixtures, only rapeseed mixed with lupine (R-Lupine) showed a significantly higher biomass than the RR monocrop (p < 0.05; Fig. 1). The DW of rapeseed grown in as the R monocrop or with legumes was similar, regardless of legume species. Additionally, biomasses of clover, lupine and vetch cultivated in mixtures were significantly lower than those grown in monocrop (Fig. 2).
Fig. 1.
Dry weights (g.pot−1) and total number of leaves (in the upper part) of rapeseed grown as monocrops with one (R) or two plants per pot (RR) and in mixtures with lupine, clover and vetch (R-Lupine, R-Clover and R-Vetch, respectively) at three month after sowing. Vertical bars indicate ± S.E. Different capital and lowercase letters indicate that the mean values of total DW, and root- and shoot-DW, respectively are significantly different at p < 0.05 (n = 5). * indicates that the total number of leaves is significantly different at p < 0.05.
Fig. 2.
Dry weights (g.plant−1) of legumes grown in monocrops or in mixtures with rapeseed (R-Lupine, R-Clover and R-Vetch, respectively) at three month after sowing. Vertical bars indicate ± S.E. ** indicates significant difference between DW of legumes in monocrop and mixture (p < 0.01).
3.2. Rapeseed grown with clover shows higher leaf SPAD values
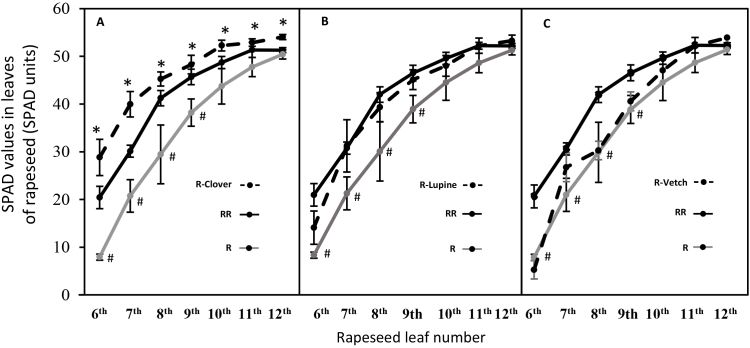
For older leaves (6th to 9th leaves), SPAD values from the RR monocrop were significantly higher than those from the R monocrop (Fig. 3). Alongside the biomass data (Fig. 1), this result confirms a different stage of growth between rapeseed in RR and R monocrops. For all leaf ranks (6th to 12th), R-Clover rapeseed leaves showed higher SPAD values than R monocrops (Fig. 3). For example, the SPAD values of 6th leaf were 28.8 ± 3.8 for R-Clover versus 7.9 ± 0.6 for R. The SPAD values of rapeseed from R-Lupine and R-Vetch were not significantly different to those determined from the rapeseed R monocrop.
Fig. 3.
SPAD values in leaves (6th to 12th leaves) from rapeseed grown in monocrops with one (R) or two plants (RR) per pot and in mixture with clover (R-Clover) (A), lupine (R-Lupine) (B) and vetch (R-Vetch) (C). Vertical bars indicate ± S.E. (n = 5). # indicates significant differences between SPAD values of leave from monocrops R and RR and * indicates significant differences between SPAD value of leaf number from rapeseed grown in monocrop R and in mixture (p < 0.05).
3.3. The BNF of legumes is increased in mixtures
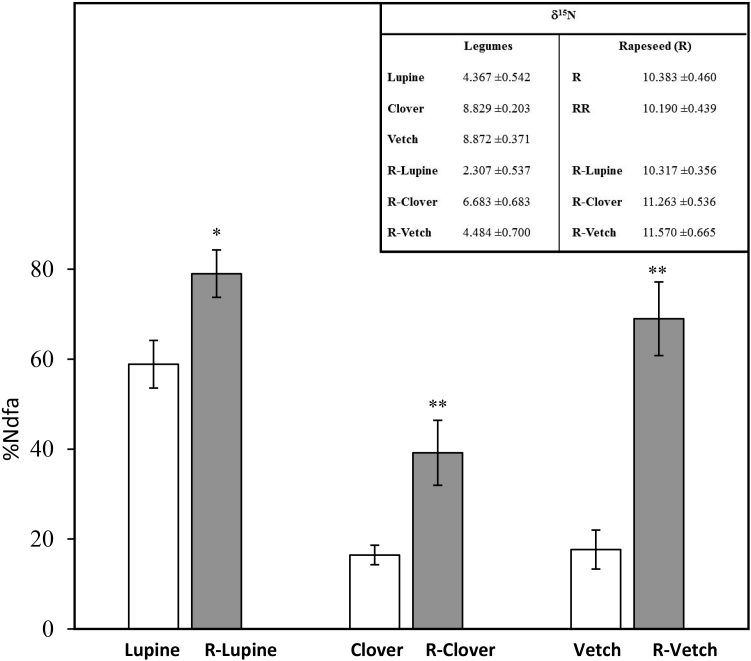
The %Ndfa expresses BNF according to the legume species (δ 15N values of rapeseed and legumes in both monocrops and mixtures are shown in insert of Fig. 4). In monocrops, it was significantly higher in lupine (58.8 ± 5.3) than clover (16.5 ± 2.1) and vetch (17.7 ± 4.3) (Fig. 4). In mixtures, %Ndfa values were 79.0 ± 5.2, 39.2 ± 7.2 and 69.0 ± 8.2 for lupine, clover and vetch, respectively. These data showed that in mixture, the BNF values were significantly increased (p < 0.01) by 34, 140 and 290% compared with their respective monocrop.
Fig. 4.
Percentage of nitrogen derived from the atmosphere (%Ndfa) in lupine, clover, and vetch grown as monocrops (white bars) or in mixtures (grey bars) with rapeseed (R-Lupine, R-Clover and R-Vetch). Vertical bars indicate ± S.E. (n = 5). * and ** indicate significant differences between monocrops and mixtures, with p < 0.05 and p < 0.01, respectively. Insert indicates δ15N values (‰) of legumes grown in monocrops (Lupine, Clover, Vetch) or in mixture with rapeseed (R-Lupine, R-Clover, R-Vetch) and δ15N of rapeseed grown in monocrops with one (R) or two (RR) plants per pot or in mixtures with legumes (R-Lupine, R-Clover and R-Vetch). Each value represents the mean ± S.E. for n = 5.
3.4. Lupine or clover maintains rapeseed N contents and preserves soil N resources
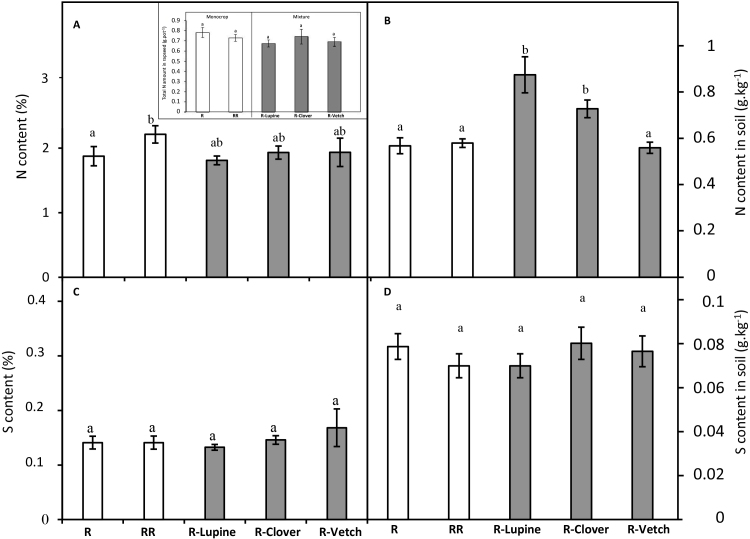
The rapeseed N content was significantly higher in the RR monocrop (2.2 ± 0.1%) than in the R monocrop (1.9 ± 0.1%: Fig. 5A) but because the RR monocrop was characterized by a significantly lower DW (Fig. 1), the total N taken up by the two monocrops was similar (Insert Fig. 5A). In mixture, regardless of the legume, the N contents of rapeseed (approximately 2%) were not significantly different from the monocrops (R or RR; Fig. 5A).
Fig. 5.
N content and total N amount (A) and S contents (C) of rapeseed and N (B) and S (D) contents in soil (g.kg−1 of soil) in monocrops (white bars) with one or two rapeseed plants per pot (R and RR, respectively) or in mixtures (grey bars) with lupine, clover and vetch (R-Lupine, R-Clover and R-Vetch, respectively) at harvest time. Vertical bars indicate ± S.E. (n = 5). Different letters indicate that mean values are significantly different at p < 0.05.
The total residual N amounts in soil from the two rapeseed monocrops (R and RR) were similar (0.5 ± 0.1 g.kg−1) and not significantly different from the soil of rapeseed-vetch (R-Vetch) mixture (Fig. 5B). Conversely, the total residual amount of N from the soils of rapeseed-lupine (R-Lupine) or rapeseed-clover (R-Clover) was significantly higher than the others (0.76 ± 0.07 and 0.63 ± 0.03 g.kg−1, respectively).
3.5. In mixtures, the total S contents decreased in legumes but not in rapeseed
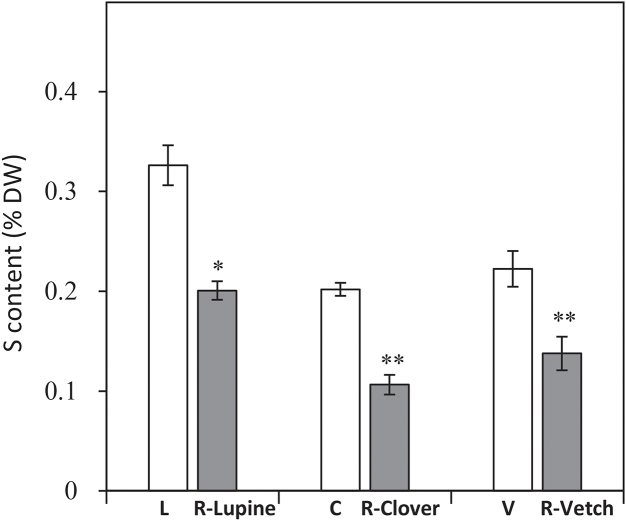
The total S contents of rapeseed were not significantly different (approximately 0.14%) between the monocrops and mixtures (Fig. 5C). The total residual amount of S in the soil was also not affected by crop type and showed the same value (approximately 0.08 g.kg−1; Fig. 5D). On the other hand, the total S contents of lupine, clover and vetch were significantly lower in mixtures than in monocrops with decreases of 40, 45 and 40%, respectively (Fig. 6). These decreases in the total legume S contents were not due to S dilution associated with an increase in biomass (Fig. 2), and therefore would be linked to a decrease in S taken up by legumes. All these results suggest that, in mixture, rapeseed is more efficient at taking up S than legumes, thereby limiting the soil S availability for the legumes, which may suffer from competition for the available S.
Fig. 6.
S content (% of DW) of lupine, clover, and vetch grown in monocrops (white bars) (L, C and V, respectively) or in mixtures with rapeseed (grey bars) (R-Lupine, R-Clover and R-Vetch). The hatched histograms represent the root DWs of legumes. Vertical bars indicate ± S.E. (n = 5). * and ** indicate significant differences between monocrops and mixtures, with p < 0.05 and p < 0.01, respectively.
4. Discussion
4.1. The rapeseed R monocrop as a relevant control
This study showed that the two rapeseed monocrops (R and RR) behave differently. Compared to R and mixtures, the RR monocrop showed a delay in development that was manifested by a reduction in the number of leaves and lower dry weights (Fig. 1). The higher SPAD values in the bottom leaves from the RR monocrop indicated that these leaves have higher chlorophyll content (compared to those from the R monocrop) and thus confirms this delay of growth. These data showed that in our growth conditions (e.g. low N input), the R monocrop seems to be the most accurate control to compare rapeseed performance without artifacts due to differences in the growth stage. The smaller biomass in the RR monocrop may be explained by intraspecific competition. Thus, nutrient availability (mineral N for example) may be limiting as the same N amount were taken up by both rapeseed monocrops (Fig. 5A and C). It could be suggest that legumes are less sensitive to this competition seeing that they can increase atmospheric dinitrogen fixation when the soil N mineral availability decrease. Numerous studies considering mixtures use RR monocrops as controls and express results per plant (e.g. dry weight per plant) without monitoring total plant biomass per pot (Cortés-Mora et al., 2010; Jamont et al., 2013). In these conditions, it is difficult to know if results indicating an increase in the performance of non-legume plants in mixtures were not caused by a delay in growth in the control monocrop due to intraspecific competition.
Therefore, by taking into account the most accurate control (being the R monocrop, which was at the same growth stage as rapeseed in mixtures), our results show the effects of mixtures on the plant-soil complex during these early developmental stages. Based on observed changes in the physiological state of rapeseed and/or the nitrogen pools of plants and soil, the potential benefits of mixtures on subsequent development stages are evident.
4.2. Effect of legumes on rapeseed growth
Our study has shown that intercrop yields were not significantly different from those of monocrops (Fig. 1). This result is in agreement with previous work showing a maintenance (or a slight increase) of rapeseed dry weight when grown in mixture with faba bean (Jamont et al., 2013). Intercropping of other Brassicaceae, such as mustard with pea or lentil, decreases biomass compared to the sole crop (Banik et al., 2000), suggesting that both for Brassicaceae and Legume species identity is a key point for intercrop functioning.
N taken up by the crop followed the same trend as DW production. Compared to control plants, no benefit of mixtures was notes for either the N contents or the plant N amounts, suggesting that the increases in N shown by other studies may depend on developmental stage and occur in later growth stages (Cortés-Mora et al., 2010; Jamont et al., 2013). However, niche separation for N acquisition between rapeseed and legumes was inherent as growing in mixtures significantly promoted BNF by the legumes.
One of the most interesting findings is the higher leaf SPAD values observed in rapeseed grown in mixtures with clover (Fig. 3). This result suggests a delay in leaf chlorophyll degradation (especially for old leaves), which can be explained by a delay in leaf senescence. As proposed by some authors for Brassica napus, preservation of leaf chlorophyll content during vegetative stage may promote an increase in the life span of leaves (Desclos-Théveniau et al., 2014) which could lead to a reduction in the asynchronism observed between the leaf remobilization of N during the pod filling (Malagoli et al., 2005).
4.3. Effect of rapeseed on the BNF of legumes and soil N resources
In this study lupine showed the greatest %Ndfa compared to clover and vetch, regardless of the crop type (mixture or monocrop). These %Ndfa values were in the same range as those reported by Howieson et al. (1998) for white lupine at maturity: 68–85% in Australia, 44–92% in Germany and 80% in France. This high BNF of lupine compared to other legumes may be explained by its low sensitivity to and low nitrogenase inhibition by mineral N available in the soil (Serrano and Chamber, 1990; Luciñski et al., 2002; Goergen et al., 2009). Furthermore, the %Ndfa of lupine, clover and vetch in mixtures with rapeseed was significantly higher than in their respective monocrops (+34%, +140% and +290%, respectively, Fig. 4). Moreover, all results agree with previous work showing an increase in legume %Ndfa in low N input systems (Jensen, 1996a; Corre-Hellou et al., 2006, 2007). For example, Corre-Hellou et al. (2006) have shown that %Ndfa increased on average by 21% in pea intercrops compared to pea as a sole crop. In our study, it can be suggested that the increase in the legume %Ndfa observed in mixtures could be due to the presence of Brassica napus, which requires higher amounts of NO3− for its growth than cereals. As a consequence, the high nitrate requirements of Brassica napus decreased the nitrate concentration in the soil and in turn increased the %Ndfa. This hypothesis is also supported by previous studies showing that N fertilization inhibits the N2 fixation process (Waterer et al., 1994; Macduff et al., 1996).
At harvest time we found that the soil N contents were significantly greater in rapeseed-lupine (R-Lupine) and rapeseed-clover (R-Clover) mixtures than in other crops. Soil N amounts were approximately 50% (0.76 g.kg−1) and 25% (0.63 g.kg−1) higher than in controls and rapeseed-Vetch (R-Vetch) intercrops, respectively (Fig. 5B). These two mixture systems maintained higher soil N pools that could mostly benefit rapeseed (as legumes are mostly dependent on N2) for later growth stages, and especially during pod filling. Such an effect on soil pools was previously demonstrated in perennial grass-legume grasslands in which legumes increased the soil N pool to the benefit of grasses growing in their neighbourhood (Gylfadóttir et al., 2007; Pirhofer-Walzl et al., 2012). Certainly, legumes can deposit significant amounts of N into the soil via N rhizodeposition leading to enrichment of N in soils and in intercropped plants (Jensen, 1996b; Jensen, 1996c; Khan et al., 2002, Galloway et al., 2003; Mahieu et al., 2007, Génard et al., 2016). Thus, from our results, it is possible that lupine and clover have the highest capacity to exude N compounds for uptake by Brassica napus, which has also been described by Cortés-Mora et al. (2010) at the early stages of growth.
Two hypotheses may explain why this high N pool in the soil associated with lupine and clover was not used by rapeseed. Firstly, it is possible that N compounds exuded from lupine or clover are taken up preferentially by rapeseed and it therefore has less need of N from the soil to satisfy its growth. Secondly, the exuded N compounds may be unavailable to rapeseed (in their entirety or partly) and may be temporarily stored in the soil before mineralization. Thus, this resulting elevated N pool may be available for later growth stages, from the bolting stage onwards, which then allows farmers to decrease N fertilization.
4.4. Competition for soil S resources
In this work, the S contents of rapeseed were the same regardless of the crop (Fig. 5C). This result showed that rapeseed, known for its high S requirement for growth, took up the same S amount irrespective of the presence of legumes. Moreover, the S contents and dry weights (Fig. 6) of legumes were lower in mixtures than in monocrops, suggesting that legumes suffer from rapeseed competition when accessing soil S resources. Indeed, rapeseed with its particularly high need for S (McGrath and Zhao, 1996), as is the case for the Brassicaceae in general, is a strong competitor for it. Nevertheless, legumes also require high S availability, especially to achieve a high level of N2 fixation (Scherer and Lange, 1996; Varin et al., 2010). Even though an increase in the %Ndfa of legumes was observed in mixtures (Fig. 4), it is possible that an increased S input could further enhance this parameter. In fact, it has been shown that S fertilization restores and even preserves N2 fixation under high N input conditions (Scherer and Lange, 1996; Habtegebrial Habtemichial et al., 2007; Tallec et al., 2008, 2009). Thus, S fertilization may be an efficient way to improve the agronomic potential of Brassica-legume mixtures.
5. Conclusions
To our knowledge this work is the first to study the effect of different legume-Brassica napus mixtures that considers the type of rapeseed monocrops (R and RR). From this comparison we observed that, in our growth conditions, the rapeseed R monocrop was more relevant than the RR monocrop, which shows a delay in growth. Rapeseed-legume mixtures maintained a high level of DW production and the uptake of both N and S in rapeseed indicated the particular benefit of lupine and clover. In fact, in both mixtures the soil N pool was substantially maintained, thanks to a significant increase in the %Ndfa of the legumes. Moreover, clover enabled an increase in SPAD values of Brassica napus, suggesting a preservation of the photosynthetic capacity and allowing N storage in these organs for a longer time. Accordingly, in low N conditions, combining legumes with rapeseed seems to be relevant for reducing the amount of N fertilizer or improving the nutrition of rapeseed during the growth cycle, especially if S inputs are optimized to minimize interspecific competition.
Declarations
Author contribution statement
Thaïs Génard, Philippe Etienne, Sylvain Diquélou and Philippe Laîné: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Cécile Revellin: Performed the experiments; Wrote the paper.
Jean-Claude Yvin: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work, conducted through the SERAPIS project, was supported by the Regional Council of Lower Normandy (grant number 12P03057), the Regional Council of Brittany, the European Regional Development Fund and CMI (Centre Mondial d’Innovation of Roullier group).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Andersen M.K., Hauggaard-Nielsen H., Ambus P., Jensen E.S. Biomass production, symbiotic nitrogen fixation and inorganic N use in dual and tri-component annual intercrops. Plant Soil. 2005;266:273–287. [Google Scholar]
- Banik P., Sasmal T., Ghosal P.K., Bagchi D.K. Evaluation of mustard (Brassica campestris Var. Toria) and legume intercropping under 1:1 and 2:1 row-replacement series systems. J. Agron. Crop Sci. 2000;185:9–14. [Google Scholar]
- Bedoussac L., Justes E. The efficiency of a durum wheat-winter pea intercrop to improve yield and wheat grain protein concentration depends on N availability during early growth. Plant Soil. 2010;330:19–35. [Google Scholar]
- Bedoussac L., Justes E. Dynamic analysis of competition and complementarity for light and N use to understand the yield and the protein content of a durum wheat–winter pea intercrop. Plant Soil. 2010;330:37–54. [Google Scholar]
- Bergersen F.J. The growth of rhizobium in synthetic media. Aust. J. Biol. Sci. 1961;14:349–360. [Google Scholar]
- Chalk P.M., Peoples M.B., McNeill A.M., Boddey R.M., Unkovich M.J., Gardener M.J., Silva C.F., Chen D. Methodologies for estimating nitrogen transfer between legumes and companion species in agro-ecosystems: A review of 15N-enriched techniques. Soil Biol. Biochem. 2014;73:10–21. [Google Scholar]
- Colnenne C., Meynard J.M., Reau R., Justes E., Merrien A. Determination of a critical nitrogen dilution curve for winter oilseed rape. Ann. Bot. 1998;81:311–317. [Google Scholar]
- Corre-Hellou G., Fustec J., Crozat Y. Interspecific competition for soil N and its interaction with N2 fixation, leaf expansion and crop growth in pea–barley intercrops. Plant Soil. 2006;282:195–208. [Google Scholar]
- Corre-Hellou G., Brisson N., Launay M., Fustec J., Crozat Y. Effect of root depth penetration on soil nitrogen competitive interactions and dry matter production in pea–barley intercrops given different soil nitrogen supplies. Field Crops Res. 2007;103:76–85. [Google Scholar]
- Cortés-Mora A., Piva G., Jamont M., Fustec J. Niche separation and nitrogen transfer in Brassica-legume intercrops. Ratar. Povrt. 2010;47:581–586. [Google Scholar]
- D’Hooghe P., Dubousset L., Gallardo K., Kopriva S., Avice J.C., Trouverie J. Evidence for proteomic and metabolic adaptations associated with alterations of seed yield and quality in sulfur- limited Brassica napus L. Mol. Cell. Proteomics. 2014;13:1165–1183. doi: 10.1074/mcp.M113.034215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclos-Théveniau M., Coquet L., Jouenne T., Etienne P. Proteomic analysis of residual proteins in blades and petioles of fallen leaves of Brassica napus. Plant Biol. 2014;17:408–418. doi: 10.1111/plb.12241. [DOI] [PubMed] [Google Scholar]
- Dubousset L., Etienne P., Avice J.C. Is the remobilization of S and N reserves for seed filling of winter oilseed rape modulated by sulphate restrictions occurring at different growth stages? J. Exp. Bot. 2010;61:4313–4324. doi: 10.1093/jxb/erq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fustec J., Lesuffleur F., Mahieu S., Cliquet J.-B. Nitrogen rhizodeposition of legumes. A review. Agron. Sustain. Dev. 2010;30:57–66. [Google Scholar]
- Galloway J.N., Aber J.D., Erisman J.W., Seitzinger S.P., Howarth R.W., Cowling E.B., Cosby B.J. The nitrogen cascade. BioScience. 2003;53:341–356. [Google Scholar]
- Garg N., Geetanjali N. Symbiotic nitrogen fixation in legume nodules: process and signaling. A review. Agron. Sustain. Dev. 2007;27:59–68. [Google Scholar]
- Génard T., Etienne P., Laîné P., Yvin J.C., Diquélou S. Nitrogen transfer from Lupinus albus L., Trifolium incarnatum L. and Vicia sativa L. contribute differently to rapeseed (Brassica napus L.) nitrogen nutrition. Heliyon. 2016;2 doi: 10.1016/j.heliyon.2016.e00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D.J., Connolly J., Hartnett D.C., Weidenhamer J.D. Designs for greenhouse studies of interactions between plants. J. Ecol. 1999;87:1–16. [Google Scholar]
- Goergen E., Chambers J.C., Blank R. Effects of water and nitrogen availability on nitrogen contribution by the legume, Lupinus argenteus Pursh. Appl. Soil Ecol. 2009;42:200–208. [Google Scholar]
- Gylfadóttir T., Helgadóttir Á., Høgh-Jensen H. Consequences of including adapted white clover in northern European grassland: transfer and deposition of nitrogen. Plant Soil. 2007;297:93–104. [Google Scholar]
- Habtegebrial Habtemichial K., Ram Singh B., Aune J.B. Wheat response to N2 fixed by faba bean (Vicia faba L:) as affected by sulfur fertilization and rhizobial inoculation in semi-arid Northern Ethiopia. J. Plant Nutr. Soil Sci. 2007;170:412–418. [Google Scholar]
- Hauggaard-Nielsen H., Ambus P., Jensen E.S. Interspecific competition, N use and interference with weeds in pea-barley intercropping. Field Crops Res. 2001;70:101–109. [Google Scholar]
- Hauggaard-Nielsen H., Ambus P., Jensen E.S. Temporal and spatial distribution of roots and competition for nitrogen in pea-barley intercrops–a field study employing 32P technique. Plant Soil. 2001;236:63–74. [Google Scholar]
- Hauggaard-Nielsen H., Jørnsgaard B., Kinane J., Jensen E.S. Grain legume–cereal intercropping: The practical application of diversity, competition and facilitation in arable and organic cropping systems. Renew. Agric. Food Syst. 2008;23:3–12. [Google Scholar]
- Howieson J.G., Fillery I.R.P., Legocki A.B., Sikorski M.M., Stepkowski T., Minchin F.R., Dilworth M.J. Nodulation, nitrogen fixation and nitrogen balance. In: Gladstones S., Atkins C.A., Hamblin J., editors. Lupins as crop plants: biology, production and utilization. Cambridge University Press; 1998. pp. 149–180. [Google Scholar]
- Jamont M., Piva G., Fustec J. Sharing N resources in the early growth of rapeseed intercropped with faba bean: does N transfer matter? Plant Soil. 2013;371:641–653. [Google Scholar]
- Jensen E.S. Grain yield, symbiotic N2 fixation and interspecific competition for inorganic N in pea-barley intercrops. Plant Soil. 1996;182:25–38. [Google Scholar]
- Jensen E.S. Barley uptake of N deposited in the rhizosphere of associated field pea. Soil Biol. Biochem. 1996;28:159–168. [Google Scholar]
- Jensen E.S. Rhizodeposition of N by pea and barley and its effect on soil N dynamics. Soil Biol. Biochem. 1996;28:65–71. [Google Scholar]
- Khan W.D.F., Peoples M.B., Herridge D.F. Quantifying below-ground nitrogen of legumes. Plant Soil. 2002;245:327–334. [Google Scholar]
- Laguerre G., Allard M.R., Revoy F., Amarger N. Rapid Identification of Rhizobia by Restriction fragment length polymorphism analysis of PCR-amplified 16S rRNA Genes. Appl. Environ. Microbiol. 1994;60:56–63. doi: 10.1128/aem.60.1.56-63.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguerre G., Mazurier S.I., Amarger N. Plasmid profiles and restriction fragment length polymorphism of Rhizobium leguminosarum bv. viciae in field populations. FEMS Microbiol. Lett. 1992;101:17–26. [Google Scholar]
- Loreau M., Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;412:72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- Luciñski R., Polcyn W., Ratajczak L. Nitrate reduction and nitrogen fixation in symbiotic association rhizobium-legumes. Acta Bioch. Pol. 2002;49:537–546. [PubMed] [Google Scholar]
- Macduff J.H., Jarvis S.C., Davidson I.A. Inhibition of N2 fixation by white clover (Trifolium repens L.) at low concentrations of NO3− in flowing solution culture. Plant Soil. 1996;180:287–295. [Google Scholar]
- Mahieu S., Fustec J., Faure M.L., Corre-Hellou G., Crozat Y. Comparison of two 15N labelling methods for assessing nitrogen rhizodeposition of pea. Plant Soil. 2007;295:193–205. [Google Scholar]
- Malagoli P., Lainé P., Rossato L., Ourry A. Dynamics of nitrogen uptake and mobilization in field-grown winter oilseed rape (Brassica napus) from stem extension to harvest. II. An 15N-labelling-based simulation model of N partitioning between vegetative and reproductive tissues. Ann. Bot. 2005;95:1187–1198. doi: 10.1093/aob/mci131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurier S. University Lyon 1; 182: 1989. Diversité de populations naturelles nodulantes de Rhizobium leguminosarum, thesis of Lyon 1. [Google Scholar]
- McGrath S.P., Zhao F.J. Sulphur uptake, yield responses and the interactions between nitrogen and sulphur in winter oilseed rape (Brassica napus) J. Agric. Sci. 1996;126:53–62. [Google Scholar]
- Naudin C., Corre-Hellou G., Pineau S., Crozat Y., Jeuffroy M.H. The effect of various dynamics of N availability on winter pea–wheat intercrops: Crop growth, N partitioning and symbiotic N2 fixation. Field Crops Res. 2010;119:2–11. [Google Scholar]
- Pedersen C.A., Knudsen L., Schnug E. Sulphur Fertilisation. In: Schnug E., editor. Sulphur agroecosystems. Springer; Netherlands: 1998. pp. 115–134. [Google Scholar]
- Pelzer E., Bazot M., Makowski D., Corre-Hellou G., Naudin C., Al Rifaï M., Baranger E., Bedoussac L., Biarnès V., Boucheny P., Carrouée B., Dorvillez D., Foissy D., Gaillard B., Guichard L., Mansard M.C., Omon B., Prieur L., Yvergniaux M., Justes E., Jeuffroy M.H. Pea–wheat intercrops in low-input conditions combine high economic performances and low environmental impacts. Eur. J. Agron. 2012;40:39–53. [Google Scholar]
- Peoples M.B., Brockwell J., Herridge D.F., Rochester I.J., Alves B.J.R., Urquiaga S., Boddey R.M., Dakora F.D., Bhattarai S., Maskey S.L., Sampet C., Rerkasem B., Khan D.F., Hauggaard-Nielsen H., Jensen E.S. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis. 2009;48:1–17. [Google Scholar]
- Pirhofer-Walzl K., Rasmussen J., Høgh-Jensen H., Eriksen J., Søegaard K., Rasmussen J. Nitrogen transfer from forage legumes to nine neighbouring plants in a multi-species grassland. Plant Soil. 2012;350:71–84. [Google Scholar]
- Rathke G.W., Behrens T., Diepenbrock W. Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): A review. Agric. Ecosyst. Environ. 2006;117:80–108. [Google Scholar]
- Ruiz-Espinoza F.H., Murillo-Amador B., Garcia-Hernandez J.L., Fenech-Larios L., Ruedi-Puente E.O., Troyo-Diégez E., Kaya C., Beltran-Morales A. Field evaluation of the relationship between Chlorophyll contents in basil leaves and portable chlorophyll meter (SPAD-502) readings. J. Plant Nut. 2010;33:423–438. [Google Scholar]
- Scherer H.W., Lange A. N2 fixation and growth of legumes as affected by sulphur fertilization. Biol. Fertil. Soils. 1996;23:449–453. [Google Scholar]
- Schjoerring J.K., Bock J.G.H., Gammelvind L., Jensen C.R., Mogensen V.O. Nitrogen incorporation and remobilization in different shoot components of field-grown winter oilseed rape (Brassica napus L.) as affected by rate of nitrogen application and irrigation. Plant Soil. 1995;177:255–264. [Google Scholar]
- Serrano A., Chamber M. Nitrate reduction in Bradyrhizobium sp. (Lupinus) strains and its effects on their symbiosis with Lupinus luteus. J. Plant Physiol. 1990;136:240–246. [Google Scholar]
- Shearer G., Kohl D.H. N2-fixation in field settings: estimations based on natural 15N abundance. Funct. Plant Biol. 1986;13:699–756. [Google Scholar]
- Tallec T., Diquélou S., Avice J.C., Lesuffleur F., Lemauviel-Lavenant S., Cliquet J.B., Ourry A. Availability of N and S affect nutrient acquisition efficiencies differently by Trifolium repens and Lolium perenne when grown in monoculture or in mixture. Environ. Exp. Bot. 2009;66:309–316. [Google Scholar]
- Tallec T., Diquélou S., Lemauviel S., Cliquet J.B., Lesuffleur F., Ourry A. Nitrogen:sulphur ratio alters competition between Trifolium repens and Lolium perenne under cutting: Production and competitive abilities. Eur. J. Agron. 2008;29:94–101. [Google Scholar]
- Vandermeer J.H. Cambridge University Press; 1989. The Ecology of Intercropping. [Google Scholar]
- Varin S., Cliquet J.B., Personeni E., Avice J.C., Lemauviel-Lavenant S. How does sulphur availability modify N acquisition of white clover (Trifolium repens L.)? J. Exp. Bot. 2010;61:225–234. doi: 10.1093/jxb/erp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterer J.G., Vessey J.K., Stobbe E.H., Soper R.J. Yield and symbiotic nitrogen fixation in a pea-mustard intercrop as influenced by N fertilizer addition. Soil Biol. Biochem. 1994;26:447–453. [Google Scholar]
- Willey R.W. Intercropping - its importance and research needs. 1. Competition and yield advantages. Field Crop Abstr. 1979;32:1–10. [Google Scholar]
- Xiao Y., Li L., Zhang F. Effect of root contact on interspecific competition and N transfer between wheat and faba bean using direct and indirect 15N techniques. Plant Soil. 2004;262:45–54. [Google Scholar]