Abstract
Parasitic infections are ubiquitous in wildlife, livestock and human populations, and healthy ecosystems are often parasite rich. Yet, their negative impacts can be extreme. Understanding how both anticipated and cryptic changes in a system might affect parasite transmission at an individual, local and global level is critical for sustainable control in humans and livestock. Here we highlight and synthesize evidence regarding potential effects of ‘system changes’ (both climatic and anthropogenic) on parasite transmission from wild host–parasite systems. Such information could inform more efficient and sustainable parasite control programmes in domestic animals or humans. Many examples from diverse terrestrial and aquatic natural systems show how abiotic and biotic factors affected by system changes can interact additively, multiplicatively or antagonistically to influence parasite transmission, including through altered habitat structure, biodiversity, host demographics and evolution. Despite this, few studies of managed systems explicitly consider these higher-order interactions, or the subsequent effects of parasite evolution, which can conceal or exaggerate measured impacts of control actions. We call for a more integrated approach to investigating transmission dynamics, which recognizes these complexities and makes use of new technologies for data capture and monitoring, and to support robust predictions of altered parasite dynamics in a rapidly changing world.
This article is part of the themed issue ‘Opening the black box: re-examining the ecology and evolution of parasite transmission’.
Keywords: infectious disease, climate change, sustainable control, stressors
1. Introduction
The current epoch of ecological time is driven by human interference [1]. Multiple anthropogenic stressors—including climate change, pollution, ocean acidification, habitat loss and fragmentation, urbanization, agricultural expansion and intensification, together with other changes in the use of water and land resources—are directly or indirectly impacting all species on earth (e.g. [2–5]). These changes may lead to the crossing or corrosion of critical thresholds, or ‘planetary boundaries’ ([6,7], see glossary), that induce physiological stress or complete system dysfunction, with negative consequences for individuals, populations and species. Such processes will have significant impacts on parasite natural history and infectious disease risks.
The anticipation of global change is not currently reflected in programmes of intervention against parasites of humans—instead the emphasis is on identifying vulnerable communities from retrospective data, and targeting those communities for intervention. In an attempt to synthesize and implement cost-effective interventions against the neglected tropical diseases (NTDs), there has been a concerted effort to distribute human medicines through mass drug administration (MDA) programmes in areas of high transmission [8], aided by donations from large pharmaceutical companies. These MDA campaigns rely largely on the presumptive treatment of putatively exposed individuals in ‘at risk’ populations [9]. The expectation, translated from the outputs of mathematical models, is that repeated MDA will reduce the size of the parasite population and simultaneously reduce levels of morbidity attributable to infection [9].
Such intervention programmes are possible because of developments in our understanding of the life cycles and ecology of parasites affecting humans and livestock, primarily gathered in the Victorian era [10]. Early optimism among health practitioners in wealthy countries that control and intervention strategies would eradicate infectious diseases continued up until the middle of the twentieth century (reviewed by [11]); yet despite early (and enduring) optimism, relatively little success, at least in terms of eradication, has been achieved. Among the NTDs, only Guinea worm is scheduled for eradication (most probably because only low-tech solutions are necessary to interrupt the transmission cycle; see §3).
Recent analysis of NTDs in Africa suggests, at first glance, that the MDA strategies have succeeded in reducing the number of infections. Where pharmaceutical interventions have had a clear effect in reducing infectious disease prevalence, the challenge now is to ensure that such success is sustainable in the context of environmental change. River blindness, caused by the nematode Onchocerca volvulus carrying the Wolbachia bacterium [12], was introduced into South America by the Simulium black fly, which has been treated with periodic ivermectin administration since 1991. Although it is unlikely that river blindness will ever be eradicated globally, prevalence has fallen from 50% to 4% of those at risk in the endemic population [13]; however, the implications of environmental changes for the long-term efficacy of this and other treatment programmes are not well understood.
Elsewhere, problems remain in terms of attributing causality to, or quantifying the success of, MDA programmes [8]. First, the historical data are imprecise and patchy; diagnosis of some infections has been characterized for decades by a lack of sensitive and/or specific tools [14]. Second, global climate models reveal an ever-changing pattern of land surface temperature, rainfall and vegetation cover across the surface of the planet [15]. Thus, contemporaneous environmental changes could potentially confound the effects of MDAs. Third, host range shifts may spread parasites into areas where monitoring and MDA are not being applied. Finally, the programmes themselves may have generated selection pressures, as has been observed in other systems such as malaria [16], leading potentially to resistance, adaptation and other evolutionary consequences.
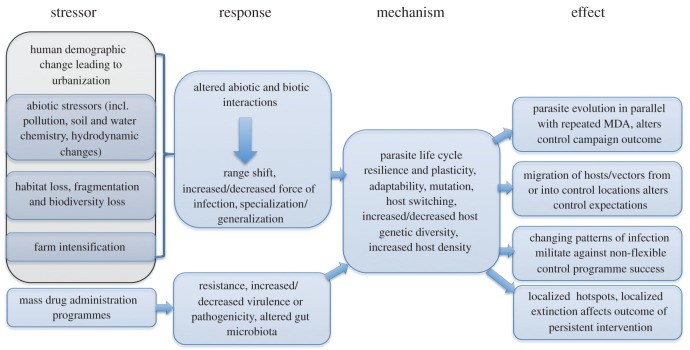
In looking to the future sustainability and success of MDA and other interventions, we posit that it is imperative to consider what factors related to global change not only impinge on current efforts, but how global changes, including those brought about by control efforts themselves, might influence the outcome of attempts at control, elimination or eradication of specific infections. Figure 1 shows the hypothesis that a combination of stressors, brought about by global anthropogenic change, will induce a set of responses that have a significant impact on control prospects of NTDs in particular and parasites of economic importance in general.
Figure 1.
Stress–response impacts on parasite control programmes. (Online version in colour.)
Given the paucity of information available from ecological studies of NTDs and other parasites of humans and livestock, a direct assessment of the evidence underpinning the hypothesis in figure 1 is challenging. We are nonetheless reminded of the value to be drawn from proxy observations from comparable systems [17]. In terms of parasitology, much of the relevant proxy information has been drawn from wildlife disease ecology, which has tended to pay more attention to the issues of global change than comparable studies on human and domestic animal parasites. In this paper we demonstrate how ecological studies of parasites in wildlife may be used to enhance our understanding of stressors arising from global change, which are likely to be important in the context of parasitic infections (both macro- and microparasites) of humans and domestic animals.
First we consider the influences of some major abiotic and biotic stressors associated with global change and, second, how these stressors might affect parasite life cycles, transmission and ecology. In doing so, we highlight that the abiotic–biotic distinction is blurred, particularly as many stressors also act simultaneously and indirectly on parasites through their hosts. Third, we explore how parasites may respond to the evolutionary pressures of such stressors. Finally, we consider how these complex impacts of global change potentially militate against the sustainable control of parasites affecting animals and humans, and make suggestions for improved understanding and control in an uncertain future world.
2. Anthropogenic abiotic and biotic stressors affecting parasite transmission
The majority of modern day ecosystem stressors are driven by industrialization combined with human population growth. These in turn are responsible for increased resource use and generation of waste products, many of which have negative impacts on the environment in complex direct and indirect ways, which may subsequently affect disease risks. For example, the combustion of fossil fuels for energy production and for powering transportation modes significantly contributes to air pollution and carbon dioxide emissions, which promote climate change. Changing land uses, including farming for food, further contribute to climate change and both are considered major drivers of biodiversity loss. Broad-scale biodiversity loss, latitudinal and altitudinal host range expansions and retractions, reduced wildlife population sizes and more limited habitat connectivity are subsequently affecting host interactions and changes in parasite transmission.
(a). Climate change
The multiple components of climate change, including temperature, precipitation and atmospheric CO2, have been extensively studied individually [18–20], but the interactions between these environmental stressors and the consequent effects on parasite transmission are complex. Thus, there is considerable uncertainty about how future climate variation and change will affect disease dynamics [21–23]. Multiple stressors might affect multiple life-history traits, potentially influencing both parasite and host fitness ([24]; see §2). In combination these stressors may counteract each other, such that the overall rate of parasite transmission remains unchanged. Higher temperatures, for example, often increase parasite growth, reproduction and infectivity [25,26], yet can also increase parasite mortality, and as such there is no change in the number of transmitted parasites [27,28]. Likewise, while temperature elevations accelerate the replication of arthropod-borne viruses in their insect or tick vectors, they simultaneously increase vector mortality and decrease biting rate, making the net effect of temperature increase on transmission difficult to predict without detailed knowledge of each component in the system [29]. In other instances, increased temperatures have more pronounced effects on the host, which may exhibit acclimation, adaptation or be forced to shunt resource investment into various life-history components, resulting in thermal preference shifts. Poikilothermic hosts are particularly vulnerable to temperature shifts, but also show remarkable adaptations and such responses by the host can be damaging for the parasite. Some fish, for example, exhibit adaptive behavioural traits to reduce transmission risk, by actively selecting thermal conditions that are detrimental to parasites (behavioural fever; [30]) or selecting flow conditions that minimize fitness costs of infection and potentially reduce transmission [31,32].
Disentangling anthropogenic environmental change from that of natural variation is problematic, particularly for indirect effects and naturally rare events such as extreme weather conditions or disease outbreaks [33,34]. The relationship between environment and transmission is also complex. Different environmental parameters may have additive, multiplicative or antagonistic and nonlinear effects on transmission, which themselves may be intercorrelated or vary at different spatial or temporal scales, with such effects difficult to measure [35,36]. Such relationships may be a consequence of transmission mode. For example, flooding events can be a key driver of some water-borne disease epidemics [37], while drought conditions cause hosts to aggregate at sites where water is available, amplifying transmission and triggering outbreaks of vector-borne diseases such as African horse sickness and Rift Valley fever [38,39]. Other environment–transmission relationships are likely to be a result of a host–parasite range shift due to climate warming. This can change the distribution of vector-borne diseases, including malaria [40] and Rift Valley fever [41]. However, climate change is not spatially homogeneous and could render previously suitable areas unsuitable for transmission and vice versa [42]. The effect of range shift can be yet more complex if the degree or rate of change differs between the host and parasite, causing host–parasite interactions to decouple across some or all regions [43]. For example, tick-borne encephalitis virus (TBEV) transmission is sustained only when temperatures result in synchronous feeding of larvae and nymphs [44]. Projected temperature rises might desynchronize feeding and shrink the area within which TBEV persists [45]. Even the immediate effects of change in temperature and rainfall on parasites are therefore complex and strongly modified by host factors.
(b). Pollution
Pollutants can cause sublethal physiological stress to hosts and hence reduce their capacity to withstand parasite invasion and/or proliferation, potentially increasing infection levels indirectly (e.g. [46]). However, pollutants also impact parasites themselves, and in aquatic ecosystems, both the infective stages of parasites and their intermediate hosts can be highly sensitive to their effects [47]. Heavy metals can inhibit the release of trematode cercariae from molluscan hosts, as well as impair their swimming behaviour and longevity [48–50]. Pharmaceutical pollutants are widespread stressors likely to affect host susceptibility to disease. The scale of this threat is increasingly apparent in aquaculture: in Chilean salmon farms alone, hundreds of tonnes of antibiotics are used annually [51]. Eutrophication—another important stressor of aquatic ecosystems, arising from excessive nutrient input—is associated with elevated intermediate host densities, parasite fecundity and increased prevalence of certain pathogen infections [52]. However, as yet, there is no overall consensus on its consequences for general patterns of infection [53,54].
Other forms of pollution are less well studied with regard to disease transmission. While it is known that light pollution can impact the structure and function of ecosystems via cascading effects [55], and that natural light cycles govern both relevant parasite life-history traits (e.g. egg hatching; [56]) and intermediate host behaviours (e.g. zooplankton diel migration; [57]), studies on the effects of light pollution on human parasite transmission remain limited [58]. Although the introduction of electricity to socio-economically developing communities has overall human health benefits, night lighting inevitably attracts certain insect vectors and increases human night-time activity. Thus vulnerability to being bitten by a vector is increased, which is implicated in higher incidences of leishmaniasis and malaria in some regions [58]. In other insect-vectored diseases, artificial lighting may have a less overt effect on transmission dynamics: triatome bugs, the vectors of Chagas' disease, typically avoid well-lit areas and artificial lighting may be driving Chagas transmission towards a sylvatic cycle [58]. Noise pollution, a known stress-induced modulator of the immune response [59] that can significantly affect behaviour and predator–prey interactions [60], has not yet been considered in terms of infectious diseases, even though it could have a major influence on farmed animals. The gaps in knowledge concerning the impacts of all types of pollution on parasite transmission are considerable, and without this information it is challenging to assess its importance across host–parasite systems.
(c). Habitat loss and fragmentation
Habitat alteration due to climate change or anthropogenic activity poses a major threat to ecosystems, often leading to substantial loss of biodiversity, ecosystem functioning and services, and reduced resilience to external stressors [61–65]. This in turn may alter host–parasite interactions, by either increasing [66–69] or decreasing [70–72] infection levels, depending on nuances of host and parasite life history (see §3). The effects of habitat change can even have contrasting effects on closely related parasite species infecting the same host; for example, sunbirds in disturbed habitats exhibited increased prevalence of Plasmodium lucens but decreased prevalence of P. megaglobularis [71]. Habitat loss and fragmentation also increase the frequency of ‘edges’—transition zones between habitats [73,74]—which are typically exposed to more extreme climatic conditions than interior sites [74]. Habitat edges often promote increased species diversity (i.e. the richness and/or relative abundances of species [75]), resulting in altered levels of interspecific competition and parasitism [76–79]. How the differential effects of edge versus interior sites impacts parasitism varies between host–parasite systems; infections may significantly increase [77], decrease [78] or be unaffected [80]. Although re-establishing connectivity may facilitate initial disease spread [81], in the long term, a larger host gene pool is likely to decrease vulnerability to disease [82], while also increasing overall biodiversity.
(d). Host density and farming intensification
Over the past 50 years, there have been unprecedented changes in farming practices and associated land use [83]. Although natural and managed forestry currently occupies about 30% of total land area, the impact of deforestation and land use intensification, especially on soil degradation, is significant. Growth in crop production and livestock has been driven by the demand for higher yields. Livestock production is the largest user of agricultural land, accounting for more than 30% of the earth's ice-free terrestrial area [83,84], but aquaculture is the fastest growing food sector [85]. Modern and large-scale farming practices typically rely on concentrating and containing inbred hosts, which can increase host exposure to and facilitate parasite transmission [86,87]. High host density is particularly important for tick-borne pathogens [88], as these vectors are relatively immobile and host–parasite contact frequencies tend to be driven by changes in host abundance and/or behaviour. Chronic stress induced by high stocking densities in aquaculture can have important implications for fish immuno-competence [89], but relationships with infection levels are variable. While high host densities can promote greater parasite population densities, the number of conspecific parasites per host may be reduced [90]. This ‘dilution effect’ (see §2f) is illustrated by a reduction in directly transmitted sea lice at the high host densities in salmonid cage aquaculture [91]. Positive effects of high host density on transmission can be attenuated by mixing susceptible and resistant hosts in rotational grazing systems [92], showing the importance of multiple hosts in modifying infection pressure. However, in aquaculture, where hundreds of thousands of hosts are contained together, this is not yet possible [93], partly because of the need to track farmed fish in the event of an accidental release, and also because of concerns about disease transmission between farmed and wild stocks (and vice versa).
(e). Urbanization
While density-dependent transmission of human parasites may be expected to increase with high population densities and ownership of companion animals, decreased human–wildlife contact and better sanitation in cities of developed countries generally point to lower levels of disease transmission among such populations (e.g. [94]), although there are exceptions. Dengue, for example, is more prevalent in urban areas due to the provision of suitable human-created microhabitats for the Aedes mosquito [95]. Urban environments with high human densities are potentially more vulnerable to water-borne or faeco-orally transmitted parasites if investment in sanitation infrastructure is neglected or disrupted due to socio-economic unrest. Poverty is an important related factor; a study of the contiguous cities of Laredo (USA) and Nuevo Laredo (Mexico) on the USA–Mexican border found that dengue transmission was strongly affected by income, and hence access to technologies such as air conditioning [96]. In developing countries, human–wildlife conflicts can be a major issue. Most emerging and re-emerging human infectious diseases (EIDs) are zoonotic, typically with origins in mammalian wildlife [97,98] or interactions between wildlife and domestic animals [99,100]. This might increase further as habitat loss forces the co-occurrence of wildlife and humans, although this could be offset by the greater effects of biodiversity loss (see below).
A major factor underpinning urbanization is demographic change. By 2050, it is estimated that almost half the world's population will live in the tropics, of which approximately 66% are likely to be living in urban contexts [101,102]. Millions of individuals are also expected to migrate during their lifetimes due to factors associated with the urban–rural cycle, extreme weather events, economic necessity, water and food security, and conflict [103]. Increased patchiness of wealth associated with urbanization, combined with disrupted social structures has already changed the entire landscape of NTDs. These diseases are no longer exclusively prevalent in less developed countries; instead they infiltrate impoverished areas of all countries, including those in the G20, giving rise to the global pattern of ‘Blue Marble Health’ [104].
Associated with urbanization is increased road building. Approximately 60% more roads are projected by 2050 compared with 2010, mostly in developing countries [105], potentially making road building one of the most significant drivers of future environmental change [106]. Road building has already increased the risk of some diseases associated with human development (e.g. agricultural intensification), with an increase in the number of human hantavirus cases reported following completion of a highway through the Brazilian Amazon [107]. Such large-scale road building will almost certainly further facilitate bushmeat hunting in the most biodiverse regions of the planet [108] and change the scale at which people are able to move wild animals out of newly exploited areas and into commodity chains, thereby increasing public health zoonosis risks.
Overall, pathogens likely to thrive as a result of urbanization tend to be either those for which transmission is strongly density-dependent, or those with vectors or reservoirs that are themselves well adapted to urban environments. The net effect on parasite burdens will be highly case-specific and difficult to predict, especially where urbanization is rapid and strong interactions with rural populations persist [109].
(f). Biodiversity loss
Current extinction rates are estimated to be 100–1000 times greater than background levels [110], with biodiversity loss being one of the hallmarks of the Anthropocene [111]. Loss of host diversity can reduce disease risk directly or indirectly through the associated loss of parasite diversity [112]. For example, reduced risk of African sleeping sickness in humans [113] has been related to the loss of wildlife host biodiversity (reviewed in [114]). Wildlife biodiversity is often correlated with human infectious disease risks. Examples include correlations between mammalian biodiversity and global biogeographic patterns of human infectious diseases [115], elevated likelihood of observing emerging infectious diseases [97] and increases in human pathogen richness and prevalence for some diseases [116]. However, in these cases it can be difficult to separate cause from correlation as areas with high levels of biodiversity are also characterized by other, unrelated, risk factors for disease transmission such as climate and poverty. Nevertheless, the fact that most human infectious diseases have origins in animals, mostly wildlife, supports suggestions that these correlations are mechanistically reasonable and that one large-scale consequence of biodiversity loss could be an overall reduction of disease transmission.
Wildlife biodiversity loss can, however, also increase disease risk. In some ecosystems the number of transmission-competent hosts is ‘diluted’ by abundant non-competent hosts, so the chance of a vector feeding on a suitable host, or of a motile infective parasite successfully contacting a transmission-competent host, may be reduced. When members of a host community are lost due to habitat loss, for example, the risk of disease to a focal host (e.g. humans) could rise. This appears to be the case for Lyme disease in North America [117–119] and there is support for generality across multiple systems [120]. In addition, generalist host species that cope more effectively with human pressure may exhibit greater reservoir competence, or the capacity to transmit pathogens, such that biodiversity loss could select for species that contribute to higher levels of parasitism [121]. Nevertheless, many studies continue to demonstrate that the dilution effect is likely to be of limited generality [122–125], and the net contributory effect of biodiversity (and its loss) to disease risks requires the balance of costs and benefits to be more thoroughly and objectively addressed [126,127]. The notion that wildlife biodiversity can provide an important service in regulating the risk of infectious disease is attractive and has received widespread exposure, although because the interactions that result in transmission events can be complex, the evidence for widespread effects continues to be mixed. In many cases, community composition including relative abundance, rather than biodiversity loss, is a greater predictor of disease risk dilution [122–125].
Biodiversity loss, and its implications for disease risks, may also be experienced at the individual host scale, with subsequent impacts on micro- and macroparasitic infection and transmission. All multicelled organisms are colonized internally and externally by communities of bacteria, eukaryotes, archaea and viruses [128]. These microbiota play a critical role in host health, particularly the gut microbiota and its involvement in immune system development and function [129,130]. In vectors such as Anopheles mosquitoes and triatome bugs, an enriched midgut microbiota stimulates upregulation of immune genes that inhibit microparasite development; however, reduced microbiota diversity arising from direct antibiotic treatment or by ingestion of antibiotics circulating in a blood meal is associated with increased microparasite infection of the insect host [131–133]. Moreover, microbiota depletion increases survival and fecundity of the vector itself, potentially exacerbating microparasite transmission [133].
The effects of anthropogenic stressors and within-host biodiversity loss on enteric helminths are highly species-dependent. Certain antibiotics remove Syphacia pinworms and other gut helminths in laboratory mice as a direct effect on the parasites themselves or through altering microbial composition, yet other antibiotics have the opposite effect on Aspiculuris pinworms, with treated hosts harbouring nearly twice as many worms as controls (reviewed in [134]). Similarly, there are direct links between the loss of bacterial diversity and truncation of helminth life cycles. Eggs of the hookworm Trichuris muris require a structural component of Gram-negative bacteria from the host's gut to trigger a signal transduction cascade to stimulate hatching [135]. The nematode Heligmosomoides polygyrus bakeri exhibits bacterial dependence for larval development; reared in axenic conditions, the nematodes do not survive beyond second-stage larvae [136]. This suggests that transmission of both T. muris and H. polygyrus is unlikely to be successful if gut microbiota diversity is inadequate, though confirmation is required from in vivo studies. These examples illustrate the potential importance of internal and external biodiversity to parasite transmission and maintenance, and support the notion of biodiversity loss being more far-reaching than is currently recognized.
(g). Altered interspecific interactions
Changes in host interactions, often linked to the stressors listed above, can drive disease emergence in new hosts. We have already highlighted this problem in association with increased human–wildlife contact, but this in turn might be altered by a range of non-human interactions. Parasites have a fundamental role in food webs [137,138]; thus, anthropogenic changes that reduce the density of higher trophic-level species that feed on larval parasite stages [139] could directly increase disease transmission to competent hosts. Parasites may also indirectly disrupt predator–prey interactions [140] and abiotic factors may affect trophic transmission by altering host foraging activity [26,141]. In addition to these altered parasite–predator–prey interactions, parasites can affect native–invasive host interactions (e.g. [142,143]); newly invading hosts either bring with them novel pathogens to which native hosts do not have resistance, or—having escaped their own native parasites—they can dilute the pool of susceptible native hosts [144].
Finally, parasite–parasite interactions affect transmission, with many studies highlighting the complex interactions of co-infecting parasites in wildlife (e.g. [145]) and livestock (e.g. [146]). As well as parasites having their own microbiota, they can serve as hosts for hyperparasites, the occurrence and life history of which are likely to be influenced by environmental changes [141,147]. Abiotic or biotic stressors may even drive symbionts to adopt parasitism, for example, where there is high competition on the host (e.g. [148]). Artificial manipulation of species interactions can be used in biocontrol, as in the case of Wolbachia infection of mosquitoes, which reduces their vectoring capacity [149].
(h). Interacting abiotic and biotic factors
The above list is not comprehensive, but rather highlights some of the key abiotic and biotic factors that may act together as ‘cocktails’ of stress, with implications for increasing or decreasing disease risks. Identifying the direct and/or indirect factors responsible for changes in disease risk is challenging because multiple stressors act simultaneously on both parasites and their hosts. Depending on habitat and season, the peak impact of different abiotic stressors can occur in or out of phase with one another; thus, while some organisms may be exposed to multiple stressors simultaneously, others will experience them sequentially. Yet, the consequences of multiple, interacting environmental threats for parasite transmission remain unclear: when they co-occur temporally and spatially, their combined effects may be additive, antagonistic or synergistic [150,151]. For example, while elevated seawater temperatures increase mortality rates of oyster larvae, this can be offset by simultaneous water acidification, which reduces the growth of pathogenic bacterial infections [152]. On coral reefs, the interaction between ocean acidification and warming contributes to coral bleaching and reduced disease resistance, leading to increased pathogenicity of existing pathogens and the emergence of new diseases [153]. These two examples are rare, because compared with terrestrial and freshwater systems, marine systems are often neglected with regard to assessing the impact of environmental stressors [154].
3. How might parasite life-history traits modulate responses to abiotic and biotic stressors?
Given the complexity of the possible effects of global change on parasite transmission, understanding the factors that drive responses across parasite taxa is essential for more general predictive ability. Here we consider the variety and complexity of parasite life cycles, as the number and diversity of hosts underpin not only how parasites might respond to environmental change, but also their relative fitness and resilience to environmental change at different life stages [155].
Parasite life cycles exhibit remarkable diversity in form and complexity. Whereas some parasites can complete their life cycle infecting a single host organism, others must negotiate their way through several host species in a particular sequence in order to achieve reproductive success. Life cycles with greater complexity rely on biodiverse and integrated communities, and as such may be highly sensitive to the loss of individual components, in the form of host, vector or species interactions required for transmission [112,156]. The level of life cycle flexibility and host specificity is also likely to influence the sensitivity of parasites to changing environments, and their ability to prosper in perturbed ecosystems.
(a). Life cycle flexibility
The use of paratenic hosts, which are not necessary for parasite development but can sustain parasites and make them available to subsequent obligate hosts, may positively influence transmission if environments become unsuitable or if non-native species outcompete and drive native obligate intermediate hosts locally extinct [157]. An example is provided by two sister species of Bothriocephalus cestode, of which only one (B. gregarius) uses a facultative paratenic host. Whereas paratenic hosts enhance the probability of B. gregarius successfully infecting definitive host fish, resource competition within paratenic hosts lowers infection intensities, and smaller progeny are produced relative to B. barbatus [157]. Consequently, reduced energy expenditure on growth enables B. gregarius to invest more in reproduction and dispersal, increasing the likelihood of re-establishment in a new population of intermediate hosts [157–159]. Alternatively, if populations of the definitive host of B. gregarius were to rapidly decline, the paratenic host might potentially replace this host [157].
Parasites that have the capacity to truncate their life cycle may be advantaged under fluctuating environments [160]. For instance, if an obligate host is temporarily unavailable due to seasonally induced migration or anthropogenic activity, developmental requirements for the absent host would be disadvantageous [161,162]. Flexibility in host use may, therefore, allow parasites to cope with seasonal variation in host availability; for example, Gymnophallus choledochus normally employs a three-host cycle in summer, but switches to a two-host cycle during winter [163]. In other species, a host may be lost permanently due to strong selection pressures, such as the lack of predators facilitating onward trophic transmission; this ‘missing host hypothesis’ could explain the two-host life cycle of schistosomes [161]. In a more extreme example, Mesostephanus haliasturis can forgo sexual reproduction by completing development, via asexual reproduction, in its snail host [164].
Other parasites employ progenesis (precocious sexual maturation) to shorten their life cycle. For example, host diet and increased temperature can induce progenesis in Stegodexamine anguillae metacercariae via secreted host-stress signals [160–162]. Thus progenesis may benefit immature trematodes when transmission to definitive hosts is compromised by the health of an intermediate host [162]. For other parasites, such as the hyperviviparous gyrodactylids, progenesis is the norm, and the first-born offspring always develops asexually from the parental worm while it is still an embryo [165]. This adaptation, together with a direct life cycle, facilitates invasion of new habitats: a host only needs to be infected with a single Gyrodactylus worm to initiate an epidemic [166].
Life cycle plasticity is particularly advantageous when facing increasing environmental and host uncertainty. Monogenean parasites of the genus Polystoma, which typically infect the urinary bladder of frogs, exhibit life cycle dimorphism, with parasites maturing in either three weeks or three years [167,168]; precocious maturation (neoteny) on the tadpole gills occurs when environmental conditions are unsuitable for normal development [169]. Although release of eggs from the slower-growing bladder morphs is induced by the mature host's gonadotropin secretions during the breeding season, both the timing of parasite egg hatching and tadpole development are sensitive to ambient temperatures and chemical environments [170]. Disrupted host chemical balance and light intensity, for instance caused by pollution, may shift the equilibrium between parasite morphs to favour either the neotenic or the slow-growing phenotype [169,170]. Similarly, phenotypic plasticity in the life cycle of the common dog parasite, Toxocara canis is dependent upon the physiological status of the host: patent infections develop only in young dogs, while larvae arrest in older hosts and are only reactivated in bitches [171], though host drug treatment might have a hidden influence.
(b). Specialist versus generalist life cycles
The evolutionary divergence of parasites has generated varying degrees of specialization in parasite traits within different habitats and hosts, some of which are more likely than others to enhance parasite success in unstable environments [155,172,173]. Although it is logical to predict that generalist parasites are more resilient to global change than specialists [121,174], this is very context-dependent [158] and includes the number of hosts in the life cycle and degree of specificity to each. Furthermore, if global change results in new conditions that are stable, parasites that are locally adapted might develop more specialist tendencies [175].
Zoonotic parasites demonstrate varying degrees of host specificity due to transmission via three, non-mutually exclusive life cycles: sylvatic, domestic and anthroponotic [4,155,176]. Host specialization arises due to parasites' investments towards infectiousness and longevity in particular hosts. For example, the nematodes Trichinella britovi and T. spiralis, both found throughout Europe, possess sylvatic and domestic (swine) host cycles. However, their epidemiology differs due to their higher adaptability to either swine (T. spiralis) or carnivore (T. britovi) hosts [4]. Nonetheless, re-establishment of T. spiralis in a red fox (Vulpes vulpes) population, decades after its elimination from domesticated swine in Northern Ireland, demonstrates how host diversity increases parasite resilience to anthropogenic farming activity; i.e. by providing alternative sylvatic reservoir hosts until preferred domestic hosts become vulnerable to infection [172,177,178].
(c). Parasite longevity
Parasite lifespan, and the time spent inhabiting different hosts, will influence the susceptibility of parasites to environmental changes, and the type of responses that are most likely to arise. Whereas short-lived parasites with rapid life cycles may be more capable of evolving adaptive response to chronic directional changes in environments, long-lived individuals may be better equipped to withstand acute, transient perturbations. The lifespan of parasitic worms can be hugely variable; among the nematodes it can range from three days in free-living Rhabdias bufonis adults to 20 years for Loa loa (reviewed by [179]); among cestodes, Taeniarhynchus saginatus can live in humans for 35 years [180]; and schistosome lifespans of 20–30 years are documented [181], though the mean longevity in optimal hosts is in the range of five years [182]. Helminth parasites with viviparous reproduction, such as Gyrodactylus spp., tend to have the shortest lifespans (few days), with age not only determining reproductive output but also reproductive mode [183]. For all species, the timing of pre-patent and patent periods varies and reproductive output typically declines with parasite age and host status (reviewed by [179]). Aside from the longevity of mature worms, it is essential to consider the persistence and resilience of environmental stages when considering how any particular parasite population will respond to global change.
(d). Parasite reproductive strategies
Long-lived parasite species tend to be iteroparous (e.g. L. loa), while other parasites exhibit semelparity (e.g. the human pinworm Enterobius vermicularis). Within a parasite species, timing of reproduction is intricately linked to biological and environmental factors, and for many species transmission is seasonal; in extreme cases this can be incredibly brief. For example, Polystoma integerrimum transmission only occurs during the host breeding season [184], and in the related species Pseudodiplorchis americanus, transmission can be restricted to just 3 h per year, being entirely dependent on monsoon rains creating suitable habitats [185]. If the rains fail, the adult parasites can reabsorb nutrients from ovoviviparous larvae held in utero and transmission is delayed [185], but the long-term implication of this strategy is unknown. Similarly, disrupted weather patterns threaten other seasonally transmitted parasites, such as brood parasitic birds, which risk phenological mismatch with their hosts [186].
Reproductive strategies of endoparasites, in particular, are determined by trade-offs in energy investments against other life-history traits [173]. Schistosomes are the only digeneans whose adult stages are exclusively dioecious and dimorphic [187,188]. Only male Schistosoma mansoni retain hermaphroditic traits, implying they are energetically costly, and may have restricted female body-form specialization required for efficient egg dispersal [158,189,190]. Evolution of dioecy in schistosomes via host–parasite coevolution demonstrates resilience to long-term environmental changes; however, slowly evolving adaptations may be disadvantageous in the face of short-term perturbations [187,190]. For both hermaphrodite and dioecious parasites, hybridization provides another tool in the parasite's ability to adapt to changing environments [191].
(e). Life cycle determinants of global change effects on parasites
Life history theory predicts that while parasites with direct life cycles have fewer energetic restrictions imposed by intermediate hosts and can invest more energy towards growth and reproduction [158,159], their dependence on a single host for reproduction might jeopardize survival. By contrast, indirect life cycles offer increased likelihood of ‘rescue’ for parasites, which may alter host specificity via the addition or exclusion of hosts [157,161,192]. Alternatively, parasites that demonstrate increased specialization of specific developmental stages, such as the dimorphic stages of Polystoma integerrimum, can inhabit a wider range of host environments and increase the probability of reproductive success [161,169,170]. Finally, dependence upon specific vectors or intermediate hosts for dispersal and reproduction renders parasites extremely vulnerable to both spatial and temporal climatic changes [2,193,194]. Recent studies suggest that parasite life-history traits may be enhanced by climate shifts and anthropogenic stressors associated with ongoing global change [195], thus providing relatively benign parasites with the potential to become increasingly pathogenic. However, while this is considered a serious threat to wildlife communities already facing mounting population pressures, conclusions are usually derived from assessments of single stressors or single parasite life stages, while the net effects to the parasite and host's whole population are rarely determined [196].
The parameters that characterize the life histories of individual parasite taxa are likely to play a critical role in determining their relative resilience in the face of changing environments. Parasite life cycles range in complexity from direct life cycles with a single host species to those with multiple intermediate and facultative paratenic hosts. The diversity of life cycles and life histories, coupled with variable flexibility and specificity of the parasite, means that there are likely to be winners and losers among parasites in perturbed environments. Whereas increased life cycle complexity might leave indirectly transmitted parasites susceptible to environmental change, if they acutely affect an obligate host population, the existence of multiple intermediate and/or reservoir hosts in a life cycle [162] and facultative paratenic hosts may provide a parasite with greater scope for adaptation [158,197]. Parasite survivorship and fecundity are the two key life history traits that impact parasite fitness, and therefore, transmission. Such traits will be subject to environmental stressors, such as drug exposure, that vary over time [198,199]. In the longer term, where stressors inhibit parasite transmission, they are likely to also impose selection pressure on life-history traits.
4. Evolutionary change of parasites
Parasites are perhaps uniquely predisposed to rapid evolution under global change. Not only are effective population sizes large and generation times typically short, but transmission imposes an exceptionally strong filter to exclude maladaptation: infective stages either find a host or die. Genotypes better suited to transmission under particular conditions will presumably be strongly selected for, with unpredictable variation in climate or host availability encouraging genetic diversity and within-genotype flexibility in key life-history traits. The potential for parasites to out-evolve their hosts suggests that increasing, rather than decreasing, parasite risks and burdens will be the norm under global change. However, the complex interactions of current stressors, as discussed thus far, can also act upon parasites at the genetic level, complicating predictions and leading to unexpected future infection patterns. Observations of parasite evolution in response to changing environments in nature are rare, but results from a few example systems are offered here to illustrate the potential diversity of parasite adaptive responses to global change.
(a). Resilience and plasticity
The complex links between existing environmental variation and disease transmission [200–202] suggest that identifying the impact of anthropogenic activities on the evolutionary responses of parasites over and above natural variation might be challenging. Models predict that increasing seasonal climate variability will drive the evolution of greater resilience of pathogens to environmental fluctuations [35]. This has been demonstrated with more extreme monsoon rainfall patterns linked to a rise in a strain of cholera, which is more resilient to water quality and quantity fluctuations [35]. Similarly, plasticity in parasite traits is likely to evolve in response to increased climatic variability, exemplified by the evolution of a plastic transmission strategy in Plasmodium relictum that has seen reproductive rates increase during periods of vector availability, thereby maximizing transmission [203].
Human management of host species and treatment strategies (see §5) are also important drivers of pathogen resilience. For example, selection pressure has resulted in altered strain dominance of the potato cyst nematode Globodera rostochiensis. Earlier planting of potatoes to allow growth in months historically too cold for larval invasion is now linked with a faster developing, more fecund strain of the parasite [204]. But by far the most pervasive evolutionary phenomenon due to intervention practices is that of increased drug resistance increasingly seen in parasites of humans [205,206] and livestock [207], including aquaculture species [208,209].
(b). Infectivity and virulence
Habitat change can strongly influence host–parasite interactions, shifting parasite diversity, abundance and transmission dynamics (discussed in §2). Evolving parasite infectivity and virulence may contribute to factors underlying these observations. Habitat fragmentation leads to smaller, patchier and more isolated populations [3]. In host–parasite interactions, infection and transmission will become more localized under such conditions. Theory and empirical data indicate that this can lead to the evolution of reduced parasite infectivity because of self-shading. This effect arises because, as individual susceptible hosts are rapidly infected locally by virulent parasites, they are surrounded by other infected hosts, which will reduce opportunities for further transmission of horizontally transmitted parasites [210,211] and parasites that use mixed transmission strategies [212]. In contrast with habitat fragmentation, intensification of farming practices is predicted to drive evolution of increased virulence; higher host availability reduces the adaptive cost of increased virulence due to host mortality [93]. Key evidence for this is the recent increase in pathology and mortality due to Flavobacterium columnare in densely stocked Finnish freshwater fish farms, linked to the emergence of more virulent, infective strains of the pathogen [213].
(c). Bet-hedging
Unpredictable conditions, such as the timing of host availability, should favour parasites that can produce offspring that vary in their life-history or transmission strategies [214]. Spreading the risk, or ‘bet-hedging’, allows parasites to increase the chances that at least some of their progeny will survive and infect a competent host. It is reasonable to expect that parasites will increasingly adopt such bet-hedging strategies to ensure survival in rapidly changing environments. The nematode Nematodirus battus historically exhibited a single generation per year with overwintered eggs hatching in spring to coincide with arrival of newborn lambs [215,216]. Evolution of multiple generations per year [217] via the production of autumn-hatching eggs that do not require vernalization has mitigated against asynchrony between larval presence and the availability of susceptible hosts in years with early warm springs [218]. However, anthropogenic changes may also hinder the evolution of parasite bet-hedging strategies. Variation in life cycle traits (e.g. rate of development, egg laying and hatching) of the fish louse Argulus foliaceus infecting farmed fish is lower than in wild populations, probably as a result of reliable host availability in fish farms compared with natural ecosystems [219].
(d). Host switching
Global change might constrain host–parasite co-evolution if the benefits of new mutations that enhance fitness (selective sweeps) are not realized in a rapidly changing environment [220]. Alternatively, host switching is a potential parasite adaptation to global change [221,222], should the availability of preferred hosts be decreased via geographical range shifts, phenological asynchrony, human management or control strategies. In some cases, switching to alternative hosts may not be optimal for parasite development, leading to reduced parasite offspring or survivorship and thereby reduced probability of transmission. This may limit how much host switching actually occurs in changing environments. However, Jones et al. [223] showed that while costs of prey switching for a parasitoid were severe in the first instance, these costs were ameliorated over successive generations. Furthermore, the force of selection will play a key role in the drive to host switch. In the case of the Guinea worm (Dracunculus medinensis), an extremely simple but effective control programme that filtered the copepod vectors from contaminated drinking water effectively blocked transmission [224] and reduced the number of human cases from an estimated 3.5 million cases in 1986 to just 126 in 2014. However, in 2015, 459 infections were recorded for the first time in dogs [13,225], suggesting a potential host-switching event, possibly driven by the effective control measures blocking transmission to humans [225].
The introduction of invasive host species generates unique opportunities for non-native parasite communities to come into contact with new hosts, and considerable potential for host switching. Classic examples of this include the introduction of squirrel parapoxvirus into UK red squirrels (Sciurus vulgaris; [142,226,227]) and crayfish plague (Aphanomyces astaci) into European crayfish (Astacus astacus [228]). Host switching from introduced to native hosts appears equally common for parasites with direct and indirect life cycles, and worryingly the majority of those reported are more virulent in native hosts than in the co-introduced invasive host [229]; however, considering that we still know very little about parasite speciation, it is difficult to predict future outcomes, and other mechanisms, such as niche specialization [230] and hybridization [231], could also affect both speciation and host range.
(e). Multiple evolutionary targets for adaptation
The above examples demonstrate how the effects of human activity and climate change are varied and far ranging with respect to parasite evolution. Targets of evolution are already altering the epidemiology of parasites; resilience, strain variation in phenology, bet-hedging in key life-history traits and host switching all demonstrate that through past unpredictability in transmission, parasites are well adapted to future changes in climate and host availability. As the evidence for the anthropogenic effects on parasite adaptive responses builds, we must now consider the evolutionary capabilities of pathogens as an integral component to predicting the future landscape of host–parasite interactions under pressures of global change. This will be particularly important when considering the consequences of parasite control programmes, arguably the greatest selective pressure faced by parasites in their evolutionary history.
5. Control programmes and predictive epidemiology in a changing world
Taken together, the observations and projections described above give a strong signal to all epidemiologists: the future is both uncertain and rapidly changing, representing a new era of health challenges in the twenty-first century that is unprecedented in human history. Multiple laboratory and field observations, modelling exercises and meta-analyses have identified key abiotic and biotic factors that govern the free-living and vector-borne stages of parasites (see §2). Seasonally variable environments are also important in determining the aggregation of animal parasites (e.g. [232]) including malaria and hookworms in some regions [233,234]. What remains unknown is how patterns of global change across decadal scales have influenced parasite transmission. While the substantial post-1997 downturn in malarial infections has undoubtedly been accelerated by large-scale control interventions, environmental changes that have reduced the vector population or climate-sensitive parasite life stages over extended periods may have also contributed. Droughts in Africa are increasingly common [235], and we cannot exclude the possibility that prolonged periods of low rainfall have contributed to the downturn in transmission of malaria and other parasitic infections, given the reliance of vectors and parasite transmission stages on water availability. This last point illustrates how cryptic factors continue to be influential. Most campaigns do not routinely collect individual patient data once the delivery programme is established, and without this it is not easily possible to differentiate the effects of MDA from those of environmental change.
(a). Evolutionary implications of control programmes
MDA programmes themselves are stressors on host–parasite systems as a result of imposing selection pressures, altering host microbiota and disrupting life cycles of vectors and intermediate hosts that have coevolved with parasites. Thus the success of these programmes should also be considered in the light of changing abiotic and biotic factors. The impetus for MDA campaigns stems from mathematical models of parasite life cycles first developed in the second half of the twentieth century (e.g. [236]), which have an underlying assumption that neither parasites nor vectors undergo significant evolution that would reduce the impact of intervention. More recent attempts that consider either diminished drug efficacy and/or greater transmission rate lead to the common output that MDA programmes do not eliminate parasite populations even after decades of intervention. Evolution of the parasite and/or vector populations along a specific life-history trajectory has not been a common feature of these models.
Long-term control programmes may do more harm than good, as any treatment imposes a selection pressure for resistance on the parasite population. Some treatments may promote the evolution of more virulent pathogen strains [237] as illustrated by evolving rodent malaria parasites in mice immunized with a candidate human malaria vaccine [238]. This in turn might lead to enhanced transmission as shown in chickens vaccinated against Marek's disease virus [239]. Poulin [198] suggests that shortening the duration of parasite infection by drug treatment negates any advantage to a parasite being long-lived, and survivorship of a parasite may therefore trade-off against other traits that affect infectivity, such as age to maturity and fecundity (see §2), resulting in enhanced transmission. Virulence–longevity trade-offs might explain increased horizontal transmission of some diseases on hospital wards [240], while nematodes of horses appear to respond to drug treatment by shortening their development period in the host [241]. According to Day & Read [242], the optimal approach for combating the evolution of drug resistance is to use the highest safe dose or the lowest, effective dose. High-dose medications are effective only if all pathogens can be killed (as in the case of HIV). If a small number of microbes are likely to evade treatment (already resistant to treatment, or if drug resistance arises de novo), then high doses of medication may allow resistant microbes to survive and spread by the very act of killing off drug-sensitive microbes [242]. On the other hand, low drug doses are more likely to enable new mutations conferring drug resistance to spread and fix in the parasite population. Whether high or low doses are optimal for combating drug resistance will depend on system-specific factors, but this study highlights that parasite evolution is rarely considered proactively in setting treatment goals and decisions.
One consequence of the ‘perfect storm’ of stressors may be the generation of so-called ‘hotspots’ of transmission [243]. This hypothetical effect does not preclude a reduction in transmission intensity, but does imply that individuals who are normally resident in areas of intervention are persistently exposed and harbouring infection. Other interpretations of the hotspot observations are possible—including the lack of engagement or disenfranchisement with control programmes [244].
(b). Adaptive management and predictive epidemiology
Changes in parasite ecology, epidemiology (§§2 and 3) and evolution (§4) have profound implications for the monitoring and control of health in managed systems, which must themselves adapt if altered challenges are to be attenuated. To keep pace, we need to develop a predictive understanding of how patterns of parasite transmission among animals and humans could change in response to the multiple, interacting stressors being placed on the global ecosystem [245]. From this understanding we need to create improved decision support systems that allow for sustainable control and management of hosts, vectors and parasites. However, the wide range of relevant anthropogenic stressors, the enormous diversity of parasite taxa, life-history traits and infection strategies, and the range of possible functional responses and interactions between them—coupled with simultaneous responses among host populations—make this a hugely challenging task. Here, we offer one approach based on co-production, knowledge transfer and wider participation.
The subtle, ‘covert’ ways in which multiple drivers of global change can affect parasite transmission are complex when considering individual stressors, while the impact of interacting stressors on future disease risk remains largely unknown. The current practice of making iterative changes in management strategy, based on accumulated evidence of infection patterns, is too static to keep up with the increasing uncertainty around transmission patterns. At the same time, advances in information and communication technology open up new data collection modalities. Organizing and applying such data streams could provide novel and powerful ways of gathering real-time understanding of changing transmission, and adapting control practices accordingly.
The zenith of adaptive management would track and react to not only parasite transmission but also evolutionary processes, including those of host populations, such that transmission functions are re-evaluated as life-history parameters change (see §§ 3 and 4 above). This would require repeated confrontation of alternative transmission models with available data, and inferring shifts in key parameters from model fits. In principle, this is already possible, but automation of the process and the availability of sufficient, robust and timely data present challenges to implementation. Citizen Science has been leveraged to gather real-time information on the distribution of invasive plant diseases [246], while mobile phone networks have been used effectively to gather data on the changing epidemiology of diseases in livestock and humans [106,247,248]. Involved professionals such as farmers and veterinary laboratories are also a source of specific surveillance data [249], which could be collated more quickly to track epidemiological patterns, and update models accordingly. For example, confirmed diagnoses of infection with the helminth N. battus in lambs are currently used to populate web-accessible maps that are updated daily during high-risk periods (www.scops.org.uk). Linking these data with models of parasite transmission dynamics and life-history parameters modified according to observations could make such models robust to parasite evolution (e.g. bet-hedging; see §4c), even pending more complete knowledge of how evolution alters epidemiology.
A major challenge in dynamic data-driven model fitting is the reliability of data collected from disparate sources, and not always by professionals using verified methods. However, the decline of expensive, centrally funded monitoring stations and systems, both for meteorological and disease data, limits the alternatives. Networks of privately collected meteorological data (e.g. forecast.io) are increasingly available and may usefully compensate for the loss of, and sometimes exceed the capability of, official sources. Similar networks for data on phenology or even parasitic infections could be envisaged. Separate observational or experimental data will always be needed and can constrain fitted parameters within plausible ranges, or select parameters most likely to be open to parasite evolution. Models of parasite transmission dynamics that are validated, updated with shifts in epidemiology and evolution, and whose outputs are accessible to end users, could form the backbone of a new wave of decision support systems that maximize the opportunities afforded by advances in modelling and new sources of data. In addition, by involving the public in disease monitoring, we can promote disease awareness, improving socio-ecological resilience [250].
We suggest that a truly predictive understanding of the effects of global change on parasite transmission will, therefore, need to incorporate the evolutionary consequences of changes imposed by combinations of abiotic and biotic stressors acting at various locations under conditions of migration, habitat loss and fragmentation. These are themselves difficult to predict, especially as the experiments required to fuel such predictions would tend to remove ‘extraneous’ variation that could actually be core to complex evolutionary trajectories under global change. Reverse engineering of models to estimate parameter alterations, which are needed to maintain parasite fitness under changing scenarios, and subsequently assessing these predictions for biological plausibility, could provide more adaptive predictions. In any case, models of parasite population dynamics that neglect the possibility of evolution of transmission strategies will have a short shelf-life under global change, and greater attention ought to be paid to this challenging area.
6. Conclusion
Differences in transmission ecology, parasite life history and the ecology of intermediate hosts and vectors will clearly play a key role in determining the sensitivity of infections to abiotic and biotic stressors [202]. Monitoring these stressors at high spatial and temporal resolution, perhaps using remotely sensed products (e.g. [251]), is likely to be of considerable help in improving our understanding of how diseases might spread in the future. However, while there is a move away from using keystone species in the general ecological field as early warning indicators of vulnerable ecosystems in favour of monitoring the balance between diversity, functional groups and connectivity, it would be naive to take this approach for infectious diseases. The impact of infectious diseases, particularly EIDs, is context-dependent (the devil is in the detail) and the importance of particular parasite species and strains will change over space and time, and so at least for the moment, targeted disease monitoring and surveillance at appropriate spatio-temporal resolution are still necessary.
Acknowledgements
We thank the BES Parasite and Pathogen Ecology and Evolution Special Interest Group for organizing the 2012 Ecology Meets Medicine Retreat and the 2015 Transmission Retreat.
Glossary
- term
definition
- parasitological terms
- definitive host
Host in or on which a parasite reaches sexual maturity.
- ectoparasite
Parasite that lives on the exterior of a host.
- endoparasite
Parasite that lives inside the body of a host.
- helminth
Parasitic worm from either the Phylum Plathyhelminthes or Nematoda.
- intermediate host
Host required for larval development or growth of a parasite before it is infective to another intermediate host or the definitive host.
- macroparasite
Parasite that produces infective stages released away from the host; typically of a larger body size than a microparasite.
- microparasite
Parasite that reproduces in situ on the host; typically microbes (viruses, bacteria, protozoa) but exceptions include viviparous helminths, such as Gyrodactylus spp.
- parasite
Organism that lives at the expense of its host and causes harm; includes both micro- and macroparasites.
- parasite intensity
Number of individual parasites of a particular species infecting a host.
- prevalence
Number of individuals of a host species infected with a species of parasite. Expressed as a percentage.
- systems biology terms
- global change
Any climatic or anthropogenic change that has an impact directly or indirectly on an ecosystem; includes all stressors associated with urbanization and human population growth (growth of urban areas, depletion of rural and natural resources, etc.).
- industrialization
Social and economic change that has transformed human societies from agrarian to industrial, resulting in urbanization, exploitation of natural resources and environmental pollution.
- planetary boundaries
Framework for identifying a ‘safe operating space for humanity’ covering nine earth system processes. Each process has a tipping point demarcating safe zones [6,7].
- social–ecological resilience
Capacity of an integrated ecosystem that includes humans to withstand perturbations and stressors.
- systems change
Biological, social and political drivers, affecting interactions, functioning and dynamic processes within ecosystems.
- urbanization
Increased numbers of people in towns and cities resulting in growth of urban areas.
Authors' contributions
All authors attended the 2015 British Ecological Society (BES) Transmission Research Retreat, where initial ideas for this manuscript were conceived. J.C. coordinated writing and drafted the article with M.B. All authors contributed examples and text, and approved the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
This work was partially funded by the Welsh Government and Higher Education Funding Council for Wales through the Sêr Cymru National Research Network for Low Carbon, Energy and Environment AquaWales Project.
References
- 1.Waters CN, et al. 2016. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 351, aad2622 ( 10.1126/science.aad2622) [DOI] [PubMed] [Google Scholar]
- 2.Mouchet J, Faye O, Julvez J, Manguin S. 1996. Drought and malaria retreat in the Sahel, West Africa. Lancet 348, 1735–1736. ( 10.1016/S0140-6736(05)65860-6) [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 4.Pozio E, Rinaldi L, Marucci G, Musella V, Galati F, Cringoli G, Boireau P, La Rosa G. 2009. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int. J. Parasitol. 39, 71–79. ( 10.1016/j.ijpara.2008.06.006) [DOI] [PubMed] [Google Scholar]
- 5.Walther GR. 2010. Community and ecosystem responses to recent climate change. Phil. Trans. R. Soc. B 365, 2019–2024. ( 10.1098/rstb.2010.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockström J, et al. 2009. Planetary boundaries: exploring the safe operating space for humanity. Ecol. Soc. 14, 32 ( 10.5751/ES-03180-140232) [DOI] [Google Scholar]
- 7.Steffen W, et al. 2015. Planetary boundaries: guiding human development on a changing planet. Science 347, 1259855 ( 10.1126/science.1259855) [DOI] [PubMed] [Google Scholar]
- 8.Smits HL. 2009. Prospects for the control of neglected tropical diseases by mass drug administration. Expert Rev. Anti Infect. Ther. 7, 37–56. ( 10.1586/14787210.7.1.37) [DOI] [PubMed] [Google Scholar]
- 9.Anderson R, Hollingsworth TD, Truscott J, Brooker S. 2012. Optimisation of mass chemotherapy to control soil-transmitted helminth infection. Lancet Lond. Engl. 379, 289–290. ( 10.1016/S0140-6736(12)60120-2) [DOI] [PubMed] [Google Scholar]
- 10.Cox FEG. 2002. History of human parasitology. Clin. Microbiol. Rev. 15, 595–612. ( 10.1128/CMR.15.4.595-612.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward J, Warren C (eds). 2006. Silent victories: the history and practice of public health in twentieth-century America. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Stephenson J. 2002. River blindness coconspirator. J. Am. Med. Assoc. 287, 1794 ( 10.1001/jama.287.14.1794-JWM20004-2-1) [DOI] [Google Scholar]
- 13.Eberhard ML, et al. 2014. The peculiar epidemiology of dracunculiasis in Chad. Am. J. Trop. Med. Hyg. 90, 61–70. ( 10.4269/ajtmh.13-0554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kongs A, Marks G, Verlé P, Van der Stuyft P. 2001. The unreliability of the Kato-Katz technique limits its usefulness for evaluating S. mansoni infections. Trop. Med. Int. Health 6, 163–169. ( 10.1046/j.1365-3156.2001.00687.x) [DOI] [PubMed] [Google Scholar]
- 15.IPCC. 2013. Climate Change 2013: The Physical Science Basis. Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press.
- 16.Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B. 2012. Malaria resurgence: a systematic review and assessment of its causes. Malar. J. 11, 122 ( 10.1186/1475-2875-11-122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uusitalo L, Lehikoinen A, Helle I, Myrberg K. 2015. An overview of methods to evaluate uncertainty of deterministic models in decision support. Environ. Model. Softw. 63, 24–31. ( 10.1016/j.envsoft.2014.09.017) [DOI] [Google Scholar]
- 18.Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162. ( 10.1126/science.1063699) [DOI] [PubMed] [Google Scholar]
- 19.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395. ( 10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 20.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145–148. ( 10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 21.Rohr JR, Dobson AP, Johnson PTJ, Kilpatrick AM, Paull SH, Raffel TR, Ruiz-Moreno D, Thomas MB. 2011. Frontiers in climate change–disease research. Trends Ecol. Evol. 26, 270–277. ( 10.1016/j.tree.2011.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barber I, Berkhout BW, Ismail Z. 2016. Thermal change and the dynamics of multi-host parasite life cycles in aquatic ecosystems. Integr. Comp. Biol 56, 561–572. ( 10.1093/icb/icw025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IPCC. 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds RK Pachauri, LA Meyer). Geneva, Switzerland: IPCC.
- 24.Paaijmans KP, Read AF, Thomas MB. 2009. Understanding the link between malaria risk and climate. Proc. Natl Acad. Sci. USA 106, 13 844–13 849. ( 10.1073/pnas.0903423106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macnab V, Barber I. 2012. Some (worms) like it hot: fish parasites grow faster in warmer water, and alter host thermal preferences. Glob. Change Biol. 18, 1540–1548. ( 10.1111/j.1365-2486.2011.02595.x) [DOI] [Google Scholar]
- 26.Studer A, Thieltges D, Poulin R. 2010. Parasites and global warming: net effects of temperature on an intertidal host–parasite system. Mar. Ecol. Prog. Ser. 415, 11–22. ( 10.3354/meps08742) [DOI] [Google Scholar]
- 27.Scott ME, Nokes DJ. 2009. Temperature-dependent reproduction and survival of Gyrodactylus bullatarudis (Monogenea) on guppies (Poecilia reticulata). Parasitology 89, 221–228. ( 10.1017/S0031182000001256) [DOI] [Google Scholar]
- 28.Lafferty KD. 2009. The ecology of climate change and infectious diseases. Ecology 90, 888–900. ( 10.1890/08-0079.1) [DOI] [PubMed] [Google Scholar]
- 29.Paaijmans KP, Blanford S, Chan BHK, Thomas MB. 2012. Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biol. Lett. 8, 465–468. ( 10.1098/rsbl.2011.1075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammed RS, Reynolds M, James J, Williams C, Mohammed A, Ramsubhag A, van Oosterhout C, Cable J. 2016. Getting into hot water: sick guppies frequent warmer thermal conditions. Oecologia 181, 911–917. ( 10.1007/s00442-016-3598-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hockley FA, Wilson CAME, Brew A, Cable J. 2014. Fish responses to flow velocity and turbulence in relation to size, sex and parasite load. J. R. Soc. Interface 11, 20130814 ( 10.1098/rsif.2013.0814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hockley FA, Wilson CAME, Graham N, Cable J. 2014. Combined effects of flow condition and parasitism on shoaling behaviour of female guppies Poecilia reticulata. Behav. Ecol. Sociobiol. 68, 1513–1520. ( 10.1007/s00265-014-1760-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone DA, Allen MR, Stott PA, Pall P, Min S-K, Nozawa T, Yukimoto S. 2009. The detection and attribution of human influence on climate. Annu. Rev. Environ. Resour. 34, 1–16. ( 10.1146/annurev.environ.040308.101032) [DOI] [Google Scholar]
- 34.Hansen G, Stone D. 2016. Assessing the observed impact of anthropogenic climate change. Nat. Clim. Change 6, 532–537. ( 10.1038/nclimate2896) [DOI] [Google Scholar]
- 35.Koelle K, Pascual M, Yunus M. 2005. Pathogen adaptation to seasonal forcing and climate change. Proc. R. Soc. B 272, 971–977. ( 10.1098/rspb.2004.3043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wearing HJ, Rohani P. 2006. Ecological and immunological determinants of dengue epidemics. Proc. Natl Acad. Sci. USA 103, 11802–11807. ( 10.1073/pnas.0602960103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cann KF, Thomas DR, Salmon RL, Wyn-Jones AP, Kay D. 2013. Extreme water-related weather events and waterborne disease. Epidemiol. Infect. 141, 671–686. ( 10.1017/S0950268812001653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baylis M, Mellor PS, Meiswinkel R. 1999. Horse sickness and ENSO in South Africa. Nature 397, 574 ( 10.1038/17512) [DOI] [PubMed] [Google Scholar]
- 39.Linthicum KJ, Anyamba A, Tucker CJ, Kelley PW, Myers MF, Peters CJ. 1999. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science 285, 397–400. ( 10.1126/science.285.5426.397) [DOI] [PubMed] [Google Scholar]
- 40.Park J-W, Cheong H-K, Honda Y, Ha M, Kim H, Kolam J, Inape K, Mueller I. 2016. Time trend of malaria in relation to climate variability in Papua New Guinea. Environ. Health Toxicol. 31, e2016003 ( 10.5620/eht.e2016003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leedale J, Jones AE, Caminade C, Morse AP. 2016. A dynamic, climate-driven model of Rift Valley fever. Geospatial Health 11, 78–93. ( 10.4081/gh.2016.394) [DOI] [PubMed] [Google Scholar]
- 42.Rogers DJ, Randolph SE. 2000. The global spread of malaria in a future, warmer world. Science 289, 1763–1766. ( 10.1126/science.289.5485.1763) [DOI] [PubMed] [Google Scholar]
- 43.Pascual M, Bouma MJ. 2009. Do rising temperatures matter. Ecology 90, 906–912. ( 10.1890/08-0730.1) [DOI] [PubMed] [Google Scholar]
- 44.Randolph SE. 2001. The shifting landscape of tick-borne zoonoses: tick-borne encephalitis and Lyme borreliosis in Europe. Phil. Trans. R. Soc. B 356, 1045–1056. ( 10.1098/rstb.2001.0893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randolph S. 2002. Predicting the risk of tick-borne diseases. Int. J. Med. Microbiol. 291, 6–10. ( 10.1016/S1438-4221(02)80002-9) [DOI] [PubMed] [Google Scholar]
- 46.France RL, Graham L. 1985. Increased microsporidian parasitism of the crayfish Orconectes virilis in an experimentally acidified lake. Water Air. Soil Pollut. 26, 129–136. ( 10.1007/BF00292063) [DOI] [Google Scholar]
- 47.Lafferty KD. 1997. Environmental parasitology: what can parasites tell us about human impacts on the environment? Parasitol. Today 13, 251–255. ( 10.1016/S0169-4758(97)01072-7) [DOI] [PubMed] [Google Scholar]
- 48.Abd Allah AT, Wanas MQS, Thompson SN. 1997. Effects of heavy metals on survival and growth of Biomphalaria glabrata Say (Gastropoda: Pulmonata) and interaction with schistosome infection. J. Molluscan Stud. 63, 79–86. ( 10.1093/mollus/63.1.79) [DOI] [Google Scholar]
- 49.Cross MA, Irwin SW, Fitzpatrick SM. 2001. Effects of heavy metal pollution on swimming and longevity in cercariae of Cryptocotyle lingua (Digenea: Heterophyidae). Parasitology 123, 499–507. ( 10.1017/S0031182001008708) [DOI] [PubMed] [Google Scholar]
- 50.Morley NJ, Crane M, Lewis JW. 2003. Cadmium toxicity and snail-digenean interactions in a population of Lymnaea spp. J. Helminthol. 77, 49–55. ( 10.1079/JOH2002148) [DOI] [PubMed] [Google Scholar]
- 51.Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dölz H, Millanao A, Buschmann AH. 2013. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 15, 1917–1942. ( 10.1111/1462-2920.12134) [DOI] [PubMed] [Google Scholar]
- 52.Johnson PTJ, Carpenter SR. 2008. Influence of eutrophication on disease in aquatic ecosystems: patterns, processes, and predictions. In Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems (eds Ostfeld RS, Keesing F, Eviner VT), pp. 71–100. Princeton, NJ: Princeton University Press. [Google Scholar]
- 53.Johnson PTJ, Townsend AR, Cleveland CC, Glibert PM, Howarth RW, McKenzie VJ, Rejmankova E, Ward MH. 2010. Linking environmental nutrient enrichment and disease emergence in humans and wildlife. Ecol. Appl. Publ. Ecol. Soc. Am. 20, 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lafferty KD, Harvell CD. 2014. The role of infectious disease in marine communities. In Marine community ecology and conservation (eds Bertness MD, Bruno J, Silliman BR, Stachowicz JJ), pp. 85–108. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 55.Bennie J, Davies TW, Cruse D, Inger R, Gaston KJ. 2015. Cascading effects of artificial light at night: resource-mediated control of herbivores in a grassland ecosystem. Phil. Trans. R. Soc. B 370, 20140131 ( 10.1098/rstb.2014.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kearn GC. 1973. An endogenous circadian hatching rhythm in the monogenean skin parasite Entobdella soleae, and its relationship to the activity rhythm of the host (Solea solea). Parasitology 66, 101–122. ( 10.1017/S0031182000044486) [DOI] [PubMed] [Google Scholar]
- 57.Lampert W. 1989. The adaptive significance of diel vertical migration of zooplankton. Funct. Ecol. 3, 21–27. ( 10.2307/2389671) [DOI] [Google Scholar]
- 58.Barghini A, de Medeiros BAS. 2010. Artificial lighting as a vector attractant and cause of disease diffusion. Environ. Health Perspect. 118, 1503–1506. ( 10.1289/ehp.1002115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monjan A, Collector M. 1977. Stress-induced modulation of the immune response. Science 196, 307–308. ( 10.1126/science.557841) [DOI] [PubMed] [Google Scholar]
- 60.Simpson SD, Radford AN, Nedelec SL, Ferrari MCO, Chivers DP, McCormick MI, Meekan MG. 2016. Anthropogenic noise increases fish mortality by predation. Nat. Commun. 7, 10 544 ( 10.1038/ncomms10544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marsh DM, Pearman PB. 1997. Effects of habitat fragmentation on the abundance of two species of Leptodactylid frogs in an Andean montane forest. Conserv. Biol. 11, 1323–1328. ( 10.1046/j.1523-1739.1997.95519.x) [DOI] [Google Scholar]
- 62.Ernst R, Linsenmair KE, Rödel M-O. 2006. Diversity erosion beyond the species level: dramatic loss of functional diversity after selective logging in two tropical amphibian communities. Biol. Conserv. 133, 143–155. ( 10.1016/j.biocon.2006.05.028) [DOI] [Google Scholar]
- 63.Ferraz G, Nichols JD, Hines JE, Stouffer PC, Bierregaard RO, Lovejoy TE. 2007. A large-scale deforestation experiment: effects of patch area and isolation on Amazon birds. Science 315, 238–241. ( 10.1126/science.1133097) [DOI] [PubMed] [Google Scholar]
- 64.Katovai E, Burley AL, Mayfield MM. 2012. Understory plant species and functional diversity in the degraded wet tropical forests of Kolombangara Island, Solomon Islands. Biol. Conserv. 145, 214–224. ( 10.1016/j.biocon.2011.11.008) [DOI] [Google Scholar]
- 65.Edwards FA, Edwards DP, Larsen TH, Hsu WW, Benedick S, Chung A, Vun Khen C, Wilcove DS, Hamer KC. 2014. Does logging and forest conversion to oil palm agriculture alter functional diversity in a biodiversity hotspot? Anim. Conserv. 17, 163–173. ( 10.1111/acv.12074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKenzie VJ. 2007. Human land use and patterns of parasitism in tropical amphibian hosts. Biol. Conserv. 137, 102–116. ( 10.1016/j.biocon.2007.01.019) [DOI] [Google Scholar]
- 67.Gillespie TR, Chapman CA, Greiner EC. 2005. Effects of logging on gastrointestinal parasite infections and infection risk in African primates. J. Appl. Ecol. 42, 699–707. ( 10.1111/j.1365-2664.2005.01049.x) [DOI] [Google Scholar]
- 68.Mbora DNM, Wieczkowski J, Munene E. 2009. Links between habitat degradation, and social group size, ranging, fecundity, and parasite prevalence in the Tana River mangabey (Cercocebus galeritus). Am. J. Phys. Anthropol. 140, 562–571. ( 10.1002/ajpa.21113) [DOI] [PubMed] [Google Scholar]
- 69.Hussain S, Ram MS, Kumar A, Shivaji S, Umapathy G. 2013. Human presence increases parasitic load in endangered lion-tailed macaques (Macaca silenus) in its fragmented rainforest habitats in Southern India. PLoS ONE 8, e63685 ( 10.1371/journal.pone.0063685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lane KE, Holley C, Hollocher H, Fuentes A. 2011. The anthropogenic environment lessens the intensity and prevalence of gastrointestinal parasites in Balinese long-tailed macaques (Macaca fascicularis). Primates J. Primatol. 52, 117–128. ( 10.1007/s10329-010-0230-6) [DOI] [PubMed] [Google Scholar]
- 71.Chasar A, Loiseau C, Valkiūnas G, Iezhova T, Smith TB, Sehgal RNM. 2009. Prevalence and diversity patterns of avian blood parasites in degraded African rainforest habitats. Mol. Ecol. 18, 4121–4133. ( 10.1111/j.1365-294X.2009.04346.x) [DOI] [PubMed] [Google Scholar]
- 72.Evans KL, Gaston KJ, Sharp SP, McGowan A, Simeoni M, Hatchwell BJ. 2009. Effects of urbanisation on disease prevalence and age structure in blackbird Turdus merula populations. Oikos 118, 774–782. ( 10.1111/j.1600-0706.2008.17226.x) [DOI] [Google Scholar]
- 73.Skole D, Tucker C. 1993. Tropical deforestation and habitat fragmentation in the Amazon: satellite data from 1978 to 1988. Science 260, 1905–1910. ( 10.1126/science.260.5116.1905) [DOI] [PubMed] [Google Scholar]
- 74.Murcia C. 1995. Edge effects in fragmented forests: implications for conservation. Trends Ecol. Evol. 10, 58–62. ( 10.1016/S0169-5347(00)88977-6) [DOI] [PubMed] [Google Scholar]
- 75.Wirth R, Meyer ST, Leal IR, Tabarelli M. 2008. Plant herbivore interactions at the forest edge. In Progress in botany (eds Lüttge U, Beyschlag W, Murata J), pp. 423–448. Berlin, Germany: Springer. [Google Scholar]
- 76.Wolf M, Batzli GO. 2001. Increased prevalence of bot flies (Cuterebra fontinella) on white-footed mice (Peromyscus leucopus) near forest edges. Can. J. Zool. 79, 106–109. ( 10.1139/z00-185) [DOI] [Google Scholar]
- 77.Chapman CA, Speirs ML, Gillespie TR, Holland T, Austad KM. 2006. Life on the edge: gastrointestinal parasites from the forest edge and interior primate groups. Am. J. Primatol. 68, 397–409. ( 10.1002/ajp.20233) [DOI] [PubMed] [Google Scholar]
- 78.Schlaepfer MA, Gavin TA. 2001. Edge effects on lizards and frogs in tropical forest fragments. Conserv. Biol. 15, 1079–1090. ( 10.1046/j.1523-1739.2001.0150041079.x) [DOI] [Google Scholar]
- 79.Ewers RM, Bartlam S, Didham RK. 2013. Altered species interactions at forest edges: contrasting edge effects on bumble bees and their phoretic mite loads in temperate forest remnants. Insect Conserv. Divers. 6, 598–606. ( 10.1111/icad.12014) [DOI] [Google Scholar]
- 80.Perea-Rodriguez JP, Milano AM, Osherov BE, Fernandez-Duque E. 2010. Gastrointestinal parasites of owl monkeys (Aotus azarai azarai) in the Argentinean Chaco. Neotrop. Primates 17, 7–11. ( 10.1896/044.017.0105) [DOI] [Google Scholar]
- 81.Hess GR. 1994. Conservation corridors and contagious disease: a cautionary note. Conserv. Biol. 8, 256–262. ( 10.1046/j.1523-1739.1994.08010256.x) [DOI] [Google Scholar]
- 82.McCallum H, Dobson A. 2002. Disease, habitat fragmentation and conservation. Proc. R. Soc. Lond. B 269, 2041–2049. ( 10.1098/rspb.2002.2079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Food and Agriculture Organization of the United Nations. 2015. FAO Statistical Pocketbook: World Food and Agriculture 2015. Food and Agriculture Organization of the United Nations. See http://www.fao.org/3/a-i4691e.pdf. [Google Scholar]
- 84.Food and Agriculture Organization of the United Nations. 2006. Livestock's long shadow. Environmental issues and options. Food and Agriculture Organization of the United Nations; See ftp://ftp.fao.org/docrep/fao/010/a0701e/a0701e.pdf. [Google Scholar]
- 85.Food and Agriculture Organization of the United Nations. 2015. Aquaculture topics and activities. Aquaculture. In FAO Fisheries and Aquaculture Department [online]. Rome, updated 14 September 2015. [Cited 5 February 2017.] http://www.fao.org/fishery/aquaculture/en. [Google Scholar]
- 86.Iguchi K, Ogawa K, Nagae M, Ito F. 2003. The influence of rearing density on stress response and disease susceptibility of ayu (Plecoglossus altivelis). Aquaculture 220, 515–523. ( 10.1016/S0044-8486(02)00626-9) [DOI] [Google Scholar]
- 87.Shaw DJ, Vercruysse J, Claerebout E, Dorny P. 1998. Gastrointestinal nematode infections of first-grazing season calves in Western Europe: associations between parasitological, physiological and physical factors. Vet. Parasitol. 75, 133–151. ( 10.1016/S0304-4017(97)00213-6) [DOI] [PubMed] [Google Scholar]
- 88.Rosà R, Pugliese A. 2007. Effects of tick population dynamics and host densities on the persistence of tick-borne infections. Math. Biosci. 208, 216–240. ( 10.1016/j.mbs.2006.10.002) [DOI] [PubMed] [Google Scholar]
- 89.Sadhu N, Sharma SRK, Joseph S, Dube P, Philipose KK. 2014. Chronic stress due to high stocking density in open sea cage farming induces variation in biochemical and immunological functions in Asian seabass (Lates calcarifer, Bloch). Fish Physiol. Biochem. 40, 1105–1113. ( 10.1007/s10695-014-9909-8) [DOI] [PubMed] [Google Scholar]
- 90.Lagrue C, Poulin R. 2015. Bottom-up regulation of parasite population densities in freshwater ecosystems. Oikos 124, 1639–1647. ( 10.1111/oik.02164) [DOI] [Google Scholar]
- 91.Samsing F, Oppedal F, Johansson D, Bui S, Dempster T. 2014. High host densities dilute sea lice Lepeophtheirus salmonis loads on individual Atlantic salmon, but do not reduce lice infection success. Aquac. Environ. Interact. 6, 81–89. ( 10.3354/aei00118) [DOI] [Google Scholar]
- 92.Mahieu M. 2013. Effects of stocking rates on gastrointestinal nematode infection levels in a goat/cattle rotational stocking system. Vet. Parasitol. 198, 136–144. ( 10.1016/j.vetpar.2013.08.029) [DOI] [PubMed] [Google Scholar]
- 93.Mennerat A, Nilsen F, Ebert D, Skorping A. 2010. Intensive farming: evolutionary implications for parasites and pathogens. Evol. Biol. 37, 59–67. ( 10.1007/s11692-010-9089-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robert V, Macintyre K, Keating J, Trape J-F, Duchemin J-B, Warren M, Beier JC. 2003. Malaria transmission in urban sub-Saharan Africa. Am. J. Trop. Med. Hyg. 68, 169–176. [PubMed] [Google Scholar]
- 95.Kearney M, Porter WP, Williams C, Ritchie S, Hoffmann AA. 2009. Integrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: the dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 23, 528–538. ( 10.1111/j.1365-2435.2008.01538.x) [DOI] [Google Scholar]
- 96.Reiter P, et al. 2003. Texas lifestyle limits transmission of dengue virus. Emerg. Infect. Dis. 9, 86–89. ( 10.3201/eid0901.020220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taylor LH, Latham SM, Woolhouse ME. 2001. Risk factors for human disease emergence. Phil. Trans. R. Soc. Lond. B 356, 983–989. ( 10.1098/rstb.2001.0888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Daszak P, Zambrana-Torrelio C, Bogich TL, Fernandez M, Epstein JH, Murray KA, Hamilton H. 2013. Interdisciplinary approaches to understanding disease emergence: the past, present, and future drivers of Nipah virus emergence. Proc. Natl Acad. Sci. USA 110, 3681–3688. ( 10.1073/pnas.1201243109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Plowright RK, et al. 2015. Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. B 282, 20142124 ( 10.1098/rspb.2014.2124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.United Nations Department of Economic and Social Affairs, Population Division. 2015. World urbanization prospects: the 2014 revision. Working Paper No. ST/ESA/SER.A/366.
- 102.United Nations Department of Economic and Social Affairs, Population Division. 2015. World population prospects: the 2015 revision, key findings and advance tables. Working Paper No. ESA/P/WP.241.
- 103.United Nations Department of Economic and Social Affairs, Population Division. 2011. Population distribution, urbanization, internal migration and development: an international perspective. Working Paper No. ESA/P/WP/223.
- 104.Hotez PJ. 2013. NTDs V.2.0: ‘Blue marble health’—neglected tropical disease control and elimination in a shifting health policy landscape. PLoS Negl. Trop. Dis. 7, e2570 ( 10.1371/journal.pntd.0002570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dulac J. 2013. Global land transport infrastructure requirements. Estimating road and railway infrastructure capacity and costs to 2050, pp. 1–50. Paris, France: International Energy Agency.
- 106.Randrianasolo L, et al. 2010. Sentinel surveillance system for early outbreak detection in Madagascar. BMC Public Health 10 ( 10.1186/1471-2458-10-31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Medeiros DB, da Rosa EST, Marques AA, Simith DB, Carneiro AR, Chiang JO, Prazeres IT, Vasconcelos PF, Nunes MR. 2010. Circulation of hantaviruses in the influence area of the Cuiabá-Santarém Highway. Mem. Inst. Oswaldo Cruz 105, 665–671. ( 10.1590/S0074-02762010000500011) [DOI] [PubMed] [Google Scholar]
- 108.Friant S, Paige SB, Goldberg TL. 2015. Drivers of bushmeat hunting and perceptions of zoonoses in Nigerian hunting communities. PLoS Negl. Trop. Dis. 9, e0003792 ( 10.1371/journal.pntd.0003792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tompkins AM, McCreesh N. 2016. Migration statistics relevant for malaria transmission in Senegal derived from mobile phone data and used in an agent-based migration model. Geospatial Health 11, 49–55. ( 10.4081/gh.2016.408) [DOI] [PubMed] [Google Scholar]
- 110.De Vos JM, Joppa LN, Gittleman JL, Stephens PR, Pimm SL. 2015. Estimating the normal background rate of species extinction. Conserv. Biol. 29, 452–462. ( 10.1111/cobi.12380) [DOI] [PubMed] [Google Scholar]
- 111.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401–406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 112.Lafferty KD. 2012. Biodiversity loss decreases parasite diversity: theory and patterns. Phil. Trans. R. Soc. B 367, 2814–2827. ( 10.1098/rstb.2012.0110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bourn D, Reid R, Rogers D, Snow B, Wint W. 2001. Environmental change and the autonomous control of tsetse and trypanosomosis in sub-Saharan Africa: case histories from Ethiopia, The Gambia, Kenya, Nigeria and Zimbabwe. Oxford, UK: Environmental Research Group Oxford (ERGO). [Google Scholar]
- 114.Reid RS, Kruska RL, Deichmann U, Thornton PK, Leak SGA. 2000. Human population growth and the extinction of the tsetse fly. Agric. Ecosyst. Environ. 77, 227–236. ( 10.1016/S0167-8809(99)00103-6) [DOI] [Google Scholar]
- 115.Murray KA, Preston N, Allen T, Zambrana-Torrelio C, Hosseini PR, Daszak P. 2015. Global biogeography of human infectious diseases. Proc. Natl Acad. Sci. USA 112, 12 746–12 751. ( 10.1073/pnas.1507442112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dunn RR. 2010. Global mapping of ecosystem disservices: the unspoken reality that nature sometimes kills us. Biotropica 42, 555–557. ( 10.1111/j.1744-7429.2010.00698.x) [DOI] [Google Scholar]
- 117.Allan BF, Keesing F, Ostfeld RS. 2003. Effect of forest fragmentation on Lyme disease risk. Conserv. Biol. 17, 267–272. ( 10.1046/j.1523-1739.2003.01260.x) [DOI] [Google Scholar]
- 118.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. 2003. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl Acad. Sci. USA 100, 567–571. ( 10.1073/pnas.0233733100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Keesing F, et al. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652. ( 10.1038/nature09575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Civitello DJ, et al. 2015. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl Acad. Sci. USA 112, 8667–8671. ( 10.1073/pnas.1506279112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang ZYX, de Boer WF, van Langevelde F, Olson V, Blackburn TM, Prins HHT. 2013. Species’ life-history traits explain interspecific variation in reservoir competence: a possible mechanism underlying the dilution effect. PLoS ONE 8, e54341 ( 10.1371/journal.pone.0054341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Randolph SE, Dobson ADM. 2012. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology 139, 847–863. ( 10.1017/S0031182012000200) [DOI] [PubMed] [Google Scholar]
- 123.Salkeld DJ, Padgett KA, Jones JH. 2013. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol. Lett. 16, 679–686. ( 10.1111/ele.12101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Young H, Griffin RH, Wood CL, Nunn CL. 2013. Does habitat disturbance increase infectious disease risk for primates? Ecol. Lett. 16, 656–663. ( 10.1111/ele.12094) [DOI] [PubMed] [Google Scholar]
- 125.Wood CL, Lafferty KD, DeLeo G, Young HS, Hudson PJ, Kuris AM. 2014. Does biodiversity protect humans against infectious disease? Ecology 95, 817–832. ( 10.1890/13-1041.1) [DOI] [PubMed] [Google Scholar]
- 126.Pongsiri MJ, Roman J, Ezenwa VO, Goldberg TL, Koren HS, Newbold SC, Ostfeld RS, Pattanayak SK, Salkeld DJ. 2009. Biodiversity loss affects global disease ecology. BioScience 59, 945–954. ( 10.1525/bio.2009.59.11.6) [DOI] [Google Scholar]
- 127.Lafferty KD, Wood CL. 2013. It's a myth that protection against disease is a strong and general service of biodiversity conservation: response to Ostfeld and Keesing. Trends Ecol. Evol. 28, 503–504. ( 10.1016/j.tree.2013.06.012) [DOI] [PubMed] [Google Scholar]
- 128.Ursell LK, Metcalf JL, Parfrey LW, Knight R. 2012. Defining the human microbiome. Nutr. Rev. 70(Suppl 1), S38–S44. ( 10.1111/j.1753-4887.2012.00493.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cebra JJ. 1999. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 69, 1046s–1051s. [DOI] [PubMed] [Google Scholar]
- 130.Round JL, Mazmanian SK. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323. ( 10.1038/nri2515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Beier MS, Pumpuni CB, Beier JC, Davis JR. 1994. Effects of para-aminobenzoic acid, insulin, and gentamicin on Plasmodium falciparum development in anopheline mosquitoes (Diptera: Culicidae). J. Med. Entomol. 31, 561–565. ( 10.1093/jmedent/31.4.561) [DOI] [PubMed] [Google Scholar]
- 132.Dong Y, Manfredini F, Dimopoulos G. 2009. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 5, e1000423 ( 10.1371/journal.ppat.1000423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gendrin M, Rodgers FH, Yerbanga RS, Ouédraogo JB, Basáñez M-G, Cohuet A, Christophides GK. 2015. Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nat. Commun. 6, 5921 ( 10.1038/ncomms6921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brown HW. 1952. The use of antibiotics in the treatment of helminthic infections. Ann. N Y Acad. Sci. 55, 1133–1138. ( 10.1111/j.1749-6632.1952.tb22676.x) [DOI] [PubMed] [Google Scholar]
- 135.Hayes KS, Bancroft AJ, Goldrick M, Portsmouth C, Roberts IS, Grencis RK. 2010. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science 328, 1391–1394. ( 10.1126/science.1187703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weinstein P, Newton W, Sawyer T, Sommerville R. 1969. Nematospiroides dubius: development and passage in the germfree mouse, and a comparative study of the free-living stages in germfree feces and conventional cultures. Trans. Am. Microsc. Soc. 88, 95–117. ( 10.2307/3224664) [DOI] [PubMed] [Google Scholar]
- 137.Marcogliese DJ, Cone DK. 1997. Food webs: a plea for parasites. Trends Ecol. Evol. 12, 320–325. ( 10.1016/S0169-5347(97)01080-X) [DOI] [PubMed] [Google Scholar]
- 138.Lafferty KD, Dobson AP, Kuris AM. 2006. Parasites dominate food web links. Proc. Natl Acad. Sci. USA 103, 11 211–11 216. ( 10.1073/pnas.0604755103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Johnson PTJ, Dobson A, Lafferty KD, Marcogliese DJ, Memmott J, Orlofske SA, Poulin R, Thieltges DW. 2010. When parasites become prey: ecological and epidemiological significance of eating parasites. Trends Ecol. Evol. 25, 362–371. ( 10.1016/j.tree.2010.01.005) [DOI] [PubMed] [Google Scholar]
- 140.Hatcher MJ, Dick JTA, Dunn AM. 2006. How parasites affect interactions between competitors and predators. Ecol. Lett. 9, 1253–1271. ( 10.1111/j.1461-0248.2006.00964.x) [DOI] [PubMed] [Google Scholar]
- 141.Thieltges DW, Jensen KT, Poulin R. 2008. The role of biotic factors in the transmission of free-living endohelminth stages. Parasitology 135, 407–426. ( 10.1017/S0031182007000248) [DOI] [PubMed] [Google Scholar]
- 142.Tompkins DM, White AR, Boots M. 2003. Ecological replacement of native red squirrels by invasive greys driven by disease. Ecol. Lett. 6, 189–196. ( 10.1046/j.1461-0248.2003.00417.x) [DOI] [Google Scholar]
- 143.Unestam T, Weiss DW. 1970. The host–parasite relationship between freshwater crayfish and the crayfish disease fungus Aphanomyces astaci: responses to infection by a susceptible and a resistant species. J. Gen. Microbiol. 60, 77–90. ( 10.1099/00221287-60-1-77) [DOI] [PubMed] [Google Scholar]
- 144.Dunn AM. 2009. Parasites and biological invasions. Adv. Parasitol. 68, 161–184. ( 10.1016/S0065-308X(08)00607-6) [DOI] [PubMed] [Google Scholar]
- 145.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M. 2010. Species interactions in a parasite community drive infection risk in a wildlife population. Science 330, 243–246. ( 10.1126/science.1190333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lello J, Boag B, Fenton A, Stevenson IR, Hudson PJ. 2004. Competition and mutualism among the gut helminths of a mammalian host. Nature 428, 840–844. ( 10.1038/nature02490) [DOI] [PubMed] [Google Scholar]
- 147.Kaunisto S, Härkönen L, Rantala MJ, Kortet R. 2015. Early-life temperature modifies adult encapsulation response in an invasive ectoparasite. Parasitology 142, 1290–1296. ( 10.1017/S0031182015000591) [DOI] [PubMed] [Google Scholar]
- 148.Skelton J, Doak S, Leonard M, Creed RP, Brown BL. 2016. The rules for symbiont community assembly change along a mutualism–parasitism continuum. J. Anim. Ecol. 85, 843–853. ( 10.1111/1365-2656.12498) [DOI] [PubMed] [Google Scholar]
- 149.Waltz E. 2016. US reviews plan to infect mosquitoes with bacteria to stop disease. Nature 533, 450–451. ( 10.1038/533450a) [DOI] [PubMed] [Google Scholar]
- 150.Folt C, Chen C, Moore M, Burnaford J. 1999. Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877. ( 10.4319/lo.1999.44.3_part_2.0864) [DOI] [Google Scholar]
- 151.Nõges P, Argillier C, Borja Á, Garmendia JM, Hanganu J, Kodeš V, Pletterbauer F, Sagouis A, Birk S. 2016. Quantified biotic and abiotic responses to multiple stress in freshwater, marine and ground waters. Sci. Total Environ. 540, 43–52. ( 10.1016/j.scitotenv.2015.06.045) [DOI] [PubMed] [Google Scholar]
- 152.Prado P, Roque A, Pérez J, Ibáñez C, Alcaraz C, Casals F, Caiola N. 2016. Warming and acidification-mediated resilience to bacterial infection determine mortality of early Ostrea edulis life stages. Mar. Ecol. Prog. Ser. 545, 189–202. ( 10.3354/meps11618) [DOI] [Google Scholar]
- 153.Anthony KRN, Connolly SR, Hoegh-Guldberg O. 2007. Bleaching, energetics, and coral mortality risk: effects of temperature, light, and sediment regime. Limnol. Oceanogr. 52, 716–726. ( 10.4319/lo.2007.52.2.0716) [DOI] [Google Scholar]
- 154.Burge CA, et al. 2014. Climate change influences on marine infectious diseases: implications for management and society. Annu. Rev. Mar. Sci. 6, 249–277. ( 10.1146/annurev-marine-010213-135029) [DOI] [PubMed] [Google Scholar]
- 155.Patz JA, Graczyk TK, Geller N, Vittor AY. 2000. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 30, 1395–1405. ( 10.1016/S0020-7519(00)00141-7) [DOI] [PubMed] [Google Scholar]
- 156.Hudson PJ, Dobson AP, Lafferty KD. 2006. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 21, 381–385. ( 10.1016/j.tree.2006.04.007) [DOI] [PubMed] [Google Scholar]
- 157.Morand S, Robert F, Connors VA. 1995. Complexity in parasite life cycles: population biology of cestodes in fish. J. Anim. Ecol. 64, 256–264. ( 10.2307/5760) [DOI] [Google Scholar]
- 158.Stearns SC. 1977. The evolution of life history traits: a critique of the theory and a review of the data. Annu. Rev. Ecol. Syst. 8, 145–171. ( 10.1146/annurev.es.08.110177.001045) [DOI] [Google Scholar]
- 159.Pianka E. 2008. r and K selection and evolution of populations. In Encyclopedia of ecology (ed. Jørgensen SE.), pp. 3113–3122. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 160.Lõhmus M, Björklund M. 2015. Climate change: what will it do to fish–parasite interactions? Biol. J. Linn. Soc. 116, 397–411. ( 10.1111/bij.12584) [DOI] [Google Scholar]
- 161.Poulin R, Cribb TH. 2002. Trematode life cycles: short is sweet? Trends Parasitol. 18, 176–183. ( 10.1016/S1471-4922(02)02262-6) [DOI] [PubMed] [Google Scholar]
- 162.Herrmann KK, Poulin R. 2011. Life cycle truncation in a trematode: does higher temperature indicate shorter host longevity? Int. J. Parasitol. 41, 697–704. ( 10.1016/j.ijpara.2011.01.009) [DOI] [PubMed] [Google Scholar]
- 163.Loos-Frank B. 1969. Zur Kenntnis der gymnophalliden Trematoden des Nordseeraumes. I. Die Alternativ-Zyklen von Gymnophallus choledochus Odhner, 1900. Z. Für Parasitenkd. 32, 135–156. ( 10.1007/BF00259976) [DOI] [PubMed] [Google Scholar]
- 164.Barker SC, Cribb TH. 1993. Sporocysts of Mesostephanus haliasturis (Digenea) produce miracidia. Int. J. Parasitol. 23, 137–139. ( 10.1016/0020-7519(93)90107-A) [DOI] [Google Scholar]
- 165.Cable J, Harris PD. 2002. Gyrodactylid developmental biology: historical review, current status and future trends. Int. J. Parasitol. 32, 255–280. ( 10.1016/S0020-7519(01)00330-7) [DOI] [PubMed] [Google Scholar]
- 166.Kennedy C. 1994. The ecology of introductions. In Parasitic diseases of fish (eds Pike A, Lewis J), pp. 189–208. Dyfed, Wales: Samara Publications Ltd., in association with the British Society for Parasitology and the Linnean Society of London. [Google Scholar]
- 167.Gallien L. 1935. Recherches expérimentales sur le dimorphisme évolutif et la biologie de ‘Polystomum integerrimum’ Fröl. Laboratoire d’évolution des êtres organisés; Les Presses universitaires de France.
- 168.Williams JB. 1961. The dimorphism of Polystoma integerrimum (Frölich) Rudolphi and its bearing on relationships within the Polystomatidae. III. J. Helminthol. 35, 181–202. ( 10.1017/S0022149X00024895) [DOI] [PubMed] [Google Scholar]
- 169.Badets M, Verneau O. 2009. Origin and evolution of alternative developmental strategies in amphibious sarcopterygian parasites (Platyhelminthes, Monogenea, Polystomatidae). Org. Divers. Evol. 9, 155–164. ( 10.1016/j.ode.2009.02.003) [DOI] [Google Scholar]
- 170.Badets M, Morrison C, Verneau O. 2010. Alternative parasite development in transmission strategies: how time flies! J. Evol. Biol. 23, 2151–2162. ( 10.1111/j.1420-9101.2010.02078.x) [DOI] [PubMed] [Google Scholar]
- 171.Traversa D. 2012. Pet roundworms and hookworms: a continuing need for global worming. Parasites Vectors 5, 91 ( 10.1186/1756-3305-5-91) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Macpherson CNL. 2005. Human behaviour and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 35, 1319–1331. ( 10.1016/j.ijpara.2005.06.004) [DOI] [PubMed] [Google Scholar]
- 173.Morgan ER, Clare EL, Jefferies R, Stevens JR. 2012. Parasite epidemiology in a changing world: can molecular phylogeography help us tell the wood from the trees? Parasitology 139, 1924–1938. ( 10.1017/S0031182012001060) [DOI] [PubMed] [Google Scholar]
- 174.Howe AD, Forster S, Morton S, Marshall R, Osborn KS, Wright P, Hunter PR. 2002. Cryptosporidium oocysts in a water supply associated with a cryptosporidiosis outbreak. Emerg. Infect. Dis. 8, 619–624. ( 10.3201/eid0806.010271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Johnson KP, Adams RJ, Page RDM, Clayton DH. 2003. When do parasites fail to speciate in response to host speciation? Syst. Biol. 52, 37–47. ( 10.1080/10635150390132704) [DOI] [PubMed] [Google Scholar]
- 176.Rafter P, Marucci G, Brangan P, Pozio E. 2005. Rediscovery of Trichinella spiralis in red foxes (Vulpes vulpes) in Ireland after 30 years of oblivion. J. Infect. 50, 61–65. ( 10.1016/j.jinf.2004.02.004) [DOI] [PubMed] [Google Scholar]
- 177.Rosenthal BM, LaRosa G, Zarlenga D, Dunams D, Chunyu Y, Mingyuan L, Pozio E. 2008. Human dispersal of Trichinella spiralis in domesticated pigs. Infect. Genet. Evol. 8, 799–805. ( 10.1016/j.meegid.2008.07.008) [DOI] [PubMed] [Google Scholar]
- 178.Zimmer IA, et al. 2009. Report of Trichinella spiralis in a red fox (Vulpes vulpes) in Northern Ireland. Vet. Parasitol. 159, 300–303. ( 10.1016/j.vetpar.2008.10.066) [DOI] [PubMed] [Google Scholar]
- 179.Gems D. 2000. Longevity and ageing in parasitic and free-living nematodes. Biogerontology 1, 289–307. ( 10.1023/A:1026546719091) [DOI] [PubMed] [Google Scholar]
- 180.Comfort A. 1955. Longevity in a tapeworm? Lancet 2, 346. [Google Scholar]
- 181.Loker ES. 1983. A comparative study of the life-histories of mammalian schistosomes. Parasitology 87, 343–369. ( 10.1017/S0031182000052689) [DOI] [PubMed] [Google Scholar]
- 182.Fulford AJC, Butterworth AE, Ouma JH, Sturrock RF. 1995. A statistical approach to schistosome population dynamics and estimation of the life-span of Schistosoma mansoni in man. Parasitology 110, 307–316. ( 10.1017/S0031182000080896) [DOI] [PubMed] [Google Scholar]
- 183.Cable J, Harris PD, Bakke TA. 2000. Population growth of Gyrodactylus salaris (Monogenea) on Norwegian and Baltic Atlantic salmon (Salmo salar) stocks. Parasitology 121, 621–629. ( 10.1017/S0031182000006971) [DOI] [PubMed] [Google Scholar]
- 184.Paul AA. 1938. Life history studies of North American fresh-water polystomes. J. Parasitol. 24, 489–510. ( 10.2307/3272275) [DOI] [Google Scholar]
- 185.Cable J, Tinsley RC. 1991. Intra-uterine larval development of the polystomatid monogeneans, Pseudodiplorchis americanus and Neodiplorchis scaphiopodis. Parasitology 103, 253–266. ( 10.1017/S0031182000059539) [DOI] [PubMed] [Google Scholar]
- 186.Saino N, Rubolini D, Lehikoinen E, Sokolov LV, Bonisoli-Alquati A, Ambrosini R, Boncoraglio G, Møller AP. 2009. Climate change effects on migration phenology may mismatch brood parasitic cuckoos and their hosts. Biol. Lett. 5, 539–541. ( 10.1098/rsbl.2009.0312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Platt TR, Brooks DR. 1997. Evolution of the schistosomes (Digenea: Schistosomatoidea): the origin of dioecy and colonization of the venous system. J. Parasitol. 83, 1035–1044. ( 10.2307/3284358) [DOI] [PubMed] [Google Scholar]
- 188.Hirai H. 2014. Chromosomal differentiation of schistosomes: what is the message? Front. Genet. 5, 301 ( 10.3389/fgene.2014.00301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Basch PF. 1990. Why do schistosomes have separate sexes? Parasitol. Today 6, 160–163. ( 10.1016/0169-4758(90)90339-6) [DOI] [PubMed] [Google Scholar]
- 190.Loker ES, Brant SV. 2006. Diversification, dioecy and dimorphism in schistosomes. Trends Parasitol. 22, 521–528. ( 10.1016/j.pt.2006.09.001) [DOI] [PubMed] [Google Scholar]
- 191.Webster JP, Borlase A, Rudge JW. 2017. Who acquires infection from whom and how? Disentangling multi-host and multi-mode transmission dynamics in the ‘elimination’ era. Phil. Trans. R. Soc. B 372, 20160091 ( 10.1098/rstb.2016.0091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Guerrant RL. 1997. Cryptosporidiosis: an emerging, highly infectious threat. Emerg. Infect. Dis. 3, 51–57. ( 10.3201/eid0301.970106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thompson RA, Wellington de Oliveira Lima J, Maguire JH, Braud DH, Scholl DT. 2002. Climatic and demographic determinants of American visceral leishmaniasis in northeastern Brazil using remote sensing technology for environmental categorization of rain and region influences on leishmaniasis. Am. J. Trop. Med. Hyg. 67, 648–655. [DOI] [PubMed] [Google Scholar]
- 194.Hakalahti T, Karvonen A, Valtonen ET. 2006. Climate warming and disease risks in temperate regions--Argulus coregoni and Diplostomum spathaceum as case studies. J. Helminthol. 80, 93–98. ( 10.1079/JOH2006351) [DOI] [PubMed] [Google Scholar]
- 195.Phillips BL, Brown GP, Shine R. 2010. Life-history evolution in range-shifting populations. Ecology 91, 1617–1627. ( 10.1890/09-0910.1) [DOI] [PubMed] [Google Scholar]
- 196.Lafferty KD, Kuris AM. 1999. How environmental stress affects the impacts of parasites. Limnol. Oceanogr. 44, 925–931. ( 10.4319/lo.1999.44.3_part_2.0925) [DOI] [Google Scholar]
- 197.Morran LT, Schmidt OG, Gelarden IA, Parrish RC II, Lively CM. 2011. Running with the Red Queen: host–parasite coevolution selects for biparental sex. Science 333, 216–218. ( 10.1126/science.1206360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Poulin R. 1998. Evolutionary ecology of parasites: from individuals to communities. London, UK: Chapman & Hall. [Google Scholar]
- 199.Skorping A, Read AF. 1998. Drugs and parasites: global experiments in life history evolution? Ecol. Lett. 1, 10–12. ( 10.1046/j.1461-0248.1998.0007d.x) [DOI] [Google Scholar]
- 200.Moss R, Watson A, Trenholm IB, Parr R. 1993. Caecal threadworms Trichostrongylus tenuis in red grouse Lagopus lagopus scoticus: effects of weather and host density upon estimated worm burdens. Parasitology 107, 199–209. ( 10.1017/S0031182000067317) [DOI] [PubMed] [Google Scholar]
- 201.Pascual M, Rodó X, Ellner SP, Colwell R, Bouma MJ. 2000. Cholera dynamics and El Nino–Southern Oscillation. Science 289, 1766–1769. ( 10.1126/science.289.5485.1766) [DOI] [PubMed] [Google Scholar]
- 202.Stenseth NC, et al. 2006. Plague dynamics are driven by climate variation. Proc. Natl Acad. Sci. USA 103, 13 110–13 115. ( 10.1073/pnas.0602447103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cornet S, Nicot A, Rivero A, Gandon S. 2014. Evolution of plastic transmission strategies in avian malaria. PLoS Pathog. 10, e1004308 ( 10.1371/journal.ppat.1004308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hominick WM. 1982. Selection of a rapidly maturing population of Globodera rostochiensis by continuous cultivation of early potatoes in Ayrshire, Scotland. Ann. Appl. Biol. 100, 345–351. ( 10.1111/j.1744-7348.1982.tb01948.x) [DOI] [Google Scholar]
- 205.Chambers HF. 2001. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7, 178–182. ( 10.3201/eid0702.700178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hyde JE. 2005. Drug-resistant malaria. Trends Parasitol. 21, 494–498. ( 10.1016/j.pt.2005.08.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Waller PJ. 1997. Anthelmintic resistance. Vet. Parasitol. 72, 391–412. ( 10.1016/S0304-4017(97)00107-6) [DOI] [PubMed] [Google Scholar]
- 208.Treasurer JW, Wadsworth S, Grant A. 2000. Resistance of sea lice, Lepeophtheirus salmonis (Krøyer), to hydrogen peroxide on farmed Atlantic salmon, Salmo salar L. Aquac. Res. 31, 855–860. ( 10.1046/j.1365-2109.2000.00517.x) [DOI] [Google Scholar]
- 209.Fallang A, Ramsay JM, Sevatdal S, Burka JF, Jewess P, Hammell KL, Horsberg TE. 2004. Evidence for occurrence of an organophosphate-resistant type of acetylcholinesterase in strains of sea lice (Lepeophtheirus salmonis Krøyer). Pest Manag. Sci. 60, 1163–1170. ( 10.1002/ps.932) [DOI] [PubMed] [Google Scholar]
- 210.Boots M, Sasaki A. 1999. ‘Small worlds’ and the evolution of virulence: infection occurs locally and at a distance. Phil. Trans. R. Soc. Lond. B 266, 1933–1938. ( 10.1098/rspb.1999.0869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Boots M, Mealor M. 2007. Local interactions select for lower pathogen infectivity. Science 315, 1284–1286. ( 10.1126/science.1137126) [DOI] [PubMed] [Google Scholar]
- 212.Berngruber TW, Lion S, Gandon S. 2015. Spatial structure, transmission modes and the evolution of viral exploitation strategies. PLoS Pathog. 11, e1004810 ( 10.1371/journal.ppat.1004810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pulkkinen K, Suomalainen L-R, Read AF, Ebert D, Rintamäki P, Valtonen ET. 2010. Intensive fish farming and the evolution of pathogen virulence: the case of columnaris disease in Finland. Proc. R. Soc. B 277, 593–600. ( 10.1098/rspb.2009.1659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fenton A, Hudson PJ. 2002. Optimal infection strategies: should macroparasites hedge their bets? Oikos 96, 92–101. ( 10.1034/j.1600-0706.2002.960110.x) [DOI] [Google Scholar]
- 215.Thomas RJ, Stevens AJ. 1960. Ecological studies on the development of the pasture stages of Nematodirus battus and N. filicollis, Nematode parasites of sheep. Parasitology 50, 31–49. ( 10.1017/S0031182000025178) [DOI] [PubMed] [Google Scholar]
- 216.Boag B, Thomas RJ. 1975. Epidemiological studies on Nematodirus species in sheep. Res. Vet. Sci. 19, 263–268. [PubMed] [Google Scholar]
- 217.Thomas DR. 1991. The epidemiology of Nematodirus battus—is it changing? Parasitology 102, 147–155. ( 10.1017/S0031182000060467) [DOI] [PubMed] [Google Scholar]
- 218.Gethings OJ, Rose H, Mitchell S, Van Dijk J, Morgan ER. 2015. Asynchrony in host and parasite phenology may decrease disease risk in livestock under climate warming: Nematodirus battus in lambs as a case study. Parasitology 142, 1306–1317. ( 10.1017/S0031182015000633) [DOI] [PubMed] [Google Scholar]
- 219.Pasternak AF, Mikheev VN, Valtonen ET. 2000. Life history characteristics of Argulus foliaceus L. (Crustacea: Branchiura) populations in Central Finland. Ann. Zool. Fenn. 37, 25–35. [Google Scholar]
- 220.Harrison E, Laine A-L, Hietala M, Brockhurst MA. 2013. Rapidly fluctuating environments constrain coevolutionary arms races by impeding selective sweeps. Proc. R. Soc. B 280, 20130937 ( 10.1098/rspb.2013.0937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hoberg EP, Brooks DR. 2008. A macroevolutionary mosaic: episodic host-switching, geographical colonization and diversification in complex host–parasite systems. J. Biogeogr. 35, 1533–1550. ( 10.1111/j.1365-2699.2008.01951.x) [DOI] [Google Scholar]
- 222.Hoberg EP, Brooks DR. 2015. Evolution in action: climate change, biodiversity dynamics and emerging infectious disease. Phil. Trans. R. Soc. B 370, 20130553 ( 10.1098/rstb.2013.0553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jones TS, Bilton AR, Mak L, Sait SM. 2015. Host switching in a generalist parasitoid: contrasting transient and transgenerational costs associated with novel and original host species. Ecol. Evol. 5, 459–465. ( 10.1002/ece3.1333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Callahan K, Bolton B, Hopkins DR, Ruiz-Tiben E, Withers PC, Meagley K. 2013. Contributions of the Guinea worm disease eradication campaign toward achievement of the millennium development goals. PLoS Negl. Trop. Dis. 7, e2160 ( 10.1371/journal.pntd.0002160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Callaway E. 2016. Dogs thwart effort to eradicate Guinea worm. Nature 529, 10–11. ( 10.1038/529010a) [DOI] [PubMed] [Google Scholar]
- 226.Tompkins DM, Sainsbury AW, Nettleton P, Buxton D, Gurnell J. 2002. Parapoxvirus causes a deleterious disease in red squirrels associated with UK population declines. Proc. R. Soc. Lond. B 269, 529–533. ( 10.1098/rspb.2001.1897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rushton SP, Lurz PWW, Gurnell J, Nettleton P, Bruemmer C, Shirley MDF, Sainsbury AW. 2006. Disease threats posed by alien species: the role of a poxvirus in the decline of the native red squirrel in Britain. Epidemiol. Infect. 134, 521–533. ( 10.1017/S0950268805005303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.James J, Nutbeam-Tuffs S, Cable J, Petrusek A, Mrugała A, Viñuela-Rodriguez Noba, Petrusek A, Oidtmann B. 2017. The prevalence of Aphanomyces astaci in invasive signal crayfish from the UK and implications for native crayfish conservation. Parasitology 12 ( 10.1017/S0031182016002419) [DOI] [PubMed] [Google Scholar]
- 229.Lymbery AJ, Morine M, Kanani HG, Beatty SJ, Morgan DL. 2014. Co-invaders: the effects of alien parasites on native hosts. Int. J. Parasitol. Parasites Wildl. 3, 171–177. ( 10.1016/j.ijppaw.2014.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Grégoir AF, Hablützel PI, Vanhove MPM, Pariselle A, Bamps J, Volckaert FAM, Raeymaekers JAM. 2015. A link between host dispersal and parasite diversity in two sympatric cichlids of Lake Tanganyika. Freshw. Biol. 60, 323–335. ( 10.1111/fwb.12492) [DOI] [Google Scholar]
- 231.Henrich T, Benesh DP, Kalbe M. 2013. Hybridization between two cestode species and its consequences for intermediate host range. Parasit. Vectors 6, 33 ( 10.1186/1756-3305-6-33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sherrard-Smith E, Perkins SE, Chadwick EA, Cable J. 2015. Spatial and seasonal factors are key determinants in the aggregation of helminths in their definitive hosts: Pseudamphistomum truncatum in otters (Lutra lutra). Int. J. Parasitol. 45, 75–83. ( 10.1016/j.ijpara.2014.09.004) [DOI] [PubMed] [Google Scholar]
- 233.Craig MH, Snow RW, le Sueur D. 1999. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol. Today 15, 105–111. ( 10.1016/S0169-4758(99)01396-4) [DOI] [PubMed] [Google Scholar]
- 234.Heukelbach J, Jackson A, Ariza L, Feldmeier H. 2008. Prevalence and risk factors of hookworm-related cutaneous larva migrans in a rural community in Brazil. Ann. Trop. Med. Parasitol. 102, 53–61. ( 10.1179/136485908X252205) [DOI] [PubMed] [Google Scholar]
- 235.Masih I, Maskey S, Mussá FEF, Trambauer P. 2014. A review of droughts on the African continent: a geospatial and long-term perspective. Hydrol. Earth Syst. Sci. 18, 3635–3649. ( 10.5194/hess-18-3635-2014) [DOI] [Google Scholar]
- 236.Gordon G, O'Callaghan M, Tallis GM. 1970. A deterministic model for the life cycle of a class of internal parasites of sheep. Math. Biosci. 8, 209–226. ( 10.1016/0025-5564(70)90151-3) [DOI] [Google Scholar]
- 237.Gandon S, Mackinnon MJ, Nee S, Read AF. 2001. Imperfect vaccines and the evolution of pathogen virulence. Nature 414, 751–756. ( 10.1038/414751a) [DOI] [PubMed] [Google Scholar]
- 238.Barclay VC, Sim D, Chan BHK, Nell LA, Rabaa MA, Bell AS, Anders RF, Read AF. 2012. The evolutionary consequences of blood-stage vaccination on the rodent malaria Plasmodium chabaudi. PLoS Biol. 10, e1001368 ( 10.1371/journal.pbio.1001368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Read AF, Baigent SJ, Powers C, Kgosana LB, Blackwell L, Smith LP, Kennedy DA, Walkden-Brown SW, Nair VK. 2015. Imperfect vaccination can enhance the transmission of highly virulent pathogens. PLoS Biol. 13, e1002198 ( 10.1371/journal.pbio.1002198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Frank SA. 1996. Models of parasite virulence. Q. Rev. Biol. 71, 37–78. ( 10.1086/419267) [DOI] [PubMed] [Google Scholar]
- 241.Molento MB, Nielsen MK, Kaplan RM. 2012. Resistance to avermectin/milbemycin anthelmintics in equine cyathostomins—current situation. Vet. Parasitol. 185, 16–24. ( 10.1016/j.vetpar.2011.10.013) [DOI] [PubMed] [Google Scholar]
- 242.Day T, Read AF. 2016. Does high-dose antimicrobial chemotherapy prevent the evolution of resistance? PLOS Comput. Biol. 12, e1004689 ( 10.1371/journal.pcbi.1004689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bousema T, et al. 2010. Identification of hot spots of malaria transmission for targeted malaria control. J. Infect. Dis. 201, 1764–1774. ( 10.1086/652456) [DOI] [PubMed] [Google Scholar]
- 244.Parker M, Allen T. 2011. Does mass drug administration for the integrated treatment of neglected tropical diseases really work? Assessing evidence for the control of schistosomiasis and soil-transmitted helminths in Uganda. Health Res. Policy Syst. 9, 3 ( 10.1186/1478-4505-9-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.McMichael AJ. 1999. Prisoners of the proximate: loosening the constraints on epidemiology in an age of change. Am. J. Epidemiol. 149, 887–897. ( 10.1093/oxfordjournals.aje.a009732) [DOI] [PubMed] [Google Scholar]
- 246.Pautasso M, Aas G, Queloz V, Holdenrieder O. 2013. European ash (Fraxinus excelsior) dieback—a conservation biology challenge. Biol. Conserv. 158, 37–49. ( 10.1016/j.biocon.2012.08.026) [DOI] [Google Scholar]
- 247.Madder M, Walker JG, Van Rooyen J, Knobel D, Vandamme E, Berkvens D, Vanwambeke SO, De Clercq EM. 2012. e-Surveillance in animal health: use and evaluation of mobile tools. Parasitology 139, 1831–1842. ( 10.1017/S0031182012000571) [DOI] [PubMed] [Google Scholar]
- 248.Mellor S.2013. Moving towards malaria elimination: developing innovative tools for malaria surveillance in Cambodia. See www.malariaconsortium.org/pages/learning-papers.htm .
- 249.van-Dijk A, Aramini J, Edge G, Moore KM. 2009. Real-time surveillance for respiratory disease outbreaks, Ontario, Canada. Emerg. Infect. Dis. 15, 799–801. ( 10.3201/eid1505.081174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Holling CS. 1973. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 4, 1–23. ( 10.1146/annurev.es.04.110173.000245) [DOI] [Google Scholar]
- 251.Ford TE, Colwell RR, Rose JB, Morse SS, Rogers DJ, Yates TL. 2009. Using satellite images of environmental changes to predict infectious disease outbreaks. Emerg. Infect. Dis. 15, 1341–1346. ( 10.3201/eid1509.081334) [DOI] [PMC free article] [PubMed] [Google Scholar]