Abstract
Network construction and reorganization is modulated by the level and pattern of synaptic activity generated in the nervous system. During the past decades, neurotrophins, and in particular brain-derived neurotrophic factor (BDNF), have emerged as attractive candidates for linking synaptic activity and brain plasticity. Thus, neurotrophin expression and secretion are under the control of activity-dependent mechanisms and, besides their classical role in supporting neuronal survival neurotrophins, modulate nearly all key steps of network construction from neuronal migration to experience-dependent refinement of local connections. In this paper, we provide an overview of recent findings showing that BDNF can serve as a target-derived messenger for activity-dependent synaptic plasticity and development at the single cell level.
Keywords: Action Potentials, Animals, Brain-Derived Neurotrophic Factor, Cell Communication, Dendrites, Synapses
Introduction
The discovery of the neurotrophins was prompted by the observation that many neurons depend on limited amounts of survival factors secreted by the target neurons. These target-derived messengers also provided a mean to refine initially coarse patterns of synaptic connections, thus contributing to the construction of neuronal networks. Subsequent studies have shown that the neurotrophins also modulate axonal and dendritic growth and remodeling, membrane receptor trafficking, neurotransmitter release, synapse formation, and function.
Key assumptions of the target-derived messengers’ model are that the neurotrophins are released from the postsynaptic targets in an activity dependent manner and act in an autocrine/paracrine manner to induce morphological or functional changes of pre- and postsynaptic elements. Four neurotrophins have been characterized in mammals: the nerve growth factor (NGF), the brain-derived neurotrophic factor (BDNF), the neurotrophin-3 (NT3), and the neurotrophic-4 (NT4). In this paper, we provide an overview of recent findings showing that BDNF fulfills these criteria in the mammalian central nervous system and can be qualified as a target-derived messenger for activity-dependent synaptic plasticity and development at the single-cell level. For further information on the biological effects of neurotrophin and underlying molecular mechanisms, interested readers are refereed to excellent reviews on these topics [1–4].
BDNF Synthesis and Sorting
BDNF is widely expressed in most brain structures, being abundant in the hippocampus, amygdala, and cerebellum [5–10]. Cell type analysis shows that it is expressed mainly by the principal glutamatergic neurons, being absent in interneurons and in astroglial cells, although astroglial expression of BDNF can be found in cell cultures [6, 7, 11, 12]. Activated microglia can also express and release BDNF, e.g., in response to ATP-dependent stimulation of P(2X) receptors [13–15].
The BDNF gene in rodents is composed of nine exons: exon I to VIII posses their own promoter and are associated by splicing mechanism to the exon IX, which is the only one to be translated into a protein. The combination of these different splicing forms with two alternative poly-adenylation sites in the 3′ untranslated region can give rise to multiple different pre-mRNA that are expressed in a developmentally regulated and tissue-specific manner [10]. The level of BDNF mRNA transcription is positively regulated by neuronal activity [16–19]. It has been proposed that the increase in BDNF expression induced by physiological activity can act as trophic positive feedback signal supporting the increased synaptic activity [1]. The increase in transcripts observed during pathological conditions [20] may either be an aberrant mechanism contributing to the pathology, e.g., epilepsy [21], or could act as a protective mechanism linked to the trophic action of BDNF [1, 22, 23]. The pre sequence of the pre-mRNA directs the translation of the BDNF gene directly into the endoplasmic reticulum (for a review, see [1]). This process occurs mainly at the level of the cell body, although under particular circumstances, the BDNF synthesis can also take place in the dendrite after translocation of the pre-mRNA [19]. The translation gives rise to a dimeric pro-BDNF protein of about 29 kDa that is cleaved to give rise to mature BDNF [24]. The pro domain is involved in the sorting of BDNF into the appropriate pathway of secretion [25]. Two different pathways of BDNF secretion do exist in neurons (Fig. 2A) the regulated and the constitutive pathway. In the regulated pathway of secretion, BDNF is packaged in large diameter vesicles/granules (Ø ~300 nm) that fuse with the plasma membrane following an intracellular Ca2+ rise. In the constitutive pathway, BDNF is packaged in smaller granules (Ø 50–100 nm) and continuously released through a Ca2+-independent fusion process that occurs in the absence of any specific triggering event [1, 26]. The two pathways also differ in the neuronal site of BDNF release. Thus, constitutive secretion occurs mainly at the soma and proximal processes, while the regulated secretion is triggered in more distal neuronal processes [27]. The sorting of BDNF into either of the two pathways requires the processing of pro-BDNF in the Golgi and trans-Golgi network. Two separate amino acid motifs, localized, respectively, in the mature and the pro domain of the protein, are involved in this process. The interaction of the motif in the mature domain with the sorting receptor carboxipeptidase E (CPE) directly targets BDNF toward the regulated secretory pathway; on the other hand, the absence of this CPE interaction seems to determine the sorting of BDNF into the constitutive pathway [2]. The interaction of the pro domain motif with the protein sortilin is also required to target BDNF toward the regulated pathway of secretion [28, 29]. The importance of the BDNF pro domain for targeting to the regulated pathway is also evident from the efficient redirection of NT-4 to secretory granules if the BDNF pro domain is attached to NT-4 [27]. Although in the case of the pro-domain-dependent targeting the underlying mechanisms are not well understood, it has been suggested that binding of the pro motif to sortilin modifies the folding of pro-BDNF, thus allowing the interaction between CPE and the mature domain [2]. An important aspect of the pro domain–sortilin interaction is the fact that a single amino acid mutation in the pro-motif (a substitution of valine in position 66 to methionine), resulting from a rather prevalent naturally occurring single nucleotide polymorphism, is sufficient to prevent the interaction of pro-BDNF with sortilin, thus impairing efficient sorting of BDNF into the regulated pathway of secretion and causing functional and cognitive deficits [28]. The unfavorable consequences of the val66met polymorphism highlight therefore the importance of a correct BDNF secretion for brain functions [30, 31]. The secretory granules budding off from the trans-Golgi network are transported into neuronal processes, either axons [32] or dendrites [33]; at this point, they can contain the pro and/or the mature BDNF. Indeed the cleavage of the pro domain can be performed by the so-called pro-protein convertases present in the granules (for further detail, see [1]). In that case, the mature form of BDNF will be released. However, recent studies seem to suggest that pro-BDNF can be secreted as such and can be cleaved extracellularly by specific extracellular proteases ([2, 34, 35], but see [36]). Furthermore, the secreted pro-BDNF can directly interact with p75NTR receptors and induce biological effects that are discussed to be opposed to those of the mature form [2]. Although both pathways of secretion can potentially release the mature and the pro-BDNF, the pro-BDNF is more abundant in the constitutive pathway [37]. This is likely due to the fact that the delay between synthesis and release is shorter in the constitutive pathway, thus reducing the probability for the pro-BDNF to be cleaved by the intracellular convertases [2].
Fig. 2.
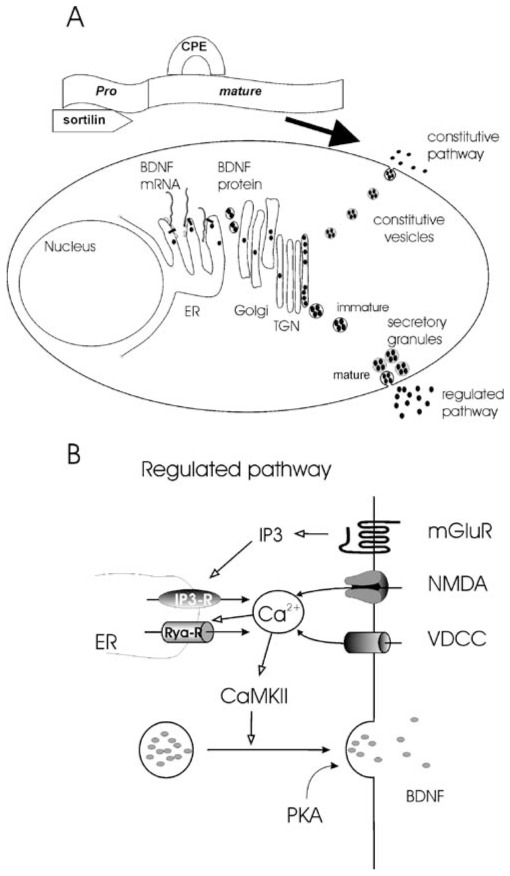
Synthesis, storage, and release of BDNF. a The pre-pro-BDNF is synthesized and sequestered in the endoplasmic reticulum (ER). Pro-BDNF then transits the Golgi apparatus and accumulates in membrane stacks of the trans-Golgi network. The trans-Golgi network can be thought of as a major protein sorting station inside the cell. At this point, two types of secretory pathways exist. Secretion via the constitutive pathway does not rely on extracellular signals or any triggering events. In the regulated pathway, fusion of the secretory granules with the plasma membrane is triggered by an intracellular rise in Ca2+. The binding of the pro form with sortiline and the mature form with carboxipeptidase E is required to address the pro-BDNF in the regulated pathway (modified from Fig. 2 in [1]; with permission. b Scheme of the signaling involved in postsynaptic regulated BDNF secretion. BDNF secretion required a postsynaptic rise in intracellular Ca2+ concentration. This Ca2+ rise can result from an influx through voltage-dependent Ca2+ channels (VDCCs) or NMDA receptors upon membrane depolarization or from activation of internal Ca2+ stores (IP3-R) following the activation of metabotropic glutamatergic receptors (mGluRs). The initial Ca2+ rise can be amplified by Ca2+-induced Ca2+ secretion via ryanodine receptors (Rya-Rs). Ca2+ activates CaMKII leading to the fusion of the secretory granules. Basal levels of PKA activation “gate” BDNF secretion
In summary, the regulation of BDNF synthesis and sorting can give rise to differences in the amount, type, and localization of secreted BDNF and determine the mechanisms of release.
Mechanisms of Regulated Dendritic BDNF Secretion
The mechanisms of neurotrophin release have been intensively studied over the past decade, employing mainly two experimental approaches (Fig. 1). The first approach relies on measuring the secreted protein using an immunochemical essay [enzyme-linked immunosorbent assay (ELISA) or Western blot) of the extracellular medium (bulk secretion; for references, see Table 1). This approach allows detecting and quantifying secretion of endogenous BDNF. However, while the bulk secretion approach is sensitive enough to detect the release of endogenous BDNF in brain slices, overexpression of BDNF is required to monitor BDNF secretion in neuronal cultures, which contain smaller numbers of neurons. Moreover, the bulk secretion approach lacks spatial information, i.e., the site of secretion, and temporal resolution. To bypass these limitations, a second approach consisting in the direct visualization of BDNF tagged with a green fluorescent protein (GFP) has been developed for neuronal cultures (for references, see Table 1). Secretion of BDNF-GFP can be directly visualized in living neurons as a decrease in intracellular fluorescence intensity. This method provides excellent spatial and temporal resolution to study the secretion of BDNF-GFP by time lapse fluorescent imaging. This approach, however, provides no information on endogenous BDNF secretion, although it has been shown that BDNF-GFP is processed and sorted in a similar way as the endogenous BDNF [33, 38, 39]. Quite recently, Nakajima et al. [40] developed a cell-based fluorescent indicator assay to visualize endogenous BDNF secretion from cultured neurons. This technique allows the detection of picomolar concentrations of BDNF with a good temporal resolution and could provide a powerful tool to study secretion of endogenous BDNF in living neurons with high resolution [40].
Fig. 1.

Different approaches to study BDNF secretion. a Released BDNF can be detected and quantified in the medium using immunochemical essays (ELISA or Western blot). b Release of BDNF can be visualized in living BDNF-GFP expressing neurons. Scale bar, 25 μm. c BDNF released from the neuron binds to TrkB receptors at the surface of the cell-based fluorescent indicator. The activated kinase domain of the TrkB receptor phosphorylates a chimeric fluorescent protein, leading to a conformational change allowing fluorescence resonance energy transfer (FRET). BDNF is therefore detected with fluorescence readout (adapted from [40])
Table 1.
Modality of BDNF release
| Authors | System | Secretion detected by (source; location of release | Secretion induced by | Secretion antagonized by | Secretion resistant to |
|---|---|---|---|---|---|
| Wetmore et al. 1994 [85] | Adult rats hippocampus | Immunohistochemistry (endogenous), unknown origin (likely somato-dendritic) | In vivo kainate injection | DNQX | |
| Goodman et al. 1996 [86] | At20 PC12, Hippocampal culture (E18) | Western blot (exogenous), unknown origin | KCl | Extracellular Ca2+-free solution | |
| Kruttgen et al. 1998 [87] | PC12 cells | ELISA (exogenous), unknown origin | KCl NT-3; NT-4/5; NGF | ||
| Griesbeck et al. 1999 [88] | Hippocampal culture (E17), Hippocampal slices adult | ELISA (endogenous and exogenous) Unknown origin | KCl | BAPTA-AM Thapsigargin/caffeine | |
| Gunther et al. 2000 [89] | Anterior pituitary At20 cell line | ELISA (endogenous and exogenous), unknown origin | KCl | Reduced by pertussis toxin | |
| Canossa et al. 2001 [90] | Hippocampal culture E17, cell line hippocampal slices adult | ELISA (exogenous) culture; (endogenous) slices, unknown origin | NGF t-ACPD (mGluR), AMPA But not: NMDA | intracellular BAPTA, caffeine thapsigargin | extracellular Ca2+-free solution |
| Gartner and Staiger 2001 [90] | Hippocampal culture E19, DIV 14-20 | ELISA (exogenous), unknown origin | Electrical stimulation, bicuculline (spontaneous bursts) | TTX, Thapsigargin/caffeine; 2ABP | |
| Hartmann et al. 2001 [33] | Hippocampal culture P0-P2, DIV 10-15 | Time lapse BDNF-GFP (exogenous), dendritic secretory granules | KCl Electrical stimulation | Tetanus toxin extracellular Ca2+-free solution Cd2+ Ni2+ (VDCC). DNQX/APV for electrical stimulation | DNQX, APV, LY 341495 (mGluR antagonist) |
| Kojima et al. 2001 [39] | Hippocampal culture E20 | Western blot; time lapse imaging BDNF-GFP (exogenous), dendritic secretory granules | KCl | TTX | |
| Balkowiec and Katz 2002 [91] | Hippocampal cultures P0, DIV 3 | ELISA (endogenous) | Electrical stimulation t-ACPD (mGluR) caffeine | TTX w-conotoxine (N-type); extracellular Ca2+-free solution Dantrolene and thapsigargin | NBQX, APV, Nimodipine (L-Type) |
| Canossa et al. 2002 [92] | Hippocampal cultures E17, DIV 5 | ELISA (exogenous), unknown origin | Spontaneous | Reduced by nitric oxide (activation of soluble guanylate cyclase increase cGMP, activation of PKG and reduction of Ca2+ release from IP3 stores) | |
| Egan et al. 2003 [28] | Hippocampal cultures E20 | ELISA BDNF-GFP (exogenous), Unknown origin | Spontaneous KCl | Val66Met substitution (KCl-induced) | |
| Aicardi et al. 2004 [79] | Cortical and hippocampal slices | ELISA (endogenous), Unknown origin | Spontaneous and electrical stimulation | ||
| Bridadski et al. 2005 [27] | Hippocampal P0-P2, DIV 10–15 | Time lapse BDNF-GFP (exogenous), dendritic secretion | KCl | ||
| Del Toro et al. 2006 [93] | Striate cell line | ELISA BDNF-GFP (exogenous), unknown origin | Spontaneous KCl | Mutation of Hungtintin gene Val66Met substitution (KCl induced) | |
| Santi et al. 2006 [94] | Hippocampal cultures E17 | ELISA (reuptake of exogenous BDNF), unknown origin | Glutamate, tACPD, AMPA caffeine NT4, NT3 electrical stimulation, KCl | TTX (the electrical induced) Reduced by nitric oxide | 0 extracellular Ca2+; reduced by intracellular BAPTA |
| Arancibia et al. 2007 [95] | Supra-optic nucleus in vivo | ELISA (endogenous), unknown origin | Osmotic pressure (1 M Nal) | ||
| Kolarow et al. 2007 [41] | Hippocampal culture P0-P2, DIV 10–15 | Time lapse BDNF-GFP (exogenous), dendritic secretory granules | KCl; NMDA But not: BDNF 8Br-cAMP | Nifedipine (L-type), Thapsigargin, Ryanodine, Rp-cAMPS (PKA antagonist), KN-62 and KN-93 (CaMKII antagonist) | Gabazine, DNQX, APV, TTX, K252a 8Br-cAMP |
| Kuczewski et al. 2008 [43] | Hippocampal culture P0, DIV 14–15 | Time lapse BDNF-GFP (exogenous), dendritic secretory granules | Spontaneous activity b-APs postsynaptic depolarization KCl | extracellular Ca2+-free solution, Cd2+ (VDCC). GDPβS (G-protein inhibitor), TTX, intracellular QX314 (Na+ blocker) Thapsigargin (KCl-induced) | NBQX/APV Thapsigargin (b-APs-induced) |
| Nakajima et al. 2008 [40] | Hippocampal culture E17 | Cell-based indicator (endogenous), unknown origin | Glutamate | TTX, CNQX/APV | |
| Bergami et al. 2008 [96] | Adult cortical slices | ELISA (endogenous), immunohistochemistry | Electrical stimulation | ||
| Kuczewski et al. 2008 [97] | Newborn rat hippocampal slice | ELISA | Spontaneous | Nifedipine (L-type) NBQX | D-AP5 Gabazine |
In spite of some differences regarding the mechanisms of BDNF secretion depending on the site investigated (i.e., axon or dendrites), the detecting method, and the stimulus used, the results obtained with these distinct assays agreed on the fact that regulated BDNF release requires an increase in intracellular Ca2+ concentration. Several sources can contribute to this Ca2+ rise. NMDA channels, voltage-dependent Ca2+ channels (VDCC), and intracellular Ca2+ stores can trigger a release of BDNF depending on the experimental conditions and the type of stimulation. Such heterogeneity in the Ca2+ source and stimuli leading to BDNF secretion (summarized in Table 1) suggest that multiple modalities of BDNF secretion can coexist. Since the goal of the present review was to summarize the observations showing that BDNF acts as a target-derived messenger, we will next focus on somato-dendritic secretion of BDNF.
Mechanistic studies of dendritic BDNF release were performed in cultured hippocampal neurons expressing GFP-tagged BDNF in response to KCl-induced depolarization [27, 33, 39, 41]. Under these circumstances, the dendritic release of BDNF requires a postsynaptic influx of Ca2+ (Fig. 2). The Ca2+ rise in turn activates the calcium calmodulin kinase II (CaMKII), leading to the fusion of BDNF containing secretory granules with the plasma membrane. Once the fusion pore is formed, BDNF is slowly released into the extracellular space (time constant≈200–300 s) [27, 33]. The slow release kinetics are partially attributable to a slow diffusion of BDNF through the pore and partially to the fact that the same secretory granule can undergo repetitive opening and closing of fusion pores (so-called kiss-and-run mechanism of release; see [42]). Moreover, delayed and asynchronous fusion events of different granules can further delay the rise of the extracellular BDNF concentration [41]. Interestingly, protein kinase A (PKA) has been described to regulate dendritic secretion of BDNF as well [41]. While activation of PKA does not trigger BDNF secretion per se, the K-induced secretion of BDNF is inhibited when the postsynaptic PKA activity is reduced, suggesting a “gating” action of PKA for the release process. This observation suggests that PKA might play a relevant role in priming dendritic BDNF-containing secretory granules, thus regulating the number of BDNF vesicles available for exocytosis.
Calcium-dependent dendritic BDNF secretion has also been shown to occur in BDNF-GFP transfected cultured neurons by either tetanic stimulation of presynaptic afferents [33] or by postsynaptic action potentials that back propagate into the dendrites (b-APs) [43]. Since neurons have multiple mechanisms for generating a dendritic Ca2+ rise, investigation on the Ca2+ sources responsible for the release has been an important issue and has highlighted differences in the mechanisms of dendritic BDNF secretion depending on the triggering stimulus (Fig. 2). Thus, while L-type VDCC are required for both long-lasting depolarization-induced and back-propagating action potentials (b-APs)-induced BDNF release, an additional Ca2+ release from intracellular stores is needed for long-lasting depolarization-induced BDNF release [41, 43]. As expected for postsynaptic BDNF release in response to tetanic synaptic stimulation, Ca2+ influx through NMDA-receptor-gated channels can substitute for L-type VGCC in triggering the postsynaptic Ca2+ influx required to initiate release [41].
Autocrine and Paracrine Action of Postsynaptically Released BDNF
Most of the studies investigating the role of BDNF in network development have examined the effects of exogenously applied BDNF or the consequences of chronic alteration of the BDNF signaling pathway. However, such spatially diffuse applications do not permit to separate the effects of pre- versus postsynaptic BDNF secretion during synaptic activity. Single-cell transfection within organotypic slice or cell cultures allows creating a defined cellular source of BDNF, thus reproducing more closely the pattern of activity-dependent release of BDNF in the brain. Still, the physiological and morphological consequences of released overexpressed BDNF-GFP could be different from that of endogenous BDNF. In spite of this limitation, these functional changes were used to demonstrate an autocrine or paracrine action of BDNF.
Particle-mediated gene transfer was used to overexpress BDNF together with green fluorescent protein in individual neurons within organotypic slice cultures. In both ferret visual cortex [44] and rat hippocampal slices cultures [45, 46], BDNF overexpressing neurons exhibited more complex dendrites when compared to control neurons. BDNF overexpression also led to the destabilization of dendritic spines. These effects were prevented by TrkB-IgG (i.e., extracellular scavengers of released BDNF), showing that they were mediated by secreted BDNF. Since these effects were not observed in control only GFP-expressing neurons, it is concluded that postsynaptically released BDNF can act in an autocrine manner to modulate neuronal architecture and synaptic function. A similar approach was used to show that BDNF can also act in a paracrine manner to affect nearby neurons. Horch and Katz [47] have produced BDNF overexpressing “donor” neurons and investigated their effect on neighboring “recipient” neurons in ferret cortex brain organotypic slices. The authors showed that BDNF released from dendrites and soma of donor BDNF-expressing cells acted directly on nearby recipient neurons and increased dendritic branching in a distance-dependent manner. This study further showed that the BDNF source had to be within 5 μm to induce dendritic modification. Thus, BDNF released from postsynaptic sites can affect neuronal dendritic structures in an autocrine/paracrine and extremely local manner.
To establish that indeed a postsynaptic source of BDNF can lead to morphological and functional modification, BDNF-GFP was expressed in a small number of neurons within hippocampal neuronal cultures obtained from bdnf−/− mice [48]. BDNF-expressing neurons, which showed a BDNF-GFP distribution characteristics of constitutively releasing neurons (compare [27] and [48]), revealed an altered dendritic morphology when compared to bdnf−/− neurons, with shorter but more numerous dendritic branches. This study further showed that the density of glutamatergic and GABAergic synapses was altered by the postsynaptic expression of BDNF. The number of glutamatergic and GABAergic terminals impinging on BDNF expressing neurons was, respectively, increased and reduced when compared to bdnf−/− neurons in the same culture. Accordingly, the frequency of miniature glutamatergic synaptic currents was increased while miniature GABAergic activity was reduced. These data therefore show that BDNF released from one single target neuron can act as a regulator of synaptic and dendritic development. Similar to the findings of Singh et al. [48] for glutamatergic synaptic development, knockout of BDNF selectively in postsynaptic neurons of neocortical cultures leads to the inability of the innervating presynaptic cells to show activity-dependent induction of new functional terminals [49]. Together, these data further highlight the importance of postsynaptically released BDNF for intact presynaptic development of glutamatergic synapses.
Whether BDNF released from postsynaptic neurons acts locally or globally has also been addressed in a loss function study. A single-cell gene knockout method was used to create a small number of BDNF-lacking neurons in organotypic slice cultures of visual cortex [50]. To estimate the number of GABAergic terminals on two postsynaptic neurons innervated by the same presynaptic interneurons, biocytin was injected into one single GABAergic cell. The number of biocytin-labeled and GAD65-positive boutons established on BDNF-KO neurons was reduced compared to neighboring control neurons. Moreover, the frequency of miniature GABAergic synaptic currents recorded in BDNF-KO neurons was also reduced. This single-cell loss of function study demonstrated that BDNF released from one target neuron can promote the formation of GABAergic synapses by acting locally on perisomatic GABAergic terminals. This observation is consistent with previous findings that single-cell BDNF overexpression in rat hippocampal cultures leads to an increase in presynaptic GAD65 expression and increased spontaneous GABAergic synaptic activity, an effect abrogated by an inhibitor of TrkB receptors and mimicked by bath-applied BDNF [51]. Both studies [50, 51] are, however, in conflict with the aforementioned negative effect of overexpressed BDNF in a BDNF−/− background [48]. These contradicting results could mean that the background of overall BDNF expression in the network (high level in the studies of Kohara et al. [50], and Ohba et al. [51]; low level in the study of Singh et al. [48]) might decide whether BDNF reduction or elevation promotes development of GABAergic terminals.
Whether constitutive or regulated release of BDNF is involved in the aforementioned effects on GABAergic synapses was addressed recently using hippocampal neurons transfected with either (Val-BDNF) or (Met-BDNF): the valine to methionine substitution in the pro-domain of BDNF impaired regulated but not constitutive secretion. However, hippocampal neurons expressing Met-BDNF or Val-BDNF showed a similar increase in presynaptic GAD65 levels [52]. This result might suggest that activity-independent constitutive release of BDNF is sufficient to regulate inhibitory synapses formation, which would be consistent also with the aforementioned results by Singh et al. [48] that are due most likely to constitutive secretion of overexpressed BDNF GFP. However, Met-BDNF secretion via secretory granules is just reduced and not blocked. It is therefore possible that a sufficient amount of Met-BDNF was released in an activity-dependent manner to regulate presynaptic GABAergic terminals. Moreover, overexpression could have led to non-physiological amount of constitutively released Met-BDNF that overcomes the impaired regulated release. In agreement with this hypothesis, other studies have shown that the blockage of neuronal activity and, consequently, of regulated secretion produces developmental alterations that are reversed by application of exogenous BDNF [53–56], thus supporting the idea that constitutive secretion of endogenous BDNF is not sufficient to sustain a correct synaptic maturation.
Further studies are clearly needed to unambiguously scrutinize the role of constitutive versus regulated release of BDNF to modulate synaptic transmission.
Activity-Dependent Release of BDNF
The first study directly addressing synaptic dendritic release of BDNF investigated the effect of tetanic stimulation, a conditioning protocol frequently used in vitro and in vivo to trigger long-term changes in synaptic efficacy [33]. Using time lapse video microscopy in living BDNF-GFP-expressing hippocampal neurons in culture, Hartmann and collaborators showed that high-frequency stimulation of glutamatergic synapses induced the release of BDNF-GFP from secretory granules that co-localized with the stimulated presynaptic terminals (FM4-64 destaining). The tetanus-induced synaptic secretion of BDNF-GFP was prevented by ionotropic glutamatergic receptor antagonists, indicating that postsynaptic secretion of BDNF-GFP is triggered by synaptically released glutamate and not by direct stimulation of the postsynaptic neurons. The postsynaptic release of BDNF occurred within a delay of tens of seconds after the start of the tetanic stimulation with an average time constant of fluorescence decrease of 200–300 s. The question whether ongoing synaptic activity can trigger dendritic BDNF-GFP release, and—if so—what would be the minimal patterns of electrical activity that are required for such release, was addressed in a recent study combining electrophysiological recording and time lapse fluorescence imaging in BDNF-GFP-expressing hippocampal neurons in culture [43]. The results provided unequivocal evidence that b-APs are necessary and sufficient to trigger a Ca2+-dependent dendritic secretion of BDNF-GFP during spontaneous synaptic activity (Fig. 3). Thus, when the potential of the recorded neuron was clamped at a hyperpolarized value to prevent action potential firing, in spite of the presence of excitatory postsynaptic potentials, dendritic release of BDNF-GFP was absent. However, when the neuron was allowed to fire action potentials as a result of ongoing spontaneous synaptic activity, secretion of dendritic BDNF-GFP was observed. This release was prevented in cells loaded with QX314, an intracellular blocker of voltage-dependent sodium channels which prevents postsynaptic action potentials, but not action-potential-triggered synaptic network activity in all other cells of the culture. These results show that postsynaptic action potentials are required to trigger synaptic dendritic release of BDNF during ongoing synaptic activity in this type of hippocampal cultures. Finally, b-APs triggered by somatic depolarization through the recording electrode-induced dendritic BDNF-GFP secretion in the presence of glutamatergic ionotropic receptor antagonists. BDNF-GFP secretion was also produced by steady-state depolarization of the neurons to −40 mV in the absence of APs. This suggests that it is the role of b-APs to produce sufficient dendritic depolarization to enable activation of VDCC, thus leading to BDNF release.
Fig. 3.
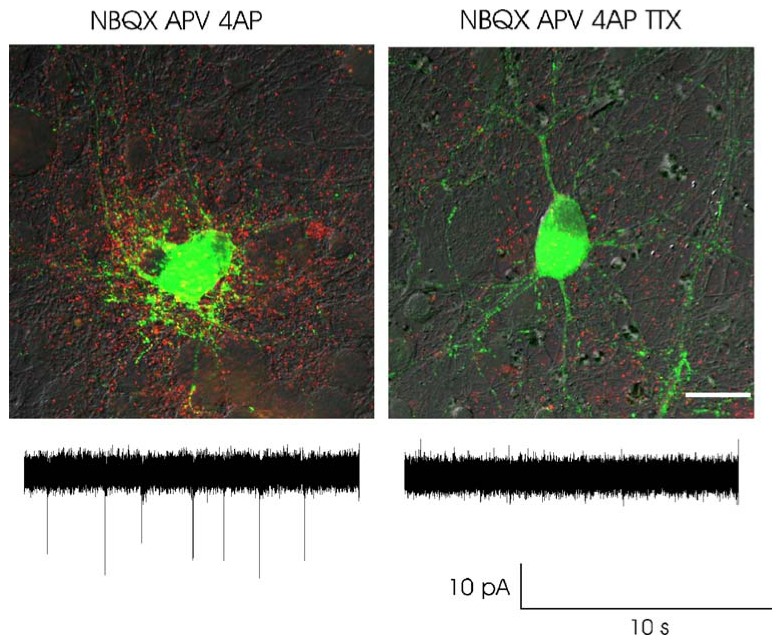
Action potential induced BDNF secretion. Merged images showing intracellular BDNF-GFP fluorescence (green) and secreted BDNF-GFP detected using an anti-GFP antibody (red) under non-permeabilizing conditions for antibody detection. BDNF-GFP secretion was induced by the firing activity produced by the K+ channel antagonist 4-amminopyridine (4AP) in the presence of glutamatergic antagonists (left). BDNF-GFP secretion was impaired by TTX (right). Lower panel: cell attached recordings showing the firing activity in the two conditions. The experiments were performed on primary hippocampal cell cultures transfected at 11 days in vitro (DIV) and stimulated at 13 DIV. Scale bar, 25 μm
An elegant approach to study activity-dependent release of endogenous BDNF at the single-cell level is to record functional changes in synaptic activity of postsynaptic neurons as an indicator of BDNF release [57]. Using this approach, these authors provided strong evidence that BDNF release can occur from individual postsynaptic hippocampal neurons in culture. Thus, when cells were depolarized to a holding potential of −40 mV by steady-state current injection through the recording electrode, the recorded neurons showed a gradual increase in miniature glutamatergic synaptic activity. This effect was prevented by scavenging extracellular BDNF, mimicked by bath-applied BDNF, abolished when chelating intracellular Ca2+ in the postsynaptic neuron, or when a mutation was introduced in the BDNF pro-domain to prevent its regulated release.
A functional approach to show release of endogenous BDNF was also used by Tanaka et al. [58] to investigate the release of endogenous BDNF at the single-cell level: they investigated the mechanisms subtending spine plasticity produced by a pairing protocol, i.e., postsynaptic firing concomitant with glutamatergic receptors activation. In hippocampal slices, such protocol induced an enlargement of dendritic spines that required endogenous BDNF. Moreover, since neither BDNF nor postsynaptic activation of glutamatergic receptors alone were sufficient to induce morphological changes, it was proposed that postsynaptically released BDNF and presynaptically released glutamate act in synergy to induce protein synthesis and, thus, spine enlargement. The authors also showed that spine plasticity can occur in the absence of postsynaptic APs if exogenous BDNF was supplied concomitantly with synaptic stimulation, suggesting that the function of b-APs in this form of plasticity is to provide sufficient levels of extracellular BDNF (i.e., release of BDNF from postsynaptic neurons). Whatever the precise mechanism underlying such functional or structural changes, these studies demonstrated a postsynaptic release of endogenous BDNF at the single-cell level induced by physiological synaptic signals [58].
Back-Propagating Action Potentials: A Key Regulator of Dendritic BDNF Release and Activity-Dependent Plasticity
In the previous sections, we reviewed several lines of evidence showing that postsynaptic secretion of BDNF participates in activity-dependent development and plasticity of neuronal circuits. Given the crucial role of b-APs in triggering dendritic BDNF release [43, 58], all conditions that affect b-APs generation should have an impact on network plasticity and development.
Dendrites express different types of voltage-gated ion channels that will boost or in contrast attenuate b-AP amplitude and associated Ca2+ rise. Thus, in cortical and hippocampal pyramidal cells, back-propagation of APs is at least in part an active process maintained by the concerted action of voltage-gated Na+ and K+ channels, determining the spread of b-APs [59–61]. Therefore, modification of the properties of these channels can affect the degree to which APs back-propagate into the dendrites and thus is likely to regulate the dendritic release of BDNF (Fig. 4). It seems worth noting that certain neuromodulators, such as acetylcholine, noradrenaline, and dopamine, through the modification of potassium conductances, can regulate the amplitude of back-propagating APs [62, 63]. Furthermore, back-propagation of APs is also sensitive to the local dendritic membrane potential [61, 64]. Thus, small amplitude b-APs in the dendrites can be boosted by appropriate and precisely timed membrane depolarization via A-type K+ channel inactivation and/or Na+ channel activation [65, 66]. Conversely, b-APs amplitude can be decreased after membrane hyperpolarization [67] or by dendritic GABAergic activity through a shunting effect [68]. Therefore, a dynamic control of dendritic back-propagation exerted by neuromodulators and local dendritic synaptic activity could act as context-dependent regulator of BDNF secretion. In this regard, it is tempting to speculate that the effects produced by selected neuromodulators on brain development, plasticity, and pathology [69, 70–72] could be, at least in part, mediated by b-APs-induced BDNF secretion (Fig. 4). Similarly, reducing the firing probability of a single neuron in a culture following expression of an inward-rectifier potassium channel affects the strength of the glutamatergic synapses impinging on this specific neuron [73]. Although the contribution of BDNF secretion was not investigated in this study, this work highlights the fact that the neuronal output, i.e. APs, can affect its own inputs.
Fig. 4.

BDNF secretion depends on the neuronal output rather than synaptic inputs. Suprathreshold excitatory postsynaptic potentials (EPSPs) trigger action potentials that back-propagate (b-APs) from the soma to the dendrites. Center, b-APs-induced BDNF secretion and synaptic strengthening. Left, this process can be reinforced by conditions that facilitate the back propagation of APs such as the action of neuromodulators. Right, when the same suprathreshold glutamatergic activity is generated in a context that prevents APs back-propagation, such as a concomitant activation of dendritic inhibition (GABA), BDNF secretion and synaptic strengthening will not occur. Modified from Comunicative & Intergrative Biology 1(2):153
Several studies have reported a contribution of released BDNF on the induction of activity-dependent synaptic plasticity, such as long-term potentiation (LTP) [74–77]. In agreement with this observation, patterns of electrical stimulation (including high-frequency tetanic stimulation or theta burst stimulation) leading to LTP at glutamatergic synapses can induce a concomitant release of BDNF [33, 78, 79].
b-APs and associated dendritic BDNF release is another signal for long-term change in synaptic efficacy. Thus, in the developing rat hippocampus, b-APs evoked by current step depolarization in one single neuron induced a BDNF-dependent long-lasting potentiation of GABAergic synapses [80]. Dendritic back-propagating APs are also associated with a particular form of synaptic plasticity called spike timing-dependent plasticity (STDP). STDP occurs when synaptic transmission is repetitively associated with postsynaptic APs [81]. The requirement for BDNF signaling in STDP was first investigated in the optic tectum where it was shown that STDP was impaired by intracellular blockade of TrkB signaling [82]. More recently, a role of BDNF secretion in STDP was suggested by the work of Tanaka et al. [58] in dendritic spines of CA1 hippocampal neurons.
The suggested involvement of b-APs-induced dendritic BDNF release in STDP raises some questions. The induction of STDP requires the repetitive and concomitant occurrence of synaptic transmission and b-APs in a temporal window of less than tens of milliseconds. However, to date, all studies addressing the kinetics of BDNF secretion suggest that dendritic release of BDNF is a slow process beginning and lasting several seconds after the triggering event (i.e., b-APs) [27, 33, 39, 41, 43]. Thus, BDNF release seems to lack the temporal characteristic to act as a coincident detector in STDP. These observations give rise to, at least, two alternative hypotheses: (a) rapid dendritic BDNF secretion, qualitatively different from the slow secretion experimentally detected so far can be triggered by STDP protocols and (b) the repeated STDP pairing is sufficient to trigger a Ca2+-dependent BDNF release, but additional mechanisms rather than BDNF secretion account for the time dependence of STDP. Further investigations are therefore required to better elucidate the role played by BDNF secretion in STDP.
Extrapolations of data obtained in vitro to a more intact system are always limited. However, recent studies have shown that action potentials generated in the soma are capable of propagating backwards to the dendritic tree in vivo, resulting in a spatial distribution of intracellular Ca2+ rise similar to that observed in vitro [83, 84]. Determining the contribution of b-APs in dendritic BDNF release in vivo therefore will be a challenging task for future investigations.
Conclusion
BDNF regulates the survival, growth, and differentiation of central and peripheral neurons. In addition to these “classical” effects, BDNF also modulates a variety of cellular function such as synapses formation and activity-dependent synaptic plasticity. Moreover, recent results show a link between several human pathological conditions and the Val66met polymorphism of the BDNF gene (impairing the process of BDNF sorting and secretion) and suggest a possible therapeutic potential for neurotrophins in treating neurodegenerative diseases. BDNF is a secreted protein, targeted to the dendrites and axons, whose expression and release are modulated by neuronal activity. Since most of these effects have been attributed to the activity-dependent release of neurotrophins from the target neurons, understanding the mode, as well as the level and pattern of synaptic activity leading to postsynaptic release of neurotrophins, has been of intense interest over the past decade.
A Ca2+- and activity-dependent dendritic BDNF release has been demonstrated in neuronal cultures. So far, at least three distinct signals sub-serving BDNF release has been directly identified: (1) tetanic stimulation of presynaptic glutamatergic fibers, (2) action potentials that propagate backwards into the dendrites, and (3) prolonged depolarization of the postsynaptic neurons. Although all three modalities of secretion share common characteristics, the biological consequences of released BDNF might be quite different. For instance, BDNF released during tetanic stimulation could locally modulate the development and plasticity of stimulated synapses, while BDNF release triggered by back-propagating action potentials could influence the development and plasticity of stimulated and non-stimulated synapses. Since BDNF does not diffuse for a long distance and is likely to act on local receptors and because neuronal calcium rises are restricted and localized events, the spatial proximity of the calcium source and BDNF release with molecular targets will determine the specificity of BDNF action and might explain why BDNF can have so widespread actions.
Acknowledgments
This work was supported by INSERM, CNRS and ANR (Agence Nationale pour la Recherche) and the DFG (SFB 553), Stiftung Rheinland-Pfalz and the Schram-Stiftung. N.K. was a recipient of a FRM (Fondation pour la Recherche Médicale) and ANR fellowships.
References
- 1.Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 2.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 3.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 5.Ernfors P, Merlio JP, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 6.Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- 7.Wetmore C, Ernfors P, Persson H, Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990;109:141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- 8.Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 9.Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorba T, Wahle P. Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures. Eur J Neurosci. 1999;11:1179–1190. doi: 10.1046/j.1460-9568.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 12.Lindholm D, Castren E, Hengerer B, Zafra F, Berninger B, Thoenen H. Differential regulation of nerve growth factor (NGF) synthesis in neurons and astrocytes by glucocorticoid hormones. Eur J Neurosci. 1992;4:404–410. doi: 10.1111/j.1460-9568.1992.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 13.Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. Ceramide activates microglia to enhance the production/secretion of brain-derived neurotrophic factor (BDNF) without induction of deleterious factors in vitro. J Neurochem. 2002;80:697–705. doi: 10.1046/j.0022-3042.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- 15.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 16.Berninger B, Marty S, Zafra F, Da Penha Berzaghi M, Thoenen H, Lindholm D. GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development. 1995;121:2327–2335. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]
- 17.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castren E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci U S A. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tongiorgi E. Activity-dependent expression of brain-derived neurotrophic factor in dendrites: facts and open questions. Neurosci Res. 2008;61:335–346. doi: 10.1016/j.neures.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- 21.Binder DK, Croll SD, Gall CM, Scharfman HE. BDNF and epilepsy: too much of a good thing. Trends Neurosci. 2001;24:47–53. doi: 10.1016/s0166-2236(00)01682-9. [DOI] [PubMed] [Google Scholar]
- 22.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 24.Seidah NG, Benjannet S, Pareek S, Savaria D, Hamelin J, Goulet B, Laliberte J, Lazure C, Chretien M, Murphy RA. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem J. 1996;314(Pt 3):951–960. doi: 10.1042/bj3140951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mowla SJ, Pareek S, Farhadi HF, Petrecca K, Fawcett JP, Seidah NG, Morris SJ, Sossin WS, Murphy RA. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosci. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brigadski T, Hartmann M, Lessmann V. Differential vesicular targeting and time course of synaptic secretion of the mammalian neurotrophins. J Neurosci. 2005;25:7601–7614. doi: 10.1523/JNEUROSCI.1776-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 29.Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Chen ZY, Bath K, McEwen B, Hempstead B, Lee F. Impact of genetic variant BDNF (Val66Met) on brain structure and function. Novartis Found Symp. 2008;289:180–188. doi: 10.1002/9780470751251.ch14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- 33.Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 35.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- 37.Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxy-peptidase e. Neuron. 2005;45:245–255. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 38.Haubensak W, Narz F, Heumann R, Lessmann V. BDNF-GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. J Cell Sci. 1998;111(Pt 11):1483–1493. doi: 10.1242/jcs.111.11.1483. [DOI] [PubMed] [Google Scholar]
- 39.Kojima M, Takei N, Numakawa T, Ishikawa Y, Suzuki S, Matsumoto T, Katoh-Semba R, Nawa H, Hatanaka H. Biological characterization and optical imaging of brain-derived neurotrophic factor-green fluorescent protein suggest an activity-dependent local release of brain-derived neurotrophic factor in neurites of cultured hippocampal neurons. J Neurosci Res. 2001;64:1–10. doi: 10.1002/jnr.1080. [DOI] [PubMed] [Google Scholar]
- 40.Nakajima T, Sato M, Akaza N, Umezawa Y. Cell-based fluorescent indicator to visualize brain-derived neurotrophic factor secreted from living neurons. ACS Chem Biol. 2008;3:352–358. doi: 10.1021/cb800052v. [DOI] [PubMed] [Google Scholar]
- 41.Kolarow R, Brigadski T, Lessmann V. Postsynaptic secretion of BDNF and NT-3 from hippocampal neurons depends on calcium calmodulin kinase II signaling and proceeds via delayed fusion pore opening. J Neurosci. 2007;27:10350–10364. doi: 10.1523/JNEUROSCI.0692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia X, Lessmann V, Martin TFJ. Imaging of evoked dense-core-vescicle exocytosis in hippocampal neurons reveals long latencies and kiss-and-run fusion events. J Cell Science. 2009;122:75–82. doi: 10.1242/jcs.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuczewski N, Porcher C, Ferrand N, Fiorentino H, Pellegrino C, Kolarow R, Lessmann V, Medina I, Gaiarsa JL. Back-propagating action potentials trigger dendritic release of BDNF during spontaneous network activity. J Neurosci. 2008;28:7013–7023. doi: 10.1523/JNEUROSCI.1673-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horch HW, Kruttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- 45.Wirth MJ, Brun A, Grabert J, Patz S, Wahle P. Accelerated dendritic development of rat cortical pyramidal cells and interneurons after biolistic transfection with BDNF and NT4/5. Development. 2003;130:5827–5838. doi: 10.1242/dev.00826. [DOI] [PubMed] [Google Scholar]
- 46.Danzer SC, Kotloski RJ, Walter C, Hughes M, McNamara JO. Altered morphology of hippocampal dentate granule cell presynaptic and postsynaptic terminals following conditional deletion of TrkB. Hippocampus. 2008;18:668–678. doi: 10.1002/hipo.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- 48.Singh B, Henneberger C, Betances D, Arevalo MA, Rodriguez-Tebar A, Meier JC, Grantyn R. Altered balance of glutamatergic/GABAergic synaptic input and associated changes in dendrite morphology after BDNF expression in BDNF-deficient hippocampal neurons. J Neurosci. 2006;26:7189–7200. doi: 10.1523/JNEUROSCI.5474-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walz C, Jungling K, Lessmann V, Gottmann K. Presynaptic plasticity in an immature neocortical network requires NMDA receptor activation and BDNF release. J Neurophysiol. 2006;96:3512–3516. doi: 10.1152/jn.00018.2006. [DOI] [PubMed] [Google Scholar]
- 50.Kohara K, Yasuda H, Huang Y, Adachi N, Sohya K, Tsumoto T. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci. 2007;27:7234–7244. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohba S, Ikeda T, Ikegaya Y, Nishiyama N, Matsuki N, Yamada MK. BDNF locally potentiates GABAergic presynaptic machineries: target-selective circuit inhibition. Cereb Cortex. 2005;15:291–298. doi: 10.1093/cercor/bhh130. [DOI] [PubMed] [Google Scholar]
- 52.Ikeda T, Tamura N, Matsuki N, Yamada MK. Conserved role of brain-derived neurotrophic factor in Val66Met: target-selective reinforcement of GABAergic synapses. Neuroreport. 2006;17:1847–1851. doi: 10.1097/WNR.0b013e328011592c. [DOI] [PubMed] [Google Scholar]
- 53.McAllister AK, Katz LC, Lo DC. Neurotrophic regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 54.Marty S, Wehrlé R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marty S, Berzaghi MD, Berninger B. Neurotrophins and activity-dependent plasticity of cortical interneurons. Trends Neurosci. 1997;20:198–202. doi: 10.1016/s0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- 56.Seil FJ, Drake-Baumann R. TrkB receptor ligands promote activity-dependent inhibitory synaptogenesis. J Neurosci. 2000;20:5367–5373. doi: 10.1523/JNEUROSCI.20-14-05367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magby JP, Bi C, Chen ZY, Lee FS, Plummer MR. Single-cell characterization of retrograde signaling by brain-derived neurotrophic factor. J Neurosci. 2006;26:13531–13536. doi: 10.1523/JNEUROSCI.4576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnston D, Hoffman DA, Colbert CM, Magee JC. Regulation of back-propagating action potentials in hippocampal neurons. Curr Opin Neurobiol. 1999;9:288–292. doi: 10.1016/s0959-4388(99)80042-7. [DOI] [PubMed] [Google Scholar]
- 60.Johnston D, Magee JC, Colbert CM, Cristie BR. Active properties of neuronal dendrites. Annu Rev Neurosci. 1996;19:165–186. doi: 10.1146/annurev.ne.19.030196.001121. [DOI] [PubMed] [Google Scholar]
- 61.Bernard C, Johnston D. Distance-dependent modifiable threshold for action potential back-propagation in hippocampal dendrites. J Neurophysiol. 2003;90:1807–1816. doi: 10.1152/jn.00286.2003. [DOI] [PubMed] [Google Scholar]
- 62.Hoffman DA, Johnston D. Neuromodulation of dendritic action potentials. J Neurophysiol. 1999;81:408–411. doi: 10.1152/jn.1999.81.1.408. [DOI] [PubMed] [Google Scholar]
- 63.Tsubokawa H, Ross WN. Muscarinic modulation of spike backpropagation in the apical dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 1997;17:5782–5791. doi: 10.1523/JNEUROSCI.17-15-05782.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsubokawa H, Miura M, Kano M. Elevation of intracellular Na+ induced by hyperpolarization at the dendrites of pyramidal neurones of mouse hippocampus. J Physiol. 1999;517(Pt 1):135–142. doi: 10.1111/j.1469-7793.1999.0135z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons [see comments] Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- 66.Stuart GJ, Hausser M. Dendritic coincidence detection of EPSPs and action potentials. Nat Neurosci. 2001;4:63–71. doi: 10.1038/82910. [DOI] [PubMed] [Google Scholar]
- 67.Tsubokawa H, Ross WN. IPSPs modulate spike back-propagation and associated [Ca2+]i changes in the dendrites of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1996;76:2896–2906. doi: 10.1152/jn.1996.76.5.2896. [DOI] [PubMed] [Google Scholar]
- 68.Lowe G. Inhibition of backpropagating action potentials in mitral cell secondary dendrites. J Neurophysiol. 2002;88:64–85. doi: 10.1152/jn.2002.88.1.64. [DOI] [PubMed] [Google Scholar]
- 69.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 70.Origlia N, Kuczewski N, Aztiria E, Gautam D, Wess J, Domenici L. Muscarinic acetylcholine receptor knockout mice show distinct synaptic plasticity impairments in the visual cortex. J Physiol. 2006;577:829–840. doi: 10.1113/jphysiol.2006.117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calabresi P, Galletti F, Saggese E, Ghiglieri V, Picconi B. Neuronal networks and synaptic plasticity in Parkinson’s disease: beyond motor deficits. Parkinsonism Relat Disord. 2007;13(Suppl 3):S259–S262. doi: 10.1016/S1353-8020(08)70013-0. [DOI] [PubMed] [Google Scholar]
- 73.Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- 74.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 75.Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 76.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci U S A. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gartner A, Staiger V. Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc Natl Acad Sci U S A. 2002;99:6386–6391. doi: 10.1073/pnas.092129699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 2004;101:15788–15792. doi: 10.1073/pnas.0406960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gubellini P, Ben Ari Y, Gaiarsa JL. Endogenous neurotrophins are required for the induction of GABAergic long-term potentiation in the neonatal rat hippocampus. J Neurosci. 2005;25:5796–5802. doi: 10.1523/JNEUROSCI.0824-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- 82.Mu Y, Poo MM. Spike timing-dependent LTP/LTD mediates visual experience-dependent plasticity in a developing retinotectal system. Neuron. 2006;50:115–125. doi: 10.1016/j.neuron.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 83.Waters J, Helmchen F. Boosting of action potential backpropagation by neocortical network activity in vivo. J Neurosci. 2004;24:11127–11136. doi: 10.1523/JNEUROSCI.2933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Waters J, Larkum M, Sakmann B, Helmchen F. Supralinear Ca2+ influx into dendritic tufts of layer 2/3 neocortical pyramidal neurons in vitro and in vivo. J Neurosci. 2003;23:8558–8567. doi: 10.1523/JNEUROSCI.23-24-08558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wetmore C, Olson L, Bean AJ. Regulation of brain-derived neurotrophic factor (BDNF) expression and release from hippocampal neurons is mediated by non-NMDA type glutamate receptors. J Neurosci. 1994;14:1688–1700. doi: 10.1523/JNEUROSCI.14-03-01688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goodman LJ, Valverde J, Lim F, Geschwind MD, Federoff HJ, Geller AI, Hefti F. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- 87.Kruttgen A, Moller JC, Heymach JV, Jr, Shooter EM. Neurotrophins induce release of neurotrophins by the regulated secretory pathway. Proc Natl Acad Sci U S A. 1998;95:9614–9619. doi: 10.1073/pnas.95.16.9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Griesbeck O, Canossa M, Campana G, Gartner A, Hoener MC, Nawa H, Kolbeck R, Thoenen H. Are there differences between the secretion characteristics of NGF and BDNF? Implications for the modulatory role of neurotrophins in activity-dependent neuronal plasticity. Microsc Res Tech. 1999;45:262–275. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<262::AID-JEMT10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 89.Gunther EC, von Bartheld CS, Goodman LJ, Johnson JE, Bothwell M. The G-protein inhibitor, pertussis toxin, inhibits the secretion of brain-derived neurotrophic factor. Neuroscience. 2000;100:569–579. doi: 10.1016/s0306-4522(00)00309-2. [DOI] [PubMed] [Google Scholar]
- 90.Canossa M, Gartner A, Campana G, Inagaki N, Thoenen H. Regulated secretion of neurotrophins by metabotropic glutamate group I (mGluRI) and Trk receptor activation is mediated via phospholipase C signalling pathways. EMBO J. 2001;20:1640–1650. doi: 10.1093/emboj/20.7.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Canossa M, Giordano E, Cappello S, Guarnieri C, Ferri S. Nitric oxide down-regulates brain-derived neurotrophic factor secretion in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99:3282–3287. doi: 10.1073/pnas.042504299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.del Toro D, Canals JM, Gines S, Kojima M, Egea G, Alberch J. Mutant huntingtin impairs the post-Golgi trafficking of brain-derived neurotrophic factor but not its Val66Met polymorphism. J Neurosci. 2006;26:12748–12757. doi: 10.1523/JNEUROSCI.3873-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Santi S, Cappello S, Riccio M, Bergami M, Aicardi G, Schenk U, Matteoli M, Canossa M. Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance. EMBO J. 2006;25:4372–4380. doi: 10.1038/sj.emboj.7601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arancibia S, Lecomte A, Silhol M, Aliaga E, Tapia-Arancibia L. In vivo brain-derived neurotrophic factor release and tyrosine kinase B receptor expression in the supraoptic nucleus after osmotic stress stimulus in rats. Neuroscience. 2007;146:864–873. doi: 10.1016/j.neuroscience.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 96.Bergami M, Santi S, Formaggio E, Cagnoli C, Verderio C, Blum R, Berninger B, Matteoli M, Canossa M. Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J Cell Biol. 2008;183:213–221. doi: 10.1083/jcb.200806137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuczewski N, Langlois A, Fiorentino H, Bonnet S, Marissal T, Diabira D, Ferrand N, Porcher C, Gaiarsa JL. Spontaneous glutamatergic activity induces a BDNF-dependent potentiation of GABAergic synapses in the newborn rat hippocampus. J Physiol. 2008;586:5119–5128. doi: 10.1113/jphysiol.2008.158550. [DOI] [PMC free article] [PubMed] [Google Scholar]


