Abstract
Objective
To assess the association between CMV IgG antibody levels, HIV disease progression, and immune activation in sub-Saharan Africa.
Design
A prospective cohort study was conducted among women enrolled in a trial that was designed to determine the effect of acyclovir on HIV disease progression in Rakai, Uganda.
Methods
The primary endpoints were progression to a CD4+ T-cell count<250 cells/μL, non-traumatic death, or initiation of ART. CD4+ T-cell counts, HIV viral load, C-reactive protein, and soluble CD14 levels were assessed biannually for 24 months. CMV IgG antibody were measured at baseline among all women, and annually among a subset of women who initiated ART during the study.
Results
There were 300 HIV/CMV coinfected participants who contributed a total of 426.4 person-years with a median follow-up time of 1.81 years. Compared to the lowest CMV IgG tertile group at baseline, the highest CMV IgG tertile group was associated with an increased risk to reach a primary endpoint independent of acyclovir use, age, CD4+ T-cell count, and HIV viral load at baseline (adjHR=1.59;[95%CI=1.05-2.39];P=0.027). Among pre-ART visits (n=1,200), women in the highest baseline CMV IgG tertile had increasing annual rates of sCD14 and CRP levels, which was not observed for the low CMV IgG tertile group. Compared to pre-ART visits, CMV IgG antibody levels were higher post-ART initiation, and concurrent levels remained associated with sCD14 and CRP during suppressive ART (n=95).
Conclusion
The magnitude of the immune response to CMV was associated with HIV disease progression and immune activation in sub-Saharan Africa.
Keywords: cytomegalovirus, HIV disease progression, humoral immune response, immune activation, inflammation
Introduction
In resource-rich settings, cytomegalovirus (CMV) co-infection, viremia, subclinical shedding, and viral diversity are associated with an increased risk of progression to AIDS and death among persons living with HIV [1-7]. The humoral immune response to CMV, as measured by CMV-specific immunoglobulin G (IgG) antibody, has also been linked to untreated HIV disease progression in men who have sex with men (MSM) [8, 9]. Despite use of antiretroviral therapy (ART), CMV co-infection is associated with increased risk of poor non-AIDS related events [10], and high CMV IgG antibody levels are associated with subclinical cardiovascular disease [11], impaired neurocognitive function [12], and decreased physical function [13]. Interestingly, similar findings have also been made among general populations in the U.S. and Europe [14-17].
The role of CMV co-infection in HIV pathogenesis is likely bidirectional [18]. HIV-induced immunosuppression might lead to increased CMV reactivation, which in turn can promote HIV replication and immune activation [18, 19]. Numerous investigations have suggested that CMV replication continues to drive CD8+ T-cell expansion and inflammation in ART-experienced individuals [20-25]. The cross-sectional evidence is conflicting in untreated and treated HIV-infected individuals, but some studies suggest CMV IgG antibody levels may also be associated with immune activation markers, such as C-reactive protein (CRP) [13], a general biomarker of systemic inflammation, and soluble CD14 (sCD14) [26],a biomarker of nonspecific monocyte activation [27].
There is limited data on the role of CMV in chronic HIV disease in sub-Saharan Africa [28-30]. In this region, primary CMV infection predominantly occurs during infancy, and is also associated with the development and maintenance of CD8+ T-cell expansion during childhood [31-33]. By adulthood, CMV seroprevalence reaches close to 100% in most populations [29, 30], so there is likely a high lifetime risk of reinfection before and during HIV infection. In addition to geographic variability in CMV shedding, CMV IgG antibody dynamics are known to differ by age, behavior, and host-genetics [22, 34]. Therefore, we aimed to assess the relationship between CMV IgG antibody levels, and HIV disease progression and immune activation in Rakai, Uganda.
Methods
Study Design
Ugandan women coinfected with HIV-1 and herpes simplex virus type 2 (HSV-2), and a CD4+ T-cell count between 300-400 cells/μL, were enrolled in a clinical trial to examine the effect of acyclovir on HIV disease progression over 24 months [35]. Individuals with AIDS-defining illnesses or those receiving ART were excluded. Participants were followed monthly for clinical screenings, drug refill, and adverse event review. Every 6-months, a physical examination was conducted and blood was drawn. Serum was stored at -80°C. Participants provided written informed consent, and the study was approved by the Uganda Virus Research Institute Science and Ethics Committee, the Uganda National Council for Science and Technology, and the Institutional Review Board of the National Institute of Allergy and Infectious Diseases. The trial was registered with ClinicalTrials.gov (NCT00405821).
Laboratory Testing
CD4+ T-cell counts, HIV viral load (VL), soluble CD14 (sCD14) and high-sensitivity CRP (hs-CRP) measurements were determined biannually [35, 36]. CMV IgG antibody levels were measured at the baseline visit among all women, and at the 12- and 24-month follow-up visits among a sub-set of women who initiated ART during the study period. CMV IgG antibody levels were measured up to a 1:200 dilution (CMV IgG Enzyme Immunoassay Test Kit; Genway Biotech; San Diego, CA).
Statistical Analysis
Kaplan-Meier analysis and Cox proportional hazard models were used to assess the association between baseline CMV IgG tertiles and HIV disease progression (composite outcome: non-traumatic death, CD4+ T-cell count ≤250 cells/μL, WHO Stage IV condition, or ART initiation by study clinicians for HIV-related symptoms). Participants were administratively censored at 24 months. The multivariable model included adjustment for study arm and potential baseline confounders: age, CD4+ T-cell count, and HIV VL. Other baseline covariates did not improve model fit as assessed by the likelihood-ratio test and Akaike's information criterion.
In a sensitivity analysis, Cox regression with robust variance estimation was performed among a propensity-matched sub-cohort (based on the baseline CD4+ T-cell count and HIV VL) [37, 38]; and the model was further adjusted for study arm and age. Greedy one-nearest neighbor 1:1 matching on the propensity score was applied to select controls to compare to women in the high CMV IgG tertile group [37].
Linear multilevel modeling with random effects for the intercept and slope were fitted to assess associations between baseline CMV IgG tertiles and annual changes in pre-ART levels of sCD14 and hs-CRP. Among women who initiated ART, random intercept models were used to examine the association of concurrent CMV IgG antibody levels (>median of post-ART visits) and sCD14 and hs-CRP.
Analyses were performed using STATA version 14.1 and R version 3.2.
Results
Of 311 women enrolled in the trial, 300 had available sera at baseline and all were HIV/HSV-2/CMV co-infected. Lower CD4+ T-cell counts at enrollment were observed among women in the higher baseline CMV IgG tertile groups (Ptrend=0.051). There was a positive association for HIV VL, sCD14, and hs-CRP with increasing CMV IgG tertiles at baseline (Ptrend<0.05;Table 1).
Table 1. Study Population Characteristics by Baseline CMV IgG Antibody Tertile.
| Enrollment Characteristics | Total | 1st Tertile (n=100) | 2nd Tertile (n=101) | 3rd Tertile (n=99) | P Valuea |
|---|---|---|---|---|---|
| Study Arm | 0.811 | ||||
| Placebo | 153 (51.0%) | 53 (53.0%) | 49 (48.5%) | 51 (51.5%) | |
| Acyclovir | 147 (49.0%) | 47 (47.0%) | 52 (51.5%) | 48 (48.5%) | |
| Age (years) | 0.650 | ||||
| 20 - 29 | 72 (24.0%) | 28 (28.0%) | 20 (19.8%) | 24 (24.2%) | |
| 30 - 39 | 130 (43.3%) | 46 (46.0%) | 42 (41.6%) | 42 (42.4%) | |
| 40 - 49 | 67 (22.3%) | 18 (18.0%) | 27 (26.7%) | 22 (22.2%) | |
| ≥ 50 | 31 (10.3%) | 8 (8.0%) | 12 (11.9%) | 11 (11.1%) | |
| BMI (kg/m2) | 0.140 | ||||
| < 18.5 | 22 (7.3%) | 4 (4.0%) | 8 (7.9%) | 10 (10.1%) | |
| 18.5 - 24.9 | 215 (71.7%) | 69 (69.0%) | 71 (70.3%) | 75 (75.8%) | |
| ≥ 25 | 63 (21.0%) | 27 (27.0%) | 22 (21.8%) | 14 (14.1%) | |
| CD4 count (cells/μL) | 0.051 | ||||
| 300 - 349 | 134 (44.7%) | 41 (41.0%) | 39 (38.6%) | 54 (54.6%) | |
| 350 - 400 | 166 (55.3%) | 59 (59.0%) | 62 (61.4%) | 45 (45.5%) | |
| HIV viral load (c/mL) | 0.006 | ||||
| < 10,000 | 107 (35.7%) | 46 (46.0%) | 38 (37.6%) | 23 (23.2%) | |
| 10,000 - 49,999 | 86 (28.7%) | 30 (30.0%) | 26 (25.7%) | 30 (30.3%) | |
| 50,000 - 99,999 | 28 (9.3%) | 10 (10.0%) | 8 (7.9%) | 10 (10.1%) | |
| ≥ 100,000 | 79 (26.3%) | 14 (14.0%) | 29 (28.7%) | 36 (36.4%) | |
| Log10 HIV viral load | <0.001 | ||||
| Mean (SD) | 4.31 (0.89) | 4.03 (0.84) | 4.36 (0.85) | 4.53 (0.92) | |
| Median (IQR) | 4.35 (3.69-5.04) | 4.10 (3.41-4.61) | 4.35 (3.76-5.07) | 4.56 (4.09-5.23) | |
| Log10 hs-CRP, mg/L | 0.029 | ||||
| Mean (SD) | 0.46 (0.40) | 0.43 (0.41) | 0.42 (0.37) | 0.52 (0.42) | |
| Median (IQR) | 0.32 (0.15-0.66) | 0.28 (0.13-0.59) | 0.29 (0.13-0.68) | 0.39 (0.20-0.75) | |
| sCD14, ng/mL | 0.002 | ||||
| Mean (SD) | 2195 (836) | 2026 (907) | 2172 (686) | 2389 (869) | |
| Median (IQR) | 2116 (1587-2766) | 1842 (1325-2644) | 2110 (1604-2651) | 2266 (1751-3059) | |
| Log10 CMV IgG Index | <0.001 | ||||
| Mean (SD) | 0.63 (0.23) | 0.42 (0.09) | 0.59 (0.03) | 0.87 (0.20) | |
| Median (range) | 0.58 (0.49-0.67)b | 0.43 (0.18-0.53) | 0.59 (0.54-0.64) | 0.87 (0.64-1.15) |
P values were calculated using the Pearson's χ2 test for categorical variables and the Cuzick test for trend across CMV IgG tertiles (low to high) for continuous variables.
The interquartile range (IQR) is shown.
Abbreviations: BMI, body mass index; IgG, immunoglobulin G; IQR, interquartile range; SD, standard deviation; hs-CRP, high sensitivity C-reactive protein; sCD14, soluble CD14.
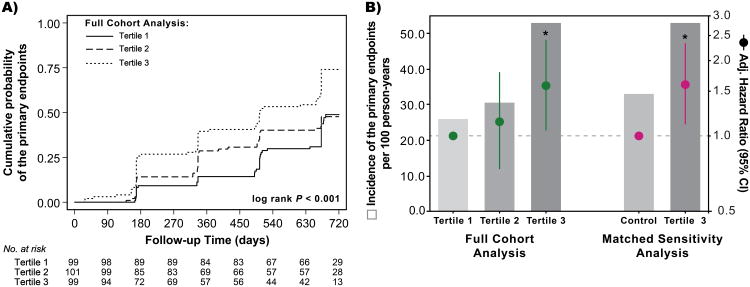
All participants contributed a total of 426.4 person-years with a median follow-up time of 1.81 years, and 150 women reached a primary endpoint (including 9 non-traumatic deaths). Ten women were lost to follow-up, of which 5 were in the highest CMV IgG tertile. Compared to women in the low CMV IgG tertile at baseline, women in the highest CMV IgG tertile had a greater cumulative probability of reaching an endpoint (Plog-rank<0.001;Fig.1A). The incidence of primary endpoints was 25.9/100 person-years (41/158.4 person-years) for the low CMV IgG tertile, 30.5/100 person-years (44/144.3 person-years) for the middle CMV IgG tertile group, and 52.6/100 person-years (65/123.6 person-years) for the high CMV IgG tertile group. Compared to women in the low CMV IgG tertile at baseline, there was an increased relative hazard of HIV disease progression for women in the high CMV IgG tertile (HR=2.21;[95%CI=1.49-3.27];P<0.001). This association was significant despite adjustment for study arm, age, baseline CD4+ T-cell count, and baseline HIV VL (adjHR=1.59;[95%CI=1.05-2.39];P=0.027;Fig.1B). A similar effect was observed when comparing women in the high CMV IgG tertile at baseline to the matched control group (adjHR=1.61;[95%CI=1.11-2.33];P=0.012;Fig.1B).
Figure 1. Baseline Cytomegalovirus IgG Antibody Levels Are Associated with HIV-1 Disease Progression.
(A) Cumulative probability of the primary endpoints by baseline CMV IgG tertiles in the full cohort. (B) Incidence of the primary endpoints and adjusted hazard ratios by baseline CMV IgG tertiles. The full cohort analysis of all 300 women was adjusted for study arm, age group, CD4+ T-cell count, and HIV viral load at baseline, and the referent was the lowest baseline CMV IgG tertile. The propensity-matched analysis accounted for CD4+ T-cell count and HIV viral load at baseline, and the Cox regression model was further adjusted for study arm and age group. See methods for derivation of the propensity-matched control group.
Note: * indicates P < 0.05.
In an analysis of 1,200 person-visits right-censored for ART initiation, baseline CMV IgG antibody levels were associated with annual changes in immune activation markers independent of time-updated CD4+ T-cell count, log10 HIV VL, and a study arm and time interaction. Women in the low CMV IgG tertile at baseline had annual decreases in sCD14 (β=-158.6 ng/mL/year;[SE=69.3];P=0.022) during follow-up, but women in the high CMV IgG tertile had an annual increase in sCD14 (β=199.1 ng/mL/year;[SE=118];P=0.091). Women in the high CMV IgG tertile at baseline also had an annual increase in hs-CRP (β=0.15 log10 mg/L/year;[SE=0.05];P=0.001), and a similar trend was observed for women in the middle CMV IgG tertile (β=0.05 log10 mg/L/year;[SE=0.05];P=0.186). Women in the low CMV IgG tertile did not have a significant annual change in hs-CRP (β=-0.15 log10mg/L/year;[SE=0.034];P=0.671). The rates of annual change in sCD14 and hs-CRP were significantly different between women in the lowest and highest CMV IgG tertiles at baseline (P<0.05).
There were 70 women with CMV IgG antibody measured at ≥1 pre-ART and post-ART visit. In a person-level analysis, post-ART log10 CMV IgG index levels were higher than pre-ART log10 CMV IgG index levels (P=0.006;Fig.S1). The median time on ART was 140 days (IQR=112-420). In a visit-specific analysis after ART initiation (n=95 person-visits), CMV IgG antibody levels above the post-ART median (>0.84 log10CMV IgG index) were associated with higher levels of sCD14 (β=421.2ng/mL;[SE=179.9];P=0.019) and hs-CRP (β=0.18 log10mg/L;[SE=0.08];P=0.028) independent of study arm, age, time-updated CD4+ T-cell count, and nadir pre-ART CD4+ T-cell count.In a sub-analysis of women with virologic suppression (<400copies/mL;n=88 person-visits), the adjusted difference in sCD14 and hs-CRP levels by the median level of CMV IgG antibody remained the same (P<0.05).
Discussion
In this prospective study, elevated CMV IgG antibody levels at baseline were associated with untreated HIV disease progression in Rakai, Uganda. Elevated CMV IgG antibody levels at baseline were also associated with higher levels and annual increases in pre-ART sCD14 and hs-CRP levels. Subclinical CMV replication during HIV infection (prior to clinical AIDS) is common [25, 39], and in a previous study among women nested in this cohort, we noted that 59% of women had detectable vaginal CMV shedding during the six-month interval prior to ART initiation [40]. Taken together, these data support the hypothesis that CMV may contribute to systemic immune activation during untreated HIV infection, and may be a co-factor in HIV disease progression in sub-Saharan Africa.
The increase in CMV IgG antibody levels after ART may indeed be due to immune reconstitution [41], but may in-part be reflective of a subclinical immune reconstitution inflammatory effect [42]. Among women nested in this cohort, we previously observed an increase in vaginal CMV shedding 2-4 months following ART initiation, and that vaginal CMV shedding was associated with elevated vaginal markers of immune activation [40, 43]. Similarly, among women on ART for a median of 4-5 months, we noted that elevated post-ART CMV IgG antibody levels were associated with higher plasma immune activation markers. There is considerable variability between studies in precisely which immune activation markers are associated with post-ART CMV IgG levels [11, 13, 26, 44-47], but our findings are consistent with the model suggesting that CMV has a role in driving persistent immune activation during suppressive ART [25]. The durability of these associations require confirmation during long-term ART use.
This study has limitations. Due to the high prevalence of CMV among adults in sub-Saharan Africa, it is difficult to directly estimate the effect of CMV co-infection on chronic HIV disease. We therefore measured CMV IgG antibody levels, however, this biomarker cannot be directly interpreted as a surrogate for CMV replication in HIV-infected individuals [48]. As discussed elsewhere [19, 22, 25], CMV IgG antibody levels may reflect one or a combination of the following: 1) lifetime burden of CMV replication and reinfection, 2) recent CMV reactivation or reinfection, or 3) strength of the host immune response to suppress low-level CMV replication. Since we had limited knowledge of other co-infections, and did not measure B-cell activation markers or total IgG levels, we cannot exclude the potential for residual confounding, particularly due to aberrant B-cell activation. Notably, however, a previous study demonstrated increases in CMV-specific IgG antibody levels during HIV disease progression that were not seen for IgG levels specific to other co-infections [49], and weak correlations have been observed between total IgG levels and CMV lysate antibody levels among HIV-infected individuals on ART [12]. It should also be noted that the results from this study may not be generalizable to men or resource-rich settings.
In summary, the magnitude of the CMV-specific humoral immune response was associated with HIV disease progression and immune activation among Ugandan women living with HIV. The role of CMV during chronic HIV infection warrants further investigation in sub-Saharan Africa.
Supplementary Material
Acknowledgments
The authors are grateful to the study participants in the trial, and to the study staff of the Rakai Health Sciences Program. Support for data management was provided in-part by the Office of Cyber Infrastructure and Computational Biology, National Institute of Allergy and Infectious Diseases.
Financial Support: This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health AI001040, and extramural funding from the National Institutes of Health AI036214.
Footnotes
Conflicts of Interest: All authors declare no conflicts of interest.
Disclaimer: The funder had no role in study design; data collection, analysis, and interpretation; or writing of the manuscript. The content of this study does not necessarily reflect the views or policies of the Dept. of Health and Human Services. The mention of trade names, commercial products or organizations does not imply endorsement by the United States Government.
Previous Presentation: Presented in-part at the Conference on Retroviruses and Opportunistic Infections (CROI), Boston, Massachusetts (Abstract ID: 16-740) on February 25th, 2016.
Author Contributions: TCQ, FN, DS, and SJR managed and designed the original trial. ADR, SG, SJR, EUP, AART, TCQ, and RHG designed the present study. EUP and ARK performed the laboratory experiments. EUP, KN, and MKG performed the statistical analysis. EUP, SG, ADR and SJR wrote the initial draft of the manuscript. All authors contributed to the overall intellectual content of the manuscript, critically reviewed and edited subsequent drafts, and approved the final version.
References
- 1.Webster A, Cook D, Emery V, Lee C, Grundy J, Kernoff P, et al. Cytomegalovirus infection and progression towards AIDS in haemophiliacs with human immunodeficiency virus infection. The Lancet. 1989;334(8654):63–66. doi: 10.1016/s0140-6736(89)90312-7. [DOI] [PubMed] [Google Scholar]
- 2.Leach CT, Detels R, Hennessey K, Liu Z, Visscher BR, Dudley JP, et al. A longitudinal study of cytomegalovirus infection in human immunodeficiency virus type 1-seropositive homosexual men: molecular epidemiology and association with disease progression. J Infect Dis. 1994;170(2):293–298. doi: 10.1093/infdis/170.2.293. [DOI] [PubMed] [Google Scholar]
- 3.Sabin CA, Devereux HL, Clewley G, Emery VC, Phillips AN, Loveday C, et al. Cytomegalovirus seropositivity and human immunodeficiency virus type 1 RNA levels in individuals with hemophilia. Journal of Infectious Diseases. 2000;181(5):1800–1803. doi: 10.1086/315476. [DOI] [PubMed] [Google Scholar]
- 4.Robain M, Boufassa F, Hubert JB, Dussaix E, Sadeg K, Meyer L. Is cytomegalovirus infection a co-factor in HIV-1 disease progression? Epidemiol Infect. 2000;125(2):415–420. doi: 10.1017/s0950268899004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robain M, Boufassa F, Hubert JB, Persoz A, Burgard M, Meyer L, et al. Cytomegalovirus seroconversion as a cofactor for progression to AIDS. AIDS. 2001;15(2):251–256. doi: 10.1097/00002030-200101260-00016. [DOI] [PubMed] [Google Scholar]
- 6.Kempen JH, Jabs DA, Wilson LA, Dunn JP, West SK, Tonascia J. Mortality risk for patients with cytomegalovirus retinitis and acquired immune deficiency syndrome. Clin Infect Dis. 2003;37(10):1365–1373. doi: 10.1086/379077. [DOI] [PubMed] [Google Scholar]
- 7.Deayton JR, Sabin CA, Johnson MA, Emery VC, Wilson P, Griffiths PD. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. The Lancet. 2004;363(9427):2116–2121. doi: 10.1016/S0140-6736(04)16500-8. [DOI] [PubMed] [Google Scholar]
- 8.Polk BF, Fox R, Brookmeyer R, Kanchanaraksa S, Kaslow R, Visscher B, et al. Predictors of the acquired immunodeficiency syndrome developing in a cohort of seropositive homosexual men. New England Journal of Medicine. 1987;316(2):61–66. doi: 10.1056/NEJM198701083160201. [DOI] [PubMed] [Google Scholar]
- 9.Detels R, Visscher BR, Fahey JL, Sever JL, Gravell M, Madden DL, et al. Predictors of clinical AIDS in young homosexual men in a high-risk area. Int J Epidemiol. 1987;16(2):271–276. doi: 10.1093/ije/16.2.271. [DOI] [PubMed] [Google Scholar]
- 10.Lichtner M, Cicconi P, Vita S, Cozzi-Lepri A, Galli M, Caputo SL, et al. Cytomegalovirus coinfection is associated with an increased risk of severe non–AIDS-defining events in a large cohort of HIV-infected patients. Journal of Infectious Diseases. 2015;211(2):178–186. doi: 10.1093/infdis/jiu417. [DOI] [PubMed] [Google Scholar]
- 11.Parrinello CM, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. Journal of Infectious Diseases. 2012;205(12):1788–1796. doi: 10.1093/infdis/jis276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunt SJ, Cysique LA, Lee S, Burrows S, Brew BJ, Price P. Short Communication: Do Cytomegalovirus Antibody Levels Associate with Age-Related Syndromes in HIV Patients Stable on Antiretroviral Therapy? AIDS Res Hum Retroviruses. 2016;32(6):567–572. doi: 10.1089/AID.2015.0328. [DOI] [PubMed] [Google Scholar]
- 13.Erlandson KM, Allshouse AA, Rapaport E, Palmer BE, Wilson CC, Weinberg A, et al. Physical function impairment of older, HIV-infected adults is associated with cytomegalovirus immunoglobulin response. AIDS research and human retroviruses. 2015;31(9):905–912. doi: 10.1089/aid.2015.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172(4):363–371. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One. 2011;6(2):e16103. doi: 10.1371/journal.pone.0016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gkrania-Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, Wareham NJ. Higher immunoglobulin G antibody levels against cytomegalovirus are associated with incident ischemic heart disease in the population-based EPIC-Norfolk cohort. J Infect Dis. 2012;206(12):1897–1903. doi: 10.1093/infdis/jis620. [DOI] [PubMed] [Google Scholar]
- 17.Gkrania-Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, Wareham NJ. Seropositivity and higher immunoglobulin G antibody levels against cytomegalovirus are associated with mortality in the population-based European prospective investigation of cancer–Norfolk Cohort. Clinical infectious diseases. 2013;56(10):1421–1427. doi: 10.1093/cid/cit083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffiths PD. CMV as a cofactor enhancing progression of AIDS. Journal of clinical virology. 2006;35(4):489–492. doi: 10.1016/j.jcv.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Gianella S, Massanella M, Wertheim JO, Smith DM. The Sordid Affair between Human Herpesvirus and Human Immunodeficiency Virus. Journal of Infectious Diseases. 2015:jiv148. doi: 10.1093/infdis/jiv148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. Journal of Infectious Diseases. 2011;203(10):1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin LY, Sheth PM, Persad D, Kovacs C, Kain T, Diong C, et al. Impact of CMV therapy with valganciclovir on immune activation and the HIV viral load in semen and blood: an observational clinical study. J Acquir Immune Defic Syndr. 2014;65(3):251–258. doi: 10.1097/01.qai.0000435256.34306.c1. [DOI] [PubMed] [Google Scholar]
- 22.Freeman ML, Lederman MM, Gianella S. Partners in Crime: The Role of CMV in Immune Dysregulation and Clinical Outcome During HIV Infection. Curr HIV/AIDS Rep. 2016;13(1):10–19. doi: 10.1007/s11904-016-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caby F, Guihot A, Lambert-Niclot S, Guiguet M, Boutolleau D, Agher R, et al. Determinants of a Low CD4/CD8 Ratio in HIV-1-Infected Individuals Despite Long-term Viral Suppression. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw076. [DOI] [PubMed] [Google Scholar]
- 24.Smith DM, Nakazawa M, Freeman ML, Anderson CM, Oliveira MF, Little SJ, et al. Asymptomatic CMV Replication During Early Human Immunodeficiency Virus (HIV) Infection Is Associated With Lower CD4/CD8 Ratio During HIV Treatment. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gianella S, Letendre S. Cytomegalovirus and HIV: A Dangerous Pas de Deux. J Infect Dis. 2016;214(2):S67–74. doi: 10.1093/infdis/jiw217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lurain NS, Hanson BA, Hotton AL, Weber KM, Cohen MH, Landay AL. AIDS research and human retroviruses. 2015. The Association of Human Cytomegalovirus with Biomarkers of Inflammation and Immune Activation in HIV-1-Infected Women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS. 2015;29(10):1263–1265. doi: 10.1097/QAD.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adland E, Klenerman P, Goulder P, Matthews PC. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antiretroviral therapy era. Frontiers in microbiology. 2015;6 doi: 10.3389/fmicb.2015.01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gronborg HL, Jespersen S, Honge BL, Jensen-Fangel S, Wejse C. Review of cytomegalovirus coinfection in HIV-infected individuals in Africa. Rev Med Virol. 2016 doi: 10.1002/rmv.1907. [DOI] [PubMed] [Google Scholar]
- 30.Bates M, Brantsaeter AB. Human cytomegalovirus (CMV) in Africa: a neglected but important pathogen. J Virus Erad. 2016;2(3):136–142. doi: 10.1016/S2055-6640(20)30456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miles DJ, van der Sande M, Jeffries D, Kaye S, Ismaili J, Ojuola O, et al. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J Virol. 2007;81(11):5766–5776. doi: 10.1128/JVI.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miles DJ, van der Sande M, Jeffries D, Kaye S, Ojuola O, Sanneh M, et al. Maintenance of large subpopulations of differentiated CD8 T-cells two years after cytomegalovirus infection in Gambian infants. PLoS One. 2008;3(8):e2905. doi: 10.1371/journal.pone.0002905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gantt S, Orem J, Krantz EM, Morrow RA, Selke S, Huang ML, et al. Prospective Characterization of the Risk Factors for Transmission and Symptoms of Primary Human Herpesvirus Infections Among Ugandan Infants. J Infect Dis. 2016 doi: 10.1093/infdis/jiw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldeck D, Larsen LA, Christiansen L, Christensen K, Hamprecht K, Pawelec G, et al. Genetic Influence on the Peripheral Blood CD4+ T-cell Differentiation Status in CMV Infection. J Gerontol A Biol Sci Med Sci. 2016;71(12):1537–1543. doi: 10.1093/gerona/glv230. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds SJ, Makumbi F, Newell K, Kiwanuka N, Ssebbowa P, Mondo G, et al. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. The Lancet infectious diseases. 2012;12(6):441–448. doi: 10.1016/S1473-3099(12)70037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redd AD, Newell K, Patel EU, Nalugoda F, Ssebbowa P, Kalibbala S, et al. Decreased monocyte activation with daily acyclovir use in HIV-1/HSV-2 coinfected women. Sexually transmitted infections. 2015 doi: 10.1136/sextrans-2014-051867. sextrans-2014-051867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837–2849. doi: 10.1002/sim.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris SR, Zhao M, Smith DR, Vargas MV, Little SJ, Gianella S. Longitudinal Viral Dynamics in Semen During Early HIV Infection. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gianella S, Redd AD, Grabowski MK, Tobian AA, Serwadda D, Newell K, et al. Vaginal Cytomegalovirus Shedding before and after Initiation of Antiretroviral Therapy in Rakai, Uganda. Journal of Infectious Diseases. 2015:jiv135. doi: 10.1093/infdis/jiv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deayton JR, Sabin CA, Britt WB, Jones IM, Wilson P, Johnson MA, et al. Rapid reconstitution of humoral immunity against cytomegalovirus but not HIV following highly active antiretroviral therapy. AIDS. 2002;16(16):2129–2135. doi: 10.1097/00002030-200211080-00004. [DOI] [PubMed] [Google Scholar]
- 42.Stone SF, Price P, Tay-Kearney ML, French MA. Cytomegalovirus (CMV) retinitis immune restoration disease occurs during highly active antiretroviral therapy-induced restoration of CMV-specific immune responses within a predominant Th2 cytokine environment. J Infect Dis. 2002;185(12):1813–1817. doi: 10.1086/340636. [DOI] [PubMed] [Google Scholar]
- 43.Nason MC, Patel EU, Kirkpatrick AR, Prodger JL, Shahabi K, Tobian AA, et al. Immunological Signaling During Herpes Simplex Virus-2 and Cytomegalovirus Vaginal Shedding After Initiation of Antiretroviral Treatment. Open Forum Infect Dis. 2016;3(2):ofw073. doi: 10.1093/ofid/ofw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunt SJ, Lee S, D'Orsogna L, Bundell C, Burrows S, Price P. The Use of Humoral Responses as a Marker of CMV Burden in HIV Patients on ART Requires Consideration of T-Cell Recovery and Persistent B-Cell Activation. Disease markers. 2014;2014 doi: 10.1155/2014/947432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Affandi JS, Montgomery J, Brunt SJ, Nolan D, Price P. The immunological footprint of CMV in HIV-1 patients stable on long-term ART. Immunity & Ageing. 2015;12(1):14. doi: 10.1186/s12979-015-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodowanec A, Williams B, Hanson B, Livak B, Keating S, Lurain N, et al. Soluble CD163 But Not Soluble CD14 Is Associated With Cytomegalovirus Immunoglobulin G Antibody Levels in Virologically Suppressed HIV+ Individuals. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2015;70(5):e171–e174. doi: 10.1097/QAI.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 47.Brunt SJ, Cysique LA, Lee S, Burrows S, Brew BJ, Price P. Do Cytomegalovirus Antibody Levels Associate with Age-Related Syndromes in HIV Patients Stable on Antiretroviral Therapy? AIDS Res Hum Retroviruses. 2016 doi: 10.1089/AID.2015.0328. [DOI] [PubMed] [Google Scholar]
- 48.Gianella S, Morris SR, Tatro E, Vargas MV, Haubrich RH, Daar ES, et al. Virologic correlates of anti-CMV IgG levels in HIV-1 infected men. Journal of Infectious Diseases. 2013:jit434. doi: 10.1093/infdis/jit434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lennette ET, Busch MP, Hecht FM, Levy JA. Potential herpesvirus interaction during HIV type 1 primary infection. AIDS Res Hum Retroviruses. 2005;21(10):869–875. doi: 10.1089/aid.2005.21.869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.