Kingella kingae has emerged as a significant cause of septic arthritis, osteomyelitis, and bacteremia in young children. A recent study examining a diverse collection of K. kingae isolates from Israel revealed four different polysaccharide capsule types in this species, designated types a to d. To determine the global distribution of K. kingae capsule types, we assembled and capsule typed an international collection of K. kingae isolates. The findings reported here show that the type a and type b capsules represent >95% of the invasive isolates, similar to the Israeli isolate collection, suggesting that a polysaccharide-based vaccine targeting these two capsules could be an attractive approach to prevent K. kingae disease.
KEYWORDS: Kingella kingae, PCR, capsule typing, clinical isolates, polysaccharide capsule
ABSTRACT
Kingella kingae is an encapsulated Gram-negative bacterium and an important etiology of osteoarticular infections in young children. A recent study examining a diverse collection of carrier and invasive K. kingae isolates from Israel revealed four distinct polysaccharide capsule types. In this study, to obtain a global view of K. kingae capsule type diversity, we examined an international collection of isolates using a multiplex PCR approach. The collection contained all four previously identified capsule types and no new capsule types. Over 95% of invasive isolates in the collection were type a or type b, similar to the findings in Israel. These results suggest that the type a and type b polysaccharide capsules may have enhanced pathogenic properties or may mark clonal groups of strains with specific virulence genes. In addition, they raise the possibility that a vaccine containing the type a and type b capsules might be an effective approach to preventing K. kingae disease.
IMPORTANCE Kingella kingae has emerged as a significant cause of septic arthritis, osteomyelitis, and bacteremia in young children. A recent study examining a diverse collection of K. kingae isolates from Israel revealed four different polysaccharide capsule types in this species, designated types a to d. To determine the global distribution of K. kingae capsule types, we assembled and capsule typed an international collection of K. kingae isolates. The findings reported here show that the type a and type b capsules represent >95% of the invasive isolates, similar to the Israeli isolate collection, suggesting that a polysaccharide-based vaccine targeting these two capsules could be an attractive approach to prevent K. kingae disease.
OBSERVATION
The use of improved culture techniques and PCR-based diagnostics in recent years has revealed that the Gram-negative bacterium Kingella kingae is a significant etiology of osteoarticular infections in children 6 to 48 months of age in countries where these sensitive detection methods are routinely employed (1). K. kingae is a normal component of the upper respiratory tract flora in young children and is present in the posterior pharynx in approximately 10% of healthy children 6 to 48 months of age at any given point in time (2–5). This organism is readily transmitted from person to person by close contact among young children, and longitudinal studies have estimated that children have an approximately 70% chance of being colonized with K. kingae during the first 2 years of life (3, 6).
In most individuals, colonization with K. kingae persists for weeks to months and is then cleared (3, 6). On occasion, the organism is able to breach the respiratory epithelial barrier, enter the bloodstream, and disseminate to distant sites, causing invasive disease. Analysis of the K. kingae population structure suggests that only some K. kingae strains are able to cause invasive disease (7). The primary clinical presentations of K. kingae disease include septic arthritis, osteomyelitis, spondylodiscitis, tenosynovitis, bacteremia without a focus, and endocarditis (8). The annual incidence of culture-proven disease among children younger than 5 years of age in Israel is 9.4 per 100,000. However, given the difficulty in cultivating K. kingae, this figure represents a minimal estimate (9). Recognizing that the use of species-specific nucleic acid amplification improves the detection of K. kingae by 500% compared to its detection by culture, the true incidence of K. kingae invasive disease in the Israeli population is likely comparable to the incidence of invasive Haemophilus influenzae type b disease before the introduction of H. influenzae type b conjugate vaccines (Hib conjugate vaccines) (i.e., >50 per 100,000) (10). The incidence of K. kingae invasive disease in other countries has not been defined but appears to be high in parts of Europe and a number of other countries around the world.
Recent studies have shown that isolates of K. kingae elaborate a polysaccharide capsule (11–13). Interestingly, elimination of encapsulation results in attenuated virulence in an infant rat model of invasive disease (14), suggesting that the capsule is an important virulence factor. Although the specific mechanism by which the capsule facilitates invasive disease has not been defined, the polysaccharide capsules of other pathogenic organisms prevent phagocytosis and block complement-mediated serum killing, promoting bacterial survival in the host. Given this information and the widespread success of polysaccharide conjugate vaccines in reducing morbidity and mortality due to a variety of encapsulated pathogenic bacteria, we recently defined the polysaccharide capsule repertoire in a diverse set of >400 Israeli K. kingae isolates (13). We found that four distinct polysaccharide capsule structures (capsule types a, b, c, and d) were present in this collection and that >95% of invasive disease isolates expressed the type a or type b capsule. Furthermore, we identified the csa, csb, csc, and csd capsule synthesis loci that are necessary for the expression of the type a, type b, type c, and type d capsules, respectively.
To gain a broader view of capsule type diversity in the global K. kingae population, in this study, we assembled an international collection of K. kingae isolates and determined the capsule type of each isolate using a multiplex PCR approach. We found that the same four capsule types identified in the Israeli isolate collection were present in the international collection and that no new capsule types were present. In addition, we established that over 95% of invasive isolates expressed the type a or type b capsule.
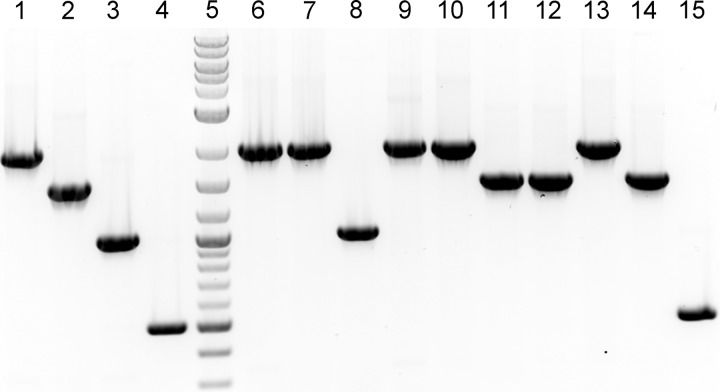
In a previous study, we developed a K. kingae capsule type PCR screening approach that relied on the use of five separate reactions: reactions specific to each of the four capsule types and a reaction that used primers flanking the capsule synthesis locus (13). To streamline the K. kingae capsule-typing process, in this work, we developed a single multiplex PCR approach for one-step identification of the capsule type. The reaction mixture contains four sets of primer pairs specific to each of the four capsule synthesis loci, designed to each produce a different size amplicon. As shown by the results in Fig. 1, the multiplex PCR produces an ~2,000-bp amplicon for the csa locus (encoding the synthesis gene for the type a capsule), an ~1,500-bp amplicon for the csb locus (encoding the synthesis genes for the type b capsule), an ~1,000-bp amplicon for the csc locus (encoding the synthesis genes for the type c capsule), and an ~500-bp amplicon for the csd locus (encoding the synthesis genes for the type d capsule).
FIG 1 .
International K. kingae isolates are represented by four capsule types. (A) A representative agarose gel of the multiplex capsule-typing PCR approach is shown. The type a amplicon is ~2,000 bp (lane 1), the type b amplicon is ~1,500 bp (lane 2), the type c amplicon is ~1,000 bp (lane 3), and the type d amplicon is ~500 bp (lane 4). The genomic DNA PCR template sources are as follows: lane 1, KK01 (type a control); lane 2, KK58 (type b control); lane 3, KK60 (type c control); lane 4, BB270 (type d control); lane 5, DNA ladder; lane 6, 16RZ2819K (type a); lane 7, 16SB9163M (type a); lane 8, ATCC 23330 (type c); lane 9, ATCC 23331 (type a); lane 10, ATCC 23332 (type a); lane 11, SW353 (type b); lane 12, SW628 (type b); lane 13, SW268 (type a); lane 14, AUS 01 (type b); lane 15, KK194 (type d).
To investigate global capsule type diversity in K. kingae, we assembled an international strain collection consisting of 150 isolates (Table 1). Genomic DNA was recovered from each isolate and was used as the template in the multiplex PCR assay. We hypothesized that any isolate that failed to produce a capsule locus-specific amplicon could potentially contain a novel capsule synthesis locus or could be a nonencapsulated strain. In total, 89 isolates were capsule type a (59.3%), 49 isolates were type b (32.7%), 8 isolates were type c (5.3%), 3 isolates were type d (2.0%), and 1 isolate (0.7%) yielded no PCR product. Further examination of the isolate that yielded no PCR product revealed that it was nonencapsulated, as assessed by alcian blue staining of surface extracts and inspection of colony morphology (Fig. 2). Using PCR primers that flank the capsule synthesis locus, a 2.5-kb product was amplified from this isolate. Sequencing of this product revealed a truncated csa locus (data not shown). Accordingly, for the purposes of this study, this isolate was considered capsule type a.
TABLE 1 .
International isolate collection used in this study
| Isolate | Location | Year | Syndromea | Capsule type |
|---|---|---|---|---|
| CA7 | Catalonia, Spain | 1997 | OA | a |
| CA20 | Catalonia, Spain | 1998 | OA | a |
| CA40 | Catalonia, Spain | 2000 | OA | b |
| CA48 | Catalonia, Spain | 2001 | OA | b |
| CA49 | Catalonia, Spain | 2001 | OA | b |
| CA55 | Catalonia, Spain | 2001 | OA | c |
| CA57 | Catalonia, Spain | 2002 | OA | a |
| CA61 | Catalonia, Spain | 2003 | OA | a |
| CA63 | Catalonia, Spain | 2004 | B | a |
| CA64 | Catalonia, Spain | 2004 | B | a |
| CA65 | Catalonia, Spain | 2004 | B | b |
| CA66 | Catalonia, Spain | 2004 | OA | a |
| CA67 | Catalonia, Spain | 2004 | B | b |
| CA68 | Catalonia, Spain | 2004 | OA | a |
| CA73 | Catalonia, Spain | 2006 | OA | b |
| CA75 | Catalonia, Spain | 2006 | B | b |
| CA77 | Catalonia, Spain | 2006 | OA | a |
| CA78 | Catalonia, Spain | 2006 | OA | a |
| CA83 | Catalonia, Spain | 2007 | OA | a |
| CA84 | Catalonia, Spain | 2007 | OA | a |
| CA85 | Catalonia, Spain | 2007 | OA | b |
| CA88 | Catalonia, Spain | 2008 | OA | a |
| CA94 | Catalonia, Spain | 2008 | OA | a |
| CA95 | Catalonia, Spain | 2008 | OA | a |
| CA99 | Catalonia, Spain | 2009 | OA | b |
| CA105 | Catalonia, Spain | 2009 | B | a |
| CA112 | Catalonia, Spain | 2009 | OA | a |
| CA151 | Catalonia, Spain | 2009 | OA | b |
| CA179 | Catalonia, Spain | 2010 | OA | a |
| CA120 | Catalonia, Spain | 2010 | B | a |
| CA122 | Catalonia, Spain | 2010 | OA | b |
| CA129 | Catalonia, Spain | 2011 | OA | a |
| CA131 | Catalonia, Spain | 2011 | OA | c |
| CA138 | Catalonia, Spain | 2012 | OA | a |
| CA139 | Catalonia, Spain | 2012 | C | d |
| CA181 | Catalonia, Spain | 2014 | OA | b |
| CA183 | Catalonia, Spain | 2014 | C | a |
| CA189 | Catalonia, Spain | 2014 | OA | a |
| CA197 | Catalonia, Spain | 2014 | OA | a |
| CA198 | Catalonia, Spain | 2014 | OA | b |
| CA199 | Catalonia, Spain | 2014 | OA | b |
| CA202 | Catalonia, Spain | 2015 | OA | a |
| CA203 | Catalonia, Spain | 2015 | OA | b |
| CA216 | Catalonia, Spain | 2016 | B | a |
| CA217 | Catalonia, Spain | 2016 | OA | b |
| CIP 73.01 | Besançon, France | 1972 | B | a |
| CIP 101722 | Grenoble, France | 1985 | B | a |
| CIP 102473 | Paris, France | 1986 | OA | b |
| SCH 25972 | Paris, France | 2007 | OA | b |
| BIA 29991 | Paris, France | 2009 | OA | a |
| SAN 6539 | Bordeaux, France | 2009 | OA | b |
| LEP 6724 | Bordeaux, France | 2009 | UNb | b |
| BOU 30672 | Paris, France | 2010 | OA | a |
| N10-6602 | Nantes, France | 2010 | OA | a |
| N10-6318 | Nantes, France | 2010 | OA | a |
| HER 31223 | Paris, France | 2010 | OA | a |
| N10-10770 | Nantes, France | 2010 | B | d |
| GRO 7183 | Bordeaux, France | 2010 | OA | a |
| N10-10419 | Nantes, France | 2010 | EC | b |
| ETI 126580 | Paris, France | 2011 | OA | a |
| HAM 137138 | Paris, France | 2011 | OA | b |
| SAI 11985 | Paris, France | 2011 | OA | a |
| POH 14284 | Paris, France | 2011 | OA | a |
| MAR 1853 | Paris, France | 2011 | OA | a |
| STF 4A | Paris, France | 2011 | C | a |
| DER 112012–1 | Paris, France | 2012 | OA | a |
| NICE 476 | Nice, France | 2012 | OA | b |
| DOR 8225 | Bordeaux, France | 2012 | UN | a |
| CAT30640171-S | Paris, France | 2013 | OA | b |
| COU 1310053120 | Sables d’Olonnes, France | 2013 | EC | a |
| FOF 3022006-S | Paris, France | 2013 | OA | b |
| DAG 31560001-S | Paris, France | 2013 | OA | a |
| ZEH 30720143-S | Paris, France | 2013 | OA | a |
| BRU32800001LA | Paris, France | 2013 | OA | a |
| KWG-1 | Paris, France | 2013 | OA | a |
| AUD31930140-S | Paris, France | 2013 | OA | a |
| BON 36648-la | Paris, France | 2013 | OA | b |
| ZUL 30220039-S | Paris, France | 2013 | C | b |
| CRA 32950107-S | Paris, France | 2013 | UN | a |
| AGO 30220109-S | Paris, France | 2013 | OA | a |
| M2003000170 | Minnesota, USA | 2003 | C | a |
| C2003003154 | Minnesota, USA | 2003 | OA | a |
| M2004000037 | Minnesota, USA | 2004 | C | b |
| C2005003818 | Minnesota, USA | 2005 | B | a |
| C2005004024 | Minnesota, USA | 2005 | B | a |
| C2005004457 | Minnesota, USA | 2005 | OA | b |
| C2005004524 | Minnesota, USA | 2005 | OA | b |
| C2006001196 | Minnesota, USA | 2006 | OA | b |
| C2006001744 | Minnesota, USA | 2006 | OA | a |
| C2006003006 | Minnesota, USA | 2006 | B | a |
| C2007000490 | Minnesota, USA | 2007 | OA | b |
| Duke 137 | North Carolina, USA | 2007 | EC | a |
| C2009000170 | Minnesota, USA | 2009 | B | b |
| C2009033016 | Minnesota, USA | 2009 | OA | a |
| C2012000896 | Minnesota, USA | 2012 | OA | a |
| 13040 | Pennsylvania, USA | 2016 | OA | a |
| 97–982 | Missouri, USA | UN | OA | a |
| SLCH 05-001-1817 | Missouri, USA | UN | OA | a |
| SLCH 002 | Missouri, USA | UN | OA | b |
| PP1 | New York, USA | UN | OA | a |
| VTK:500285 | USA | UN | UN | c |
| VTK:500287 | USA | UN | UN | c |
| VTK:501585 | USA | UN | UN | a |
| VTK:501586 | USA | UN | UN | c |
| VTK:500615 | USA | UN | UN | a |
| VTK:501628 | USA | UN | UN | a |
| VTK:501629 | USA | UN | UN | a |
| VTK:501804 | USA | UN | UN | a |
| CAN1 | Montreal, Canada | 2003 | OA | a |
| CAN2 | Montreal, Canada | 2005 | OA | a |
| CAN3 | Montreal, Canada | 2005 | OA | a |
| CAN7 | Montreal, Canada | 2006 | OA | b |
| CAN8c | Montreal, Canada | 2007 | OA | a |
| CAN9 | Montreal, Canada | 2007 | OA | a |
| CAN13 | Montreal, Canada | 2009 | OA | b |
| CAN16 | Montreal, Canada | 2010 | OA | b |
| CAN21 | Montreal, Canada | 2012 | OA | b |
| CAN22 | Montreal, Canada | 2012 | B | b |
| CAN24 | Montreal, Canada | 2013 | OA | a |
| CAN25 | Montreal, Canada | 2013 | OA | a |
| 9508+31135 | Reykjavíc, Iceland | 1995 | OA | b |
| 9911+17199 | Reykjavíc, Iceland | 1999 | OA | a |
| 0111+28183 | Reykjavíc, Iceland | 2001 | OA | b |
| 0211+12480 | Reykjavíc, Iceland | 2002 | OA | b |
| 0303+28260 | Reykjavíc, Iceland | 2003 | OA | b |
| 0309+30201 | Reykjavíc, Iceland | 2003 | OA | c |
| 0405+30002 | Reykjavíc, Iceland | 2004 | OA | b |
| 0410+23083 | Reykjavíc, Iceland | 2004 | B | a |
| 0601+26281 | Reykjavíc, Iceland | 2006 | OA | a |
| 0604+12258 | Reykjavíc, Iceland | 2006 | OA | b |
| S0910230213 | Reykjavíc, Iceland | 2009 | OA | b |
| S1010080184 | Reykjavíc, Iceland | 2010 | OA | c |
| 09WG5552P | Christchurch, New Zealand | 2009 | B | a |
| 09WT1836F | Christchurch, New Zealand | 2009 | B | a |
| 11DC5983H | Christchurch, New Zealand | 2011 | B | a |
| 15JS24141R | Christchurch, New Zealand | 2015 | B | a |
| 15RB7013L | Christchurch, New Zealand | 2015 | OA | a |
| 15RJ0022G | Christchurch, New Zealand | 2015 | OA | a |
| 15R43594M | Christchurch, New Zealand | 2015 | C | a |
| 16RZ0774E | Christchurch, New Zealand | 2016 | C | a |
| 16RZ2819K | Christchurch, New Zealand | 2016 | C | a |
| 16SB9163M | Christchurch, New Zealand | 2016 | C | a |
| ATCC 23330 | Norway | 1960s | C | c |
| ATCC 23331 | Norway | 1960s | B | a |
| ATCC 23332 | Norway | 1960s | B | a |
| SW353 | Switzerland | 2013 | C | b |
| SW628 | Switzerland | 2014 | OA | b |
| SW268 | Switzerland | 2015 | OA | a |
| AUS 01 | Geelong, Australia | 2013 | EC | b |
| KK194 | St. Petersburg, Russia | 2003 | C | d |
B, bacteremia; C, carriage; EC, endocarditis; OA, osteoarticular.
UN, unknown.
Isolate CAN8 is nonencapsulated due to a truncated csaA gene but was included as a type a isolate for the purposes of this analysis.
FIG 2 .
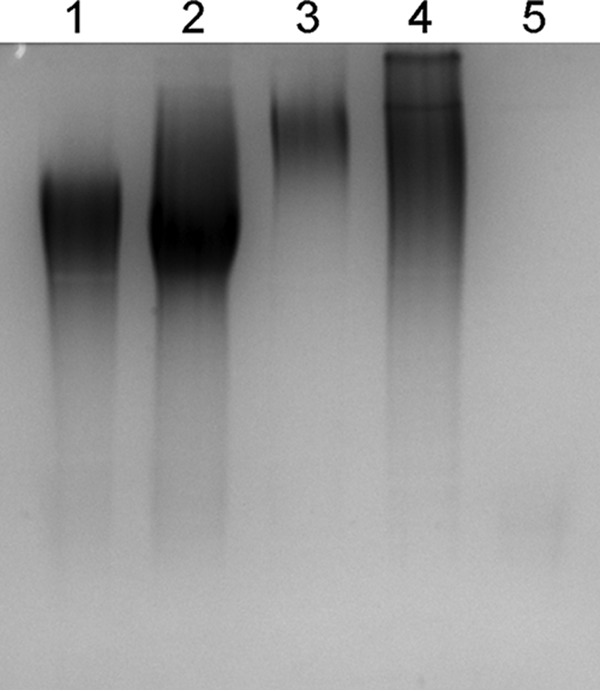
Isolate CAN8 is nonencapsulated. Mild-acid surface extracts from capsule type a strain KK01 (lane 1), type b strain KK58 (lane 2), type c strain KK60 (lane 3), type d strain BB270 (lane 4), and isolate CAN8 (lane 5) were analyzed following SDS-PAGE and alcian blue staining. The lack of alcian blue-stained material in the extract of CAN8 confirms that this isolate is nonencapsulated.
A detailed summary of the K. kingae capsule-typing results by country and syndrome (invasive isolate, carrier isolate, or unknown) at the time of isolation is shown in Table 2. Among the 126 isolates that were known to be recovered from systemic sites in patients with invasive K. kingae disease (osteoarticular infections, bacteremia, or endocarditis), 76 were type a (60.3%), 45 were type b (35.7%), 4 were type c (3.2%), and 1 was type d (0.8%), similar to the breakdown among invasive isolates in Israel (13). Of the 13 carrier isolates from oropharyngeal swabs, 7 were type a (53.8%), 3 were type b (23.1%), 1 was type c (7.7%), and 2 were type d (15.4%). While the small number of carrier isolates precludes any meaningful analysis of capsule type distribution, the relative representation of each type was similar to the distribution in the previously published Israeli carrier isolate collection (49.0% type a, 19.2% type b, 12.1% type c, and 19.7% type d) (13). With regard to the countries with at least 10 isolates, >80% of isolates were type a or type b, and in all countries except Iceland, the most common capsule type was type a. Interestingly, all 10 of the New Zealand isolates were type a, including the 4 carrier isolates.
TABLE 2 .
Summary of capsule-typing results based on country of isolation and associated clinical condition
| Country (no. of isolates) | No. of isolates from indicated clinical condition with indicated capsule type |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Invasive |
Carrier |
Unknown |
|||||||||||||
| Total no. | a | b | c | d | Total no. | a | b | c | d | Total no. | a | b | c | d | |
| Spain (45) | 43 | 25 | 16 | 2 | 2 | 1 | 1 | ||||||||
| France (36) | 30 | 20 | 9 | 1 | 2 | 1 | 1 | 3 | 2 | 1 | |||||
| USA (28) | 18 | 12 | 6 | 2 | 1 | 1 | 8 | 5 | 3 | ||||||
| Canada (12) | 12 | 7a | 5 | ||||||||||||
| Iceland (12) | 12 | 3 | 7 | 2 | |||||||||||
| New Zealand (10) | 6 | 6 | 4 | 4 | |||||||||||
| Norway (3) | 2 | 2 | 1 | 1 | |||||||||||
| Switzerland (3) | 2 | 1 | 1 | 1 | 1 | ||||||||||
| Australia (1) | 1 | 1 | |||||||||||||
| Russia (1) | 1 | 1 | |||||||||||||
| Total (150) | 126 | 76 | 45 | 4 | 1 | 13 | 7 | 3 | 1 | 2 | 11 | 7 | 1 | 3 | |
Isolate CAN8 is nonencapsulated due to a truncated csaA gene but was included as a type a isolate for the purposes of this analysis.
The four previously identified K. kingae capsule types that were originally characterized in a diverse collection of Israeli isolates were also identified in the international collection of isolates presented here. No new capsule types were identified in this international collection, confirming the limited capsular repertoire of K. kingae compared to those of other pathogens that reside in the respiratory tract, such as Neisseria meningitidis (13 capsule types) and Streptococcus pneumoniae (over 90 capsule types). The study has the limitation that carrier isolates were poorly represented, reflecting the lack of K. kingae colonization studies using culture-based methods outside Israel. As a consequence, we cannot exclude the possibility that other capsule types or nonencapsulated strains exist in the countries examined in this study, keeping in mind the likelihood that some K. kingae strains are able to colonize the oropharynx but are unable to cause invasive disease. In addition, there is little information on the epidemiology of K. kingae disease in developing countries, which may harbor unidentified capsule types. Another limitation of this study is the lack of complete epidemiological data on every isolate. Therefore, the capsule type distribution may be skewed due to the presence of epidemiologically related strains in this collection.
Given that the capsule-typing multiplex PCR approach is specific for the four known K. kingae capsule types, we anticipated that new capsule types in this international collection of isolates would be associated with the lack of a PCR amplicon. The only isolate that failed to yield an amplicon in the multiplex PCR was strain CAN8. Using PCR primers that flank the capsule synthesis locus, we were able to amplify a 2.5-kb amplicon from this strain. Nucleotide sequencing of this amplicon revealed a truncated csaA gene with a large, 1,085-bp deletion in the 3′ region, resulting in a nonencapsulated phenotype. Thus, CAN8 is a type a strain that presumably experienced a deletion event and does not have a novel capsule type.
In conclusion, we found that 95.1% of the international invasive disease isolates were either capsule type a or b, virtually identical to the 96.0% of Israeli invasive disease isolates that were either type a or type b (13). This finding is similar to the situation with H. influenzae, where isolates expressing the serotype b capsule were responsible for >95% of all invasive H. influenzae disease in the pre-Hib vaccine era, despite the presence of 6 different polysaccharide capsule types (capsule types a to f) (15). It is possible that the K. kingae type a and type b polysaccharide capsules have enhanced pathogenic properties. Alternatively, these capsules may be associated with clonal groups of strains that harbor important virulence genes. Because anticapsular antibody protects against disease caused by organisms such as H. influenzae type b and S. pneumoniae, it is intriguing to speculate that a type a-type b capsular polysaccharide conjugate vaccine might be an effective strategy to prevent disease by K. kingae.
Bacterial strains.
The international K. kingae isolates examined in this study are listed in Table 1. The capsule type a (KK01), capsule type b (KK58), capsule type c (KK60), and capsule type d (BB270) control strains were described previously (13). The K. kingae isolates were grown on chocolate II agar (BD, Sparks, MD) for 17 to 18 h at 37°C in a humidified 5% CO2 atmosphere. The isolates were stored in brain heart infusion (BHI) broth with 20% glycerol at −80°C.
Capsule typing.
A multiplex PCR strategy was developed to screen for the presence of each of the four unique capsule synthesis loci in a single PCR assay. The primers were designed to produce an ~2,000-bp amplicon for the csa locus (csamultiF, 5′ AGTACAGAACACTTGTTGTTGC 3′, and csamultiR, 5′ AACATTGGCGCAGACAAATTC 3′), an ~1,500-bp amplicon for the csb locus (csbmultiF, 5′ AGATTGGTGGACTTTATATGGTAATTATG 3′, and csbmultiR, 5′ AAATAGAATATTGCGACTGTGCG 3′), an ~1,000-bp amplicon for the csc locus (cscmultiF, 5′ CATTAGCATTGATGCCATTTATGAAC 3′, and cscmultiR, 5′ CGATTGATGACTATTAAACCTTCGG 3′), and an ~500-bp amplicon for the csd locus (csdmultiF, 5′ AAAGGTAAATATCAATTTGCAATTATTTGC 3′, and csdmultiR, 5′ CTTAATAGGACATCATCACCCAAATC 3′). Genomic DNA from the K. kingae isolates was prepared using the Wizard genomic DNA purification kit (Promega, Madison, WI). Each PCR mixture contained 5 μl of 2.0× Taq red Apex master mix (Genesee Scientific, San Diego, CA), 0.5 μl of genomic DNA template, each of the eight primers at a final concentration of 125 nM, and sterile PCR-grade H2O in a total reaction mixture volume of 10 μl. The cycling conditions were as follows: 2 min at 94°C, 30 cycles of 15 s at 94°C, 20 s at 58°C, and 1 min at 72°C, and a final 5-min extension at 72°C. Three microliters of each PCR mixture was analyzed using agarose gel electrophoresis, and the capsule type was determined by the size of the amplicon (Fig. 1). The accuracy of this multiplex PCR strategy in determining capsule type was confirmed by comparing the results with the previously published results of the original PCR-based capsule-typing system (13).
Alcian blue staining.
Surface extracts were prepared using Tris-acetate, pH 5.0, separated using SDS-PAGE, and stained with alcian blue as described previously (11).
ACKNOWLEDGMENTS
We extend our sincerest thanks to Carmen Muñoz-Almagro, Stephane Bonacorsi, Nataliya Balashova, Tony Walls, Anja Werno, Joanne Mitchell, and Jacques Schrenzel for contributing isolates to the international collection. This study would not have been possible without their generous contributions.
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health under grant numbers R56AI103125 and 1R01AI121015 to J.W.S.G.
The authors declare that there are no conflicts of interest.
This work has not been presented at any meetings.
REFERENCES
- 1.Chometon S, Benito Y, Chaker M, Boisset S, Ploton C, Bérard J, Vandenesch F, Freydiere AM. 2007. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J 26:377–381. doi: 10.1097/01.inf.0000259954.88139.f4. [DOI] [PubMed] [Google Scholar]
- 2.Anderson de la Llana R, Dubois-Ferriere V, Maggio A, Cherkaoui A, Manzano S, Renzi G, Hibbs J, Schrenzel J, Ceroni D. 2015. Oropharyngeal Kingella kingae carriage in children: characteristics and correlation with osteoarticular infections. Pediatr Res 78:574–579. doi: 10.1038/pr.2015.133. [DOI] [PubMed] [Google Scholar]
- 3.Yagupsky P, Dagan R, Prajgrod F, Merires M. 1995. Respiratory carriage of Kingella kingae among healthy children. Pediatr Infect Dis J 14:673–678. doi: 10.1097/00006454-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Yagupsky P, Peled N, Katz O. 2002. Epidemiological features of invasive Kingella kingae infections and respiratory carriage of the organism. J Clin Microbiol 40:4180–4184. doi: 10.1128/JCM.40.11.4180-4184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceroni D, Dubois-Ferrière V, Anderson R, Combescure C, Lamah L, Cherkaoui A, Schrenzel J. 2012. Small risk of osteoarticular infections in children with asymptomatic oropharyngeal carriage of Kingella kingae. Pediatr Infect Dis J 31:983–985. doi: 10.1097/INF.0b013e31825d3419. [DOI] [PubMed] [Google Scholar]
- 6.Slonim A, Walker ES, Mishori E, Porat N, Dagan R, Yagupsky P. 1998. Person-to-person transmission of Kingella kingae among day care center attendees. J Infect Dis 178:1843–1846. doi: 10.1086/314488. [DOI] [PubMed] [Google Scholar]
- 7.Amit U, Porat N, Basmaci R, Bidet P, Bonacorsi S, Dagan R, Yagupsky P. 2012. Genotyping of invasive Kingella kingae isolates reveals predominant clones and association with specific clinical syndromes. Clin Infect Dis 55:1074–1079. doi: 10.1093/cid/cis622. [DOI] [PubMed] [Google Scholar]
- 8.Yagupsky P. 2015. Kingella kingae: carriage, transmission, and disease. Clin Microbiol Rev 28:54–79. doi: 10.1128/CMR.00028-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubnov-Raz G, Ephros M, Garty BZ, Schlesinger Y, Maayan-Metzger A, Hasson J, Kassis I, Schwartz-Harari O, Yagupsky P. 2010. Invasive pediatric Kingella kingae infections: a nationwide collaborative study. Pediatr Infect Dis J 29:639–643. doi: 10.1097/INF.0b013e3181d57a6c. [DOI] [PubMed] [Google Scholar]
- 10.Yagupsky P. 2016. Advances in diagnosis of Kingella kingae disease, p 49–63. In St Geme JW III (ed), Advances in understanding Kingella kingae, 1st ed. Springer, Cham, Switzerland. [Google Scholar]
- 11.Porsch EA, Kehl-Fie TE, St Geme JW III. 2012. Modulation of Kingella kingae adherence to human epithelial cells by type IV pili, capsule, and a novel trimeric autotransporter. mBio 3:e00372-12. doi: 10.1128/mBio.00372-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendaoud M, Vinogradov E, Balashova NV, Kadouri DE, Kachlany SC, Kaplan JB. 2011. Broad-spectrum biofilm inhibition by Kingella kingae exopolysaccharide. J Bacteriol 193:3879–3886. doi: 10.1128/JB.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starr KF, Porsch EA, Seed PC, Heiss C, Naran R, Forsberg LS, Amit U, Yagupsky P, Azadi P, St Geme JW III. 2016. Kingella kingae expresses four structurally distinct polysaccharide capsules that differ in their correlation with invasive disease. PLoS Pathog 12:e1005944. doi: 10.1371/journal.ppat.1005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starr KF, Porsch EA, Seed PC, St Geme JW III. 2016. Genetic and molecular basis of Kingella kingae encapsulation. Infect Immun 84:1775–1784. doi: 10.1128/IAI.00128-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turk DC. 1984. The pathogenicity of Haemophilus influenzae. J Med Microbiol 18:1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]