Abstract
Aim:
Toxoplasma gondii is an ubiquitous apicomplexan parasite which causes toxoplasmosis in humans and animals. Felids especially cats are definitive hosts and almost all warm-blooded mammals, including livestock and human can serve as intermediate hosts. Food animals can be reservoirs for T. gondii and act as one of the sources for parasite transmission to humans. The objective of this study is to collect serological data on the prevalence of anti-T. gondii antibody, and risk factors for certain food animals from Africa to provide a quantitative estimate of T. gondii infection among these species from different African countries.
Materials and Methods:
Four databases were used to search seroepidemiological data on the prevalence of anti-T. gondii antibody in food animals between 1969 and 2016 from African countries. The search focused on data obtained by serologic test in food animals and meta-analyses were performed per species.
Results:
A total of 30,742 individual samples from 24 countries, described in 68 articles were studied. The overall estimated prevalence for toxoplasmosis in chicken, camel, cattle, sheep, goat, pig were 37.4% (29.2-46.0%), 36% (18-56%), 12% (8-17%), 26.1% (17.0-37.0%), 22.9% (12.3-36.0%), and 26.0% (20-32.0%), respectively. Moreover, major risk factor of infection was age, farming system, and farm location.
Conclusions:
A significant variation in the seroepidemiological data was observed within each species and country. The results can aid in an updated epidemiological analysis but also can be used as an important input in quantitative microbial risk assessment models. Further studies are required for a better and continual evaluation of the occurrence of this zoonotic infection.
Keywords: animal health, meta-analysis, Toxoplasmosis, zoonosis
Introduction
Toxoplasma gondii is a coccidian parasite that is globally widespread and causes a common infection in animal and human. The parasite was described for the first time in a North African rodent (Ctenodactylus gondii) independently by Nicolle, Manceaux, and Splendore in 1908 [1]. Felids especially cats are definitive hosts and represent the key element in the epidemiology of disease caused by this parasite. Almost all warm-blooded mammals, including livestock, and human can serve as intermediate hosts [2]. T. gondii can infect all homeotherms and is responsible for many abortions and fetal malformations in human and animal [3].
According to estimates, approximately 1/3 of the world’s population would be infected [4] and T. gondii infection represent the most prevalent parasitic zoonotic disease worldwide [5]. This parasite is present on all continents, and the rate of infection vary highly according to areas [2]. However, climate change has led to an increase of T. gondii infections in different regions of the world as a result of changing environmental conditions [6].
Humans get infected after ingesting undercooked or raw meat, by ingesting cat-shed oocysts via contaminated soil, food, water or congenitally by transplacental transmission of tachyzoites [5]. However, the clinical disease is seen only in few cases with serious consequences in immunocompromised people and pregnant women [7]. Toxoplasmosis is a major cause of reproductive failure in sheep, goats, and pigs [8,9] and also recognized as a serious problem in immunocompromised patients particularly AIDS patient [10,11]. Furthermore, recent studies have shown that toxoplasmosis is a risk factor for schizophrenia [12], epilepsy [13], and traffic accidents [14] and highly virulent atypical strains of T. gondii have been incriminated with pneumonia, even in immunocompetent people [15].
Toxoplasmosis, especially cerebral toxoplasmosis has become the most common opportunistic infection of the central nervous system during HIV infection in the world [10,11]. Africa is the most continent affected by HIV/AIDS infection that affects about 30 million people on the continent [16]. Unluckily countries most affected are those least able to meet the cost of prevention and treatment of disease. Thus, toxoplasmosis has become an important public health problem on the continent account to the severity of the infection in AIDS patients more frequent in Africa. The absence of public health schemes to manage the spread of this disease places African populations at risk of ongoing and possibly increasing incidence and prevalence, as well as a corresponding increase in mortality and morbidity due to toxoplasmosis [17].
Food animals are important livestock species, especially in developing countries and their products (meat and milk) are used in various parts of the world. Pork and chicken are the most consumed meat in the world with global production estimated at 115.5 and 108.7 million tons in 2014 [18]. In Africa; cattle, chicken, sheep, goat, pig, and camel represent the most consumed animal species. According to estimate, the meat production on the continent was estimated at 17352 thousands of tons in 2013 and increasing every year [18]. Food animals can be reservoirs for T. gondii and act as one of the sources for parasite transmission to humans. Many epidemiologic studies have found an association between consumption of undercooked or raw meat and T. gondii infection in human [19,20]. Based on limited population-based data, the Food and Agriculture Organization and World Health Organization estimated that approximately 22% of human T. gondii infections are meatborne [21].
To detect T. gondii in meat animal, three methods have been used. These methods include serological assays, bioassay, and polymerase chain reaction (PCR) [22]. Among these three methods, serological assays are rapid and have good accuracy for detecting anti-T. gondii antibodies in food animals [23-25] and the modified agglutination test (MAT) and enzyme-linked immunosorbent assay (ELISA), are the most commonly used serological test.
Compared to other continents, few studies have been conducted on toxoplasmosis in Africa. Studies available on the seroprevalence of toxoplasmosis in African countries are still fragmented, except some countries including Ethiopia where the infection is well documented. Therefore, there have been a few studies on seroprevalence rates of T. gondii in animal species on the continent, and the results of the available studies are sometimes contradictory.
Meta-analysis is a method to synthesize the results of various studies for a given question and was applied to a wide range of food safety questions [26]. The quantitative results obtained from meta-analysis were used as inputs in risk assessment models [27]. According to Gliner al. [28], the advantages of performing a meta-analysis include providing summary statistics based on multiple individual studies, increasing precision in estimating effects, and taking the size of studies into account.
The aim of this systematic review and meta-analysis study is to collect serological data on the prevalence of anti-T. gondii antibody, and risk factors for most consumed food animals from Africa to provide a quantitative estimate of T. gondii infection among these species.
Materials and Methods
Ethical approval
This study did not require an ethical approval as it was based on information/data retrieved from published studies already available in the public domain.
Data sources and searches
We conducted a systematic literature review on the seroprevalence of T. gondii among food animals in African countries as per preferred reporting items for systematic reviews and meta-analyses criteria [29]. Relevant studies were identified by searching four literature databases including PubMed, Web of Science, Scopus, and Google Scholar. No time limitation was imposed. The search criteria were specified in advance and the search was executed on 11/12/2015 and last updated on 01/04/2016. The search string used was the following: “toxoplasma” OR “toxoplasmosis” AND “seroprevalence” OR “seroepidemiology” AND “sheep” OR “goat” OR “pig” OR “cattle” OR “chicken” OR “camel” AND “Africa”.
Data collection and eligibility criteria
For this review, only articles written in English and French were considered. Two investigators studied titles and abstract of all the articles and retrieved data. Several criteria were used to select eligible studies (1) study were performed in animals raised in different African countries; (2) the prevalence of T. gondii had to be detected by serologic methods (ELISA, MAT, direct agglutination test [DAT], modified direct agglutination test [MDAT], indirect fluorescent antibody test [IFAT], latex agglutination test [LAT], and Sabin and Feldman test [SFT]); (3) samples had to originate from food animals (cattle, chicken, camel, pigs, sheep and goat); (4) samples had to be collected from animals which were naturally infected; (5) sampling strategy had to be directed toward a random population; (6) the sample size was <35. The extracted data included: Year of publication, host, country of the study, sample size, number of cases, diagnostic test, and risk factors. Reference lists of full-text publications and textbooks were also examined to identify studies not retrieved by the original search. All studies were coded according to the previously chosen parameters, and data were recorded in Microsoft Excel table.
Quality and bias assessment of eligible studies
Each eligible study was assessed for quality and bias using the risk of bias tool, which is a methodological quality assessment checklist for prevalence studies [30]. 10 questions were contained in this checklist, and each of the 10 questions was scored 1 or 0 based on the quality of each eligible study [30]. This questions were as follows:
Q1: Was the study’s target population a close representation of the national population in relation to relevant variables?
Q2: Was the sampling frame a true or close representation of the target population?
Q3: Was some form of random selection used to select the samples, or, was a census undertaken?
Q4: Was the likelihood of non-response bias minimal?
Q5: Were data collected directly from the subjects (as opposed to a proxy)?
Q6: Was an acceptable case definition used in the study?
-
Q7: Was the study instrument that measured the parameter of interest shown to have reliability and validity (if necessary)?
- Yes (if using MAT, ELISA, DAT, and MDAT),
- No (using other serologic detection methods).
Q8: Was the same mode of data collection used for all subjects?
Q9: Was the length of the shortest prevalence period for the parameter of interest appropriate? Q10: Were the numerator(s) and denominator(s) for the parameter of interest appropriate?
Eight different detection methods were used in these eligible studies. For question 7, which was to determine the reliability and validity of the measurement, MAT, ELISA, DAT and MDAT were considered as reliable diagnostic methods (score 1) [24,25], and other diagnostic tests such as LAT, indirect immunoflourescent assay (IFA), indirect hemagglutination assay (IHA), SFT, were determined as unreliable methods (score 0). A quality score was determined by rescaling the sum of scores of each eligible study between 0 and 1 [30]. Quality assessment was completed independently by two assessors, and a table of quality score computation for each eligible study is provided in the Supplementary Table-S1.
Supplementary Table-S1.
Quality score assessment based on the “risk of bias tool“ (Hoy et al., 2012).
| Species | Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Summary |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pig | Bamba et al. [36] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Chicken | Gebremedhin et al. [37] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Goat | Abdel-Hafeez et al. [38] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Sheep | Dechicha et al. [39] | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Goat | Dechicha et al. [39] | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Cattle | Dechicha et al. [39] | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Cattle | Onyiche and Ademola [40] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Pig | Onyiche and Ademola [40] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Cattle | Elfahal et al. [41] | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| Pig | Gebremedhin et al. [42] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Camel | Hadush et al. [43] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Sheep | Lahmar et al. [44] | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Sheep | Hammond-Aryee et al. [45] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Chicken | Boughattas et al. [46] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Chicken | Ayinmode and Olaosebikan [47] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Goat | Davoust et al. [48] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Sheep | Davoust et al. [48] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Sheep | Gebremedhin and Gizaw [49] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Goat | Gebremedhin and Gizaw [49] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Sheep | Gebremedhin et al. [50] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Goat | Gebremedhin et al. [50] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Cattle | Medani and Kamil [51] | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Sheep | Medani and Kamil [51] | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Camel | Kadle [52] | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 | |
| Camel | Gebremedhin et al. [53] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Chicken | Tilahun et al. [54] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Chicken | Aboelhadid et al. [55] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Goat | Zwedu et al. [56] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Goat | Swai and Kaaya [57] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Cattle | Ndou et al. [58] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Pig | Ayinmode and Olaosebikan [59] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Sheep | Gebremedhin et al. [60] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Sheep | Bamba et al. [61] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Sheep | Al-mabruk et al. [62] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 9 |
| Sheep | Gharbi et al. [63] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Chicken | Barakat et al. [64] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Pig | Rakotoharinome et al. [65] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Cattle | Swai and Schoonman [66] | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Camel | Khalil and Abdel Gadir [67] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Sheep | Khalil and Abdel Gadir [67] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Sheep | Boughattas and Bouratbine [68] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Sheep | Kamani et al. [69] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Goat | Kamani et al. [69] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Cattle | Ibrahim et al. [70] | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Chicken | Dubey et al., [71] | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Chicken | Lindstrom et al. [72] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Sheep | Shapaan et al. [73] | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Goat | Teshale and Dumaitre [74] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Sheep | Samra et al. [75] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Chicken | Dubey et al. [76] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Chicken | Deyab and Hassanein [77] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Goat | Hove et al. [78] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 9 |
| Cattle | Schoonman et al. [79] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Pig | Hove et al. [80] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Sheep | Sawadogo et al. [81] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Sheep | Negash and Tilahun [82] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Goat | Negash and Tilahun [82] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Chicken | Dubey et al. [83] | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Cattle | Joshua and Akinwumi [84] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Chicken | El-Massry et al. [85] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Sheep | Van der Puije et al. [86] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Goat | Van der Puije et al. [86] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Goat | Bisson et al. [87] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Pig | Arkoh Mensah et al. [88] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Pig | Hove and Dubey [89] | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Camel | Hilali et al. [90] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Chicken | Hassanain and Elfadaly [91] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Sheep | Deconinck et al. [92] | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Sheep | Achu-Kwi and Ekue [93] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Sheep | El-Ghaysh and Mansour [94] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Goat | Amin and Silsmore [95] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Sheep | Pangui et al. [96] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Camel | Elamin et al. [97] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Sheep | Pandley and Mansour [98] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Sheep | Weitzman et al. [99] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Sheep | Bekele and Kasali [100] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Goat | Bekele and Kasali [100] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Cattle | Bekele and Kasali [100] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Chicken | Aganga and Belino [101] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Goat | Falade [102] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Chicken | Rifaat et al. [103] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
Data analysis
Data were recorded in Microsoft Excel spreadsheet and analysed by MetaXL version 4.0 software (EpiGear Int Pty Ltd., Wilston) [31] for the meta-analyses and graphed as forest plot. For pooled prevalence analysis, random effects model was adopted over fixed effect model because there is more robust when analyzing heterogeneous studies [32]. Data were transformed by a double arcsine transformation as described by Barendregt et al. [33] to stabilize the variance. Publication bias was assessed by funnel plots representing the double arcsine transformation of the prevalence against the standard error [34]. Heterogeneity among studies was evaluated by Cochrane Q and I2 statistical methods. A significant value (p<0.05) in the Cochrane Q method suggests a real effect difference in the meta-analysis. A value of I2 was used to measure the inconsistency across studies. Values of 25%, 50%, and 75% were considered as having a low, moderate, and high degree of heterogeneity, respectively [35].
Results
Schematic flow diagram describing the selection of relevant studies Figure-1.
Figure-1.
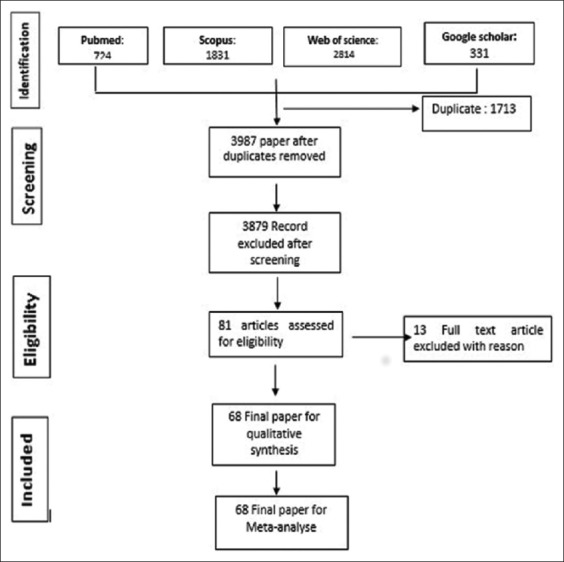
Schematic flow diagram describing the selection of relevant studies.
Characteristics of eligible studies
Figure-1 shows the flow diagram of the selection of eligible studies. A total of 5700 papers published between 1969 and 2016 were identified by literature search among the four database searched. After duplicate removed and irrelevant studies based on titles and abstracts, 81 articles were retrieved for detailed full-text analysis. 13 were excluded due to the following reasons: Two were not available; the sample size was lower than 35 in four study; the diagnosis was established on the basis of other methods than serologic test in seven studies. Table-1 shows the characteristics of included studies [36-103]. Finally, a total of 68 articles from 24 countries were included in this systematic review and meta-analysis study. Approximately, 60% (41/68) of the studies were published within the last 10 decade (2007-2016) of the review period. The regional distribution of studies was west Africa (18), East Africa (17), North Africa (21), Southern Africa (8), and Central Africa (4). Our analysis included a totally 30,742 individual samples distributed as follows: 14,272 sheep, 6355 goats, 3366 cattle, 2798 chickens, 2080 pigs, and 1621 camels. Eight different types of diagnostic tests were employed to evaluate T. gondii infection. These diagnostic methods were MAT, ELISA, IHA, DAT, MDAT, IFA, LAT, and SFT. The most used diagnostic tests in 47 year surveys were ELISA and MAT in 24 and 20 studies, which was followed by LAT (14), IHA (13), DAT (6), IFA (6), MDAT (3) and SFT (1). Sensitivity and specificity of diagnostic test are described in Table-2 as reported in literature.
Table-1.
Characteristics of included studies.
| Study No | Country | Author | Year | Hosts | Method | Sample size | Positive (%) | Quality score |
|---|---|---|---|---|---|---|---|---|
| 1 | Burkina-Faso | Bamba et al. [36] | 2016 | Pig | MAT | 300 | 87 (29) | 8 |
| 2 | Ethiopia | Gebremedhin et al. [37] | 2015 | Chicken | MAT | 601 | 183 (30.50) | 9 |
| 3 | Egypt | Abdel-Hafeez et al. [38] | 2015 | Goat | IHAT | 100 | 64 (64) | 7 |
| 4 | Algeria | Dechicha et al. [39] | 2015 | Sheep, Goat, Cattle | IFAT | 714 | 59 (8.26) | 6 |
| 5 | Nigeria | Onyiche et al. [40] | 2015 | Cattle, Pig | ELISA | 512 | 117 (22.85) | 9 |
| 6 | Sudan | Elfahal et al. [41] | 2015 | Cattle | ELISA | 181 | 24 (13.30) | 6 |
| 7 | Ethiopia | Gebremedhin et al. [42] | 2015 | Pig | DAT | 402 | 129 (32.10) | 9 |
| 8 | Ethiopia | Hadush et al. [43] | 2015 | Camel | DAT | 384 | 262 (68.20) | 9 |
| 9 | Tunisia | Lahmar et al. [44] | 2015 | Sheep, Goat, Cattle | MAT | 261 | 82 (36.78) | 7 |
| 10 | South-Africa | Hammond-Aryee et al. [45] | 2015 | Sheep | ELISA | 292 | 23 (8.00) | 9 |
| 11 | Tunisia | Boughattas et al. [46] | 2014 | Chicken | MAT | 40 | 40 (100) | 8 |
| 12 | Nigeria | Ayinmode et al. [47] | 2014 | Chicken | MAT | 225 | 81 (40.40) | 9 |
| 13 | Senegal | Davoust et al. [48] | 2014 | Cattle, Goat, Horse, Sheep | MAT | 419 | 148 (35.33) | 8 |
| 14 | Ethiopia | Gebremedhin and Gizaw [49] | 2014 | Sheep, Goat | ELISA | 184 | 48 (26.08) | 9 |
| 15 | Ethiopia | Gebremedhin et al. [50] | 2014 | Sheep, Goat | DAT | 628 | 50 (17.62) | 9 |
| 16 | Sudan | Medani and Kamil [51] | 2014 | Cattle, Sheep | ELISA | 540 | 153 (28.33) | 7 |
| 17 | Somalia | Kadle [52] | 2014 | Camel | LAT | 64 | 4 (6.3) | 7 |
| 18 | Ethiopia | Gebremedhin et al. [53] | 2014 | Camel | DAT | 455 | 220 (49.62) | 9 |
| 19 | Ethiopia | Tilahun et al. [54] | 2013 | Chicken | MAT | 64 | 41 (64.00) | 9 |
| 20 | Egypt | Aboelhadid et al. [55] | 2013 | Chicken | MAT | 215 | 30 (13.95) | 8 |
| 21 | Ethiopia | Zwedu et al. [56] | 2013 | Goat | ELISA | 927 | 183 (19.70) | 9 |
| 22 | Tanzania | Swai and Kaaya [57] | 2013 | Goat | LAT | 337 | 65 (19.30) | 8 |
| 23 | South-Africa | Ndou et al. [58] | 2013 | Cattle | ELISA | 178 | 37 (20.8) | 8 |
| 24 | Nigeria | Ayinmode and Olaosebikan [59] | 2013 | Pig | ELISA | 100 | 25 (25) | 8 |
| 25 | Ethiopia | Gebremedhin et al. [60] | 2013 | Sheep | ELISA | 1130 | 357 (31.59) | 9 |
| 26 | Burkina-Faso | Bamba et al. [61] | 2013 | Sheep | MAT | 339 | 96 (28.3) | 8 |
| 27 | Libya | Al-Mabruk et al. [62] | 2013 | Sheep | LAT | 5806 | 4120 (71.00) | 9 |
| 28 | Tunisia | Gharbi et al. [63] | 2013 | Sheep | ELISA | 350 | 38 (10.85) | 8 |
| 29 | Egypt | Barakat et al. [64] | 2012 | Chicken | ELISA | 125 | 48 (38.40) | 8 |
| 30 | Madagascar | Rakotoharinome et al. [65] | 2012 | Pig | ELISA | 250 | 57 (22.80) | 8 |
| 31 | Tanzania | Swai and Schoonman [66] | 2012 | Cattle | LAT | 51 | 06 (12.80) | 6 |
| 32 | Sudan | Khalil and Abdel Gadir [67] | 2011 | Cattle, Camel, Sheep | LAT | 200 | 76 (38.00) | 7 |
| 33 | Tunisia | Boughattas and Bouratbine [68] | 2011 | Sheep | MAT | 158 | 28 (17.70) | 9 |
| 34 | Nigeria | Kamani et al. [69] | 2010 | Sheep, Goat | ELISA | 744 | 42 (5.45) | 8 |
| 35 | Egypt | Ibrahim et al. [70] | 2009 | Cattle | ELISA | 93 | 10 (10.75) | 5 |
| 36 | Ghana | Dubey et al., [71] | 2008 | Chicken | MAT | 85 | 40 (47.00) | 7 |
| 37 | Uganda | Lindstrom et al. [72] | 2008 | Chicken | MAT | 50 | 25 (50.00) | 8 |
| 38 | Egypt | Shapaan et al. [73] | 2008 | Sheep | MAT | 300 | 131 (43.70) | 7 |
| 39 | Ethiopia | Teshale and Dumaitre [74] | 2007 | Goat | MDAT | 641 | 480 (74.80) | 9 |
| 40 | South-Africa | Samra et al. [75] | 2007 | Sheep | ELISA | 600 | 26 (4.30) | 9 |
| 41 | Egypt | Dubey et al. [76] | 2003 | Chicken | MAT | 108 | 51 (47.20) | 8 |
| 42 | Egypt | Deyab and Hassanein [77] | 2005 | Chicken | MAT | 150 | 28 (18.1) | 9 |
| 43 | Zimbabwe | Hove et al. [78] | 2005 | Goat | IFAT | 312 | 214 (68.58) | 9 |
| 44 | Tanzania | Schoonman et al. [79] | 2010 | Cattle | LAT | 665 | 24 (3.60) | 8 |
| 45 | Zimbabwe | Hove et al. [80] | 2005 | Pig | IFAT | 238 | 47 (26.79) | 8 |
| 46 | Morocco | Sawadogo et al. [81] | 2005 | Sheep | ELISA | 261 | 72 (27.60) | 9 |
| 47 | Ethiopia | Negash and Tilahun [82] | 2004 | Sheep, Goat | MDAT | 174 | 79 (45.40) | 9 |
| 48 | RDC, Mali, Burkina-Faso and Kenya | Dubey et al. [83] | 2005 | Chicken | MAT | 80 | 29 (36.25) | 7 |
| 49 | Nigeria | Joshua and Akinwumi [84] | 2003 | Cattle | LAT | 586 | 99 (16.9) | 8 |
| 50 | Egypt | El-Massry et al. [85] | 2000 | Chicken | MAT | 150 | 28 (18.70) | 8 |
| 51 | Ghana | Van der Puije et al. [86] | 2000 | Sheep, Goat | ELISA | 1258 | 384 (30.52) | 10 |
| 52 | Uganda | Bisson et al. [87] | 2000 | Goat | ELISA | 784 | 240 (31.00) | 10 |
| 53 | Ghana | Arkoh Mensah et al. [88] | 2000 | Pig | ELISA | 641 | 260 (40.60) | 10 |
| 54 | Zimbabwe | Hove and Dubey [89] | 1999 | Pig | MAT | 97 | 9 (09.30) | 7 |
| 55 | Egypt | Hilali et al. [90] | 1998 | Camel | DAT | 166 | 29 (17.40) | 9 |
| 56 | Egypt | Hassanain and Elfadaly [91] | 1997 | Chicken | IHAT | 600 | 200 (33.33) | 7 |
| 57 | Burkina-Faso, Ivory-Coast, Djiboutia, Ethiopia, Niger, Senegal | Deconinck et al. [92] | 1996 | Sheep | IHAT | 1042 | 15 (23.00) | 6 |
| 58 | Cameroon | Achu-Kwi and Ekue [93] | 1994 | Sheep | LAT | 211 | 67 (31.80) | 7 |
| 59 | Egypt | El-Ghaysh and Mansour [94] | 1994 | Sheep | MAT | 102 | 50 (49.00) | 8 |
| 60 | Nigeria | Amin and Silsmore [95] | 1993 | Sheep, Goat | LAT | 465 | 37 (7.95) | 7 |
| 61 | Senegal | Pangui et al. [96] | 1993 | Sheep | IFAT | 190 | 88 (46.30) | 7 |
| 62 | Sudan | Elamin et al. [97] | 1992 | Camel | LAT | 482 | 323 (67.00) | 7 |
| 63 | Zimbabwe | Pandley and Van Knapen [98] | 1992 | Sheep | ELISA | 216 | 13 (06.00) | 10 |
| 64 | Niger | Weitzman and Stem [99] | 1991 | Sheep | LAT | 70 | 10 (14.00) | 8 |
| 65 | Ethiopia | Bekele and Kasali [100] | 1989 | Sheep, Goat, Cattle | IHAT | 2437 | 349 (14.32) | 8 |
| 66 | Nigeria | Aganga and Belino [101] | 1984 | Chicken | IHAT | 250 | 112 (44.80) | 7 |
| 67 | Nigeria | Falade [102] | 1978 | Goat | LAT | 751 | 23 (3.06) | 7 |
| 68 | Egypt | Rifaat et al. [103] | 1969 | Chicken | DAT | 85 | 17 (20.00) | 7 |
MAT: Modified agglutination test, DAT: Direct agglutination test, MDAT: Modified direct agglutination test, ELISA: Enzyme-linked immunosorbent assay, LAT: Latex agglutination test, IFAT: Indirect fluorescent antibody test, IHAT: Indirect hemagglutination test
Table-2.
Comparing diagnostic methods.
| Diagnostic test | Study (%) N=68 | Sensitivity (%) | Specificity (%) | References |
|---|---|---|---|---|
| MAT, DAT, MDAT | 38.23 | 82.9 | 92.29 | Dubey et al. [23] |
| ELISA | 29.41 | 72.9 | 85.90 | Dubey et al. [23] |
| LAT | 17.64 | 45.9 | 96.90 | Dubey et al. [23] |
| IHA | 07.35 | 29.4 | 98.30 | Dubey et al. [23] |
| IFA | 05.88 | 80.40 | 91.40 | Arthur and Blewett [103] |
| SFT | 01.47 | 54,4 | 90,80 | Dubey et al. [23] |
MAT: Modified agglutination test, DAT: Direct agglutination test, MDAT: Modified direct agglutination test, ELISA: Enzyme-linked immunosorbent assay, LAT: Latex agglutination test, IHA: Indirect hemagglutination assay, IFA: Indirect immunoflourescent assay, SFT: Sabin and Feldman test
Quality and bias assessments
Supplementary Table-S1 (Appendix) represents the quality score of different eligible study. The quality score in 54/84 eligible studies ranged from 6 and 8 (Table-S1) [36-103]. It shows that the risk of bias in these studies was moderate. Besides, many of the eligible studies were conducted in regional and local farms or slaughterhouses, which were not representative of the national population of animals sampled in these countries. Only 5 of the 84 studies were conducted at the national level (Table-S1). Moreover, studies on animal toxoplasmosis were available only in 24 countries out of 54 of African continent. The risk of bias due to quality deficiency in eligible studies was mainly due to external validity criteria, while the flaws internal validity recorded in eligible studies concerned the use of diagnostic tests other than reference methods such as ELISA and MAT (Table-2) [104]. Finally, the symmetry in the funnel plots ruled out substantial publication bias (Figure-2).
Figure-2.
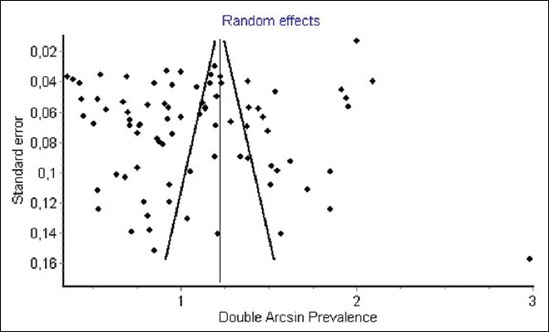
Funnel plot of double arcsinus seroprevalence estimates in food animals.
Population prevalence in food animals
Prevalence of anti-T. gondii antibody in sheep
Data from 27 studies from 17 countries were obtained among sheep. 10 studies used ELISA, 6 studies used MAT, 5 used LAT, 2 used IHA and IFA, DAT and MDAT were used in 1 study, respectively. A total number of individual samples was 14,272. The prevalence of toxoplasmosis in sheep varied from 4.30% to 68.00%. The random effect model used in the meta-analysis (Figure-3) gave an overall estimated prevalence of 26.1% (95% confidence interval [CI] 17.0-37.0%). The result of heterogeneity was also 96.83% (95% CI 96.18-97.38%) for the degree of inconsistency.
Figure-3.
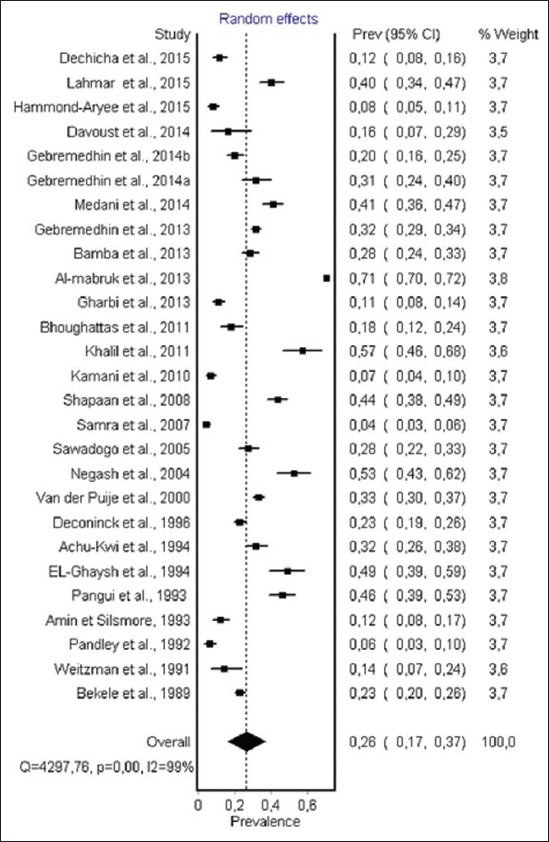
Forest plot of Toxoplasma gondii infection prevalence in sheep (random effect model). In a forest plot, each study is represented by a line, the width of the line represents the confidence intervals for effect estimate of each study, and area of the box indicates the weight given to each study. This description of forest plot is applied to all forest plots presented in Figures-3-8.
Prevalence of anti-T. gondii antibody in goats
The data obtained from T. gondii infection in goat result from 17 studies from 9 countries. The reported prevalence ranged from 3.6% to 74.8%. For diagnostic methods, 5 studies performing ELISA, 2 studies performing LAT, 2 studies, performing MDAT, IFAT, IHA, respectively, and 1 study performing MAT and DAT, respectively. The total number of individual samples was 6355. The random effect model (Figure-4) gave an overall estimated prevalence of 22.9% (95% CI 12.3-36.0%). The result of heterogeneity was also 99.1% (95% CI 99.0-99.3%) for the degree of inconsistency.
Figure-4.
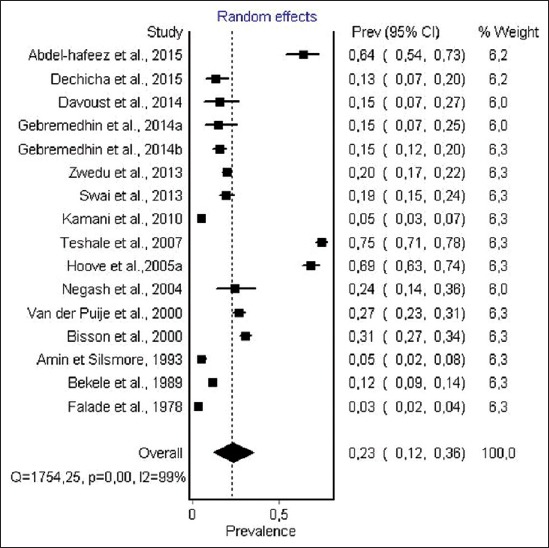
Forest plot of Toxoplasma gondii infection prevalence in goat (random-effects model).
Prevalence of anti-T. gondii antibody in cattle
Information on T. gondii infection in cattle was obtained from 11 studies from 8 countries. 5 studies performing LAT; 4 studies performing ELISA; 4 studies performing IFAT and IHA, respectively. The total number of individual samples was 3366. T. gondii infection prevalence among cattle ranged from 3.6% to 32%. The random effect model (Figure-5) gave an overall estimated prevalence of 12% (95% CI 8-17%, p<0.001). The result of heterogeneity was also 92.56% (95% CI 88.65-95.12) for the degree of inconsistency. A detailed description of each study is given in Figure-5.
Figure-5.
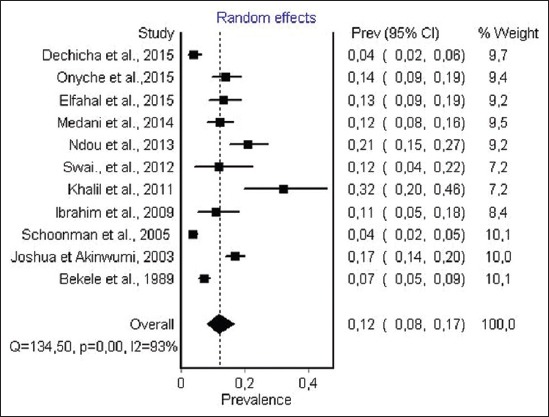
Forest plot of Toxoplasma gondii infection prevalence in cattle (random-effects model).
Prevalence of anti-T. gondii antibody in camels
For camels, 6 studies from 4 African countries were obtained. Most countries concerned were East African countries: Sudan, Ethiopia, and Somalia. For diagnostic tests, 3 studies used LAT and 3 used DAT. The total number of individual samples was 1621. Prevalence varied from 6.3 to 68.2. The overall estimated prevalence (Figure-6) for toxoplasmosis in camel by random-effect model was 36% (95% CI 18-56%). The result of heterogeneity was also 98.28% (95% CI 97.47-98.81%) for the degree of inconsistency.
Figure-6.
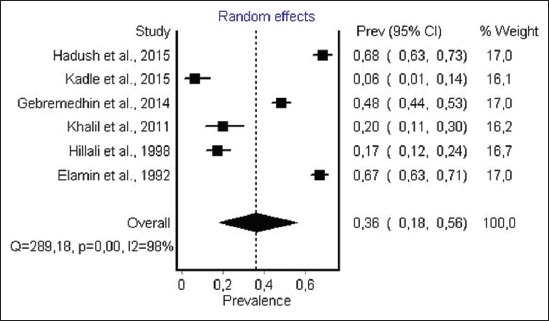
Forest plot of Toxoplasma gondii infection prevalence in camel (random-effects model).
Prevalence of anti-T. gondii antibody in pig
Data on T. gondii infection in pig were obtained from 8 studies from 6 countries in Africa. 4 studies, performing ELISA, 2 studies, performing MAT and 1 study performing DAT and IFAT respectively. A total number of individual sampled was 2330. Prevalence varied from 9.3 to 40.6. Overall estimated prevalence for anti-T. gondii antibody in pig (Figure-7) was 26.0% (95% CI 20.0-32.2). The result of heterogeneity was also 91.3% (95% CI 85.26-94.8) for the degree of inconsistency. Detailed description of each study is given in Figure-7.
Figure-7.
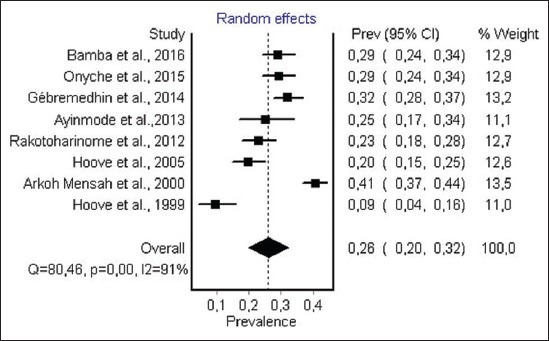
Forest plot of Toxoplasma gondii infection prevalence in pig (random-effects model).
Prevalence of anti-T. gondii antibody in chicken
Out of the 16 sero-epidemiological studies from 8 countries in the African continent, 12 studies used MAT, 2 used IHA and 1 study used ELISA and SFT, respectively, for diagnostic of anti-T. gondii antibody in chicken. The total number of individual chicken samples for serological testing was 2948. The prevalence of anti-T. gondii antibody ranged from 6.3% to 100%. The random effect model gave an overall estimated prevalence (Figure-8) of 37.4% (95% CI 29.2-46.0). The result of heterogeneity was also 95.2% (95% CI 93.6-96.6) for the degree of inconsistency.
Figure-8.
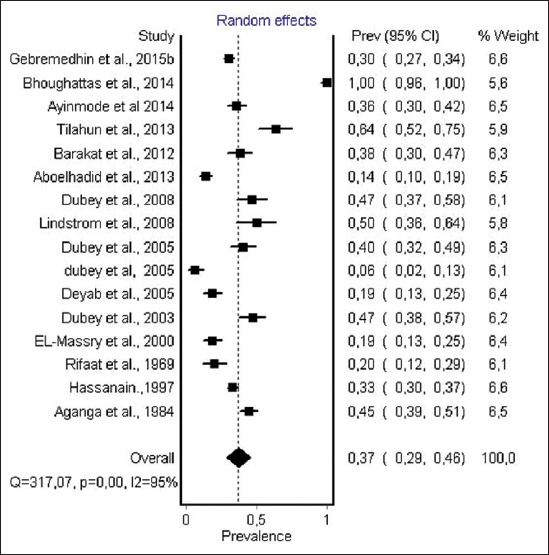
Forest plot of Toxoplasma gondii infection prevalence in chicken (random-effects model).
Risk factor
About 18 papers out of 68 selected articles for this systematic review reported statistically significant risk factors for the presence of anti-T. gondii antibody in different food animals.
Among sheep and goat, six main risk factors for the presence of anti-T. gondii antibody were identified from different studies. It was: Age (Table-1) [49,56,69,86], management farming system (Table-1) [56,75,78], farm location (Table-1) [57,60,69,86], climatic condition (Table-1) [49,74], sex [48,49], and breed (Table-1) [50,78]. Moreover, three of this main risk factors were also identified in cattle namely: Age (Table-1) [40], management system (Table-1) [79], and sex (Table-1) [40].
Among pigs, in addition to age (Table-1) [88]; management system (Table-1) [40,80] and breed [88]; the main risk factor identified was feeding type containing bio products (Table-1) [42].
Otherwise, among chicken, the major risk factor for presence of anti-T. gondii were cats density (Table-1) [37] and management system (Table-1) [64].
Discussion
Toxoplasmosis is one of the most widespread zoonoses in warm-blooded animals. The results of this review allowed us to compare estimates of infection with T. gondii and exposure to the parasite in different food animals from Africa. T. gondii infection is widespread in some food animals, especially chicken, camel, pig, sheep, and goats which represent the most consumed animal species in Africa for their meat, and there is a wide disparity between the levels of infection in different animal species considered.
The estimated prevalence of anti-T. gondii antibody in ruminants was significantly different: Camels, 36% (95% CI 18-56%); sheep, 26.1% (95% CI 17.0-37.0) and goat, 22.9% (95% CI 12.3-36.0%) were the most infected hosts, while the lowest seroprevalence were recorded in cattle 12% (95% CI 8-17%). The highest infection levels are recorded in chickens 37.4% (95% CI 29.2-46.0%), while moderate pooled seroprevalence were obtained in pigs 26% (95% CI 20.0-32.0). However, within each animal species a visible heterogeneity was observed, with a seroprevalence of antibodies ranging from 3.6% to 100% (Table-1) [46,79], as shown in the forest plots (Figures-3-8).
The overall pooled estimate in small ruminants was significant and the infection is more common in sheep which represents the most sensitive species to infection [8]. The highest prevalence were obtained in Ethiopia, 74.80% (Table-1) [74] and Zimbabwe, 68.58% (Table-1) [78]. This result shows the variability of infection rates from one region to another within the same species. In most serological studies from sheep and goats included in the meta-analysis, age is considered an important risk factor, as higher seropositivity is found in older animals (Table-1) [49,56,69,86]. This result is in agreement with the results of studies conducted in France and Iran but in all the world [105-107]. According to many authors, the highest prevalence were reported in farms with epizootic abortions (Table-1) [58,108], while lower seroprevalence was recorded in intensively managed sheep systems (Table-1) [56,78,109]. Toxoplasmosis causes heavy economic losses to sheep industry worldwide and losses are mainly due to abortion and other reproductive failure [110-111]. The ingestion of undercooked meat from infected sheep, especially lamb is considered an important source of infection for humans [112]. Therefore, the estimate demonstrates the risk associated with the consumption of raw products derived from small ruminants in countries where the infection rate is high (Table-1) [50,68]. Usually, raw or undercooked lamb meat is considered a delicacy in some countries and is therefore considered an important source of infection. On the other hand, adult sheep meat is often well cooked, and therefore, probably poses a lower risk of infection to the consumer than lamb meat [112].
In pigs, T. gondii infection prevalence ranged from 26.80 to 40.60 excluding one study from Zimbabwe in 1999 reporting a prevalence of 09.60 (Table-1) [89], and lower prevalence rates were recorded in other regions around the world. Thus, prevalence of 28.9% was found in fattening pigs in Serbia [113], 20% in Argentina [114], and 15.6% in Portugal [115]. Poljak et al. [116] reported prevalence in pig farms from Canada of 11.6 in 2001, 0% in 2003 and 1.2% in 2004. High infection rate recorded in some African countries may be due to an extensive management system of pigs which is very widespread in Africa. Studies conducted in Ghana, Ethiopia and Zimbabwe have shown that a high prevalence of T. gondii was observed in extensively managed pig or backyard scavenging pigs than an intensively managed pig, hence the importance of modern intensive farming systems in reducing the prevalence of T. gondii infection in domestic pigs (Table-1) [36,80]. According to Gamble et al., the prevalence of T. gondii in pigs is also influenced by management systems [117]. In poorly managed non-confinement systems, seroprevalence in pigs was as high as 68% [8]. Moreover, most studies conducted in Ghana, Zimbabwe and Ethiopia revealed that, the age of the animal, the Breed, and the management practices appeared to be the major determinants of prevalence of antibodies against T. gondii (Table-1) [40,80,88]. Most pigs acquire T. gondii infection postnatally by ingestion of oocysts from contaminated environment or ingestion of infected tissues of animals. Few pigs become infected prenatally by transplacental transmission of the parasite. Raising pigs indoors in confinement has greatly reduced T. gondii infection in pigs, but the recent trend of organic farming is likely to increase T. gondii infection in pigs [8]. The consumption of pork infected by T. gondii is one of the main risk factors for human infection [5,112]. Pork is known as one of the most important sources of T. gondii infection in many countries such as China and USA, most human infections were associated with Pork consumption [3].
The highest estimated prevalence of anti T. gondii antibody was record in chickens 37.41% (95% CI 29.20-46.00%) with seroprevalence that ranged from 6.32% to 100% (Table-1) [46,76]. Chickens are considered one of the most important hosts in the epidemiology of T. gondii infection because they are an efficient source of infection for cats that excrete the environmentally resistant oocysts and because humans may become infected with this parasite after eating undercooked infected chicken meat [118]. Studies from Tunisia, Ethiopia, and Uganda revealed very high prevalence of anti-T. gondii antibody among chicken, not encountered in any African country (Table-1) [46,54,72], suggesting high environmental contamination by oocysts of T. gondii excreted by cats in these countries. the prevalence of 24.4% was reported in free-range (FR) chickens from Indonesia, 12.5% in chickens from Italy, 30% in chickens from Poland, and 24.2% in chickens from Vietnam by Dubey et al. (Table-1) [71]. In rural areas from Brazil, a prevalence higher than 50% in free ranging chickens was identified, indicating also a widespread contamination of rural environment of that country with T. gondii oocysts [119]. Furthermore, the prevalence rates were higher among FR than commercial farm chickens according to many authors (Table-1) [37,64]. Higher seroprevalence particularly in free range chickens (house-reared) refers to the public health importance of chickens as source of zoonotic toxoplasmosis to human (Table-1) [47,64]. In developing sub-Saharan countries, chickens are killed at home or in unsupervised slaughter facilities and the viscera are left for scavengers or are improperly disposed and T. gondii infection can be transmitted to human if care is not taken to wash hands thoroughly after cutting meat and during cooking of meat [120].
Results indicate that the estimated prevalence of toxoplasmosis in cattle from Africa is the lowest obtained 12% (95% CI 8-17%, p<0.001) among different food animals. The highest and the lowest prevalence were recorded in Sudan, 32%, and Tanzania, 4%, respectively (Table-1) [67,78]. This overall estimate is higher than the infection rate reported in North of Portugal that was estimated at 7.5% in cattle [121]. In West Indies, a prevalence of 8.4% was reported [122]. In Brazil, the reported sero-prevalence was 49.4% in cattle from a highly endemic area of human toxoplasmosis [123]. Whereas in Malaysia and Vietnam, lower seroprevalence of 7.9% and 10.5% were, respectively, reported in cattle [124,125]. High prevalence of toxoplasmosis of cattle in some areas may be due to the following factors: Humid and temperate climate; the absence of routine treatment against feline toxoplasmosis, considerable cat abundance and last but not least exposure to cats and their oocysts. Several epidemiological studies have mentioned that the consumption of raw or undercooked beef could be considered as a risk for T. gondii infection in humans [126]. But according to Kijlstra and Jongert [112] and Dubey and Jones [3] transmission from cattle is not important for human infection. Given the low level of infection in cattle from Africa, we can assume that the risk for T. gondii infection in humans from beefs is low as compared to other hosts of T. gondii. Among ruminants, camels are the most infected species by T. gondii, 36% (95% CI 18-56%). T. gondii infection rate in Africa ranged from 17% to 68% and the highest rates were obtained in Sudan (Table-1) [97]. A higher prevalence has been reported from Turkey (90.9%) [127], while lower seroprevalence was recorded earlier from Iran 3.12% [128] and Saudi Arabia 6.5% [129].
Overall, the variation of seroprevalence of T. gondii infection among different species might be due to the difference in density of cats and wild felids around farm, climatic conditions [130], farming and management practices [3], sample size, cutoff titer, duration of studies, and sensitivity difference in the serological tests employed. According to Guo et al. [131], the heterogeneity in prevalence could also be related to the presence of risk factors including farm type, feeding practices, presence of cats, rodent control and bird control methods, farm management, carcasses handling and disposal, and water source and quality. Moreover, studies carried out in distinct countries and various climatic conditions affect the results that could be another reason for this heterogeneity.
Results from some studies showed significant relation between animal age and T. gondii infection among all hosts. It shows a higher prevalence in adults animals than young which may be resulted from more exposure during animal growth. Animals acquire Toxoplasma infection merely via ingestion of oocyst and when prevalence is considerably high. There is a widespread oocyst contamination of the environment because of fecal contamination of soil and groundwater either by domestic or feral cats. Understanding prevalence rate of animal toxoplasmosis will help us to estimate the rate of human toxoplasmosis and it can be a good indicator of environment and final host contamination [107]. This point is extremely important to mention that it is not easy to consider prevention and control program without enough information about prevalence of toxoplasmosis in animal since they are a major source of transmission to human.
Given the vital role of animals in the transmission of T. gondii to humans via their products (meat and milk) and the prominent role of cats in disseminating and contamination of the environment by oocysts [1], more emphasis should be placed on the prevention of animal toxoplasmosis in Africa.
Caution is warranted in the interpretation of results of T. gondii prevalence in camel. Regarding such species, the prevalence data used in this study were analyzed based on a limited number of national studies, and nationwide surveys are not available in these meat animals, which resulted in a wide 95% CI of the estimated prevalence.
Conclusion
This systematic review was performed to evaluate the prevalence of T. gondii infection among sheep, goat, cattle, pig, camel, and chicken which represent the most consumed food animal species in different African countries. The Random-effects meta-analysis approach in this current study provided an estimate of T. gondii prevalence in various meat animals with an increased level of precision. The widespread prevalence of T. gondii in sheep, chicken, camel, pig, and goats indicates a food safety concern in different African countries, especially countries where the infection is more important. Other studies are required for a better and continual evaluation of the occurrence of this zoonotic infection.
Authors’ Contributions
The study was conceptualized and protocols were carried out by YA. ABNT and PS were involved in the database search, data extraction, statistical analysis, and manuscript written. CA and EY Studied titles and abstract of all the articles and retrieved data. Quality assessment of each study was completed independently by YGH and IY. MNA and SF oversaw data collection and analysis of statistical results. All authors have read and approved the content.
Acknowledgments
The authors are grateful to the Ministry of Higher Education and Scientific Research of Benin, Through the Doctoral Support Program No. 125 MESRS/CAB/DC/SGM/DRFM/DRH/REGIE of 24 February 2015.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Ferguson D. J. Toxoplasma gondii 1908-2008, homage to Nicolle, Manceaux and Splendore. Mem. Inst. Oswaldo Cruz. 2009;104(2):133–148. doi: 10.1590/s0074-02762009000200003. [DOI] [PubMed] [Google Scholar]
- 2.Dubey J. P. Toxoplasmosis of Animals and Humans. 2nd ed. Florida, USA: CRC Press; 2010. [Google Scholar]
- 3.Dubey J. P, Jones J. L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008;38(11):1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Moncada P. A, Montoya J. G. Toxoplasmosis in the fetus and newborn: An update on prevalence, diagnosis and treatment. Expert. Rev. Anti Infect. Ther. 2012;10(7):815–828. doi: 10.1586/eri.12.58. [DOI] [PubMed] [Google Scholar]
- 5.Tenter A. M, Heckerotha A. R, Weiss L. M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patz J. A, Graczyk T. K, Geller N, Vittor A. Y. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 2000;30(12):1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 7.Torgerson P. R, Mastroiacovo P. The global burden of congenital toxoplasmosis: A systematic review. Bull. World Health Organ. 2013;91(7):501–508. doi: 10.2471/BLT.12.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubey J. P. Toxoplasmosis in pigs: The last 20 years. Vet. Parasitol. 2009a;164:89–103. doi: 10.1016/j.vetpar.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Dubey J. P. Toxoplasmosis in sheeps: The last 20 years. Vet. Parasitol. 2009b;152:25–80. doi: 10.1016/j.vetpar.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Bourée P, Dumazedier D, Magdeleine C, Sobesky G. Toxoplasmose cérébrale et SIDA àla martinique. Méd. Trop. 1997;57(3):259–261. [PubMed] [Google Scholar]
- 11.Weiss L. M, Dubey J. P. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009;39(8):895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torrey E. F, Bartko J. J, Lun Z. R, Yolken R. H. Antibodies to Toxoplasma gondii in patients with schizophrenia: A meta-analysis. Schizophr. Bull. 2007;33:729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer B. S. Meta-analysis of three case controlled studies and an ecological study into the link between cryptogenic epilepsy and chronic toxoplasmosis infection. Seizure. 2007;16(8):657–663. doi: 10.1016/j.seizure.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Flegr J, Klose J, Novotná M, Berenreitterová M, Havlíček J. Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study. BMC Infect. Dis. 2009;9:72. doi: 10.1186/1471-2334-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leal F. E, Cavazzana C. L, de Andrade H. F. J, Galisteo A, Jr, de Mendonca J. S, Kallas E. G. Toxoplasma gondii pneumonia in immunocompetent subjects: Case report and review. Clin. Infect. Dis. 2007;44(6):e62–e66. doi: 10.1086/511871. [DOI] [PubMed] [Google Scholar]
- 16.Pasquali P. Infections au VIH etzoonoses. Food Agric. Organ. 2007;163 [Google Scholar]
- 17.Hammond-Aryee K, Esser M, Van Helden P. D. Toxoplasma gondii seroprevalence studies on humans and animals in Africa. S. Afr. Fam. Pract. 2015;56(2):119–124. [Google Scholar]
- 18. [Accessed on 20-06-2016]. Available from: http://www.faostat.fao.org/site/569/default.aspx#ancor .
- 19.Cook A. J. C, Gilbert R. E, Buffolano W, Zufferey J, Petersen E, Jenum P. A, Foulon W, Semprini A. E, Dunn D. T. Sources of Toxoplasma infection in pregnant women: European multicentre case-control study. Br. Med. J. 2000;321:142–147. doi: 10.1136/bmj.321.7254.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyer K. M, Holfels E, Roizen N, Swisher C, Mack D, Remington J, Withers S, Meier P, McLeod R. Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: Implications for prenatal management and screening. Am. J. Obstet. Gynecol. 2005;192:564–571. doi: 10.1016/j.ajog.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 21.FAO-OMS. Joint FAO/WHO food standards program codex committee on food hygiene. Forty-seventh session Boston, Massachusetts, United States of America, 9-13 November 2015. 2015 [Google Scholar]
- 22.Montoya J. G. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J. Infect. Dis. 2002;185(1):S73–S82. doi: 10.1086/338827. [DOI] [PubMed] [Google Scholar]
- 23.Dubey J. P, Thulliez P, Weigel R. M, Andrews C. D, Lind P, Powell E. C. Sensitivity and specificity of various serologic tests for detection of Toxoplasma gondii infection in naturally infected sows. Am. J. Vet. Res. 1995;56(8):1030–1036. [PubMed] [Google Scholar]
- 24.Villena I, Durand B, Aubert D, Blaga R, Geers R, Thomas M, Perret C, Alliot A, Escotte-Binet S, Thebault A, Boireau P, Halos L. New strategy for the survey of Toxoplasma gondii in meat for human consumption. Vet. Parasitol. 2012;183(3-4):203–208. doi: 10.1016/j.vetpar.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Gamble H. R, Dubey J. P, Lambillotte D. N. Comparison of a commercial ELISA with the modified agglutination test for detection of Toxoplasma infection in the domestic pig. Vet. Parasitol. 2005;128:177–181. doi: 10.1016/j.vetpar.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Gonzales-Barron U, Butler U. The use of meta-analytical tools in risk assessment for food safety. Food Microbiol. 2011;28:823–827. doi: 10.1016/j.fm.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Barron U. G, Soumpasis I, Butler F, Prendergast D, Duggan S, Duffy G. Estimation of prevalence of Salmonella on pig carcasses and pork joints, using a quantitative risk assessment model aided by meta-analysis. J. Food Prot. 2009;72:274–285. doi: 10.4315/0362-028x-72.2.274. [DOI] [PubMed] [Google Scholar]
- 28.Gliner G. A, Morgan N. L, Leech O. Research Methods in Applied Settings: An Integrated Approach to Design and Analysis. 2nd ed. Abingdon, UK: Routledge; 2009. [Google Scholar]
- 29.Hutton B, Salanti G, Caldwell D. M, Chaimani A, Schmid C. H, Cameron C, Ioannidis J. P, Straus S, Thorlund K, Jansen J. P, Mulrow C, Catalá-López F, Gøtzsche P. C, Dickersin K, Boutron I, Altman D. G, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 30.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of inter rater agreement. J. Clin. Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 31. [Accessed on 22 May 2016, 11h 55 min]. Available from: http://www.epigear.com/index_files/metaxl.html .
- 32.Doi S. A, Barendregt J. J, Mozurkewich E. L. Meta-analysis of heterogeneous clinical trials: An empirical example. Contemp. Clin. Trials. 2011;32:288–298. doi: 10.1016/j.cct.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Barendregt J. J, Doi S. A, Lee Y. Y, Norman R. E, Vos T. Meta-analysis of prevalence. J. Epidemiol. Community Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 34.Sterne J. A, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 35.Higgins J. P, Thompson S. G, Deeks J. J, Altman D. G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bamba S, Halos F, Tarnagda Z, Alanio A, Macé E, Moukoury S, Sangaré I, Guiguemdé R, Costa J. M, Bretagne S. Seroprevalence of Toxoplasma gondii and direct genotyping using mini-sequencing in free-range pigs in Burkina Faso. Int. J. Food Microbiol. 2016;230:10–15. doi: 10.1016/j.ijfoodmicro.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Gebremedhin E. Z, Tesfamaryam G, Yunus H. A, Duguma R, Tilahun G, Di Marco V, Vitale M. Seroepidemiology of Toxoplasma gondii infection in free-range chickens (Gallus domesticus) of Central Ethiopia. Epidemiol. Infect. 2015b;143(3):608–617. doi: 10.1017/S0950268814000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Hafeez E. H, Kamal A. M, Abdelgelil N. H, Abdel-Fatah M. Parasites transmitted to human by ingestion of different types of meat, El-Minia City, El-Minia Governorate, Egypt. J. Egypt. Soc. Parasitol. 2015;45(3):671–680. doi: 10.12816/0017935. [DOI] [PubMed] [Google Scholar]
- 39.Dechicha A. S, Bachi F, Gharbi I, Gourbdji E, Baazize-Ammi D, Guetarni D. Sero-epidemiological survey on toxoplasmosis in cattle, sheep and goats in Algeria. Afr. J. Agric. Res. 2015;10(20):2113–2119. [Google Scholar]
- 40.Onyiche T. G. E, Ademola I. O. Seroprevalence of anti- Toxoplasma gondii antibodies in cattle and pigs in Ibadan, Nigeria. J. Parasit. Dis. 2015;39(2):309–314. doi: 10.1007/s12639-013-0350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elfahal A. M, Elhassan A. M, Hussien M. O, Enan K. A, Musa A. B, El Hussein A. M. Seroprevalence of Toxoplasma gondii in dairy cattle with reproductive problems in Sudan. ISRN Vet. Sci. 2013;89:51–65. doi: 10.1155/2013/895165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gebremedhin E. Z, Kebeta M. M, Asaye M, Ashenafi H, Di Marco V, Vitale M. First report on seroepidemiology of Toxoplasma gondii infection in pigs in central Ethiopia. BMC Vet. Res. 2015a;11:59. doi: 10.1186/s12917-015-0384-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hadush A, Gebru M. U, Zeru F, Hadush T, Tesfamaryam G, Feleke A. Sero-epidemiology of camel toxoplasmosis and public awareness on its zoonotic importance in central afar region, North East Ethiopia. World Appl. Sci. J. 2015;33(12):1880–1887. [Google Scholar]
- 44.Lahmar I, Lachkhem A, Slama D, Sakly W, Haouas N, Gorcii M, Babba H. Prevalence of toxoplasmosis in sheep, goats and cattle in Southern Tunisia. J. Bacteriol. Parasitol. 2015;6:245. [Google Scholar]
- 45.Hammond-Aryee K, Van Helden L. S, Van Helden P. D. The prevalence of antibodies to Toxoplasma gondii in sheep in the Western Cape, South Africa. Onderstepoort J. Vet. Res. 2015;82(1):E1–E5. doi: 10.4102/ojvr.v82i1.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boughattas S, Ayari K, Sa T, Aoun K, Bouratbine A. Survey of the parasite Toxoplasma gondii in human consumed ovine meat in Tunis city. PLoS One. 2014;9(1):e85044. doi: 10.1371/journal.pone.0085044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ayinmode A. B, Olaosebikan R. I. Seroprevalence of Toxoplasma gondii infection in free ranged chicken from rural and urban settlements in Oyo State, Nigeria. Afr. J. Med. Med. Sci. 2014;43(Suppl51):57. [PubMed] [Google Scholar]
- 48.Davoust B, Mediannikov O, Roqueplo C, Perret C, Demoncheaux J. P, Sambou M, Guillot J, Blaga R. Serological survey of animal toxoplasmosis in Senegal. Bull. Soc. Pathol. Exot. 2015;108(1):73–77. doi: 10.1007/s13149-014-0403-4. [DOI] [PubMed] [Google Scholar]
- 49.Gebremedhin E. Z, Gizaw D. Seroprevalence of Toxoplasma gondii Infection in sheep and goats in three districts of Southern nations, nationalities and peoples region of Ethiopia. World Appl. Sci. J. 2014a;31(11):1891–1896. [Google Scholar]
- 50.Gebremedhin E. Z, Abdurahaman M, Hadush T, Tessema T. S. Seroprevalence and risk factors of Toxoplasma gondii infection in sheep and goats slaughtered for human consumption in Central Ethiopia. BMC Res. Notes. 2014b;7:696. doi: 10.1186/1756-0500-7-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medani M. Y. I, Kamil I. Y. Serosurvey of Toxoplasma gondii in sheep and cattle for human consumption in Khartoum slaughterhouses, Sudan. Int. J. Infect. Dis. 2014;21:157. [Google Scholar]
- 52.Kadle A. A. H. Sero-prevalence of toxoposis in domestic animals in Benadir Region, Somalia. Open. J. Vet. Med. 2014;4(08):170. [Google Scholar]
- 53.Gebremedhin E. Z, Yunus H. A, Tesfamaryam G, Tessema T. S, Dawo F, Terefe G, Di Marco V, Vitale M. First report of Toxoplasma gondii in camels (Camelus dromedarius) in Ethiopia: Bioassay and seroepidemiological investigation. BMC Vet. Res. 2014;10:222. doi: 10.1186/s12917-014-0222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tilahun G, Tiao N, Ferreira L. R, Choudhary S, Oliveira S, Verma S. K, Kwok O. C, Molla B, Saville W. J, Medhin G, Kassa T, Aleme H, Gebreyes W. A, Su C, Dubey J. P. Prevalence of Toxoplasma gondii from free-range chickens (Gallus domesticus) from Addis Ababa, Ethiopia. J. Parasitol. 2013;99(4):740–741. doi: 10.1645/12-25.1. [DOI] [PubMed] [Google Scholar]
- 55.Aboelhadid S. M, Abdel-Ghany A. E, Ibrahim M. A, Mahran H. A. Seroprevalence of Toxoplasma gondii infection in chickens and humans in Beni Suef, Egypt. Glob. Vet. 2013;11:139–144. [Google Scholar]
- 56.Zewdu E, Agonafir A, Tessema T. S, Tilahun G, Medhin G, Vitale M, Di Marco V, Cox E, Vercruysse J, Dorny P. Seroepidemiological study of caprine toxoplasmosis in East and West Shewa Zones, Oromia Regional State, Central Ethiopia. Res. Vet. Sci. 2013;94(1):43–48. doi: 10.1016/j.rvsc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 57.Swai E. S, Kaaya J. E. A survey of Toxoplasma gondii antibodies by latex agglutination assay in dairy goats in Northern Tanzania. Trop. Anim. Health Prod. 2013;45(1):211–217. doi: 10.1007/s11250-012-0193-2. [DOI] [PubMed] [Google Scholar]
- 58.Ndou R. V, Pelele W. P. S, Dzoma B. M, Nyirenda M, Motsei L. E, Bakunzi F. R. An investigation into the prevalence of Toxoplasma gondii among indigenous, communally reared goats in the Mafikeng Area of the North West Province of South Africa. Life Sci. J. 2013;8(Suppl 1):38–41. [Google Scholar]
- 59.Ayinmode A. B, Olaosebikan R. I. Antibodies to Toxoplasma gondii in backyard and wandering pigs in Ibadan, Nigeria: Implications for pork consumption. Bull. Anim. Health Prod. Afr. 2014;61(3):493–497. [Google Scholar]
- 60.Gebremedhin E. Z, Agonafir A, Tessema T. S, Tilahun G, Medhin G, Vitale M, Di Marco V, Cox E, Vercruysse J, Dorny P. Seroepidemiological study of ovine toxoplasmosis in East and West Shewa Zones of Oromia Regional State, Central Ethiopia. BMC Vet. Res. 2013;9:117. doi: 10.1186/1746-6148-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bamba S, Kamga-Waladjo A. R, Cissé M, Tarnagda Z. (2013) Serological survey on bovine toxoplasmosis in Bobo-Dioulasso, Burkina Faso. Rev. Med. Vet. Trop. 2012;65:63–66. [Google Scholar]
- 62.Al-Mabruk A. A, Alkhunfas S. R, El-Buni A. A, Annajar B. B, Elsaid M. M. A. Seroprevalence of Toxoplasma gondii antibodies in sheep from Libya. Int. J. Adv. Res. 2013;1(9):148–154. [Google Scholar]
- 63.Gharbi M, Zribi L, Jedidi M, Chakkhari H, Hamdi S, R’hayem S, Darghouth M. A. Prevalence of Toxoplasma gondii infection in sheep from Tunisia. Bull. Soc. Pathol. Exot. 2013;106(3):184–187. doi: 10.1007/s13149-013-0290-4. [DOI] [PubMed] [Google Scholar]
- 64.Barakat A. M, Salem L. M, El-Newishy A. M, Shaapan R. M, El-Mahllawy E. K. Zoonotic chicken toxoplasmosis in some Egyptians Governorates. Pak. J. Biol. Sci. 2012;15(17):821–826. doi: 10.3923/pjbs.2012.821.826. [DOI] [PubMed] [Google Scholar]
- 65.Rakotoharinome M, Andriamanivo H, Blaga R, Perret C, Lacour S, Grasset-Chevillot A, Mace P, Thomas M, Villena I, Aubert D, Boireau P, Porphyre V. Toxoplasmosis and trichinellosis: An epidemiological survey of pig population in Madagascar. European Multicolloquium on Parasitology (EMOP) XI, Cluj Napoca. Roumanie: 2012. [Google Scholar]
- 66.Swai E. S, Schoonman L. A survey of zoonotic diseases in trade cattle slaughtered at Tanga city abattoir: A cause of public health concern. Asian. Pac. J. Trop. Biomed. 2012;2(1):55–60. doi: 10.1016/S2221-1691(11)60190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khalil K. M, Abdel Gadir A. E. Prevalence of Toxoplasma gondii antibodies in camels and their herders in three ecologically different areas in Sudan. J. Camel Pract. Res. 2007;14(1):11–13. [Google Scholar]
- 68.Boughattas S, Bouratbine A. Prevalence of food-borne Toxoplasma gondii in free-ranging chickens sold in Tunis, Tunisia. J. Food Qual. Hazards Control. 2014;1(3):89–92. [Google Scholar]
- 69.Kamani J, Mani A. U, Egwu G. O. Seroprevalence of Toxoplasma gondii infection in domestic sheep and goats in Borno state, Nigeria. Trop. Anim. Health Prod. 2010;42(4):793–797. doi: 10.1007/s11250-009-9488-3. [DOI] [PubMed] [Google Scholar]
- 70.Ibrahim H. M, Huang P, Salem T. A, Talaat R. M, Nasr M. I, Xuan X, Nishikawa Y. Short report: Prevalence of Neospora caninum and Toxoplasma gondii antibodies in northern Egypt. Am. J. Trop. Med. Hyg. 2009;80(2):263–267. [PubMed] [Google Scholar]
- 71.Dubey J. P, Huong L. T, Lawson B. W, Subekti D. T, Tassi P, Cabaj W, Sundar N, Velmurugan G. V, Kwok O. C, Su C. Seroprevalence and isolation of Toxoplasma gondii from free-range chickens in Ghana, Indonesia, Italy, Poland, and Vietnam. J. Parasitol. 2008;94(1):68–71. doi: 10.1645/GE-1362.1. [DOI] [PubMed] [Google Scholar]
- 72.Lindstrom I, Sundar N, Lindh J, Kironde F, Kabasa J. D, Kwok O. C, Dubey J. P, Smith J. E. Isolation and genotyping of Toxoplasma gondii from Ugandan chickens reveals frequentmultiple infections. Parasitology. 2008;135:39–45. doi: 10.1017/S0031182007003654. [DOI] [PubMed] [Google Scholar]
- 73.Shaapan R. M, El-Nawawi F. A, Tawfik M. A. Sensitivity and specificity of various serological tests for the detection of Toxoplasma gondii infection in naturally infected sheep. Vet. Parasitol. 2008;153(3-4):359–362. doi: 10.1016/j.vetpar.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 74.Teshale S, Dumaitre A. Serological survey of caprine toxopiasmosis in Ethiopia: Prevalence and risk factors. Parasite. 2007;14(2):155–159. doi: 10.1051/parasite/2007142155. [DOI] [PubMed] [Google Scholar]
- 75.Samra N. A, McCrindle C. M, Penzhorn B. L, Cenci-Goga B. Seroprevalence of toxoplasmosis in sheep in South Africa. J. S. Afr. Vet. Assoc. 2007;78(3):116–120. doi: 10.4102/jsava.v78i3.301. [DOI] [PubMed] [Google Scholar]
- 76.Dubey J. P, Graham D. H, Dahl E, Hilali M, El-Ghaysh A, Sreekumar C, Kwok O. C, Shen S. K, Lehmann T. Isolation and molecular characterization of Toxoplasma gondii from chickens and ducks from Egypt. Vet. Parasitol. 2003;114(2):89–95. doi: 10.1016/s0304-4017(03)00133-x. [DOI] [PubMed] [Google Scholar]
- 77.Deyab A. K, Hassanein R. Zoonotic toxoplasmosis in chicken. J. Egypt. Soc. Parasitol. 2005;147(5):341–350. [PubMed] [Google Scholar]
- 78.Hove T, Lind P, Mukaratirwa S. Seroprevalence of Toxoplasma gondii infection in goats and sheep in Zimbabwe. Onderstepoort J. Vet. Res. 2005b;72(4):267–272. doi: 10.4102/ojvr.v72i4.181. [DOI] [PubMed] [Google Scholar]
- 79.Schoonman L. B, Wilsmore T, Swai E. S. Sero-epidemiological investigation of bovine toxoplasmosis in traditional and smallholder cattle production systems of Tanga Region, Tanzania. Trop. Anim. Health Prod. 2010;42(4):579–587. doi: 10.1007/s11250-009-9460-2. [DOI] [PubMed] [Google Scholar]
- 80.Hove T, Lind P, Mukaratirwa S. Seroprevalence of Toxoplasma gondii infection in domestic pigs reared under different management systems in Zimbabwe. Onderstepoort J. Vet. Res. 2005a;72(3):231–237. doi: 10.4102/ojvr.v72i3.200. [DOI] [PubMed] [Google Scholar]
- 81.Sawadogo P, Hafid J, Bellete B, Sung R. T, Chakdi M, Flori P, Raberin H, Hamouni I. B, Chait A, Dalal A. Seroprevalence of T. gondii in sheep from Marrakech, Morocco. Vet. Parasitol. 2005;130(1-2):89–92. doi: 10.1016/j.vetpar.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 82.Negash T, Tilahun G. Serological survey on toxoplasmosis in sheep and goats in Nazareth, Ethiopia. Re. Med. Vet. 2004;155(10):486–487. [Google Scholar]
- 83.Dubey J. P, Karhemere S, Dahl E, Sreekumar C, Diabate A, Dabire K. R, Vianna M. C, Kwok O. C, Lehmann T. First biologic and genetic characterization of Toxoplasma gondii isolates from chickens from Africa (Democratic Republic of Congo, Mali, Burkina Faso, and Kenya) J. Parasitol. 2005;91(1):69–72. doi: 10.1645/GE-410R. [DOI] [PubMed] [Google Scholar]
- 84.Joshua R. A, Akinwumi K. A. Prevalence of antibodies to Toxoplasma gondii in four breeds of cattle at Ibadan, Nigeria. Trop. Vet. 2004;21(3):134–137. [Google Scholar]
- 85.El-Massry A, Mahdy O. A, El-Ghaysh A, Dubey J. P. Prevalence of Toxoplasma gondii antibodies in sera of turkeys, chickens, and ducks from Egypt. J. Parasitol. 2000;86(3):627–628. doi: 10.1645/0022-3395(2000)086[0627:POTGAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 86.Van der Puije W. N, Bosompem K. M, Canacoo E. A, Wastling J. M, Akanmori B. D. The prevalence of anti- Toxoplasma gondii antibodies in Ghanaian sheep and goats. Acta Trop. 2000;76(1):21–26. doi: 10.1016/s0001-706x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 87.Bisson A, Maley S, Rubaire-Akiiki C. M, Wastling J. M. The seroprevalence of antibodies to Toxoplasma gondii in domestic goats in Uganda. Acta Trop. 2000;76(1):33–38. doi: 10.1016/s0001-706x(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 88.Arko-Mensah J, Bosompem K. M, Canacoo E. A, Wastling J. M, Akanmori B. D. The seroprevalence of toxoplasmosis in pigs in Ghana. Acta Trop. 2000;76(1):27–31. doi: 10.1016/s0001-706x(00)00085-1. [DOI] [PubMed] [Google Scholar]
- 89.Hove T, Dubey J. P. Prevalence of Toxoplasma gondii antibodies in sera of domestic pigs and some wild game species from Zimbabwe. J. Parasitol. 1999;85(2):372–337. [PubMed] [Google Scholar]
- 90.Hilali M, Romand S, Thulliez P, Kwok O. C, Dubey J. P. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in sera from camels from Egypt. Vet. Parasitol. 1998;75(42-43):269–271. doi: 10.1016/s0304-4017(97)00181-7. [DOI] [PubMed] [Google Scholar]
- 91.Hassanain M. A, Elfadaly H. A. Biological assay of Toxoplasma gondii Egyptian mutton isolates. Int. J. Zool. Res. 1997;7(4):330–337. [Google Scholar]
- 92.Deconinck P, Pangui L. J, Akakpo J, Garrouste A, Ouattara L, Roger F, Tibayrenc R, Dorchies P. Prevalence of toxoplasmosis in small ruminants in tropical Africa: Results of a sero-epidemiological survey of 1042 animals. Rev. Méd. Vét. 1996;19:30–36. [Google Scholar]
- 93.Achu-Kwi M. D, Ekue N. F. Prevalence of Toxoplasma gondii antibodies in Djallonke sheep flocks in the Vina Division, Cameroon. Bull. Anim. Health Prod. Afr. 1994;42(2):89–92. [Google Scholar]
- 94.El-Ghaysh A. A, Mansour M. M. Detection of antibodies to Toxoplasma gondii in an Egyptian sheep-herd using modern serological techniques. J. Egypt. Assoc. Immunol. 1994;1:117–121. [Google Scholar]
- 95.Amin J. D, Silsmore A. J. A serological survey of some abortifacient diseases of sheep and goats in the Maiduguri area of Nigeria. Bull. Anim. Health Prod. Afr. 1993;41(2):123–128. [Google Scholar]
- 96.Pangui L. J, Lahamdi A, Samb F. Use of IFI and ELISA in a serological survey of toxoplasmosis in sheep in Dakar-Senegal. Rev. Méd. Vét. 1993;16:25–29. [Google Scholar]
- 97.Elamin E. A, Elias S, Daugschies A, Rommel M. Prevalence of Toxoplasma gondii antibodies in pastoral camels (Camelus dromedarius) in the Butana plains, mid-Eastern Sudan. Vet. Parasitol. 1992;43(3-4):171–175. doi: 10.1016/0304-4017(92)90158-6. [DOI] [PubMed] [Google Scholar]
- 98.Pandey V. S, Van Knapen F. The seroprevalence of toxoplasmosis in sheep, goats and pigs in Zimbabwe. Ann. Trop. Med. Parasitol. 1992;86(3):313–315. doi: 10.1080/00034983.1992.11812671. [DOI] [PubMed] [Google Scholar]
- 99.Weitzman G. L, Stem E. C. Preliminary serological survey for bluetongue and toxoplasmosis in sheep in Niger. Trop. Anim. Health Prod. 1991;23(4):258. doi: 10.1007/BF02357112. [DOI] [PubMed] [Google Scholar]
- 100.Bekele T, Kasali O. B. Toxoplasmosis in sheep, goats and cattle in central Ethiopia. Vet. Res. Commun. 1989;13(5):371–375. doi: 10.1007/BF00346069. [DOI] [PubMed] [Google Scholar]
- 101.Aganga A. O, Belino E. D. Toxoplasmosis in local breed of chicken in Zaria, Nigeria. Int. J. Zoonoses. 1984;11(2):170–172. [PubMed] [Google Scholar]
- 102.Falade S. Toxoplasma gondii antibodies in Nigerian goats. Trop. Anim. Health Prod. 1978;10(1):175–177. doi: 10.1007/BF02235335. [DOI] [PubMed] [Google Scholar]
- 103.Rifaat M. A, Morsy T. A, Sadek M. S. M. Toxoplasmosis in chickens and pigeons in U.A.R. (Preliminary Report) J. Trop. Med. Hyg. 1969;72:193–194. [PubMed] [Google Scholar]
- 104.Arthur M. J, Blewett D. A. IFAT detection of IgG specific to Toxoplasma in thoracic fluids from aborted lambs: Evaluation on routine diagnostic submissions. Vet. Rec. 1988;122:29–31. doi: 10.1136/vr.122.2.29. [DOI] [PubMed] [Google Scholar]
- 105.Dumetre A, Daniel A, Luc R, Aurelien M, Marie-Laure D. Toxoplasma gondii infection in sheep from Haute-Vienne, France: Seroprevalence and isolate genotyping by microsatellite analysis. Vet. Parasitol. 2006;142(3-4):376–379. doi: 10.1016/j.vetpar.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 106.Sharif M, Sarvi S, Shokri A, Teshnizi S. H, Rahimi M. T, Mizani A, Daryani A. Toxoplasma gondii infection among sheep and goats in Iran: A systematic review and meta-analysis. Parasitol. Res. 2015;114(1):1–16. doi: 10.1007/s00436-014-4176-2. [DOI] [PubMed] [Google Scholar]
- 107.Guo M, Dubey J. P, Hill D, Buchanan R. L, Gamble H. R, Jones J. L, Pradhan A. K. Prevalence and risk factors for Toxoplasma gondii infection in meat animals and meat products destined for human consumption. J. Food Prot. 2015;78:457–476. doi: 10.4315/0362-028X.JFP-14-328. [DOI] [PubMed] [Google Scholar]
- 108.Dubey J. P, Kirkbride C. A. Enzootic toxoplasmosis in sheep in North-Central United-States. J. Parasitol. 1989;75:673–676. [PubMed] [Google Scholar]
- 109.Ragozo A. M. A, Yai L. E. O, Oliveira L. N, Dias R. A, Dubey J. P, Gennari S. M. Seroprevalence and isolation of Toxoplasma gondii from sheep from São Paulo state, Brazil. J. Parasitol. 2008;94(6):1259–1263. doi: 10.1645/GE-1641.1. [DOI] [PubMed] [Google Scholar]
- 110.Buxton D, Maley S. W, Wright S. E, Rodger S, Bartley P, Innes E. A. Toxoplasma gondii and ovine toxoplasmosis: New aspects of an old story. Vet. Parasitol. 2007;149(1):25–28. doi: 10.1016/j.vetpar.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 111.da Silva A. F, Brandão F. Z, Oliveira F. C. R, Ferreira A. M. R. Toxoplasma gondii in the sheep industry: A global overview and the situation in Brazil. Rev. Bras. Ciên. Vet. 2013;20(4):179–188. [Google Scholar]
- 112.Kijlstra A, Jongert E. Control of the risk of human toxoplasmosis transmitted by meat. Int. J. Parasitol. 2008;38:1359–1370. doi: 10.1016/j.ijpara.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 113.Klun I, Djurković-Djaković O, Katić-Radivojević S, Nikolić A. Cross-sectional survey on Toxoplasma gondii infection in cattle, sheep and pigs in Serbia: Seroprevalence and risk factors. Vet. Parasitol. 2006;135(2):121–131. doi: 10.1016/j.vetpar.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 114.Venturini M. C, Bacigalupe D, Venturini L, Rambeaud M, Basso W, Unzaga J. M, Perfumo C. J. Seroprevalence of Toxoplasma gondii in sows from slaughterhouses and in pigs from an indoor and an outdoor farm in Argentina. Vet. Parasitol. 2004;124(3):161–165. doi: 10.1016/j.vetpar.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 115.de Sousa S, Ajzenberg D, Canada N, Freire L, da Costa J. C, Dardé M. L, Dubey J. P. Biologic and molecular characterization of Toxoplasma gondii isolates from pigs from Portugal. Vet. Parasitol. 2006;135(2):133–136. doi: 10.1016/j.vetpar.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 116.Poljak Z, Dewey C. E, Friendship R. M, Martin S. W, Christensen J, Ojkic D, Chow E. Pig and herd level prevalence of Toxoplasma gondii in Ontario finisher pigs in 2001 2003, and 2004. Can. J. Vet. Res. 2008;72(4):303–310. [PMC free article] [PubMed] [Google Scholar]
- 117.Gamble H. R, Brady R. C, Dubey J. P. Prevalence of Toxoplasma gondii infection in domestic pigs in the New England states. Vet. Parasitol. 1999;82(2):129–136. doi: 10.1016/s0304-4017(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 118.Jones J. L, Dubey J. P. Foodborne toxoplasmosis. Clin. Infect. Dis. 2012;55(6):845–851. doi: 10.1093/cid/cis508. [DOI] [PubMed] [Google Scholar]
- 119.de Oliveira L. N, Costa Junior L. M, de Melo C. F, Ramos Silva J. C, Bevilaqua C. M, Azevedo S. S, Gennari S. M. Toxoplasma gondii isolates from free-range chickens from the northeast region of Brazil. J. Parasitol. 2009;95(1):235–237. doi: 10.1645/GE-1730.1. [DOI] [PubMed] [Google Scholar]
- 120.Dubey J. P. Toxoplasma gondii infections in chickens (Gallus domesticus): Prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health. 2010;57(1):60–73. doi: 10.1111/j.1863-2378.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- 121.Lopes A. P, Dubey J. P, Neto F, Rodrigues A, Martins T, Rodrigues M, Cardoso L. Seroprevalence of Toxoplasma gondii infection in cattle, sheep, goats and pigs from the North of Portugal for human consumption. Vet. Parasitol. 2013;193(1):266–269. doi: 10.1016/j.vetpar.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 122.Chikweto A, Kumthekar S, Tiwari K, Nyack B, Deokar M. S, Stratton G, Dubey J. P. Seroprevalence of Toxoplasma gondii in pigs, sheep, goats, and cattle from Grenada and Carriacou, West Indies. J. Parasitol. 2011;97(5):950–951. doi: 10.1645/GE-2811.1. [DOI] [PubMed] [Google Scholar]
- 123.Costa G. H. N, Cabral D. D, Varandas N. P, de Almeida Sobral E, de Almeida Borges F, Castagnolli K. C. Frequency of anti-Neospora caninum and anti-Toxoplasma gondii antibodies in bovine sera from the states of São Paulo and Minas Gerais. Sem. Ciên. Agrárias. 2001;22(1):61–66. [Google Scholar]
- 124.Chandrawathani P, Nurulaini R, Zanin C, Premaalatha B, Adnan M, Jamnah O, Seah T. C. Research note seroprevalence of Toxoplasma gondii antibodies in pigs, goats, cattle, dogs and cats in Peninsular Malaysia. Trop. Biomed. 2008;25:257–258. [PubMed] [Google Scholar]
- 125.Huong L. T. T, Ljungström B. L, Uggla A, Björkman C. Prevalence of antibodies to Neospora caninum and Toxoplasma gondii in cattle and water buffaloes in Southern Vietnam. Vet. Parasitol. 1998;75(1):53–57. doi: 10.1016/s0304-4017(97)00178-7. [DOI] [PubMed] [Google Scholar]
- 126.Baril L, Ancelle T, Goulet V, Thulliez P, Tirard-Fleury V, Carme B. Risk factors for Toxoplasma infection in pregnancy: A case-control study in France. Scand. J. Infect. Dis. 1999;31(3):305–309. doi: 10.1080/00365549950163626. [DOI] [PubMed] [Google Scholar]
- 127.Utuk A. E, Kirbas A, Babur C, Balkaya I. Detection of Toxoplasma gondii antibodies and some helminthic parasites in camels from Nevsehir Province of Turkey. Isr. J. Vet. Med. 2012;67(2):106–108. [Google Scholar]
- 128.Dehkordi F. S, Borujeni M. R. H, Rahimi E, Abdizadeh R. Detection of Toxoplasma gondii in raw caprine, ovine, buffalo, bovine, and camel milk using cell cultivation, cat bioassay, capture ELISA, and PCR methods in Iran. Foodborne Pathog. Dis. 2013;10(2):120–125. doi: 10.1089/fpd.2012.1311. [DOI] [PubMed] [Google Scholar]
- 129.Al-Anazi A. D. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in sera from camels (Camelus dromedarius) in Riyadh Province, Saudi Arabia. J. Egypt. Soc. Parasitol. 2011;41:245–250. [PubMed] [Google Scholar]
- 130.Montoya J. G, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 131.Guo M, Mishra A, Buchanan R. L, Dubey J. P, Hill D. E, Gamble H. R. A systematic meta-analysis of Toxoplasma gondii prevalence in food animals in the United States. Foodborne. Pathog. Dis. 2016;13(3):109–118. doi: 10.1089/fpd.2015.2070. [DOI] [PubMed] [Google Scholar]


