Abstract
Using a flow cell biofilm model, we showed that a sub-MIC of azithromycin (AZM) can delay but not inhibit Pseudomonas aeruginosa biofilm formation and results in the development of a stable AZM resistance phenotype. Furthermore, mature biofilms were not affected by AZM.
The opportunistic pathogen Pseudomonas aeruginosa is the leading cause of morbidity and mortality in patients with cystic fibrosis (CF) (1). P. aeruginosa infections can be difficult to eradicate due to their propensity to form biofilms (10) and their inherent resistance to antibiotics. Treatment regimens generally involve a rigorous and aggressive antibiotic assault to minimize the detrimental cycle of infection, inflammation, and subsequent scar tissue formation. The use of azithromycin (AZM), a macrolide antibiotic, in treating chronic infections of P. aeruginosa in the lungs of CF patients has been gaining favor due to the improved outcome of CF patients treated with this antibiotic (5, 15, 20). AZM is approved for treatment of acute pulmonary bacterial infections but not against P. aeruginosa, as MICs are significantly higher than the 8-μg/ml concentration achievable in the lung tissue (7). Although the exact mechanism by which AZM may be acting to improve CF patient outcome remains elusive, both the anti-inflammatory and antimicrobial characteristics of the drug have been implicated (14, 16).
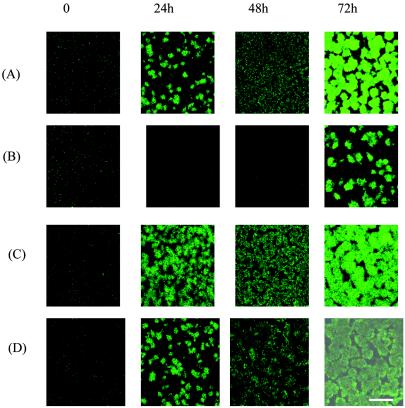
Previous studies using short-term static biofilm models suggest that a sub-MIC of AZM can alter or inhibit biofilm development by P. aeruginosa (3, 6, 11, 13). We investigated the effectiveness of a sub-MIC of AZM against both nascent and mature P. aeruginosa PAO1 biofilms by a previously described method of flow cells and confocal microscopy (4) over an extended time frame. FAB medium (8) amended with 20 μM KNO3 and, when specified, AZM (a generous gift from Pfizer, Groton, Conn.) at 2 or 8 μg/ml was used for all flow cell studies. Addition of AZM (2 μg/ml) delayed initial biofilm formation in comparison to that of the unexposed control (Fig. 1A and B) and corroborated the results of previous static biofilm studies (3, 6, 11). This finding was particularly impressive since the MIC for our PAO1 strain was 128 μg/ml. Interestingly, the effects of a sub-MIC of AZM appear to be specific to the initial stages of biofilm development since, after 48 h, a resistance phenotype was able to subvert the inhibitory effect of AZM and result in a very robust biofilm (Fig. 1B). This result is in contrast to previous reports suggesting biofilm inhibition by this drug (11, 19). These previous observations may have been biased by the use of static biofilm models in which nutrients are finite, thereby limiting the practical application of this technique. Thus, the static model system may not have provided sufficient time for a biofilm variant to develop and be detected.
FIG. 1.
Scanning confocal micrographs of P. aeruginosa PAO1 biofilms fluorescing green fluorescent protein cultivated in flow cells with or without continuous exposure to 2 μg of AZM/ml. Images are representative of one area of the biofilm monitored throughout the duration of the experiment. (A) PAO1 without AZM; (B) PAO1 with AZM; (C) PAO1-BV without AZM; (D) PAO1-BV with AZM. Bar, 20 μm.
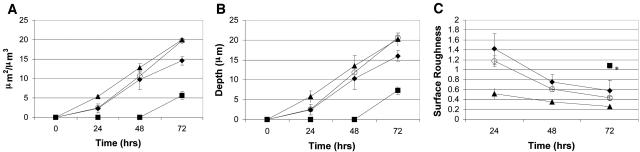
To determine if the resistance phenotype we observed was a stable inheritable trait, cells derived from a biofilm developed in the presence of 2 μg of AZM/ml (PAO1-BV) were harvested and passaged 10 times planktonically in Luria-Bertani broth containing 200 μg of carbenicillin/ml (to maintain the pTdK-borne gfp). In the absence of AZM, biofilm formation by PAO1-BV (Fig. 1C) appeared to be both temporally and architecturally similar to that of PAO1 (Fig. 1A). In the presence of 2 μg of AZM/ml, biofilm formation by PAO1-BV was not delayed (Fig. 1D) in contrast to that which we observed for PAO1 (Fig. 1B). The stability of the PAO1-BV phenotype even after repeated passage in the absence of AZM implies the presence of a stably inherited trait. As the images shown in Fig. 1 are merely representative of a single image area, we monitored five randomly selected areas per biofilm and performed a quantitative comparison of PAO1 and PAO1-BV architectures by previously described methods (9). We measured the total biomass, average thickness, and surface roughness parameters of each image area (Fig. 2). These compiled data quantitatively support our microscopic observations in that they clearly indicate that AZM delays biofilm formation in PAO1, as evidenced by decreased biomass and depth and increased surface roughness. Conversely, the measurements of these parameters for PAO1-BV grown in the presence of drug were similar to the data observed for PAO1 in the absence of drug.
FIG. 2.
Comparison of characteristics of PAO1 and PAO1-BV biofilms calculated from image stacks cultivated in flow cells and imaged by confocal laser scanning microscopy. (A) Total biomass; (B) average thickness; (C) surface roughness. Symbols: ⧫, PAO1; ▪, PAO1 exposed to 2 μg of AZM/ml; ▴, PAO1-BV; ○, PAO1-BV exposed to 2 μg of AZM/ml. Each data point represents the average of five image stacks collected from randomly selected areas ± 1 standard deviation. An asterisk indicates that surface roughness could not be calculated at earlier time points when only monolayers of biofilm thickness existed.
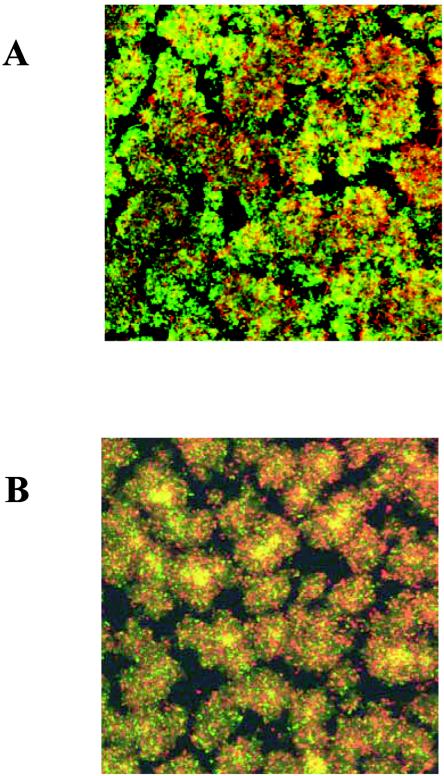
While exposure to 2 μg of AZM/ml causes a delay in initial PAO1 biofilm development, mature biofilms were not affected by the presence of 8 μg of AZM/ml (Fig. 3), the highest clinically achievable level in lung tissue (7). PAO1 biofilms were cultivated in flow cells for 3 days as described above and then exposed to 8 μg of AZM/ml for 24 h. Biofilms were stained identically with BacLight Live/Dead stain (12), examined by confocal microscopy, and compared to non-AZM-exposed biofilms treated in the same way. In this experiment, plasmid pTdK was not present in the strains tested in order to alleviate interference between green fluorescent protein and the Live/Dead stain fluorophores. In addition, viable plate counts of bacteria scraped from biofilms of PAO1 either exposed (6.5 ± 1.9 109 CFU [mean ± standard deviation]) or not exposed (1.9 ± 0.5 × 109) to 8 μg of AZM/ml showed no significant difference. Taken together, our data lead to us to conclude that 8 μg of AZM/ml is not effective in killing mature P. aeruginosa biofilms.
FIG. 3.
Scanning confocal micrographs of 4-day PAO1 biofilms cultivated in flow cells and then stained with BacLight Live/Dead stain. (A) Exposure to 8 μg of AZM/ml for 24 h; (B) no exposure to AZM. Images represent overlays of green (Live) and red (Dead) signals collected by confocal laser scanning microscopy at a magnification of ×400.
Using biofilm methods that allow for observations over longer periods of time, we have demonstrated that AZM, at a sub-MIC, has the ability to retard, but not prevent, biofilm formation. Although the exact mechanism by which AZM affects P. aeruginosa during this stage of biofilm development is unknown, AZM has been shown to affect production of P. aeruginosa outer membrane proteins, pili, and alginate (11, 18, 21). Subtle alterations in these components may influence the initial adherence of the bacteria, resulting in altered biofilm development. A link between AZM sensitivity and quorum sensing has also been suggested (6, 17).
Investigations into the potential mechanisms of AZM resistance by the biofilm variant are ongoing. The stability of PAO1-BV supports the acquisition of a stable mutation(s), or the presence of an inherent persistent population, defined as a naturally hyperresistant subset of the population (2). Although AZM was not effective against mature biofilms, it does appear to be initially effective against nascent biofilms, which may occur during acute stages of infection when biofilm bacteria are sloughing and colonizing new regions of the lung. Thus, AZM may aid in limiting the spread of the infection within the lung.
Acknowledgments
We gratefully acknowledge L. Passador and M. J. Filiatrault for review of this manuscript.
This work was supported by grants from the Cystic Fibrosis Foundation Therapeutics (IGLEWS03FG1 and IGLEWSS00G0) and the National Institutes of Health (NIH R37AI33713) to B.H.I. R.J.G was supported by a National Institutes of Health Training Grant (5T32A107362).
REFERENCES
- 1.Brennan, A. L., and D. M. Geddes. 2002. Cystic fibrosis. Curr. Opin. Infect. Dis. 15:175-182. [DOI] [PubMed] [Google Scholar]
- 2.Brooun, A., S. Liu, and K. Lewis. 2000. A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 44:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carfartan, G., P. Gerardin, D. Turck, and M. O. Husson. 2004. Effect of subinhibitory concentrations of azithromycin on adherence of Pseudomonas aeruginosa to bronchial mucins collected from cystic fibrosis patients. J. Antimicrob. Chemother. 53:686-688. (First published 3 March 2004.) [DOI] [PubMed] [Google Scholar]
- 4.De Kievit, T. R., R. Gillis, S. Marx, C. Brown, and B. H. Iglewski. 2001. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl. Environ. Microbiol. 67:1865-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Equi, A., I. M. Balfour-Lynn, A. Bush, and M. Rosenthal. 2002. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet 360:978-984. [DOI] [PubMed] [Google Scholar]
- 6.Favre-Bonte, S., T. Kohler, and C. Van Delden. 2003. Biofilm formation by Pseudomonas aeruginosa: role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. J. Antimicrob. Chemother. 52:598-604. [DOI] [PubMed] [Google Scholar]
- 7.Girard, A. E., D. Girard, A. R. English, T. D. Gootz, C. R. Cimochowski, J. A. Faiella, S. L. Haskell, and J. A. Retsema. 1987. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob. Agents Chemother. 31:1948-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heydorn, A., B. K. Ersboll, M. Hentzer, M. R. Parsek, M. Givskov, and S. Molin. 2000. Experimental reproducibility in flow-chamber biofilms. Microbiology 146:2409-2415. [DOI] [PubMed] [Google Scholar]
- 9.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 10.Hoiby, N., H. Krogh Johansen, C. Moser, Z. Song, O. Ciofu, and A. Kharazmi. 2001. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect. 3:23-35. [DOI] [PubMed] [Google Scholar]
- 11.Ichimiya, T., K. Takeoka, K. Hiramatsu, K. Hirai, T. Yamasaki, and M. Nasu. 1996. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy 42:186-191. [DOI] [PubMed] [Google Scholar]
- 12.Mah, T. F., B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O'Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 13.Moskowitz, S. M., J. M. Foster, J. Emerson, and J. L. Burns. 2004. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J. Clin. Microbiol. 42:1915-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen, T., S. G. Louie, P. M. Beringer, and M. A. Gill. 2002. Potential role of macrolide antibiotics in the management of cystic fibrosis lung disease. Curr. Opin Pulm. Med. 8:521-528. [DOI] [PubMed] [Google Scholar]
- 15.Saiman, L., B. C. Marshall, N. Mayer-Hamblett, J. L. Burns, A. L. Quittner, D. A. Cibene, S. Coquillette, A. Y. Fieberg, F. J. Accurso, and P. W. Campbell III. 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749-1756. [DOI] [PubMed] [Google Scholar]
- 16.Swords, W. E., and B. K. Rubin. 2003. Macrolide antibiotics, bacterial populations and inflammatory airway disease. Neth. J. Med. 61:242-248. [PubMed] [Google Scholar]
- 17.Tateda, K., R. Comte, J. C. Pechere, T. Kohler, K. Yamaguchi, and C. Van Delden. 2001. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45:1930-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tateda, K., Y. Ishii, Y. Hirakata, T. Matsumoto, A. Ohno, and K. Yamaguchi. 1994. Profiles of outer membrane proteins and lipopolysaccharide of Pseudomonas aeruginosa grown in the presence of sub-MICs of macrolide antibiotics and their relation to enhanced serum sensitivity. J. Antimicrob. Chemother. 34:931-942. [DOI] [PubMed] [Google Scholar]
- 19.Vranes, J. 2000. Effect of subminimal inhibitory concentrations of azithromycin on adherence of Pseudomonas aeruginosa to polystyrene. J. Chemother. 12:280-285. [DOI] [PubMed] [Google Scholar]
- 20.Wolter, J., S. Seeney, S. Bell, S. Bowler, P. Masel, and J. McCormack. 2002. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 57:212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolter, J. M., and J. G. McCormack. 1998. The effect of subinhibitory concentrations of antibiotics on adherence of Pseudomonas aeruginosa to cystic fibrosis (CF) and non-CF-affected tracheal epithelial cells. J. Infect. 37:217-223. [DOI] [PubMed] [Google Scholar]