Abstract
To address the public health problem of antibiotic resistance, the European Union (EU) founded the European Antimicrobial Resistance Surveillance System. A network of 40 hospitals that serve approximately 30% of the Spanish population (about 12 million) participated. Each laboratory reported data on antimicrobial susceptibility testing using standard laboratory procedures that were evaluated by an external quality control program. The antibiotic consumption data were obtained from the National Health System. We compared the antibiotic susceptibility of Spanish isolates of invasive Streptococcus pneumoniae (2001 to 2003) with antibiotic consumption. Invasive S. pneumoniae was isolated from 1,968 patients, 20% of whom were children at or below the age of 14 years. Of non-penicillin-susceptible strains (35.6%; 95% confidence interval, 34 to 37.2), 26.4% were considered intermediate and 9.2% were considered resistant. Between 2001 and 2003, penicillin resistance decreased from 39.5 to 33% overall and from 60.4 to 41.2% in children at or below the age of 14 years (P = 0.002). Resistance to erythromycin was at 26.6%, and coresistance with penicillin was at 19.1%. Of total isolates, the ciprofloxacin MIC was >2 μg/ml for 2.1%, with numbers increasing from 0.4% (2001) to 3.9% (2003). Total antibiotic use decreased from 21.66 to 19.71 defined daily doses/1,000 inhabitants/day between 1998 and 2002. While consumption of broad-spectrum penicillins, cephalosporins, and erythromycin declined, use of amoxicillin-clavulanate and quinolones increased by 17.5 and 27%, respectively. The frequency of antibiotic resistance in invasive S. pneumoniae in Spain was among the highest in the EU. However, a significant decrease in penicillin resistance was observed in children. This decrease coincided with the introduction of a heptavalent conjugate pneumoccocal vaccine (June 2001) and with a global reduction in antibiotic consumption levels.
Antimicrobial resistance in pathogenic bacteria is a public health problem that has become increasingly acute in recent years, and Spain is one of the most affected European countries (1, 19, 22). An international approach to the management of antimicrobial resistance is essential for its control (34). Consequently, the World Health Organization (WHO), the European Commission (EC), and the Centers for Disease Control and Prevention (CDC) have recognized the importance of studying the determinants of resistance as well as the need for control strategies (23, 29, 34).
In Europe, antimicrobial resistance of invasive pathogens has been monitored by the European Antimicrobial Resistance Surveillance System (EARSS). Its U.S. counterpart, the International Network for the Study and Prevention of Emerging Antimicrobial Resistance (INSPEAR), was launched by the CDC with a similar goal (23). Funded by the EC, EARSS is an international network of national surveillance systems that attempts to collect reliable and comparable data. The purpose of EARSS is to document variations in antimicrobial resistance over time and space so as to provide the basis for and assess the effectiveness of prevention programs and policy decisions. More than 20 countries and 600 European laboratories provide antibiotic susceptibility data to national databases and to a central database.
Streptococcus pneumoniae is the most frequent cause of bacterial respiratory tract infections, including otitis, sinusitis, pneumonia, and infectious exacerbation of chronic bronchitis, as well as potentially life-threatening systemic infections such as meningitis and bacteremia. Until recently, penicillin was the treatment of choice for S. pneumoniae infections. However, pneumococci possessing structural changes in the penicillin binding proteins have been discovered in most countries in which studies have been performed. This change results in reduced susceptibility to penicillin as well as to other β-lactam antibiotics (1, 27, 31). The penicillin MICs for many of these strains are 0.1 to 1.0 μg/ml, allowing extrameningeal infections to be treated with high doses of this antibiotic (21). However, the existence of strains for which the MIC is ≥2 μg/ml and the frequent cross-resistance with other antibiotics create a serious therapeutic problem (1, 20, 31).
The rates of antimicrobial resistance and antimicrobial consumption in Spain are among the highest (1, 4, 19). The goal of this study was to describe and analyze antibiotic resistance trends in invasive isolates of S. pneumoniae collected by Spanish hospitals participating in the EARSS network between 2001 and 2003. Since we found that penicillin resistance was decreasing, we also sought to determine whether general antibiotic consumption was also decreasing.
MATERIALS AND METHODS
Participating hospitals.
To fulfill the goal of obtaining representative data, participating hospitals were chosen to meet the following criteria: (i) total coverage of at least 20% of the Spanish population, (ii) representation of different areas of the country, and (iii) inclusion of different kinds of hospitals (size and category). Each participating hospital was coded in order to preserve its anonymity.
Isolates studied.
We included all clinical isolates of S. pneumoniae obtained from blood and cerebrospinal fluid (CSF) in Spanish laboratories participating in EARSS between 2001 and 2003. For this study, invasive infection is considered to be infection with an S. pneumoniae strain isolated from blood or CSF. Only the first invasive isolate per patient was reported.
Data collection.
A questionnaire concerning hospital characteristics (coverage, hospital type, number of beds, number of patients admitted per year, hospital departments), methods of antimicrobial susceptibility study, and interpretation criteria was submitted to each participating center. One isolate record was completed for each patient. This form included the patient's personal data (code, age, sex), hospital and departmental data, and antibiotic susceptibility data.
Participating hospitals sent prospective standardized results to the Centro Nacional de Microbiología of the Instituto de Salud Carlos III (Ministry of Health), where results were analyzed and validated by using the laboratory-based WHONET 5 program (WHO Collaborating Centre for the Surveillance of Antibiotic Resistance). All records were carefully analyzed by a medical microbiologist. Duplicate isolates from a single patient were identified and deleted. Discrepancies and atypical results were resolved by telephone, and the corresponding database records were updated if necessary. An annual report of all the data stored in the central database was sent to each participating laboratory in order to avoid possible disagreements.
Susceptibility studies.
The protocol for S. pneumoniae susceptibility testing included study of susceptibility to penicillin by means of the oxacillin disk and/or the MIC of penicillin G (recommended in cases where resistance is found by the oxacillin disk test), to third-generation cephalosporins, to erythromycin, and to ciprofloxacin as a marker of fluoroquinolone susceptibility. Moreover, data on antimicrobial susceptibility to other antibiotics were also considered when a participating laboratory routinely tested them. For this reason, the number of strains studied for each antibiotic and method was not always the same as the total number of strains.
Antimicrobial susceptibility was tested by using the disk-plate diffusion method combined with the use of E-test strips (AB-Biodisk, Solna, Sweden) in 25 of the 40 participating laboratories. The remaining 15 laboratories used different commercial microdilution systems: 7 used Wider (Fco. Soria Melguizo, Madrid, Spain), 4 used MicroScan (Dade-Behring, Deerfield, Ill.), 3 used Sensititre (Radiometer/Copenhagen Company, Copenhagen, Denmark) and 1 used Vitek (bioMérieux, Marcy l'Etoile, France).
Most laboratories (39 of 40) applied the norms and criteria of the NCCLS for interpretation of antibiotic susceptibility. One laboratory used the Neosensitab commercial system (Rosco, Taastrup, Sweden; available at http://www.rosco.dk) for the disk diffusion method and NCCLS criteria for the E-test method. No significant differences in susceptibility results were found between the laboratory using Neosensitab system criteria and the laboratories using NCCLS criteria. The quality control results of this laboratory also agreed with those obtained by reference laboratories (see below).
For cefotaxime, the NCCLS breakpoints for meningitis (≤0.5 to ≥2 μg/ml) were considered in all isolates. Because clinical infection data generated by blood isolates were not always available, some of the blood isolates were probably implicated in meningitis cases. In addition, cefotaxime susceptibility in strains from blood was also calculated with the new NCCLS cefotaxime breakpoints (≤1 to ≥4 μg/ml) (18).
Quality control.
To assess the comparability of susceptibility test results, a quality assurance exercise was performed annually for the 40 Spanish laboratories participating in EARSS. The United Kingdom National External Quality Assessment Scheme (NEQAS) designed this quality control system. Altogether, testing included 24 highly characterized control invasive strains, including 6 S. pneumoniae strains with different resistance phenotypes. It was recommended that laboratories include these external quality control strains in the regular internal quality control procedures performed by each laboratory. Data on susceptibility to penicillin G, cefotaxime, ciprofloxacin, and erythromycin were required. In addition, each laboratory completed a questionnaire concerning the methods used for determining susceptibility and applying interpretation criteria.
Antibiotic consumption.
Due to the high rates of antibiotic resistance in S. pneumoniae in Spain (1, 20, 22), we studied the evolution of antibiotic consumption in the community between 1998 and 2002. The Spanish Ministry of Health and Consumer Affairs maintains a drug database with all retail pharmacy sales of medicines acquired with National Health System prescriptions, covering nearly 100% of the Spanish population (15, 24). This database was used to gather information on sales during the period studied. The information was tabulated, and the number of units sold was converted into defined daily doses (DDD) of active drug ingredients by use of the WHO methodology (33). The number of DDD per 1,000 inhabitants per day for each of the active drug ingredients was then calculated; however, these data were not available in correlation to patient age.
Statistical analyses.
Differences in the prevalence of antibiotic resistance between different groups were assessed by the Fisher exact test. Association was determined by calculation of odds ratios (OR) with 95% confidence intervals (95% CI). The null hypothesis was rejected for P values of <0.05. Statistical analyses were performed by using EPI-Info (version 6.04).
RESULTS
Characteristics of participating laboratories.
Between 2001 and 2003, 40 laboratories reported data on invasive S. pneumoniae isolates. The estimated average coverage of the Spanish population was 30%, corresponding to approximately 12,000,000 persons. The annual median numbers of hospital beds and patients admitted were about 17,800 and 730,000, respectively. Four hospitals (10%) had more than 1,000 beds, 12 (30%) had 500 to 1,000, 17 (42.5%) had 250 to 500, and 7 (17.5%) had fewer than 250.
Quality control results.
Among participating laboratories, the overall concordance of antimicrobial susceptibility for the six S. pneumoniae control isolates tested in the three years was 94 to 97% for penicillin, 97 to 100% for erythromycin, and 82 to 100% for cefotaxime. Several Spanish laboratories did not report the categorization of ciprofloxacin susceptibility due to the lack of breakpoints in the majority of microbiology societies. However the MICs reported by more than 90% of Spanish hospitals were within the range of the reference MIC ± 1 dilution.
In the very few cases of disagreement between the expected quality control results and the performance of individual laboratories, each individual case was analyzed and discussed with the participants. Measures to improve the laboratory procedures were proposed when necessary, including dispatch of the isolates to the Spanish S. pneumoniae reference laboratory.
Patient data and incidence of invasive infections.
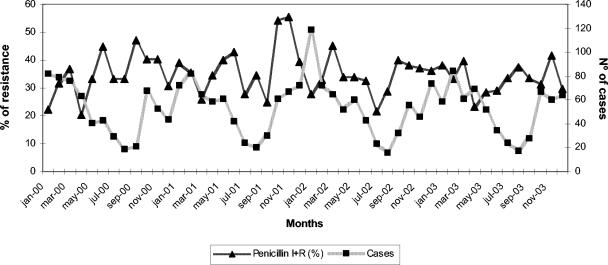
Over the study period, the 40 participating hospitals reported data for 1,968 invasive isolates of S. pneumoniae, corresponding to the same number of patients: 656 in 2001, 657 in 2002, and 655 in 2003. Of total isolates, 1,247 (63.4%) were isolated from males and 721 (36.6%) were isolated from females. There were 1,847 strains (93.9%) isolated from blood and 121 (6.1%) isolated from CSF. Of the total number of isolates, 404 (20%) were from children at or below the age of 14 years, and 356 (17.4%) were from children at or below the age of 4 years. The numbers of invasive cases in children were 139 (2001), 128 (2002), and 137 (2003). The incidence of invasive S. pneumoniae infections in the general population was estimated to be 5.8 cases/100,000 patients. Strains were isolated more frequently in the winter months (718 strains [36.5%]) than in the summer months (203 strains [10.3%]; P < 0.01). The number of invasive infections by month is shown in Fig. 1. A trend toward less penicillin susceptibility and lower case numbers during the summer months was noted, in contrast with less penicillin resistance and higher case numbers during the winter months (Fig. 1).
FIG. 1.
Number of invasive infections by S. pneumoniae and prevalence of decreased susceptibility to penicillin by month according to data from EARSS-Spain, 2001 to 2003.
Antimicrobial susceptibility.
A total of 679 of the 1,968 strains reported (34.5%; 95% CI, 32.9 to 35.5%) had decreased penicillin susceptibility. Quantitative results (MICs) were reported in 1,869 cases (95%), while penicillin susceptibility was derived from the 1-μg oxacillin disk in 99 cases (Table 1). Of the 1,869 isolates for which quantitative information was available, 665 (35.6%; 95% CI, 34 to 37.2%) were not susceptible to penicillin: 9.2% were resistant (MIC, >1 μg/ml) and 26.4% were intermediate (Table 1). As a function of the method used, decreased susceptibility to penicillin was found to be 36.6% by microdilution methods and 34.2% by the E-test (P = 0.17). Compared to other European countries participating in the EARSS network that provided susceptibility results for at least 400 invasive isolates during the study period, the rate of decreased susceptibility to penicillin in Spain was among the highest; corresponding rates were 21.7% for Portugal, 12.8% for Belgium, 11.9% for Ireland, 11.2% for Italy, 2.2% for Germany, and 1.1% for The Netherlands (http://www.earss.rivm.nl).
TABLE 1.
Antimicrobial susceptibilities of invasive S. pneumoniae isolates (EARSS-Spain, 2001 to 2003)
| Antibiotic | No. (%) of strains
|
|||
|---|---|---|---|---|
| Total | Susceptible | Intermediate | Resistant | |
| Penicillin | 1,869 | 1,204 (64.4) | 493 (26.4) | 172 (9.2) |
| Cefotaximea | 1,869 | 1,688 (90.3) | 153 (8.2) | 28 (1.5) |
| Oxacillin | 99 | 85 (85.9) | 14 (14.1) | |
| Erythromycin | 1,873 | 1,355 (72.3) | 20 (1.1) | 498 (26.6) |
| Clindamycin | 1,294 | 1,026 (79.3) | 17 (1.3) | 251 (19.4) |
| Tetracycline | 1,096 | 852 (77.8) | 20 (1.8) | 224 (20.4) |
| Rifampin | 960 | 949 (98.9) | 4 (0.4) | 7 (0.7) |
| Ciprofloxacinb | 792 | 775 (97.9) | 17 (2.1) | |
| Levofloxacin | 488 | 485 (99.4) | 1 (0.2) | 2 (0.4) |
Breakpoints used, ≤0.5 to ≥2 μg/ml.
Breakpoint used, >2 μg/ml.
Of the 1,869 pneumococcal isolates for which cefotaxime susceptibility data were reported, 9.7% showed reduced susceptibility; the majority of these (8.2%) were intermediate (MIC = 1) (Table 1). The prevalence of blood isolates for which cefotaxime MICs were ≥2 μg/ml was 1.4%. The activity loss of cefotaxime was parallel to that noted for penicillin. None of the 1,204 penicillin-susceptible isolates had reduced susceptibility to cefotaxime, while 181 out of 665 (27.2%) isolates not susceptible to penicillin also had reduced cefotaxime susceptibility (P < 0.001) (Table 2). All the cefotaxime-resistant isolates, 1.5% of the total, were also resistant to penicillin.
TABLE 2.
Susceptibility to erythromycin and cefotaxime according to penicillin susceptibility
| Group and antibiotic | % of isolatesa
|
||
|---|---|---|---|
| S | I | R | |
| Penicillin G susceptible | |||
| Cefotaximeb | 100 | 0 | 0 |
| Erythromycin | 87.1 | 0.5 | 12.4 |
| Penicillin G intermediate | |||
| Cefotaximeb | 87 | 12.7 | 0.3 |
| Erythromycin | 39.9 | 1.5 | 58.6 |
| Penicillin G resistant | |||
| Cefotaximeb | 25.8 | 60.2 | 14.8 |
| Erythromycin | 48.9 | 2.1 | 49 |
S, susceptible; I, intermediate; R, resistant.
Breakpoints used; ≤0.5 to ≥2 μg/ml.
Erythromycin resistance was also more common among non-penicillin-susceptible isolates (55.6%) than among penicillin-susceptible isolates (12.4%) (P < 0.001) (Table 2). The rate of erythromycin and penicillin coresistance was 19.1%. Frequencies of erythromycin and clindamycin resistance were 27.4 and 19.4%, respectively, in the 1,294 isolates for which both antibiotics were studied; 19.4% of these isolates were resistant to both erythromycin and clindamycin, while 8% were resistant to erythromycin alone. No annual differences in the erythromycin-clindamycin susceptibility patterns were detected.
Resistance to penicillin, erythromycin, cefotaxime, and clindamycin was higher in females than in males, although the difference was not statistically significant. However, combined trend analysis of these four antibiotics showed that antimicrobial resistance was significantly more prevalent in females than in males (P < 0.01; OR, 1.17; 95% CI, 1.06 to 1.29).
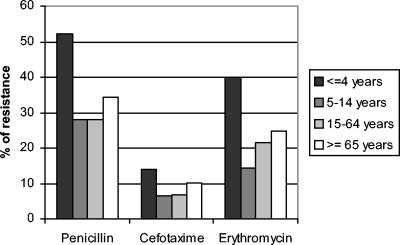
For children ≤4 years old, 52.3% of isolates were intermediate or resistant to penicillin, while 31.8% of isolates from patients ≥5 years old were intermediate or resistant (P < 0.001; OR, 2.35; 95% CI, 1.85 to 3). The corresponding percentages for cefotaxime were 14.1 versus 8.6% (P < 0.001; OR, 1.75; 95% CI, 1.19 to 2.57), and those for erythromycin were 40.1 versus 24.4% (P < 0.001; OR, 1.75; 95% CI, 1.19 to 2.57) (Fig. 2).
FIG. 2.
Antimicrobial resistance of invasive S. pneumoniae isolates in relation to patient age (EARSS-Spain, 2001 to 2003).
The ciprofloxacin MIC was reported for 792 strains, and the MICs at which 50 and 90% of isolates were inhibited were 1 and 2 μg/ml, respectively. We also found 17 (2.1%) strains for which the MIC was >2 μg/ml. There were no significant differences in penicillin or erythromycin resistance between ciprofloxacin-resistant and ciprofloxacin-susceptible strains. Levofloxacin susceptibility was tested for 488 isolates, 3 of which (0.6%) were not susceptible. Decreased susceptibilities to tetracycline and rifampin were found for 244 of 1,096 (22.2%) and 11 of 960 (1.1%) isolates, respectively. Resistance to tetracycline was more prevalent among non-penicillin-susceptible isolates (39.2%) than among penicillin-susceptible isolates (9.5%) (P < 0.001). No vancomycin-resistant strains were found.
Antimicrobial resistance trends.
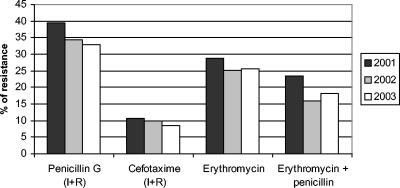
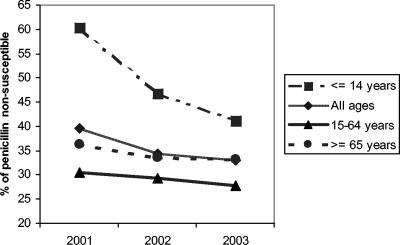
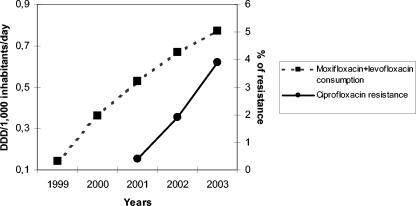
Changes in antimicrobial resistance over time are detailed in Fig. 3. Overall rates of reduced penicillin susceptibility (intermediate plus resistant isolates) decreased from 39.5% in 2001 to 33% in 2003 (P = 0.05; OR, 1.26; 95% CI, 1 to 1.59). This change was principally due to changes in isolates from children ≤14 years old. In this age group, the frequency of reduced penicillin susceptibility fell from 60.4% in 2001 to 41.2% in 2003 (P = 0.002; OR, 2.16; 95% CI, 1.29 to 3.63) (Fig. 4). The decrease in the frequency of reduced penicillin susceptibility among adults (>14 years old) was not statistically significant (P = 0.36) (Fig. 4). Rates of erythromycin resistance and reduced susceptibility to cefotaxime showed slight decreases, from 28.7% in 2001 to 25.7% in 2003 (P = 0.22) and from 10.7% in 2001 to 8.4% in 2003 (P = 0.19), respectively (Fig. 3). The frequency of coresistance to penicillin and erythromycin was 23.4% in 2001 compared to 18.1% in 2003 (P = 0.02; OR, 1.38; 95% CI, 1.04 to 1.82) (Fig. 3). In children ≤14 years old, the frequency of coresistance decreased 11.1%, from 29.1% in 2001 to 18% in 2003 (P = 0.03; OR, 1.87; 95% CI, 1 to 3.51). The rate of resistance to ciprofloxacin (MIC, >2 μg/ml) increased from 0.4% in 2001 to 3.9% in 2003 (Fig. 5).
FIG. 3.
Annual evolution of antimicrobial resistance in invasive S. pneumoniae strains isolated by Spanish laboratories participating in EARSS (2001 to 2003). I, intermediate; R, resistant.
FIG. 4.
Annual evolution of non-penicillin-susceptible invasive S. pneumoniae in relation to patient age.
FIG. 5.
Annual evolution of levofloxacin consumption plus moxifloxacin consumption and ciprofloxacin resistance in invasive S. pneumoniae.
Antibiotic consumption.
Antibiotic consumption in Spain from 1998 to 2002 is detailed in Table 3. Overall antibiotic use decreased from 21.66 DDD/1,000 inhabitants/day in 1998 to 19.71 DDD/1,000 inhabitants/day in 2002 (9% decrease; P < 0.001). Specifically, there were decreases in the consumption of broad-spectrum penicillins (from 6.02 to 4.58 DDD/1,000 inhabitants/day), cephalosporins (from 2.65 to 2.12 DDD/1,000 inhabitants/day), and macrolides (from 3.38 to 3.09 DDD/1,000 inhabitants/day) (P < 0.001). The use of amoxicillin-clavulanate and quinolones increased 17.5 and 27%, respectively (P < 0.001). Consumption of levofloxacin and moxifloxacin started in Spain in 1998 and 2000, respectively, and increased constantly to 0.34 DDD/1,000 inhabitants/day in 2002 for each. In the case of macrolide antibiotics, erythromycin consumption underwent a decrease from 0.45 to 0.20 DDD/1,000 inhabitants/day (P < 0.001), while clarithromycin and azithromycin consumption increased from 1998 to 2002 (P < 0.001) (Table 3). Figure 5 shows the annual evolution of levofloxacin and moxifloxacin consumption in relation to the percentage of S. pneumoniae isolates for which the MIC was >2 μg/ml.
TABLE 3.
Community antimicrobial consumption in Spain, 1998 to 2002
| Antibiotics | Consumption (DDD/1,000 inhabitants/day)
|
||||
|---|---|---|---|---|---|
| 1998 | 1999 | 2000 | 2001 | 2002 | |
| Global | 21.66 | 21.25 | 20.38 | 19.85 | 19.71 |
| Broad-spectrum penicillins | 6.02 | 5.64 | 5.29 | 4.78 | 4.58 |
| Amoxicillin-clavulanate | 4.67 | 4.95 | 4.71 | 4.75 | 5.49 |
| Cephalosporins | 2.65 | 2.59 | 2.39 | 2.15 | 2.12 |
| Macrolides | 3.38 | 3.30 | 3.17 | 3.04 | 3.09 |
| Erythromycin | 0.45 | 0.36 | 0.30 | 0.24 | 0.20 |
| Clarithromycin | 1.39 | 1.39 | 1.46 | 1.55 | 1.54 |
| Azithromycin | 0.64 | 0.81 | 0.85 | 0.87 | 0.92 |
| Quinolones | 1.96 | 2.14 | 2.20 | 2.36 | 2.49 |
| Levofloxacin | 0.01 | 0.14 | 0.20 | 0.29 | 0.34 |
| Moxifloxacin | 0.16 | 0.24 | 0.34 | ||
DISCUSSION
The increasing prevalence of antibiotic resistance is a serious public health concern. Epidemiological surveillance of antibiotic resistance is indispensable for empirical treatment of infections, implementation of resistance control measures, and prevention of antibiotic-resistant microorganisms (12). The EARSS network, which includes more than 600 laboratories, is an epidemiological surveillance system based on susceptibility data provided by each microbiology laboratory according to standard methods based mainly on the NCCLS rules. EARSS methodology has been evaluated previously, and EARSS is considered the official European network of national surveillance systems. It aims to collect comparable and reliable antimicrobial resistance data. This European network has some important characteristics as an antibiotic resistance surveillance system (5), including the aggregation of data for individual countries and for the whole of Europe, rapid analysis and diffusion of data, early detection systems for antimicrobial resistance in pathogens of great clinical and public health relevance, and basic support for public health decision making.
The primary laboratory receives the clinical specimens and performs routine antimicrobial susceptibility testing for clinical treatment. The resulting information has the disadvantages of possible variability of the antimicrobial agents assayed, the study methods performed, and the interpretative criteria employed, although in our experience, the vast majority of laboratories used NCCLS-recommended methodologies. EARSS researchers have performed validation of antimicrobial susceptibility results from 22 European countries, including Spain (3). Cross-validation of routine data gathering and centralized surveys has been performed (17). For example, for invasive Escherichia coli, the same trends in resistance to ampicillin, ciprofloxacin, gentamicin, and trimethoprim were found in routine sample results and in a centrally tested sample (17).
The prevalence of non-penicillin-susceptible invasive isolates of S. pneumoniae found in this study (34.5%) is similar to that reported from other studies in Spain (1, 22). However, this result is lower than the global prevalence if all types of clinical isolates, especially those of respiratory origin, are accounted for (1, 20, 22). In a national multicenter study performed in Spain between 1998 and 1999, 34.1% of 314 isolates from blood had reduced susceptibility to penicillin, in contrast with 53.2% of 1,169 isolates from respiratory samples (22). High rates of resistance have also been reported for Asian countries (13, 26, 27). In Taiwan, the prevalences of non-penicillin-susceptible invasive isolates from children ≤18 years old and from adults reached 76 and 45%, respectively (26). In the United States, the prevalence of decreased susceptibility of S. pneumoniae to penicillin was 38% among 10,103 respiratory tract isolates sampled between 2000 and 2001 (8), and 32% of 1,345 blood isolates isolated in 2002 had decreased penicillin susceptibility (14). Notably, variation in regional antimicrobial penicillin resistance has also been described (30).
In this study a constant and significant decrease in the prevalence of penicillin-resistant pneumococcal strains occurred between 2001 and 2003 (Fig. 3). Previously, two other Spanish studies (9, 10) described a similar reduction among isolates from all types of clinical sources and reported a high initial prevalence of penicillin resistance, >52% in both cases. This trend was observed before the introduction of the heptavalent pneumococcal conjugate vaccine in Spain (June 2001).
The serotypes included in the heptavalent pneumococcal conjugate vaccine (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) covered 60% of the 11,165 Spanish pneumococci isolated from all sources between January 1997 and June 2001 and 75.6% of the strains from children (9). In addition, penicillin resistance has been associated mainly with certain serotypes, principally serotypes 6, 9, 14, 19, and 23 (9), all of which are included in the vaccine. It is believed that about 40% of Spanish children ≤2 years old have been vaccinated privately, since the vaccine is not yet included in the official vaccination calendar. In the present study the reduction in penicillin resistance was particularly significant among children (Fig. 4), suggesting that vaccination may play a key role in preventing antimicrobial resistance in S. pneumoniae (32), as has been the case for Haemophilus influenzae type b (28).
We also found a reduction in antibiotic consumption in recent years, except for amoxicillin-clavulanic acid, the new fluoroquinolones (levofloxacin and moxifloxacin), and the new macrolides (clarithromycin and azithromycin). Although multiple factors influence the emergence and spread of antimicrobial resistance, antimicrobial consumption is one of the most important. It has been demonstrated that β-lactam antibiotic consumption levels correlate strongly with rates of pneumococcal penicillin resistance in various European countries as measured by EARSS data (2). It is likely that the reduction in levels of reduced penicillin susceptibility, which was reported for Spanish pneumococcal isolates before the conjugate vaccine was available, could be associated with variations in antibiotic consumption.
In this study, about 70% of erythromycin-resistant strains were also resistant to clindamycin. This probably implies a ribosomal methylation mechanism of resistance mediated by erm genes, the mechanism reported most frequently for European S. pneumoniae isolates (1, 25). The remaining strains showed a pattern of resistance (M phenotype) compatible with the presence of an active expulsion pump encoded by the mef genes. This mechanism predominates in the United States and generates resistance to macrolides of 14 and 15 atoms (7) but not to lincosamides and macrolides of 16 atoms. The same resistance phenotype could also be generated by the action of an inducible ribosomal methylation mechanism (1, 25). In the present study, no relevant changes in the prevalences of profiles of resistance to macrolides were noted.
Ciprofloxacin susceptibility is a good marker of susceptibility to other fluoroquinolones, such as levofloxacin and moxifloxacin, which currently represent good therapeutic alternatives for S. pneumoniae. In fact, the combined consumption of levofloxacin and moxifloxacin increased in Spain from 0.14 DDD/1,000 inhabitants/day in 1999 to 0.67 DDD/1,000 inhabitants/day in 2002. Despite this, fluoroquinolone-resistant pneumococci were rare in this study, and ciprofloxacin demonstrated good in vitro activity against the majority of pneumococci tested, in agreement with the results of previous studies (1, 7). The percentage of strains for which the ciprofloxacin MIC was >2 μg/ml (2.1%) agrees with the results of other recent studies, in which a reduction in susceptibility to fluoroquinolones was noted for approximately 2% of strains (6, 16). This is less than the 5.3 and 7.0% of strains isolated from respiratory tract infections reported in two recent Spanish studies (11, 22). However, an important increase in the prevalence of ciprofloxacin resistance has been observed in our study. Some authors have found a statistically significant association between a reduction in penicillin susceptibility and a reduction in ciprofloxacin susceptibility (MIC, >2 μg/ml) (6, 16, 22) or between a reduction in ciprofloxacin susceptibility and resistance to macrolides (11, 22). Neither of these associations was noted in our study.
In summary, although penicillin resistance levels among invasive Spanish S. pneumoniae strains were among the highest in Europe, they have declined significantly in recent years, especially in children. This trend occurred in the contexts of increased heptavalent pneumococcal conjugate vaccination of children and reduced global antibiotic use in the community. Although quinolone resistance remained low, a rapid increase was noted. Therefore, properly designed and conducted surveillance systems will continue to be essential for detecting changes in antimicrobial resistance.
Acknowledgments
EARSS is funded by the European Commission, Directorate General for Health and Consumer Affairs (agreement SI2.123794). This work was supported by research grants MPY 1012/04 (Instituto de Salud Carlos III, Ministry of Health) and SBVI1284/02-13 (Dirección General de Salud Pública, Ministry of Health).
Spanish members of EARSS are José Lite and Javier Garau (Hospital [H.] Mutua de Terrassa, Terrassa), Dionisia Fontanals (Corporació Parc Taulí, Barcelona), Pilar Berdonces and M. José L. De Goicoetxea (H. Galdakao, Galdakao), Oscar del Valle-Ortiz (H. Vall d'Hebron, Barcelona), Isabel Wilhemi (H. Severo Ochoa, Leganés), Francisco J. Vasallo-Vidal (H. do Meixoeiro, Vigo), Elena Loza (H. Ramón y Cajal, Madrid), Pilar Peña and Avelino Gutiérrez-Altés (H. La Paz, Madrid), Gregoria Megías-Lobón and Eva Ojeda (H. General Yagüe, Burgos), Carmina Martí (Hospital General [H.G.] de Granollers, Granollers), Maria José Gastañares (H. San Millán, Logroño), Mercedes Menéndez-Rivas (H. Infantil del Niño Jesús, Madrid), Pilar Bermudez and Marta García-Campello (Complejo Hospitalario de Pontevedra, Pontevedra), Rosario Moreno and Alfonso García-del Busto (H.G. de Castellón, Castellón), María del Mar Pérez-Moreno and Ignacio Buj (H. Verge de la Cinta, Tortosa), Gloria Royo and Matilde Elia (Hospital General Universitario [H.G.U.] de Elche, Elche), Francisco Merino and Ángel Campos (H. de Soria, Soria), María Teresa Pérez-Pomata (H.G.U. de Guadalajara, Guadalajara), Almudena Tinajas (H.G. Cristal Piñor, Orense), Consuelo Miranda and María Dolores Pérez (Hospital Universitario [H.U.] Virgen de la Nieves, Granada), Ana Fleites (H.G. de Asturias, Oviedo), Carmen Amores (H. San Agustín, Linares), Pilar Teno (H. San Pedro de Alcántara, Cáceres), A. Gimeno and Ramona Jiménez (H. Infanta Cristina, Badajoz) Carmen Raya (H. del Bierzo, Ponferrada), Begoña Fernandez (H. Sta. María Nai, Orense), María Fe Brezmes (H. Virgen de la Concha, Zamora), María Teresa Cabezas (H. de Poniente, El Ejido), Rafael Carranza (H.G. La Mancha-Centro, Ciudad Real), Alberto Gil-Setas, José Javier García-Irure, and Luis Torroba (Grupo Hospitales Ambulatorio General Solchaga, Pamplona), Alberto Yagüe (H. Vega Baja, Orihuela), Natalia Montiel (H. Costa del Sol, Marbella), Alfonso Pinedo (H. Virgen de la Victoria, Málaga), José Antonio Lepe (H. Río Tinto, Huelva), Juan Carlos Alados (H. de Jerez, Cádiz), Manuel Rodríguez (H. Puerto Real, Cádiz), Estrella Martín and Samuel Bernal (H. Valme, Seville), Inocente Cuesta (H. Ciudad de Jaén, Jaén), MŞ Dolores Crespo and Juan José Palomar (Complejo Hospitalario de Albacete, Albacete), MŞ José Revillo (H. Miguel Servet, Zaragoza), and Virtudes Gallardo (Consejería de Salud de la Junta de Andalucía, Seville).
REFERENCES
- 1.Baquero, F., J. A. García-Rodriguez, J. C. García de Lomas, L. Aguilar, and the Spanish Surveillance Group for Respiratory Pathogens. 1999. Antimicrobial resistance of 1,113 Streptococcus pneumoniae isolates from patients with respiratory tract infections in Spain: results of a 1-year (1996-1997) multicenter surveillance study. Antimicrob. Agents Chemother. 43:357-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronzwaer, S., O. Carss, U. Buchholz, S. Mölstad, W. Goettsch, I. K. Veldhuijzen, J. L. Kool, M. J. W. Sprenger, J. E. Degener, and participants in the European Antimicrobial Resistance Surveillance System. 2002. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg. Infect. Dis. 8:278-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronzwaer, S., U. Buchholz, P. Courvalin, J. Snell, G. Cornaglia, A. de Neeling, H. Aubrey-Damon, J. Degener, and EARSS participants. 2002. Comparability of antimicrobial susceptibility test results from 22 European countries and Israel: an external quality assurance exercise of the European Antimicrobial Resistance Surveillance System (EARSS) in collaboration with the United Kingdom National External Quality Assurance Scheme (UK NEQAS). J. Antimicrob. Chemother. 50:953-964. [DOI] [PubMed] [Google Scholar]
- 4.Carss, O., S. Mölstad, and A. Melander. 2002. Variation in antibiotic use in the European Union. Lancet 357:1851-1853. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. 1988. Guidelines for evaluating surveillance systems. Morb. Mortal. Wkly. Rep. 37:S5. [Google Scholar]
- 6.Chen, D. K., A. McGeer, J. C. de Azevedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 7.Doern, G. V., K. P. Heilmann, H. K. Huynh, P. R. Rhomberg, S. L. Coffman, and A. B. Brueggemann. 2001. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999-2000, including a comparison of resistance rates since 1994-1995. Antimicrob. Agents Chemother. 45:1721-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doern, G. V., and S. D. Brown. 2004. Antimicrobial susceptibility among community-acquired respiratory pathogens in the USA: data from PROTEK US 2000-01. J. Infect. 48:56-65. [DOI] [PubMed] [Google Scholar]
- 9.Fenoll, A., G. Asensio, I. Jado, S. Berrón, M. T. Camacho, M. Ortega, and J. Casal. 2002. Antimicrobial susceptibility and pneumococcal serotypes. J. Antimicrob. Chemother. 50:13-19. [DOI] [PubMed] [Google Scholar]
- 10.García-Rey, C., E. Bouza, L. Aguilar, J. García de Lomas, F. Baquero, and the Spanish Surveillance Group for Respiratory Pathogens. 2003. Evolution of penicillin and erythromycin co-resistance in Streptococcus pneumoniae in Spain. Int. J. Antimicrob. Agents 22:541-544. [DOI] [PubMed] [Google Scholar]
- 11.García-Rey, C., L. Aguilar, F. Baquero, and the Spanish Surveillance Group for Respiratory Pathogens. 2000. Influences of different factors on prevalence of ciprofloxacin resistance in Streptococcus pneumoniae in Spain. Antimicrob. Agents Chemother. 44:3481-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goosens, H., and M. J. W. Sprenger. 1998. Community acquired infections and bacterial resistance. BMJ 317:654-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsueh, P. R., Y. C. Liu, J. M. Shyr, T. L. Wu, J. J. Yan, J. J. Wu, H. S. Leu, Y. C. Chuang, Y. J. Lau, and K. T. Luh. 2000. Multicenter surveillance of antimicrobial resistance of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in Taiwan during the 1998-1999 respiratory season. Antimicrob. Agents Chemother. 44:1342-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karlowsky, J. A., M. E. Jones, D. C. Draghi, C. Thornsberry, D. F. Sahm, and G. A. Volturo. 2004. Prevalence and antimicrobial susceptibilities of bacteria isolated from blood cultures of hospitalized patients in the United States in 2002. Ann. Clin. Microbiol. Antimicrob. 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lázaro, E., M. Madurga, and F. J. de Abajo. 2002. Evolución del consumo de antibióticos en España, 1985-2000. Med. Clin. 118:561-568. [DOI] [PubMed] [Google Scholar]
- 16.Liñares, J., A. G. de la Campa, and R. Pallarés. 1999. Fluoroquinolone resistance in Streptococcus pneumoniae. N. Engl. J. Med. 341:1546-1547. [DOI] [PubMed] [Google Scholar]
- 17.Livermore, D. M., E. J. Threlfall, M. H. Reacher, A. P. Johnson, D. James, T. Cheasty, A. Shah, F. Warburton, A. V. Swan, J. Skinner, A. Graham, and D. C. Speller. 2000. Are routine sensitivity test data suitable for the surveillance of resistance? Resistance rates amongst Escherichia coli from blood and CSF from 1991-1997, as assessed by routine and centralized testing. J. Antimicrob. Chemother. 45:205-211. [DOI] [PubMed] [Google Scholar]
- 18.NCCLS. 2002. Performance standards for antimicrobial susceptibility testing, 12th informational supplement. Approved standard M100-S12. NCCLS, Wayne, Pa.
- 19.Oteo, J., J. Campos, F. Baquero, and the Spanish members of the European Antimicrobial Resistance Surveillance System. 2002. Antibiotic resistance in 1962 invasive isolates of Escherichia coli in 27 Spanish hospitals participating in the European Antimicrobial Resistance Surveillance System (2001). J. Antimicrob. Chemother. 50:945-952. [DOI] [PubMed] [Google Scholar]
- 20.Oteo, J., J. I. Alós, and J. L. Gómez-Garcés. 2001. Antimicrobial resistance of Streptococcus pneumoniae in 1999-2000 in Madrid (Spain): multicenter surveillance study. J. Antimicrob. Chemother. 47:215-218. [DOI] [PubMed] [Google Scholar]
- 21.Pallarés, R., J. Liñares, M. Vadillo, C. Cabellos, F. Manresa, P. F. Viladrich, R. Martin, and F. Gudiol. 1995. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N. Engl. J. Med. 333:474-480. [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Trallero, E., C. Fernández-Mazarrasa, C. García-Rey, E. Bouza, L. Aguilar, J. García-de-Lomas, F. Baquero, and the Spanish Surveillance Group for Respiratory Pathogens. 2001. Antimicrobial susceptibilities of 1,684 Streptococcus pneumoniae and 2,039 Streptococcus pyogenes isolates and their ecological relationships: results of a 1-year (1998-1999) multicenter surveillance study in Spain. Antimicrob. Agents Chemother. 45:3334-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richet, H. M., J. Mohammed, L. C. McDonald, W. R. Jarvis, and INSPEAR. 2001. Building communication networks: International Network for the Study and Prevention of Emerging Antimicrobial Resistance. Emerg. Infect. Dis. 7:319-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-Bremon, A., M. Ruiz-Tovar, B. Pérez-Gorricho, P. Díaz de Torres, and R. López-Rodriguez. 2000. Non-hospital consumption of antibiotics in Spain: 1987-1997. J. Antimicrob. Chemother. 45:395-400. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz, F. J., J. Verhoef, A. C. Fluit, and the SENTRY Participants Group. 1999. Comparative activity of 27 antimicrobial compounds against 698 Streptococcus pneumoniae isolates originating from 20 European university hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 18:450-453. [DOI] [PubMed] [Google Scholar]
- 26.Siu, L. K., M. L. Chu, M. Ho, Y. S. Lee, and C. C. Wang. 2002. Epidemiology of invasive pneumococcal infection in Taiwan: antibiotic resistance, serogroup distribution, and ribotype analysis. Microb. Drug Resist. 8:201-208. [DOI] [PubMed] [Google Scholar]
- 27.Song, J. H., N. Y. Lee, S. Ichiyama, R. Yoshida, Y. Hirakata, W. Fu, A. Chongthaleong, N. Aswapokee, C. H. Chiu, M. K. Lalitha, K. Thomas, J. Perera, T. T. Yee, F. Jamal, U. C. Warsa, B. X. Vinh, M. R. Jacobs, P. C. Appelbaum, and C. H. Pai. 1999. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Clin. Infect. Dis. 28:1206-1211. [DOI] [PubMed] [Google Scholar]
- 28.Tamargo, I., K. Fuentes, A. Llop, J. Oteo, and J. Campos. 2003. High levels of multiple antibiotic resistance among 938 Haemophilus influenzae type b meningitis isolates in Cuba (1990-2002). J. Antimicrob. Chemother. 52:695-698. [DOI] [PubMed] [Google Scholar]
- 29.Thamdrup, V., and K. Borge (ed.). 1998. The microbial threat. Recommendations of the European Union Conference on the Microbial Threat. Ministry of Health and Ministry of Food, Agriculture and Fisheries, Copenhagen, Denmark.
- 30.Thornsberry, C., D. F. Sahm, L. J. Kelly, I. A. Critchley, M. E. Jones, A. T. Evangelista, and J. A. Karlowsky. 2002. Regional trends in antimicrobial resistance among clinical isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States: results from the TRUST Surveillance Program, 1999-2000. Clin. Infect. Dis. 34(Suppl. 1):S1-S3. [DOI] [PubMed] [Google Scholar]
- 31.Thornsberry, C., M. E. Jones, M. L. Hickey, Y. Mauriz, J. Kahn, and D. F. Sahm. 1999. Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in the United States, 1997-1998. J. Antimicrob. Chemother. 44:749-759. [DOI] [PubMed] [Google Scholar]
- 32.Whitney, C. G., and K. P. Klugman. 2004. Vaccines as tools against resistance: the example of pneumococcal conjugate vaccine. Semin. Pediatr. Infect. Dis. 15:86-93. [DOI] [PubMed] [Google Scholar]
- 33.WHO Collaborating Centre for Drug Statistics Methodology. 1999. Anatomical therapeutic chemical (ATC) classification index including defined daily doses (DDDs) for plain substances. WHO Collaborating Centre for Drug Statistics Methodology, Oslo, Norway.
- 34.Williams, R. J., and D. L. Heymann. 1998. Containment of antimicrobial resistance. Science 279:1153-1154. [DOI] [PubMed] [Google Scholar]