Abstract
From June to October of 2002, a cluster of Escherichia coli isolates producing extended-spectrum β-lactamases (ESBLs) was detected in Stockholm. The isolates were grouped into two clones, one of which had already circulated in the same area before the outbreak. CTX-M-type ESBLs and coresistance to ciprofloxacin were identified in the strains.
Extended-spectrum β-lactamases (ESBLs) are enzymes capable of hydrolyzing oxyimino cephalosporins, thereby causing resistance to these drugs (14, 17). Since the first ESBL was identified in Germany in the 1980s (7), they have been found worldwide (2), and outbreaks of hospital-acquired ESBL-producing enterobacteria have been reported in some European countries, Asia, and the United States. ESBL-producing enterobacteria have also been identified in the Nordic countries (5, 8), but nosocomial outbreaks have not yet been reported.
From June to October of 2002, a cluster of ESBL-producing Escherichia coli was recognized in two adjacent hospitals, South-Stockholm General Hospital and Rosenlund Hospital, in southern Stockholm. This finding prompted an epidemiological analysis and a retrospective investigation of previously collected resistant E. coli from southern Stockholm. The objectives of this study were to investigate whether the isolates from the cluster were genetically related, thereby verifying nosocomial transmission, and furthermore to characterize the resistance mechanisms of the strains.
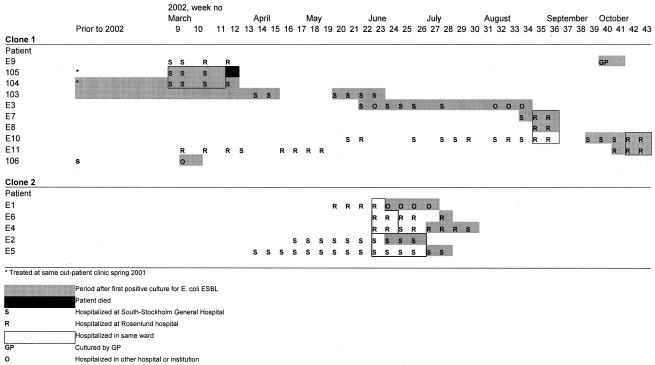
All resistant E. coli isolates collected from late 1999 to October 2002 were included. The presence of ESBL was tested by both the double-disk method (10) and Etest (ABBIODISK, Solna, Sweden). In total, 18 isolates produced ESBLs, including the 11 isolates (E 1 to E 11) found during the outbreak and seven (101 to 107) detected between September 2000 and March 2002 (Fig. 1; Table 1). Patients' transfer among health-care facilities before and during outbreak is illustrated in Fig. 1.
FIG. 1.
Patients' transfer among health-care facilities, with focus on the involved hospitals S and R.
TABLE 1.
ESBL-producing E. coli identified from patients in the southern Stockholm area
| Patient/ strain | Age | Sex | Hospital contacta | Sampling date (yr-mo-day) | Source | β-Lactamase gene
|
PFGE identification | ||
|---|---|---|---|---|---|---|---|---|---|
| TEM | OXA | CTX-M-1-type | |||||||
| 101 | 80 | Female | No contact | 2000-09-29 | Urine | + | − | + | Unrelated |
| 102 | 56 | Male | Outpatient visit (S) | 2001-04-25 | Urine | + | − | − | Unrelated |
| 103 | 85 | Female | S | 2001-05-30 | Urine | + | + | + | Clone 1, subtype a |
| 104 | 85 | Male | Same outpatient clinic (S) as 105 | 2001-09-25 | Urine | + | + | + | Clone 1, subtype a |
| 105 | 83 | Female | Same outpatient clinic (S) as 104 | 2002-03-01 | Nephrostomic drainage | + | + | + | Clone 1, subtype a |
| 106 | 80 | Female | S | 2002-03-08 | Urine | + | + | + | Clone 1, subtype b |
| 107 | 65 | Female | No contact | 2002-03-25 | Urine | + | − | + | Unrelated |
| E 1 | 91 | Female | R | 2002-06-12 | Urine | − | + | + | Clone 2 |
| E 2 | 51 | Female | S | 2002-06-13 | Wound | − | + | + | Clone 2 |
| E 3 | 59 | Female | S | 2002-06-25 | Urine | + | + | + | Clone 1, subtype a |
| E 4 | 85 | Female | S and R | 2002-07-01 | Wound | − | + | + | Clone 2 |
| E 5 | 57 | Male | S | 2002-07-03 | Wound | − | + | + | Clone 2 |
| E 6 | 84 | Female | R | 2002-07-05 | Urine | − | + | + | Clone 2 |
| E 7 | 78 | Female | S and R | 2002-08-28 | Urine | + | + | + | Clone 1, subtype a |
| E 8 | 93 | Female | R | 2002-08-29 | Urine | + | + | + | Clone 1, subtype a |
| E 9 | 90 | Female | S and R | 2002-09-30 | Urine | − | + | + | Clone 1, subtype c |
| E 10 | 84 | Female | S and R | 2002-10-02 | Urine | + | + | + | Clone 1, subtype a |
| E 11 | 82 | Female | S and R | 2002-10-11 | Urine | + | + | + | Clone 1, subtype a |
S, South-Stockholm General Hospital; R, Rosenlund Hospital.
These strains showed resistance to aztreonam, piperacillin, cefotaxime, and ciprofloxacin, with variable levels of susceptibility to ceftazidime. Notably, the MICs of cefotaxime were higher than those of ceftazidime.
Real-time PCR assays were developed in this study for detection of CTX-M-1-type and SHV-type β-lactamases, while TEM and OXA PCR were performed according to the methods of Yano et al. (20) and Ouellette et al. (12), respectively. SHV primers (SHV1, 5′-CTTTATCGGCCCTCACTCAA-3′; SHV2, 5′-AGGTGCTCATCATGGGAAAG-3′) were designed on the basis of the published SHV-1 sequence (15), and CTX-M-1-type primers (CM1, 5′-CGTCACGCTGTTGTTAGGAA-3′; CM2, 5′-TCGGTTCGCTTTCACTTTTC-3′) were based on the published sequences of CTX-M-1 (1), CTX-M-3 (4), CTX-M-10 (11), CTX-M-11 (GenBank accession no. AY005110), and CTX-M-12 (6). The CTX-M-1-type gene was detected in all isolates except for isolate 102. The OXA gene was detected in 15 of the isolates, the TEM gene in 12 of the isolates, while the SHV gene could not be detected in any isolate (Table 1).
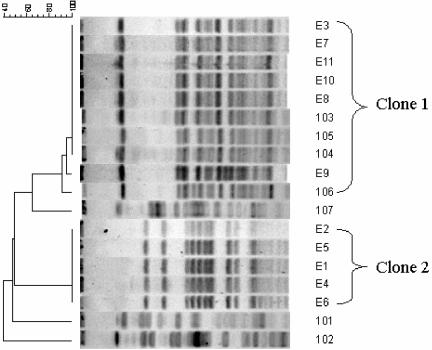
The strains were genotyped by pulsed-field gel electrophoresis (PFGE) after macrorestriction with XbaI, generating two clonal types and three unique patterns (Fig. 2). Isolates E 1 to E 11 were clustered into two clones (clone 1 and 2); four earlier isolates also belonged to clone 1 with the earliest isolated in May 2001, and the remaining (101, 102, and 107) belonged to three unrelated types (Fig. 2). Clone 1 isolates could be further divided into three subtypes: 1a, 1b, and 1c.
FIG. 2.
Dendrogram and XbaI PFGE profiles of the ESBL-producing E. coli investigated in the study. The scale at the top is the Dice index.
β-Lactamase analysis was carried out on representative isolates of each PFGE pattern. Analytical isoelectric focusing of crude cell-free sonicates was performed by the method of Matthew et al. (9). The ESBL activity of each β-lactamase band detected was confirmed by a cefotaxime-hydrolyzing disk diffusion assay. Briefly, the bands were cut out from the gel and placed, at a distance of approximately 15 mm from a paper disk containing 30 μg of cefotaxime, on an agar plate previously seeded with E. coli ATCC 25922 (cefotaxime susceptible). Indentation of the inhibition zone towards a β-lactamase band indicated hydrolysis of cefotaxime by that enzyme. Clone 1 isolates (1a, 1b, and 1c) yielded a β-lactamase band, focusing at pI 8.9, which was proved to be an ESBL by the cefotaxime-hydrolyzing assay, and also bands at pI 5.4 (except clone 1c) and 7.4, indicative of TEM and OXA enzymes, respectively, but with no ESBL activity. Clone 2 isolates produced two ESBLs, focusing at pIs 7.4 and 8.9. ESBL bands with pIs 8.4, 8.9, and 6.1/7.9 were discovered from sonic extracts from strains 101, 107, and 102, respectively.
The fact that clone 1 and clone 2 isolates harbored the CTX-M-1-type β-lactamase gene and expressed an ESBL at pI 8.9, together with their resistance profile, indicated that these clones produced CTX-M-1 ESBLs. Besides, clone 2 isolates also expressed OXA-type ESBLs according to the β-lactamase analysis and the presence of the specific β-lactamase genes. The TEM and OXA β-lactamases produced by clone 1 isolates had no ESBL activity.
With regard to the three PFGE-unrelated strains, strain 101 produced a CTX-M-3 ESBL (a member of the CTX-M-1-type β-lactamases), and strain 107 produced a CTX-M-1 ESBL based on the findings in PCR and β-lactamase characterization. Strain 102 produced a TEM-type ESBL (pI 6.1) and another ESBL with pI 7.9, which was probably a CTX-M-2-type or CTX-M-9-type enzyme.
In accordance with the laboratory findings, patients 101 and 107 had no contact with the outbreak-involved hospitals, and patient 102 had only outpatient visits at the hospital.
In recent years, a new family of ESBLs, called CTX-M, that preferentially hydrolyze cefotaxime has arisen (2, 19). Strains expressing CTX-M-type β-lactamases have, earlier, most often been associated with focal outbreaks in Eastern Europe, South America, and Japan (2). Recently CTX-M-producing strains have been reported in Western Europe as well (3, 16), and now, also in Sweden.
The ESBL-producing strains, in the present study, were coresistant to ciprofloxacin, which was in accordance to the report by Tolun et al. (18). The study by Paterson et al. (13) showed that 11 of the 15 ciprofloxacin-resistant ESBL-producing Klebsiella pneumoniae isolates belonged to just four genotypes, similarly 15 of the 18 ciprofloxacin-resistant ESBL-producing E. coli isolates in the present study were grouped into only two clones, suggesting that patient-to-patient transmission of such strains occurred. The close relationship between ESBL production and ciprofloxacin resistance is worrisome since ciprofloxacin resistance severely limits already restricted treatment options.
In conclusion, this study confirmed the nosocomial outbreak of ESBL-producing E. coli in southern Stockholm in 2002, which is the first documented hospital-acquired ESBL outbreak in Sweden. Through molecular epidemiological investigations it was disclosed that the outbreak actually consisted of two clones, one of which had already circulated in the same area 1 year before the outbreak. The study also revealed that both clones were CTX-M-type ESBL producers; besides, one of the clones expressed OXA-type ESBLs as well. The close relationship between ESBL production and ciprofloxacin resistance observed in the study poses the necessity of continued surveillance on these clinically important coresistant strains.
Acknowledgments
We gratefully acknowledge Lena Gezelius for technical assistance, Bengt Wretlind for providing Pseudomonas aeruginosa PAO 25 as a control strain in PCR, and Carl Erik Nord for helpful discussion.
REFERENCES
- 1.Bauernfeind, A., I. Stemplinger, R. Jungwirth, S. Ernst, and J. M. Casellas. 1996. Sequences of beta-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other beta-lactamases. Antimicrob. Agents Chemother. 40:509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford, P. A. 2001. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doucet-Populaire, F., J. C. Ghnassia, R. Bonnet, and J. Sirot. 2000. First isolation of a CTX-M-3-producing Enterobacter cloacae in France. Antimicrob. Agents Chemother. 44:3239-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gniadkowski, M., I. Schneider, A. Palucha, R. Jungwirth, B. Mikiewicz, and A. Bauernfeind. 1998. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing beta-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob. Agents Chemother. 42:827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen, D. S., D. Sirot, and H. J. Kolmos. 1998. Extended spectrum betalactamases in Danish Klebsiella isolates. Ugeskr. Læger. 160:2261-2262. [PubMed] [Google Scholar]
- 6.Kariuki, S., J. E. Corkill, G. Revathi, R. Musoke, and C. A. Hart. 2001. Molecular characterization of a novel plasmid-encoded cefotaximase (CTX-M-12) found in clinical Klebsiella pneumoniae isolates from Kenya. Antimicrob. Agents Chemother. 45:2141-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knothe, H., P. Shah, V. Krcmery, M. Antal, and S. Mitsuhashi. 1983. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11:315-317. [DOI] [PubMed] [Google Scholar]
- 8.Manninen, R., H. Auvinen, and P. Huovinen. 1997. Resistance to second- and third-generation cephalosporins among Escherichia coli and Klebsiella species is rare in Finland. Clin. Microbiol. Infect. 3:408-413. [DOI] [PubMed] [Google Scholar]
- 9.Matthew, A., A. M. Harris, M. J. Marshall, and G. W. Ross. 1975. The use of analytical isoelectrical focusing for detection and identification of β-lactamases. J. Gen. Microbiol. 88:169-178. [DOI] [PubMed] [Google Scholar]
- 10.NCCLS. 2002. Screening and confirmatory tests for ESBLs in Klebsiella pneumoniae, K. oxytoca, and Escherichia coli, 5th ed., vol. 22. Document M2-A7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.Oliver, A., J. C. Perez-Diaz, T. M. Coque, F. Baquero, and R. Canton. 2001. Nucleotide sequence and characterization of a novel cefotaxime-hydrolyzing beta-lactamase (CTX-M-10) isolated in Spain. Antimicrob. Agents Chemother. 45:616-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouellette, M., L. Bissonnette, and P. H. Roy. 1997. Precise insertion of antibiotic resistance determinants into Tn21-like transposons: nucleotide sequence of the OXA-1 beta-lactamase gene. Proc. Natl. Acad. Sci. USA 84:7378-7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paterson, D. L., L. Mulazimoglu, J. M. Casellas, W. C. Ko, H. Goossens, A. Von Gottberg, S. Mohapatra, G. M. Trenholme, K. P. Klugman, J. G. McCormack, and V. L. Yu. 2000. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum beta-lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin. Infect. Dis. 30:473-478. [DOI] [PubMed] [Google Scholar]
- 14.Rasheed, J. K., C. Jay, B. Metchock, F. Berkowitz, L. Weigel, J. Crellin, C. Steward, B. Hill, A. A. Medeiros, and F. C. Tenover. 1997. Evolution of extended-spectrum beta-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob. Agents Chemother. 41:647-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice, L. B., L. L. Carias, A. M. Hujer, M. Bonafede, R. Hutton, C. Hoyen, and R. A. Bonomo. 2000. High-level expression of chromosomally encoded SHV-1 beta-lactamase and an outer membrane protein change confer resistance to ceftazidime and piperacillin-tazobactam in a clinical isolate of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 44:362-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabate, M., R. Tarrago, F. Navarro, E. Miro, C. Verges, J. Barbe, and G. Prats. 2000. Cloning and sequence of the gene encoding a novel cefotaxime-hydrolyzing beta-lactamase (CTX-M-9) from Escherichia coli in Spain. Antimicrob. Agents Chemother. 44:1970-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah, A. A., F. Hasan, S. Ahmed, and A. Hameed. 2004. Extended-spectrum beta-lactamases (ESBLs): characterization, epidemiology and detection. Crit. Rev. Microbiol. 30:25-32. [DOI] [PubMed] [Google Scholar]
- 18.Tolun, V., O. Kucukbasmaci, D. Torumkuney-Akbulut, C. Catal, M. Ang-Kucuker, and O. Ang. 2004. Relationship between ciprofloxacin resistance and extended-spectrum beta-lactamase production in Escherichia coli and Klebsiella pneumoniae strains. Clin. Microbiol. Infect. 10:72-75. [DOI] [PubMed] [Google Scholar]
- 19.Tzouvelekis, L. S., E. Tzelepi, P. T. Tassios, and N. J. Legakis. 2000. CTX-M-type beta-lactamases: an emerging group of extended-spectrum enzymes. Int. J. Antimicrob. Agents 14:137-142. [DOI] [PubMed] [Google Scholar]
- 20.Yano, H., A. Kuga, K. Irinoda, R. Okamoto, T. Kobayashi, and M. Inoue. 1999. Presence of genes for beta-lactamases of two different classes on a single plasmid from a clinical isolate of Serratia marcescens. J. Antibiot. (Tokyo) 52:1135-1139. [DOI] [PubMed] [Google Scholar]