Abstract
Portal hypertension is a common clinical syndrome, defined by a pathologic increase in the portal venous pressure. Increased resistance to portal blood flow, the primary factor in the pathophysiology of portal hypertension, is in part due to morphological changes occurring in chronic liver diseases. This results in rerouting of blood flow away from the liver through collateral pathways to low-pressure systemic veins. Through a variety of computed tomographic, sonographic, magnetic resonance imaging and angiographic examples, this article discusses the appearances and prevalence of both common and less common portosystemic collateral channels in the thorax and abdomen. A brief overview of established interventional radiologic techniques for treatment of portal hypertension will also be provided. Awareness of the various imaging manifestations of portal hypertension can be helpful for assessing overall prognosis and planning proper management.
Keywords: Portal hypertension, Diagnostic imaging, Portosystemic collaterals, Image-guided therapy
Core tip: Pathologic resistance to portal venous blood flow results in elevated portal pressure, forcing blood to decompress through various portosystemic collaterals. Blood may circumvent the liver via intrathoracic, intraabdominal, abdominal wall and pelvic collateral pathways - resulting in variceal bleeding, ascites and encephalopathy. Our objective is to provide a comprehensive review of the commonly recruited portosystemic collaterals in portal hypertension and demonstrate its multimodality appearance on ultrasound, computed tomography and magnetic resonance imaging. Additionally, we will review several image-guided therapies which either aim to decrease portal venous pressure or mitigate the sequelae of portal hypertension.
INTRODUCTION
The portal venous system is a unique circulatory system which connects two systems of capillary beds; one in the gastrointestinal tract and splenic parenchyma, and the second in the hepatic sinusoids. The portal vein transports blood from abdominal viscera and ramifies - much like an artery - at the liver, ending into the hepatic sinusoids. The main portal vein is typically formed by the confluence of splenic vein and the superior mesenteric vein, posterior to the neck of the pancreas. The inferior mesenteric vein usually drains into the splenic vein and does not directly connect to the main portal vein (Figure 1). Other tributaries of the portal vein that make up the portal venous system are the left gastric, right gastric, paraumbilical, and cystic veins.
Figure 1.
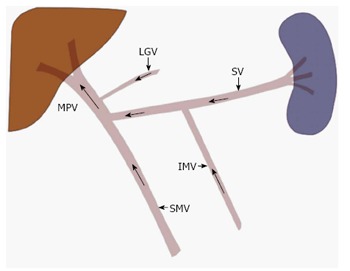
Normal portal venous anatomy and direction of blood flow. The main portal vein (MPV) is most commonly formed when the splenic vein (SV) and the superior mesenteric vein (SMV) join. While variable, the inferior mesenteric vein (IMV) most commonly drains in to the splenic vein, at the level of the pancreatic body. Other tributaries may also join the MPV, such as the left gastric vein (LGV) as depicted here.
Normal portal venous pressure is between 5 to 10 mmHg, while the normal pressure gradient between the portal vein and the inferior vena cava, known as the hepatovenous pressure gradient (HVPG), is typically 1 to 5 mmHg[1]. Pathologic increase in portal venous pressure is primarily caused by resistance to portal inflow, which can occur either at the level of the portal vein, hepatic sinusoids or hepatovenous outflow. In addition to an increase in hepatic vascular resistance to portal blood flow, there is progressive splanchnic vasodilatation that aggravates the portal hypertension syndrome by augmenting portal blood flow[2]. Recent updates in pathophysiologic understanding of portal hypertension have also highlighted the contribution of hepatic sinusoidal endothelial dysfunction elevating portal pressure[1]. Ongoing portal hypertension eventually leads to formation of collateral circulation that directly connects the portal blood vessels to systemic circulation, bypassing the liver and thus constituting the clinical syndrome of portal hypertension[3-5]. Clinically significant portal hypertension is defined as an increase in HVPG to ≥ 10 mmHg; above this threshold, the complications of portal hypertension may begin to appear[6]. Formation of portosystemic collaterals is a complex process involving the opening, dilatation and hypertrophy of pre-existing vascular channels in order to decompress the portal system[2,4,5,7,8]. Some have also postulated that a component of angiogenesis is also involved in collateral formation[9].
In this review, we discuss the entire spectrum of portosystemic collateral pathways in the abdomen and thorax, through examples of computed tomography (CT), ultrasonography (US), magnetic resonance imaging (MRI) and angiographic examples. A brief overview of established interventional techniques for treatment of portal hypertension and related complications will also be provided.
IMAGING MODALITIES FOR THE ASSESSMENT OF PORTAL HYPERTENSION AND COLLATERAL PATHWAYS
A variety of imaging modalities are available to provide early diagnosis, prognostication and treatment planning for patients with advanced liver cirrhosis and portal hypertension. Catheter-based hepatic venography allows for measurement of HVPG which is the difference between the wedge and the free hepatic venous pressures. Measurement of HVPG is currently the best available method to evaluate the presence and severity of portal hypertension, however, this minimally invasive technique is not without risks - including bleeding, infection, possible contrast reaction, arrhythmias and need for intravenous sedation[8,10]. US, CT, and MRI offer diagnostic information complementary to catheter-based techniques.
US is typically the initial, first-line modality choice for the diagnosis and follow-up of portal hypertension[8,11]. Spectral and colour Doppler US can provide accurate and specific detection of certain portosystemic collaterals (recanalized paraumbilical vein, splenorenal collaterals, dilated left and short gastric veins), as well as, directionality of flow within the portal vein[8,11]. US is also very accurate in the detection of portal and hepatic venous thrombosis and can be used for the surveillance of transjugular intrahepatic portosystemic shunt (TIPS) patency. In addition, US can provide cheap and readily accessible assessment of hepatic parenchymal nodularity, splenomegaly, provide screening for liver tumors including metastasis and hepatocellular carcinoma (HCC), as well as, detect the presence of abdominal ascites[8]. Unfortunately, the clinical usefulness of US in portal hypertension remains unsettled as it is plagued by the lack of reproducibility and accuracy secondary to intra- and interobserver variation and patient related factors (body habitus, respiratory motion, etc.). Transient elastography is a novel but well-validated sonographic technique that has emerged in the evaluation of hepatic fibrosis. The reported correlation between liver stiffness and HWVP makes elastography a potential helpful tool for the non-invasive evaluation of portal hypertension[12].
Both CT and MRI provide excellent cross-sectional visualization of the portal venous system with superior spatial and contrast resolution when combined with intravenous contrast agents (non-iodinated contrast and gadolinium chelate agents, respectively)[8]. CT and MRI can outline the full extent of portal vein thrombosis, portosystemic collateral mapping, and treatment planning in cases of complex anatomy. Although endoscopy is the gold standard, CT can also be useful in the detection of esophageal/gastric varices. In a prospective evaluation, Perri et al[13] demonstrated that CT had a 90% sensitivity in the identification of esophageal varices determined to be large on endoscopy, but only about 50% specific. The sensitivity of CT in detecting gastric varices was 87%. CT however carries with it the burden of ionizing radiation and there is a small risk of both allergic reaction and nephrotoxicity with both CT and MR contrast agents[8,14].
Four-dimensional flow MRI (4-D flow MR) with complete volumetric acquisition of the hepatic arterial and portal venous system also offers a promising non-invasive approach for characterization and follow-up of portal hypertension. Studies have demonstrated the utility of 4-D flow MR for the evaluation of the entire splanchnic system with high spatial resolution, as well as, validated the technique for quantification of flow velocities and volume using Doppler US data as reference standards[15-17]. Future clinical applications may include the evaluation of patients with portal hypertension for the purpose of interventional treatment planning, as well as, post-procedural TIPS assessment and follow-up[15].
ANATOMIC SITES OF PORTOSYSTEMIC CONFLUENCE
To ensure consistency in terminology, it should be noted that varices are dilated end-organ veins that have the propensity to bleed whereas shunts are dilated collateral channels that simply connect the portal and systemic vascular beds. The portosystemic collateral channels that can develop in portal hypertension are numerous, widespread and varied in appearance. Intrathoracic manifestations of portosystemic collateral vessels characteristically develop by way of the coronary vein into esophageal or paraesophageal (22%-38%) varices and cardiophrenic varices (18%)[10,18,19]. Other common pathways of portosystemic shunting involve gastroesophageal, paraumbilical, splenorenal, and inferior mesenteric collateral vessels. Pleuro-pericardial-peritoneal, pancreaticoduodenal, splenoazygos and mesocaval collaterals are less common pathways for decompression of portal vein (Figure 2).
Figure 2.
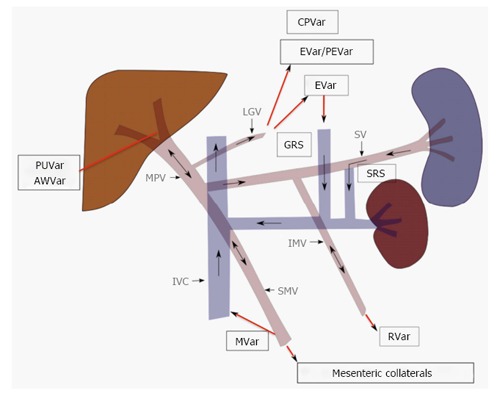
Portosystemic collateral pathways and direction of blood flow in portal hypertension. Progressive resistance to hepatopetal flow results in slowed and eventually reversed flow in the main portal vein (MPV). Portal venous system decompresses by recruiting several pre-exiting collateral pathways, the selection of which is partly dictated by the location of the portal venous resistance. Paraumbilical (PUVar), abdominal wall varices (AWVar), esophageal (EVar), paraesophageal (PEVar), gastric (GVar), cardiophrenic (CPVar), mesenteric (MVar) and rectal (RVar) varices may be created in order to allow the passage the portal venous blood into systemic circulation. LGV: Left gastric vein; SV: Splenic vein; IMV: Inferior mesenteric vein; IVC: Inferior vena cava; SRS: Splenorenal shunt; GRS: Gastrorenal shunt.
CORONARY, ESOPHAGEAL, PARAESOPHAGEAL AND CARDIOPHRENIC VARICES
Coronary (or left gastric) veins within the lesser omentum are the most frequently depicted varices, seen in 80% of cross-sectional and 86% of angiographic studies in patients with portal hypertension[16,20,21]. With CT, the cephalic portion of the coronary vein is clearly delineated, often as multiple channels near the gastroesophageal junction. A coronary vein larger than 5-6 mm in diameter on Doppler sonograms or CT is considered abnormal and is an indicator of portal hypertension[20,22-24].
Coronary venous collaterals are usually accompanied by esophageal or paraesophageal varices. Esophageal varices are usually supplied by the anterior branch of the left gastric vein, whereas the posterior branch of this vein supplies paraesophageal varices[25]. On CT, varices appear as well-defined round, tubular, or serpentine structures that are smooth, have homogeneous attenuation, and enhance with contrast material to the same degree as adjacent portal and mesenteric veins (Figure 3).
Figure 3.
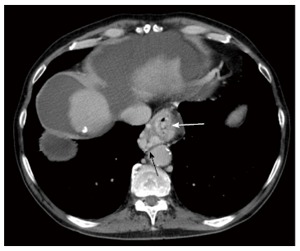
Axial enhanced computed tomography of the upper abdomen in portal venous phase demonstrates multiple large tubular and serpiginous esophageal (white arrow) and paraesophageal (black arrow) varices at the level of the esophageal hiatus of the diaphragm.
Esophageal varices, the most common and clinically important collateral vessels, consist of dilated subepithelial and submucosal veins in the wall of the lower esophagus (Figure 4). They usually drain into the azygos or the hemiazygos system[20]. The reported rate of variceal hemorrhage in patients with esophageal varices is estimated at 10%-30% per year, with the mortality from variceal hemorrhage high at 20%-35%[26]. CT is useful for detection and grading of esophageal varices, with a detection rate of more than 92% of large varices which have an elevated risk of bleeding[18]. Typical CT appearance is nodular thickening of the esophageal wall and enhancing nodular intraluminal protrusions with scalloped borders[18].
Figure 4.
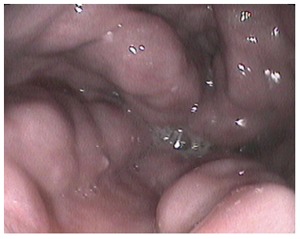
Endoscopic image of large tortuous submucosal esophageal varices protruding into the esophageal lumen.
Paraesophageal varices are venous collaterals surrounding the esophagus through a network of multiple veins and connect the coronary vein with the azygos, hemiazygos veins and the vertebral plexus. They are seen in 22%-38% of CT scans as dilated collateral vessels surrounding the esophagus and the descending thoracic aorta (Figure 5)[10,18,19]. They are located outside the walls of the esophagus and thus cannot be seen with endoscopy. Their clinical significance is not entirely clear, however, Lin et al[27] demonstrated that paraesophageal varices revealed on chest CT suggested a poor prognosis for patients with esophageal variceal hemorrhage who underwent sclerotherapy.
Figure 5.
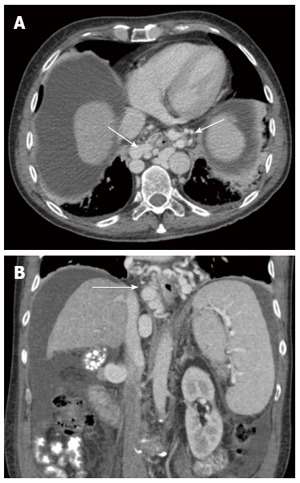
Axial (A) and coronal (B) cross-sectional enhanced computed tomography images show large paraesophageal varices (white arrow) surrounding the esophagus circumferentially in a patient with liver cirrhosis and portal hypertension. Note the nodular and shrunken liver, low-density abdominal ascites and splenomegaly.
Cardiophrenic angle varices consist of dilated pericardiacophrenic veins, which frequently occur in patients with cirrhosis caused by membranous obstruction of the inferior vena cava (IVC). Their reported prevalence is estimated at 18%[10]. At radiography, they may manifest as undulating masses along the cardiac borders, simulating a tumor (Figure 6).
Figure 6.
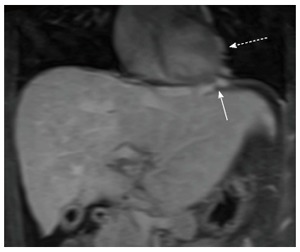
Coronal post-gadolinium T1-weighted fat-suppressed magnetic resonance image shows prominent cardiophrenic (white arrow) and pericardial collateral veins (dashed white arrow) in a patient with Budd-Chiari syndrome.
GASTRIC VARICES AND GASTRORENAL SHUNTS
Gastric varices, along with esophageal varices, are by far the most common portosystemic pathways seen in portal hypertension[21]. The reported prevalence of gastric varices ranges from 2% to 70%. Esophageal and gastric varices frequently coexist, as noted in the widely used Sarin endoscopic grading classification for gastric varices (Table 1)[28]. Esophageal varices are more likely to be supplied by the left gastric or the coronary vein, whereas gastric varices are more likely to be supplied by the short gastric and posterior gastric veins[29]. Dilated short gastric veins appear as a tangle of vessels along the medial aspect of the spleen near the hilum, making it often difficult to distinguish between the gastric fundus and individual vessels (Figure 7). Gastric varices are known to simulate tumors or thickened rugae at endoscopy or barium radiography (Figure 8).
Table 1.
Sarin endoscopic grading classification for gastric varices
| Category | Description |
| Gastroesophageal varix type 1 | Continuation from esophageal varices which extend along the lesser curve |
| Gastroesophageal varix type 2 | Continuation from esophageal varices which extend along the lesser curve but are more tortuous than GOV-1 |
| Isolated gastric varix type 1 | Occur in the absence of esophageal varices and are located at the gastric fundus. Varices are tortuous and complex |
| Isolated gastric varix type 2 | Occur in the absence of esophageal varices and are located at the gastric body, antrum or pylorus |
Figure 7.
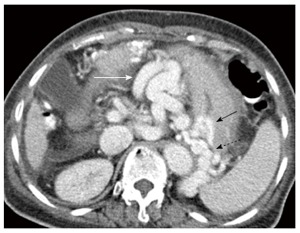
Enhanced axial computed tomography image acquired in portal venous phase demonstrates large upper abdominal omental varices (white arrow). Additionally, several enlarged submucosal gastric (black arrow) and short gastric varices (dashed black arrow).
Figure 8.
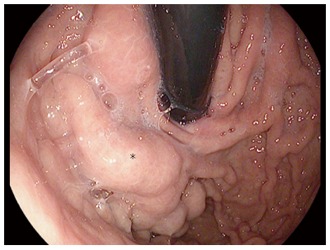
Retroflexed endoscopic image of large tortuous submucosal gastric varices (black star).
Gastric varices that usually drain into the esophageal or paraesophageal veins but can occasionally drain into the left renal vein via a gastrorenal shunt (Figure 9). A gastrorenal shunt appears as a large left-sided retroperitoneal venous channel, associated with dilatation of the left renal vein[30]. These shunts may arise from pre-existing tiny portosystemic communications or from the adrenal and periadrenal venous system[30]. In patients with gastrorenal shunts, large gastric varices may be encountered in the absence of esophageal varices.
Figure 9.
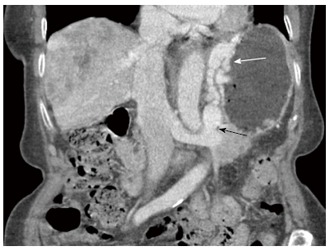
Coronal enhanced computed tomography image demonstrates a large gastrorenal shunt (black arrow) and perigastric, as well as, gastric submucosal varices (white arrow) in a patient with liver cirrhosis and portal hypertension.
PERISPLENIC VARICES AND SPLENORENAL AND SPLENOCAVAL/SPLENOAZYGOS SHUNTS
Splenic varices usually traverse the splenocolic ligament and are seen as dilated veins in the anteroinferior aspect of the spleen[20]. Perisplenic collaterals can communicate with the gastric veins. It should be noted that the tortuous splenic veins frequently seen at the hilum of the enlarged spleen should not be called perisplenic varices.
A spontaneous splenorenal shunt can also develop, which on CT, is seen as large, tortuous veins in the region of the splenic and left renal hilum that drain into an enlarged left renal vein (Figure 10)[30]. These shunts can be so tortuous that the exact origin of the connection along the splenic vein is sometimes difficult to discern.
Figure 10.
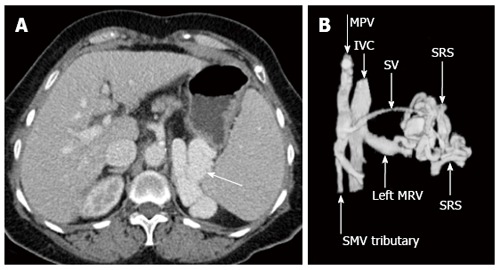
Axial enhanced computed tomography acquired in portal venous phase demonstrates a prominent splenorenal shunt (A, white arrow), left anterior oblique three dimensional computed tomography reconstruction re-demonstrates spontaneous splenorenal shunt draining portal venous blood into the left extra-hilar main renal vein (B). MPV: Main portal vein; SMV: Superior mesenteric vein; IVC: Inferior vena cava; SV: Splenic vein; SRS: Spontaneous splenorenal shunt; MRV: Main renal vein.
In the rare case of a splenocaval shunt, large veins may be seen extending from the inferior aspect of the spleen to the pelvis and draining into the inferior vena cava via the left internal iliac vein or gonadal vein[20]. Splenoazygos shunts involve portal decompression via splenic vein to hemiazygos vein or posterior abdominal wall veins[20]. CT is the best modality for demonstrating these deep portosystemic collateral shunts[20].
PARAUMBILICAL AND ABDOMINAL WALL COLLATERALS
The paraumbilical vein arises from the left portal vein and usually courses between the lateral and medial segments of the left hepatic lobe, along the anterior edge of the falciform ligament. The course and number of the paraumbilical collaterals is variable[31,32]. On cross-sectional imaging, paraumbilical varices appear as tubular structures more than 2-3 mm in diameter (Figure 11) and usually anastomose with the superior epigastric or internal thoracic veins. From there, drainage is typically either into the superior vena cava or anastomose with inferior epigastric vein and then drain into the inferior vena cava through the external iliac vein[31]. Occasionally, the paraumbilical vein drains into the abdominal veins, creating a “Medusa’s head” appearance (Figure 12). The paraumbilical system is considered a frequent abdominal portosystemic pathway, with reported prevalence of 30%-35%[20,22].
Figure 11.
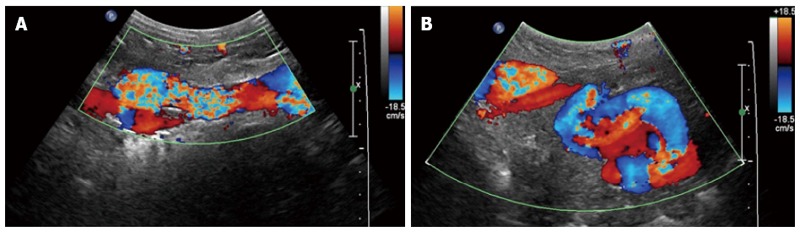
Sagittal (A) and transverse midline (B) Doppler sonographic images demonstrate turbulent flow within a recanalized paraumbilical vein.
Figure 12.

Axial enhanced computed tomography image acquired in portal venous phase (A) and coronal-oblique three-dimensional computed tomography reconstruction (B) demonstrating large paraumbilical varices with large associated caput medusa.
OMENTAL AND MESENTERIC COLLATERALS
Omental collateral vessels are infrequently included in list of common portosystemic pathways because they are not well visualized with angiography or other modalities. However, Cho et al[20] demonstrated a 20% prevalence of omental varices in their series of 60 patients with cirrhosis. Omental varices tend to be small but are usually numerous throughout the greater omentum and should not be mistaken for metastases on CT imaging.
Mesenteric collateral vessels usually appear as dilated and tortuous branches of the superior mesenteric vein within the mesenteric fat[20]. These collateral vessels ultimately drain into the systemic venous system via the retroperitoneal or pelvic veins[32]. Mesenterorenal shunts also exist, although rare. They manifest as communication between superior mesenteric vein and the right renal vein[33]. The presence of collateral vessels through the inferior mesenteric vein has also been documented, with authors theorizing that such vessels usually flow into systemic circulation via middle and inferior rectal (or hemorrhoidal) varices[34]. Occasionally, blood shunts from the inferior mesenteric vein directly into the inferior vena cava, which is called an inferior mesenteric-caval shunt (or mesocaval shunt) (Figure 13). This may cause encephalopathy, although there is less of a risk of hemorrhage from rectal varices[34].
Figure 13.
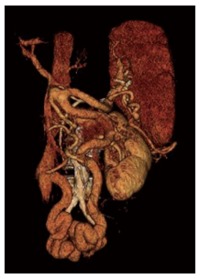
Right anterior oblique three-dimensional computed tomography reconstruction shows mesocaval collateral veins shunting blood from the inferior mesenteric vein to the inferior vena cava via the right ovarian vein.
OTHER COLLATERAL VESSELS
Intrahepatic portal veins may form collateral pathways with hepatic venous branches or direct communication with the left gastric vein, usually in the left lobe[32]. A loose collateral plexus over the hepatic surfaces sometimes is widely distributed over the parietal peritoneum, with branches piercing the diaphragm to join pericardial, pleural, and pulmonary veins (pleuropericardial-peritoneal collaterals)[35]. The term vein of Sappey has been used in the past to indicate these small diaphragmatic collaterals, but is now regarded as synonymous for the paraumbilical vein[36,37].
RADIOLOGIC INTERVENTIONS IN PORTAL HYPERTENSION
Interventions involving the portal venous system were conceived as early as 1969, when Rosch and colleagues reported creating a portosystemic shunt within the liver parenchyma in a series of dog experiments using Teflon tubes as stents[38]. Subsequent decades have seen an expansion in the variety of therapeutic interventions to treat portal hypertension. The primary goal in treating portal hypertension is reduction in the portal venous pressure itself, in order to mitigate complications such as variceal hemorrhage, congestive gastroenteropathy, refractory ascites, hepatic hydrothorax, and hepatorenal syndrome[39]. In cases where it is not possible to achieve this primary goal, various procedures can be offered to palliate or control the symptoms related to portal hypertension.
REDUCING PORTAL VENOUS PRESSURE: TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT
Transjugular intrahepatic portosystemic shunt (TIPS) is an image-guided, minimally invasive procedure in which polytetrafluoroethylene (PTFE)-covered stents are deployed through the hepatic parenchyma, bridging the hepatic vein and portal vein[40]. Briefly, frequently under general anaesthesia, the right internal jugular vein is accessed and under fluoroscopic guidance, a catheter-trocar set is typically advanced from the right hepatic vein to the right portal vein. Once appropriate position is confirmed, angioplasty and a PTFE-covered stent is deployed - bridging the portal venous system with the system venous system (Figure 14)[39].
Figure 14.
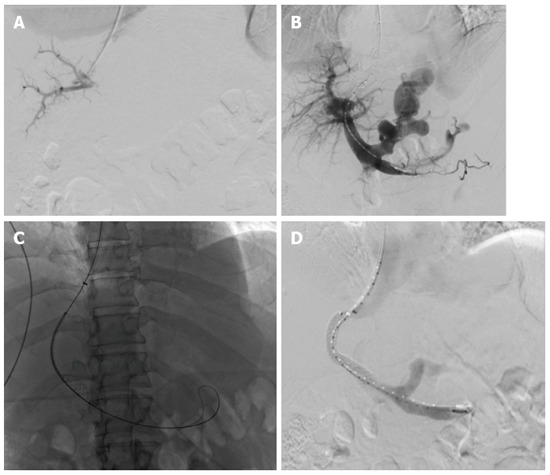
Transjugular intrahepatic portosystemic shunt procedure performed on a 54-year-old male with alcohol-induced cirrhosis and portal hypertension who presents with intractable ascites. A: Rosch-Uchida transjugular intrahepatic portosystemic shunt (TIPS) trochar-needle set (Cook Medical, Bloomington, United States) was advanced into an anterior branch of the right portal vein successfully after four attempts; B: Subsequently a wire and pigtail catheter were advanced into the main portal vein and subtraction angiography was performed demonstrating large dilated left gastric varices; C: Viatorr polytetrafluoroethylene (PTFE)-covered self-expanding stent (Gore Medical, Flagstaff, United States) was deployed over the tract; D: Completion portography demonstrates patency of the TIPS with decreased overall flow into the gastric varices.
TIPS reduces the portosystemic pressure gradient by functioning as a side-to-side portocaval shunt. The strongest evidence in favor of performing a TIPS procedure exists for the secondary prevention of the onset or recurrence of variceal bleeding[41,42]. A recent meta-analysis published in 2008 found more than threefold decrease in the risk of recurrent bleeding after insertion of a TIPS compared with various forms of endoscopic therapy[43]. TIPS is also indicated for treatment of refractory ascites[44]. A recent trial also showed improved survival in the group of patients who received a TIPS, on top of reduced risk of recurrent ascites[44,45]. Despite limited evidence, TIPS has also found a wider clinical use than just secondary prevention of variceal bleeding and treatment of refractory ascites. Additional indications include: hydrothorax, acute gastropathy, hepatorenal syndrome, Budd-Chiari Syndrome and hepatorenal syndrome[39].
Disadvantages of TIPS include: deterioration of hepatic function caused by diversion of portal venous blood flow and shunt dysfunction, requiring routine imaging surveillance and shunt maintenance procedures. TIPS is contraindicated in patients with congestive heart failure, severe pulmonary hypertension, severe tricuspid regurgitation, and hepatic failure[39]. Moreover, creating an unimpeded shunt to carry un-detoxified blood to systemic circulation may result in worsening of existing hepatic encephalopathy[46].
Direct intrahepatic portocaval shunt (DIPS) is a variation of the TIPS procedure, where intravascular ultrasound (IVUS) is utilized to assist in the transhepatic puncture, passing directly from the IVC to the portal vein, through the caudate lobe[47]. DIPS may be helpful in cases of hepatic vein thrombosis, challenging anatomy or in the presence of an ill-suited hepatic parenchymal tract (presence of hepatocellular carcinoma for example) [47].
PALLIATING SYMPTOMS OF PORTAL HYPERTENSION: BRTO AND VARIANTS
For the past two decades, balloon-occluded retrograde transvenous obliteration of varices (BRTO) has become common practice in Asia for the management of gastric varices. Studies have shown that gastric varices may bleed despite HVPG being less than 12 mmHg, below the treatment target pressure for TIPS[48,49]. The conventional technique involves advancing a catheter from the femoral vein into the outlet of the gastrorenal shunt, typically in the region of the left renal vein. Following balloon occlusion of the shunt, a sclerosant is injected retrograde to fill the gastric varices (Figure 15). Balloon-catheter and sclerosing agent are typically left in place from 4-48 h before completing the procedure[39,48-50].
Figure 15.
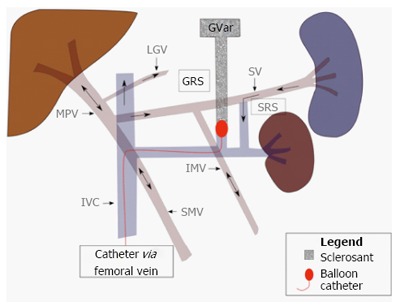
Retrograde balloon occlusion of gastro-renal shunt via femoral venous approach coursing through the inferior vena cava and left renal vein. After balloon occlusion, a sclerosing agent is injected into the gastric varices (GVar). Black arrows indicate directional blood flow. MPV: Main portal vein; IMV: Inferior mesenteric vein; IVC: Inferior vena cava; GRS: Gastrorenal shunt.
BRTO has been shown to be effective in controlling gastric variceal bleeding with low re-bleed rates[48,50]. The efficacy of BRTO for treating gastric varices has been described as excellent and some authors have insisted that the procedure could be used as a prophylactic treatment technique before the rupture of gastric varices[49]. BRTO is also thought to improve portal venous flow as it diverts the blood flow from a collateral shunt pathway to the liver. As such, BRTO is also an effective treatment for those patients with refractory hepatic encephalopathy[46,48-51].
BRTO is advantageous to TIPS in that it does not require general anesthesia and can be performed on patients with poor hepatic reserve or those with encephalopathy[49]. However, occlusion of the natural shunts results in increase hepatopetal flow through the portal vein, increasing portal venous pressure. This may inadvertently aggravate esophageal varices and ascites. In patients with poor hepatic reserve, risks and benefits of BRTO should be weighted very carefully[39,49]. Additionally, the sclerosing agents themselves also have a potential serious side effects including, pulmonary edema, disseminated intravascular coagulopathy, portal vein thrombosis, renal dysfunction and anaphylaxis[49,50,52].
Coil-assisted retrograde transvenous obliteration (CARTO) of gastric varices is a modified version of the original BRTO procedure. Utilizing dual microcatheter technique, one microcatheter is placed within the proximal shunt while the second is advanced and placed distally closer to gastric varices. Coil embolization is performed via the proximally placed microcatheter, while gelfoam embolization of the gastric varices is performed via the distal microcatheter. After variceal embolization is complete, the entire microcatheter system is removed[52]. A recent study has demonstrated similar procedural efficacy to BRTO, while alleviating the complications related to an indwelling balloon catheter (balloon-rupture, access site complication, intensive care admission and monitoring), as well as, avoiding the potential systemic side-effects of the sclerosing agent[52]. A similar procedure, using a vascular plug instead of a coil pack (vascular plug-assisted transvenous obliteration of gastric varices or “PARTO”), has also been validated demonstrating high technical success and clinical efficacy for the treatment of gastric varices and hepatic encephalopathy[53] (Figure 16).
Figure 16.
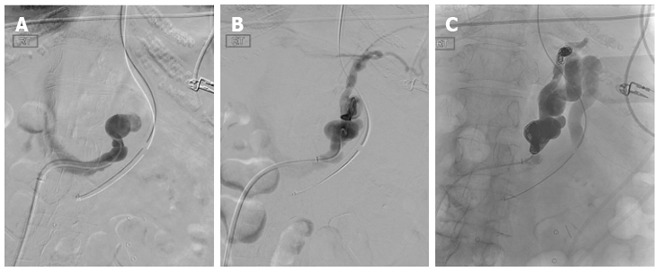
Coil-assisted retrograde transvenous obliteration of gastric varices in a 55-year-old patient who presented with recurrent gastric variceal bleeding refractory to endoscopic therapy. A: Angled catheter was navigated into the left gastrorenal shunt after obtaining access from the right common femoral vein and advancing 5-french sheath into the inferior vena cava; B: Digital subtraction angiography performed through the gastrorenal shunt demonstrating large dilated shunt vessels, as well as, secondary outflow through pericardiophrenic collaterals; C: Both the proximal and distal shunts were coil-occluded and gelfoam was utilized to embolize and thrombose the gastric varices.
CONCLUSION
Portal hypertension is a common clinical syndrome characterized by pathologic increase in portal venous pressure and the formation of portosystemic collaterals that divert blood away from the liver. The imaging appearance can be quite variable and knowledge of the various collateral pathways is essential for diagnosis and treatment planning. The role of radiology continues to evolve with increased utilization of advanced imaging techniques in detecting and diagnosing portal hypertension and liver disease, as well as, image-guided interventions to treat complications of portal hypertension.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Authors declare no potential conflicts of interests. No financial support.
Peer-review started: November 15, 2016
First decision: January 10, 2017
Article in press: February 17, 2017
P- Reviewer: Guo XZ, Kreisel W S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Bosch J, Groszmann RJ, Shah VH. Evolution in the understanding of the pathophysiological basis of portal hypertension: How changes in paradigm are leading to successful new treatments. J Hepatol. 2015;62:S121–S130. doi: 10.1016/j.jhep.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch J, Abraldes JG, Fernández M, García-Pagán JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53:558–567. doi: 10.1016/j.jhep.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Plessier A, Darwish-Murad S, Hernandez-Guerra M, Consigny Y, Fabris F, Trebicka J, Heller J, Morard I, Lasser L, Langlet P, et al. Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. 2010;51:210–218. doi: 10.1002/hep.23259. [DOI] [PubMed] [Google Scholar]
- 4.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch J, Navasa M, Garcia-Pagán JC, DeLacy AM, Rodés J. Portal hypertension. Med Clin North Am. 1989;73:931–953. doi: 10.1016/s0025-7125(16)30646-0. [DOI] [PubMed] [Google Scholar]
- 6.Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573–582. doi: 10.1038/nrgastro.2009.149. [DOI] [PubMed] [Google Scholar]
- 7.Harmanci O, Bayraktar Y. Clinical characteristics of idiopathic portal hypertension. World J Gastroenterol. 2007;13:1906–1911. doi: 10.3748/wjg.v13.i13.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7:141–155. doi: 10.1586/egh.12.83. [DOI] [PubMed] [Google Scholar]
- 9.Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976–980. doi: 10.1016/j.jhep.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Wachsberg RH, Yaghmai V, Javors BR, Levine CD, Simmons MZ, Maldjian PD. Cardiophrenic varices in portal hypertension: evaluation with CT. Radiology. 1995;195:553–556. doi: 10.1148/radiology.195.2.7724782. [DOI] [PubMed] [Google Scholar]
- 11.Berzigotti A, Piscaglia F. Ultrasound in portal hypertension--part 2--and EFSUMB recommendations for the performance and reporting of ultrasound examinations in portal hypertension. Ultraschall Med. 2012;33:8–32; quiz 30-31. doi: 10.1055/s-0031-1299145. [DOI] [PubMed] [Google Scholar]
- 12.Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol. 2012;56:696–703. doi: 10.1016/j.jhep.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Perri RE, Chiorean MV, Fidler JL, Fletcher JG, Talwalkar JA, Stadheim L, Shah ND, Kamath PS. A prospective evaluation of computerized tomographic (CT) scanning as a screening modality for esophageal varices. Hepatology. 2008;47:1587–1594. doi: 10.1002/hep.22219. [DOI] [PubMed] [Google Scholar]
- 14.Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48 Suppl 1:S68–S92. doi: 10.1016/j.jhep.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Stankovic Z, Allen BD, Garcia J, Jarvis KB, Markl M. 4D flow imaging with MRI. Cardiovasc Diagn Ther. 2014;4:173–192. doi: 10.3978/j.issn.2223-3652.2014.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stankovic Z, Csatari Z, Deibert P, Euringer W, Blanke P, Kreisel W, Abdullah Zadeh Z, Kallfass F, Langer M, Markl M. Normal and altered three-dimensional portal venous hemodynamics in patients with liver cirrhosis. Radiology. 2012;262:862–873. doi: 10.1148/radiol.11110127. [DOI] [PubMed] [Google Scholar]
- 17.Roldán-Alzate A, Frydrychowicz A, Niespodzany E, Landgraf BR, Johnson KM, Wieben O, Reeder SB. In vivo validation of 4D flow MRI for assessing the hemodynamics of portal hypertension. J Magn Reson Imaging. 2013;37:1100–1108. doi: 10.1002/jmri.23906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YJ, Raman SS, Yu NC, To’o KJ, Jutabha R, Lu DS. Esophageal varices in cirrhotic patients: evaluation with liver CT. AJR Am J Roentgenol. 2007;188:139–144. doi: 10.2214/AJR.05.1737. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Lee KS, Kim SA, Kim TS, Hwang JH, Lim JH. Computed radiography of the chest in patients with paraesophageal varices: diagnostic accuracy and characteristic findings. AJR Am J Roentgenol. 1998;170:1527–1531. doi: 10.2214/ajr.170.6.9609167. [DOI] [PubMed] [Google Scholar]
- 20.Cho KC, Patel YD, Wachsberg RH, Seeff J. Varices in portal hypertension: evaluation with CT. Radiographics. 1995;15:609–622. doi: 10.1148/radiographics.15.3.7624566. [DOI] [PubMed] [Google Scholar]
- 21.Hoevels J, Lunderquist A, Tylén U, Simert G. Porto-systemic collaterals in cirrhosis of the liver. Selective percutaneous transhepatic catheterization of the portal venous system in portal hypertension. Acta Radiol Diagn (Stockh) 1979;20:865–877. doi: 10.1177/028418517902000602. [DOI] [PubMed] [Google Scholar]
- 22.Subramanyam BR, Balthazar EJ, Madamba MR, Raghavendra BN, Horii SC, Lefleur RS. Sonography of portosystemic venous collaterals in portal hypertension. Radiology. 1983;146:161–166. doi: 10.1148/radiology.146.1.6849040. [DOI] [PubMed] [Google Scholar]
- 23.Wachsberg RH, Simmons MZ. Coronary vein diameter and flow direction in patients with portal hypertension: evaluation with duplex sonography and correlation with variceal bleeding. AJR Am J Roentgenol. 1994;162:637–641. doi: 10.2214/ajr.162.3.8109512. [DOI] [PubMed] [Google Scholar]
- 24.Lafortune M, Marleau D, Breton G, Viallet A, Lavoie P, Huet PM. Portal venous system measurements in portal hypertension. Radiology. 1984;151:27–30. doi: 10.1148/radiology.151.1.6701328. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi N, Kuroki K, Yanaga K. Percutaneous Transhepatic Mechanical Thrombectomy for Acute Mesenteric Venous Thrombosis. J Endovasc Ther. 2005;12:508–511. doi: 10.1583/04-1335MR.1. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Tsao G. Current Management of the Complications of Cirrhosis and Portal Hypertension: Variceal Hemorrhage, Ascites, and Spontaneous Bacterial Peritonitis. Dig Dis. 2001;34:382–386. doi: 10.1159/000444551. [DOI] [PubMed] [Google Scholar]
- 27.Lin CY, Lin PW, Tsai HM, Lin XZ, Chang TT, Shin JS. Influence of paraesophageal venous collaterals on efficacy of endoscopic sclerotherapy for esophageal varices. Hepatology. 1994;19:602–608. doi: 10.1002/hep.1840190310. [DOI] [PubMed] [Google Scholar]
- 28.Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343–1349. doi: 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
- 29.Feldman M, Feldman M. Gastric varices. Gastroenterology. 1956;30:318–321. [PubMed] [Google Scholar]
- 30.Mitty HA, Cohen BA, Sprayregen S, Schwartz K. Adrenal pseudotumors on CT due to dilated portosystemic veins. AJR Am J Roentgenol. 1983;141:727–730. doi: 10.2214/ajr.141.4.727. [DOI] [PubMed] [Google Scholar]
- 31.Morin C, Lafortune M, Pomier G, Robin M, Breton G. Patent paraumbilical vein: anatomic and hemodynamic variants and their clinical importance. Radiology. 1992;185:253–256. doi: 10.1148/radiology.185.1.1523319. [DOI] [PubMed] [Google Scholar]
- 32.Okuda K, Matsutani S. Portal-systemic collaterals: anatomy and clinical implications. 1st ed. Tokyo, Japan: Springer Japan; 1991. [Google Scholar]
- 33.Gould H, Benjamin S, Alter A. Retroperitoneal varices simulating masses. Gastrointest Radiol. 1982;7:335–9. doi: 10.1007/BF01887667. [DOI] [PubMed] [Google Scholar]
- 34.Malde H, Nagral A, Shah P, Joshi MS, Bhatia SJ, Abraham P. Detection of rectal and pararectal varices in patients with portal hypertension: efficacy of transvaginal sonography. AJR Am J Roentgenol. 1993;161:335–337. doi: 10.2214/ajr.161.2.8333372. [DOI] [PubMed] [Google Scholar]
- 35.Kim M, Mitchell DG, Ito K. Portosystemic collaterals of the upper abdomen: review of anatomy and demonstration on MR imaging. Abdom Imaging. 2000;25:462–470. doi: 10.1007/s002610000014. [DOI] [PubMed] [Google Scholar]
- 36.William P. Grey’s Anatomy. New York, NY: Churchill Livingstone; 1999. Veins of the abdomen and pelvis: hepatic portal system; pp. 1602–1604. [Google Scholar]
- 37.Uflacker R. Atlas of vascular anatomy. Baltimore, MA: Lippincott Williams & Wilson; 1997. Veins of the abdomen and pelvis; pp. 699–803. [Google Scholar]
- 38.Rösch J, Hanafee WN, Snow H. Transjugular portal venography and radiologic portacaval shunt: an experimental study. Radiology. 1969;92:1112–1114. doi: 10.1148/92.5.1112. [DOI] [PubMed] [Google Scholar]
- 39.Kirby JM, Cho KJ, Midia M. Image-guided intervention in management of complications of portal hypertension: more than TIPS for success. Radiographics. 2013;33:1473–1496. doi: 10.1148/rg.335125166. [DOI] [PubMed] [Google Scholar]
- 40.Fidelman N, Kwan SW, LaBerge JM, Gordon RL, Ring EJ, Kerlan RK. The transjugular intrahepatic portosystemic shunt: an update. AJR Am J Roentgenol. 2012;199:746–755. doi: 10.2214/AJR.12.9101. [DOI] [PubMed] [Google Scholar]
- 41.Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, Perarnau JM, Berger E, Blum U, Gabelmann A, Hauenstein K. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165–171. doi: 10.1056/NEJM199401203300303. [DOI] [PubMed] [Google Scholar]
- 42.García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 43.Zheng M, Chen Y, Bai J, Zeng Q, You J, Jin R, Zhou X, Shen H, Zheng Y, Du Z. Transjugular intrahepatic portosystemic shunt versus endoscopic therapy in the secondary prophylaxis of variceal rebleeding in cirrhotic patients: meta-analysis update. J Clin Gastroenterol. 2000;42:507–516. doi: 10.1097/MCG.0b013e31815576e6. [DOI] [PubMed] [Google Scholar]
- 44.Rössle M, Ochs A, Gülberg V, Siegerstetter V, Holl J, Deibert P, Olschewski M, Reiser M, Gerbes AL. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701–1707. doi: 10.1056/NEJM200006083422303. [DOI] [PubMed] [Google Scholar]
- 45.Narahara Y, Kanazawa H, Fukuda T, Matsushita Y, Harimoto H, Kidokoro H, Katakura T, Atsukawa M, Taki Y, Kimura Y, et al. Transjugular intrahepatic portosystemic shunt versus paracentesis plus albumin in patients with refractory ascites who have good hepatic and renal function: a prospective randomized trial. J Gastroenterol. 2011;46:78–85. doi: 10.1007/s00535-010-0282-9. [DOI] [PubMed] [Google Scholar]
- 46.Uflacker R, Silva Ade O, d’Albuquerque LA, Piske RL, Mourão GS. Chronic portosystemic encephalopathy: embolization of portosystemic shunts. Radiology. 1987;165:721–725. doi: 10.1148/radiology.165.3.3685350. [DOI] [PubMed] [Google Scholar]
- 47.Petersen BD, Clark TW. Direct intrahepatic portocaval shunt. Tech Vasc Interv Radiol. 2008;11:230–234. doi: 10.1053/j.tvir.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Saad WE, Khaja MS, Hirota S. Balloon-occluded retrograde transvenous obliteration of gastric varices: conception, evolution, and history. Tech Vasc Interv Radiol. 2012;15:160–164. doi: 10.1053/j.tvir.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Cho SK, Shin SW, Lee IH, Do YS, Choo SW, Park KB, Yoo BC. Balloon-occluded retrograde transvenous obliteration of gastric varices: outcomes and complications in 49 patients. AJR Am J Roentgenol. 2007;189:W365–W372. doi: 10.2214/AJR.07.2266. [DOI] [PubMed] [Google Scholar]
- 50.Ninoi T, Nishida N, Kaminou T, Sakai Y, Kitayama T, Hamuro M, Yamada R, Nakamura K, Arakawa T, Inoue Y. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol. 2005;184:1340–1346. doi: 10.2214/ajr.184.4.01841340. [DOI] [PubMed] [Google Scholar]
- 51.Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001;12:327–336. doi: 10.1016/s1051-0443(07)61912-5. [DOI] [PubMed] [Google Scholar]
- 52.Lee EW, Saab S, Gomes AS, Busuttil R, McWilliams J, Durazo F, Han SH, Goldstein L, Tafti BA, Moriarty J, et al. Coil-Assisted Retrograde Transvenous Obliteration (CARTO) for the Treatment of Portal Hypertensive Variceal Bleeding: Preliminary Results. Clin Transl Gastroenterol. 2014;5:e61. doi: 10.1038/ctg.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gwon DI, Kim YH, Ko GY, Kim JW, Ko HK, Kim JH, Shin JH, Yoon HK, Sung KB. Vascular Plug-Assisted Retrograde Transvenous Obliteration for the Treatment of Gastric Varices and Hepatic Encephalopathy: A Prospective Multicenter Study. J Vasc Interv Radiol. 2015;26:1589–1595. doi: 10.1016/j.jvir.2015.07.011. [DOI] [PubMed] [Google Scholar]


