Abstract
Ghrelin, as a kind of multifunctional protein polypeptide, is mainly produced in the fundus of the stomach and can promote occurrence and development of many tumors, including gastrointestinal tumors, which has been proved by the relevant researches. Most gastrointestinal stromal tumors (GISTs, about 80%), as the most common mesenchymal tumor, also develop in the fundus. Scientific research has confirmed that ghrelin, its receptors and mRNA respectively can be found in GISTs, which demonstrated the existence of a ghrelin autocrine/paracrine loop in GIST tissues. However, no reports to date have specified the mechanism whether ghrelin can promote the occurrence and development of GISTs. Studies of pulmonary artery endothelial cells in a low-oxygen environment and cardiac muscle cells in an ischemic environment have shown that ghrelin can activate the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin (PI3K/AKT/mTOR) signaling pathway. Moreover, some studies of GISTs have confirmed that activation of the PI3K/AKT/mTOR pathway can indeed promote the growth and progression of GISTs. Whether ghrelin is involved in the development or progression of GISTs through certain pathways remains unknown. Can we find a new target for the treatment of GISTs? This review explores and summaries the relationship among ghrelin, the PI3K/AKT/mTOR pathway and the development of GISTs.
Keywords: Gastrointestinal stromal tumor, PI3K/AKT/mTOR pathway, Ghrelin, Occurrence, Development
Core tip: Ghrelin has been proven to promote the occurrence and development of gastrointestinal tumors. Some gastrointestinal stromal tumors (GISTs) express ghrelin and its receptors. However, no previous reports have specified whether ghrelin is involved in the occurrence and development of GISTs. Through a review of the literature, this paper is the first to summarize and discuss the correlation between ghrelin and GISTs.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) originate from pacemaker cells (interstitial cells of Cajal, or Cajal cells). They are the most common mesenchymal tumors in the gastrointestinal tract, with an annual incidence of approximately 10-20 per million. Although GISTs can occur in any part of the digestive tract, about 70% are found in the stomach, 10%-25% in the small intestine[1], and a small percentage in the rectum. However, GISTs rarely affect the esophagus or colon[2].
GISTs result from acquired functional changes caused by mutations in the KIT and PDGFRA genes located on chromosome 4q12. These mutations result in expression of activated forms of the protein products [c-KIT, which is a receptor tyrosine kinase (RTK), and platelet-derived growth factor receptor-α (PDGFRA)], leading to inhibition of apoptosis, activation of cell proliferation, and promotion of tumorigenesis[3,4].
At present, treatment strategies for GISTs mainly focused on the KIT and PDGFRA genes and their RTK products. Although introduction of the c-KIT and PDGFRA inhibitor imatinib (Gleevec®) has greatly improved treatment efficacy, the median progression-free survival time of patients with GISTs is only about 2 years. The incidence of secondary (acquired) drug resistance within the first 2 years of imatinib treatment is approximately 40%-50%[5]. Patients who display primary resistance to first-line therapy with imatinib can be treated with the multiple-kinase inhibitor sunitinib malate[6]. However, one trial revealed that the objective response rate to sunitinib malate was only 65% (7% partial response and 58% stable disease without progression)[7]. Moreover, the clinical effect is short and drug resistance soon appears. Therefore, although there are many advantages in the current targeted therapies for GISTs, there are also drawbacks, highlighting the urgent need for new ways to treat GISTs.
Clinical observations indicate that most GISTs originate at the base of the stomach, which is also the main secretion site of gastric ghrelin. As described in the following sections, ghrelin is a protein with a variety of functions[8]. Ghrelin receptors are expressed in several types of tumors, including gastric and colon cancer, and are able to promote tumor growth[9-11]. The Cajal cells from which GISTs arise both produce ghrelin and express the ghrelin receptor[12]. Whether ghrelin is involved in the development or progression of GISTs through certain pathways remains unknown. Can we find a new target for the treatment of GISTs? We herein review the relevant literature on this topic.
GHRELIN
Ghrelin is a 28-amino-acid peptide that also exists as des-Gln(14)-ghrelin[13-15]. Ghrelin is currently considered to be the main endogenous ligand of growth receptors[16]. The ghrelin coding gene is located on chromosome 3 (3p25-26)[17].
Approximately 80% of ghrelin in serum is produced by cells at the base of the stomach (“A-X-like” cells), most of which are distributed in acid-secreting glands[18]. These “A-X-like” cells constitute approximately 20% of gastric endocrine cells[19]. Ghrelin is also secreted by the hypothalamus, pituitary gland, kidneys[20], placenta[21], intestinal tract, thyroid[22], heart[13], Leydig cells[23], neutrophils, lungs[24,25], and ovarian tissues[26,27].
Some of the many functions of ghrelin include regulation of growth hormone secretion, energy balance, gastrointestinal motility, gastric acid secretion, cardiovascular activity, pancreatic hormone secretion, glucose metabolism, prolactin and adrenocorticotropic hormone secretion, sleep[15,23], and gonadal hormone secretion. Several studies[9-11] have shown that ghrelin can promote the development of malignant tumors through a variety of signaling pathways that increase cell proliferation and metastasis, including the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin (PI3K/AKT/mTOR), Ras/RAF/extracellular signal-regulated kinases (ERK1/2), Janus kinase/signal transducers and activators of transcription (JAK/STAT), and Src kinase pathways.
GISTs EXPRESS GHRELIN AND GHRELIN RECEPTORS
To the best of our knowledge, the only study that has examined the expression of ghrelin and its receptors in GIST tissues is a Japanese study[28] in which ghrelin, ghrelin receptors, and their respective mRNA were detected in all 17 GIST tissues examined, although the extent of ghrelin and ghrelin receptor expression differed in each GIST tissue. The study demonstrated the existence of a ghrelin autocrine/paracrine loop in GIST tissues, suggesting that ghrelin may play a role in the occurrence and development of GISTs.
In contrast, the same study[28] found no statistically significant differences between positive ghrelin expression and tumor location (P = 0.426), tumor size (P = 0.590), KIT genotype (P = 0.935), a mitotic number of > 5 (P = 0.210), a Ki67 index of < 5 (P = 0.659), or risk of stromal tumor recurrence (P = 0.420). Additionally, ghrelin receptor expression was not correlated with the tumor grade (P = 0.208), Ki67 index (P = 0.717), mitotic count (P = 0.264), tumor location (P = 0.392), tumor size (P = 1), or tumor morphological type (P = 0.223). However, because the study sample was small (17 cases), the significance of these results remains unclear.
ROLE OF PI3K/AKT/mTOR PATHWAY IN GISTs
RTKs such as c-KIT and PDGFRA can activate a variety of intracellular signaling pathways, such as PI3K/AKT/mTOR, Ras/RAF/ERK, JAK/STAT, and Src kinases, which can in turn activate signaling axes downstream of c-KIT. These RTKs are therefore key factors in the treatment of GISTs, especially refractory GISTs[29]. PI3K/AKT/mTOR pathway activation is associated with the development and invasion of a number of tumors[9-11]. Indeed, c-KIT activation via autophosphorylation leads to excessive activation of PI3K/AKT/mTOR, which promotes the development and progression of GISTs[29,30]. Thus, blocking excessive activation of this signaling pathway may provide a new therapeutic strategy for GISTs.
The PI3K/AKT/mTOR signaling pathway regulates cell growth, proliferation, and differentiation. The initiating signal molecule, PI3K[31], can be activated by RTKs, G protein-coupled receptors, and oncogenic Ras (Figure 1). Phosphorylation of the RTK recruits PI3K to the inner surface of the cell membrane, where it catalyzes the phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2) to phosphatidylinositol (3,4,5)-trisphosphate (PIP3). This reaction is reversed by phosphatase and tensin homolog (PTEN), which dephosphorylates PIP3 to PIP2[32]. Generation of PIP3 promotes translocation of the serine/threonine protein kinase AKT to the plasma membrane, and activated AKT indirectly leads to the accumulation of the Ras homology protein Rheb and activation of mTORC1[33]. mTORC1 then activates the downstream kinases S6 kinase 1 (S6K1) and S6K2, which stimulate protein synthesis, cell survival, and growth signal release[34-36]. This pathway is essential for the continued growth of tumors and it is therefore being intensely investigated as a source of new therapeutic strategies for GISTs.
Figure 1.
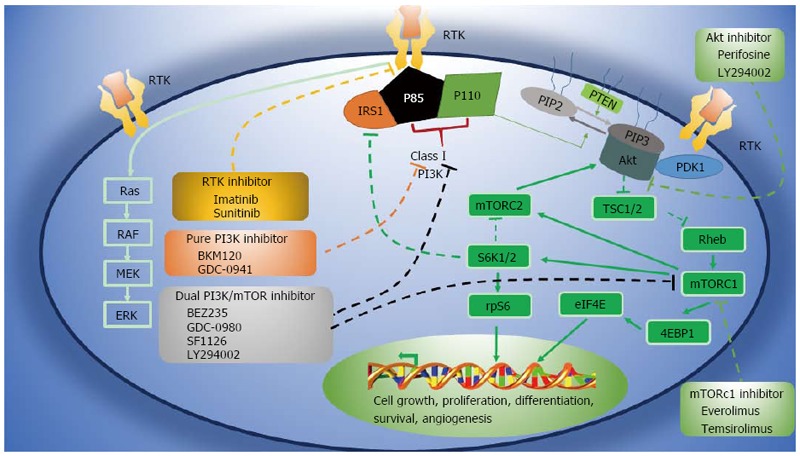
PI3K/AKT/mTOR pathway and inhibitors in clinical development[29]. Solid lines represent activating actions, and dotted lines represent inhibitory actions. 4EBP1: 4E-binding protein 1; PKB: Protein kinase B; ERK: Extracellular signal-related kinase; IRS1: Insulin receptor substrate 1; MEK: Mitogen-activated protein/ERK kinase; mTOR: Mammalian target of rapamycin; mTORC: mTOR complex; PDK1: Pyruvate dehydrogenase lipoamide kinase isozyme 1; PI3K: Phosphatidylinositol 3-kinase; PIP2: Phosphatidylinositol 4,5-bisphosphate; PIP3: Phosphatidylinositol 3,4,5-trisphosphate; PTEN: Phosphatase and tensin homolog; Rheb: Ras homolog enriched in brain; rpS6: Ribosomal protein S6; RTK: Receptor tyrosine kinase; S6K: Ribosomal S6 kinase; TSC1/2: Tuberous sclerosis protein.
The PI3K/AKT/mTOR signaling pathway is blocked by a number of inhibitors. Rapamycin derivatives such as temsirolimus, the first clinically applied inhibitor, mainly inhibit mTORC1. Dual inhibitors such as GDC-0980, BEZ235, and SF1126 inhibit PI3K and mTORC1/2[37], whereas BKM120 and GDC-0941 specifically inhibit PI3K but not mTOR[38]. In addition, Alkyl phosphoric acid choline compound can effectively inhibit AKT[39,40].
Studies have indicated that loss of the PTEN gene and dysregulation of the PI3K/ATK/mTOR signaling pathway may play an important role in the progression and drug resistance of GISTs. In cells with absent or low-level expression of PTEN, tumor progression is more rapid[41]. PTEN gene silencing also leads to abnormal activation of the PI3K/AKT/mTOR pathway[42].
The expression of mTOR and phosphorylated mTOR is much higher in GISTs measuring > 5 cm in diameter with a medium to high risk of recurrence than in GISTs measuring < 5 cm in diameter with a low or very low recurrence risk[43]. However, few reports have focused on the role of mTOR in the pathogenesis of GISTs.
Collectively, these studies indicate that RTK-targeted therapy can benefit about 85% patients with GISTs, but drug resistance is a major hindrance to its application. The PI3K/ATK/mTOR pathway is believed to be an important pathway for the development and progression of GISTs, suggesting that signaling molecules in this pathway may be useful targets for the development of novel therapies.
GHRELIN ACTIVATES PI3K/ATK/mTOR PATHWAY
Ghrelin activates the PI3K/AKT/mTOR pathway through phosphorylation of AKT and mTOR. Two studies respectively investigated the protective effects of ghrelin on pulmonary artery endothelial cells in a low-oxygen environment and on cardiac muscle cells in an ischemic environment[44,45] and found that ghrelin could promote myocardial cell proliferation, inhibited apoptosis, reduced myocardial fibrosis, improved cardiac function, and significantly increased the survival rate of adipose-derived mesenchymal stem cells and pulmonary artery endothelial cells exposed to hypoxia. Western blot analysis showed that ghrelin reduced expression of the pro-apoptotic protein Bax, increased the level of the anti-apoptotic protein Bcl-2, and significantly increased phosphorylation of AKT and mTOR[44,45]. The PI3K inhibitor LY294002 suppressed phosphorylation events, indicating that ghrelin activates the PI3K/AKT/mTOR pathway through phosphorylation. Two other studies, one examined the modulatory effect of ghrelin on gastric mucosal prostaglandin and nitric oxide and another examined gastric mucosal inflammatory responses to Helicobacter pylori, also showed that ghrelin activates the PI3K/AKT/mTOR pathway in different ways[46,47] (Figure 2). Whether ghrelin promotes the development and progression of GISTs through this or other mechanisms remains unclear.
Figure 2.
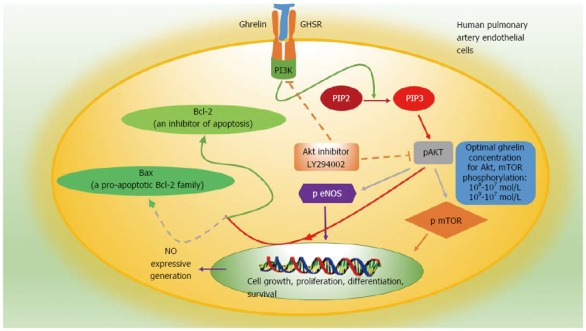
Specific mechanism of ghrelin involved in the protection of pulmonary artery endothelial cells and activation of the PI3K/AKT/mTOR pathway in human pulmonary artery endothelial cells[44-47]. Solid lines represent activating actions, and dotted lines represent inhibitory actions. PIP2: Phosphatidylinositol 4,5-bisphosphate; PIP3: Phosphatidylinositol 3,4,5-trisphosphate.
CONCLUSION
GISTs express ghrelin and ghrelin receptors. Ghrelin can activate the PI3K/ATK/mTOR signaling pathway in cardiac muscle cells and pulmonary artery endothelial cells, which plays an important role in the occurrence and development of GISTs. It is possible that ghrelin may promote GISTs by activating this pathway. However, this remains to be fully elucidated.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare that there is no conflict of interest related to this study.
Peer-review started: September 28, 2016
First decision: October 10, 2016
Article in press: January 11, 2017
P- Reviewer: Hegardt FG, Navarrete A, Slomiany BL, Unger M S- Editor: Gong ZM L- Editor: Ma JY E- Editor: Wang CH
References
- 1.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 2.Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S, McCarter MD, Polikoff JA, Tan BR, Owzar K; American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 4.Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003;125:660–667. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 5.Gramza AW, Corless CL, Heinrich MC. Resistance to Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumors. Clin Cancer Res. 2009;15:7510–7518. doi: 10.1158/1078-0432.CCR-09-0190. [DOI] [PubMed] [Google Scholar]
- 6.Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JV, Booth BP, Verbois SL, Morse DE, Liang CY, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13:1367–1373. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 7.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 8.De Ambrogi M, Volpe S, Tamanini C. Ghrelin: central and peripheral effects of a novel peptydil hormone. Med Sci Monit. 2003;9:RA217–RA224. [PubMed] [Google Scholar]
- 9.Chopin L, Walpole C, Seim I, Cunningham P, Murray R, Whiteside E, Josh P, Herington A. Ghrelin and cancer. Mol Cell Endocrinol. 2011;340:65–69. doi: 10.1016/j.mce.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Nikolopoulos D, Theocharis S, Kouraklis G. Ghrelin: a potential therapeutic target for cancer. Regul Pept. 2010;163:7–17. doi: 10.1016/j.regpep.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Nikolopoulos D, Theocharis S, Kouraklis G. Ghrelin’s role on gastrointestinal tract cancer. Surg Oncol. 2010;19:e2–e10. doi: 10.1016/j.suronc.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Yang CG, Liao ZF, Qiu WC, Yan J, Wang ZG. Function of ghrelin and ghrelin receptors in the network regulation of gastric motility. Mol Med Rep. 2014;10:2453–2458. doi: 10.3892/mmr.2014.2571. [DOI] [PubMed] [Google Scholar]
- 13.Casanueva FF, Dieguez C. Ghrelin: the link connecting growth with metabolism and energy homeostasis. Rev Endocr Metab Disord. 2002;3:325–338. doi: 10.1023/a:1020901624103. [DOI] [PubMed] [Google Scholar]
- 14.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 15.Muccioli G, Tschöp M, Papotti M, Deghenghi R, Heiman M, Ghigo E. Neuroendocrine and peripheral activities of ghrelin: implications in metabolism and obesity. Eur J Pharmacol. 2002;440:235–254. doi: 10.1016/s0014-2999(02)01432-2. [DOI] [PubMed] [Google Scholar]
- 16.Beaumont NJ, Skinner VO, Tan TM, Ramesh BS, Byrne DJ, MacColl GS, Keen JN, Bouloux PM, Mikhailidis DP, Bruckdorfer KR, et al. Ghrelin can bind to a species of high density lipoprotein associated with paraoxonase. J Biol Chem. 2003;278:8877–8880. doi: 10.1074/jbc.C200575200. [DOI] [PubMed] [Google Scholar]
- 17.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- 18.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 19.Rindi G, Necchi V, Savio A, Torsello A, Zoli M, Locatelli V, Raimondo F, Cocchi D, Solcia E. Characterisation of gastric ghrelin cells in man and other mammals: studies in adult and fetal tissues. Histochem Cell Biol. 2002;117:511–519. doi: 10.1007/s00418-002-0415-1. [DOI] [PubMed] [Google Scholar]
- 20.Mori K, Yoshimoto A, Takaya K, Hosoda K, Ariyasu H, Yahata K, Mukoyama M, Sugawara A, Hosoda H, Kojima M, et al. Kidney produces a novel acylated peptide, ghrelin. FEBS Lett. 2000;486:213–216. doi: 10.1016/s0014-5793(00)02308-5. [DOI] [PubMed] [Google Scholar]
- 21.Horvath TL, Diano S, Sotonyi P, Heiman M, Tschöp M. Minireview: ghrelin and the regulation of energy balance--a hypothalamic perspective. Endocrinology. 2001;142:4163–4169. doi: 10.1210/endo.142.10.8490. [DOI] [PubMed] [Google Scholar]
- 22.Kanamoto N, Akamizu T, Hosoda H, Hataya Y, Ariyasu H, Takaya K, Hosoda K, Saijo M, Moriyama K, Shimatsu A, et al. Substantial production of ghrelin by a human medullary thyroid carcinoma cell line. J Clin Endocrinol Metab. 2001;86:4984–4990. doi: 10.1210/jcem.86.10.7891. [DOI] [PubMed] [Google Scholar]
- 23.Barreiro ML, Gaytán F, Caminos JE, Pinilla L, Casanueva FF, Aguilar E, Diéguez C, Tena-Sempere M. Cellular location and hormonal regulation of ghrelin expression in rat testis. Biol Reprod. 2002;67:1768–1776. doi: 10.1095/biolreprod.102.006965. [DOI] [PubMed] [Google Scholar]
- 24.Volante M, Allìa E, Gugliotta P, Funaro A, Broglio F, Deghenghi R, Muccioli G, Ghigo E, Papotti M. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J Clin Endocrinol Metab. 2002;87:1300–1308. doi: 10.1210/jcem.87.3.8279. [DOI] [PubMed] [Google Scholar]
- 25.Volante M, Fulcheri E, Allìa E, Cerrato M, Pucci A, Papotti M. Ghrelin expression in fetal, infant, and adult human lung. J Histochem Cytochem. 2002;50:1013–1021. doi: 10.1177/002215540205000803. [DOI] [PubMed] [Google Scholar]
- 26.Gaytan F, Barreiro ML, Chopin LK, Herington AC, Morales C, Pinilla L, Casanueva FF, Aguilar E, Diéguez C, Tena-Sempere M. Immunolocalization of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in the cyclic human ovary. J Clin Endocrinol Metab. 2003;88:879–887. doi: 10.1210/jc.2002-021196. [DOI] [PubMed] [Google Scholar]
- 27.Korbonits M, Bustin SA, Kojima M, Jordan S, Adams EF, Lowe DG, Kangawa K, Grossman AB. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J Clin Endocrinol Metab. 2001;86:881–887. doi: 10.1210/jcem.86.2.7190. [DOI] [PubMed] [Google Scholar]
- 28.Ekeblad S, Nilsson B, Lejonklou MH, Johansson T, Stålberg P, Nilsson O, Ahlman H, Skogseid B. Gastrointestinal stromal tumors express the orexigen ghrelin. Endocr Relat Cancer. 2006;13:963–970. doi: 10.1677/erc.1.01201. [DOI] [PubMed] [Google Scholar]
- 29.Patel S. Exploring novel therapeutic targets in GIST: focus on the PI3K/Akt/mTOR pathway. Curr Oncol Rep. 2013;15:386–395. doi: 10.1007/s11912-013-0316-6. [DOI] [PubMed] [Google Scholar]
- 30.Bauer S, Duensing A, Demetri GD, Fletcher JA. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene. 2007;26:7560–7568. doi: 10.1038/sj.onc.1210558. [DOI] [PubMed] [Google Scholar]
- 31.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 33.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sridharan S, Basu A. S6 kinase 2 promotes breast cancer cell survival via Akt. Cancer Res. 2011;71:2590–2599. doi: 10.1158/0008-5472.CAN-10-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pastor MD, García-Yébenes I, Fradejas N, Pérez-Ortiz JM, Mora-Lee S, Tranque P, Moro MA, Pende M, Calvo S. mTOR/S6 kinase pathway contributes to astrocyte survival during ischemia. J Biol Chem. 2009;284:22067–22078. doi: 10.1074/jbc.M109.033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- 37.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway--beyond rapalogs. Oncotarget. 2010;1:530–543. doi: 10.18632/oncotarget.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conley AP, Araujo D, Ludwig J, Ravi V, Samuels BL, Choi H, Thall PF, Patel S, Benjamin R, Trent J. A randomized phase II study of perifosine (P) plus imatinib for patients with imatinib-resistant gastrointestinal stromal tumor (GIST) J Clin Oncol. 2009;27:10563. [Google Scholar]
- 40.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 41.Ricci R, Maggiano N, Castri F, Rinelli A, Murazio M, Pacelli F, Potenza AE, Vecchio FM, Larocca LM. Role of PTEN in gastrointestinal stromal tumor progression. Arch Pathol Lab Med. 2004;128:421–425. doi: 10.5858/2004-128-421-ROPIGS. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Ikezoe T, Nishioka C, Takezaki Y, Hanazaki K, Taguchi T, Yokoyama A. Long-term exposure of gastrointestinal stromal tumor cells to sunitinib induces epigenetic silencing of the PTEN gene. Int J Cancer. 2012;130:959–966. doi: 10.1002/ijc.26095. [DOI] [PubMed] [Google Scholar]
- 43.Li JC, Zhu HY, Chen TX, Zou LY, Wang XY, Zhao HC, Xu J. Roles of mTOR and p-mTOR in gastrointestinal stromal tumors. Asian Pac J Cancer Prev. 2013;14:5925–5928. doi: 10.7314/apjcp.2013.14.10.5925. [DOI] [PubMed] [Google Scholar]
- 44.Yang D, Liu Z, Zhang H, Luo Q. Ghrelin protects human pulmonary artery endothelial cells against hypoxia-induced injury via PI3-kinase/Akt. Peptides. 2013;42:112–117. doi: 10.1016/j.peptides.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Han D, Huang W, Ma S, Chen J, Gao L, Liu T, Zhang R, Li X, Li C, Fan M, et al. Ghrelin improves functional survival of engrafted adipose-derived mesenchymal stem cells in ischemic heart through PI3K/Akt signaling pathway. Biomed Res Int. 2015;2015:858349. doi: 10.1155/2015/858349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slomiany BL, Slomiany A. Induction in gastric mucosal prostaglandin and nitric oxide by Helicobacter pylori is dependent on MAPK/ERK-mediated activation of IKK-β and cPLA2: modulatory effect of ghrelin. Inflammopharmacology. 2013;21:241–251. doi: 10.1007/s10787-013-0169-5. [DOI] [PubMed] [Google Scholar]
- 47.Slomiany BL, Slomiany A. Modulation of gastric mucosal inflammatory responses to Helicobacter pylori via ghrelin-induced protein kinase Cδ tyrosine phosphorylation. Inflammopharmacology. 2014;22:251–262. doi: 10.1007/s10787-014-0206-z. [DOI] [PubMed] [Google Scholar]


