Abstract
AIM
To investigate the association between serum human epidermal growth factor receptor 2 (HER2) extracellular domain (ECD) and tissue HER2 status, and the prognostic value of serum HER2 ECD in patients with gastric cancer.
METHODS
A total of 239 patients with gastric cancer were enrolled from December 2012 to June 2013. Serum HER2 ECD was determined by chemiluminescent assay, and tissue HER2 status was evaluated by immunohistochemistry and fluorescence in situ hybridization assay. A receiver operating characteristic (ROC) curve was plotted to identify the optimal cut-off value for serum HER2 ECD assay for predicting survival in gastric cancer patients.
RESULTS
Serum HER2 ECD was significantly correlated with tissue HER2 status (P < 0.001), tumor size (P < 0.001), and intestinal type of gastric cancer (P = 0.021). Serum HER2 ECD levels differed significantly between patients with HER2-positive tissue expression and those with HER2-negative tissue expression. ROC analysis yielded an area under the curve value of 0.79 (95%CI: 0.71-0.87, P < 0.001), with a sensitivity and specificity of 0.54 (95%CI: 0.37-0.70) and 0.93 (95%CI: 0.88-0.96), respectively. With a cut-off value of 24.75 ng/mL, high serum HER2 ECD had a negative impact on overall survival of the patients (HR: 1.93, 95%CI: 1.32-4.38, P = 0.006).
CONCLUSION
Serum HER2 ECD could be a highly specific surrogate biomarker for tissue HER2 status in gastric cancer. Optimal cut-off criteria for predicting survival should be established.
Keywords: Gastric cancer, Serum HER2 extracellular domain, Tissue HER2 status, Prognosis
Core tip: Precise determination of HER2 status is crucial for the appropriate use of HER2-targeted therapy in gastric cancer patients. Our study investigated the association between serum HER2 extracellular domain (ECD) and tissue HER2 status, and also determined the prognostic value of serum HER2 ECD in a large cohort of patients. Serum HER2 ECD could provide an easily accessible surrogate marker for tissue HER2 status, with high specificity. Furthermore, high serum HER2 ECD had a negative impact on overall survival in patients with gastric cancer.
INTRODUCTION
Patients with advanced gastric cancer positive for human epidermal growth factor receptor 2 (HER2) expression or gene amplification may benefit from HER2-targeted therapy[1]. Accurate determination of HER2 status is therefore crucial for the appropriate use of such therapy, as emphasized in the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology[2]. These guidelines recommend the use of immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) to assess HER2 overexpression. However, these recommended methods have some limitations. First, a 13% discordancy rate has been reported between the results of IHC and FISH assays[3]. This discrepancy might be partly due to the fact that IHC detects protein expression on the tumor cell surface, while FISH detects gene amplification in the tumor cell nucleus. Second, IHC is semi-quantitative and notoriously subjective, while FISH is time-consuming and relatively expensive. Third, HER2 status might change during trastuzumab therapy[4], and neither IHC nor FISH are effective assays for reflecting the real-time scenario of HER2 status[5]. More convenient and reproducible detection methods are therefore needed to identify HER2-positive gastric cancer, thus facilitating the appropriate and effective use of HER2-targeted therapy[6].
Noninvasive serum HER2 extracellular domain (ECD) assay has the potential to supplement existing HER2 tests[5]. HER2 ECD is the extracellular fragment of the HER2 protein, which is located on the surface of tumor cells and may be released into the circulation by shedding. Detection of serum HER2 ECD could thus provide additional information on HER2 status. Compared with tissue specimens, blood samples are readily accessible and can be obtained repeatedly, making serum HER2 ECD assay an ideal tool for the dynamic monitoring of tumor phenotype. Moreover, the approved automatic detection platforms[7], could be used to measure serum HER2 ECD levels objectively and reliably. The clinical relevance of serum HER2 ECD has been investigated extensively in breast cancer, and good concordance has been reported between serum HER2 ECD levels and HER2 status in the primary tumor site[8-10]. Generally, high serum HER2 ECD levels in patients with metastatic breast cancer were correlated with high risk of disease progression, decreased survival, and reduced response to treatment[11-13]. Several studies have also investigated the clinical significance of serum HER2 ECD in gastric cancer[14-16]. However, differences in detection methods and patient enrollment across studies have led to inconsistent results regarding the associations among serum HER2 ECD concentrations, tissue HER2 status, and patient outcome. Further studies are therefore urgently needed to provide more solid data[17].
Here, we conducted a retrospective study in a large cohort of patients with gastric cancer to investigate the relationship between serum HER2 ECD concentration and tissue HER2 status, and to determine the prognostic value of serum HER2 ECD.
MATERIALS AND METHODS
Patient enrollment
This study was approved by the Research Ethics Committee of the Chinese People’s Liberation Army General Hospital. A total of 239 consecutive patients with histologically and pathologically confirmed gastric cancer treated between December 2012 and June 2013 were included. Written informed consent was obtained from the patients. Fasting blood samples were collected the day after admission to hospital, and then stored at -80 °C until analysis. Clinicopathological variables including age, gender, tumor location, tumor size, tissue HER2 status, and survival time were collected. Patients were followed up every 3 mo for the first 2 years, every 6 mo for the next 3 years, and every 1 year after 5 years.
Tissue HER2 status evaluated by IHC and FISH
HER2 IHC was performed as described previously[18]. In brief, tumor specimens were fixed in 10% neutral-buffered formalin overnight and embedded in paraffin blocks. Sections (4 μm) were cut from the tissue paraffin blocks, and IHC was performed using HercepTest II™ (Dako, Denmark) according to the manufacturer’s instructions. This process was conducted at the Department of Pathology, Chinese People’s Liberation Army General Hospital. IHC sections were reviewed by experienced pathologists and were scored for HER2 expression according to the criteria used in the ToGA trial[1]. If tissue HER2 expression was scored as 2+, a further FISH assay was performed with Abbott-Vysis PathVysion™ (Abbott Laboratories, United States) according to the manufacturer’s protocol. Tumor specimens with a HER2:CEP17 signal ratio ≥ 2.0 or 3+ IHC staining intensity were judged as HER2-positive.
Serum HER2 ECD assay
Serum HER2 ECD concentrations were measured by chemiluminescence immunoassays using the ADVIA Centaur System (Siemens Diagnostics, United States) with a detection range of 0.5-350 ng/mL. The assays were conducted in strict adherence with the manufacturer’s instructions and blinded to clinical outcomes.
Statistical analysis
Overall survival (OS) was defined as the period from surgery to death from any cause, and patients who were alive were censored at the last follow-up. Survival curves were plotted by the Kaplan-Meier method, and log-rank tests were used to compare curves. Receiver operating characteristic (ROC) curves were plotted to investigate the diagnostic role of serum HER ECD for tissue HER2 status. Youden’s index (sensitivity + specificity - 1) was used to determine the optimal cut-off value from the ROC plot. The associations between serum HER2 ECD concentrations and clinicopathological variables were investigated using χ2 tests. Student’s t test and Mann-Whitney test were used for analysis of continuous variables. Statistical analyses were performed and presented using GraphPad Prism 6. A two-sided P value < 0.05 was considered significant.
RESULTS
Patient characteristics
A total of 239 patients with gastric cancer were enrolled in the study. The patient characteristics are shown in Table 1. The median age was 61 years (range: 23-89 years), and there were 167 (69.9%) men and 72 (30.1%) women. All tumors were adenocarcinomas. According to the American Joint Committee on Cancer 7th edition, 39 (16.3%) patients had stage I, 78 (32.6%) had stage II, 102 (42.7%) had stage III, and 20 (8.4%) had stage IV tumors. Tumors were classified as intestinal- and diffuse-type in 104 (43.5%) and 135 (56.5%) patients, respectively.
Table 1.
Clinicopathological characteristics
| Variables | No. of patients | Percent |
| Age (range, 23-89, yr) | ||
| ≥ 60 | 126 | 52.7 |
| < 60 | 113 | 47.3 |
| Gender | ||
| Male | 167 | 69.9 |
| Female | 72 | 30.1 |
| Smoking status | ||
| Never | 61 | 25.5 |
| Ever | 178 | 74.5 |
| Tumor location | ||
| GEJ | 41 | 17.2 |
| Non-GEJ | 198 | 82.8 |
| Tumor size | ||
| ≥ 5 cm | 102 | 42.7 |
| < 5 cm | 137 | 57.3 |
| pTNM stage | ||
| I | 39 | 16.3 |
| II | 78 | 32.6 |
| III | 102 | 42.7 |
| IV | 20 | 8.4 |
| Lauren classification | ||
| Intestinal type | 104 | 43.5 |
| Diffuse type | 135 | 56.5 |
| Tissue HER2 status (IHC/FISH) | ||
| Positive | 39 | 16.3 |
| Negative | 200 | 83.7 |
| Serum HER2 ECD (ng/mL), median (range) | 10.5 (4.2-190.2) |
GEJ: Gastroesophageal junction; HER2: Human epidermal growth factor receptor-2; ECD: Extracellular domain; IHC: Immunohistochemistry; FISH: Fluorescence in situ hybridization.
Regarding tissue HER2 status, 150 patients were IHC 1+/0 according to IHC staining, 70 were IHC 2+, and 19 were IHC 3+. Patients with IHC 2+ expression were subjected to FISH assay, and 20 of these patients demonstrated gene amplification (Figure 1). According to the criteria in the ToGA trial, there were 200 (83.7%) HER2-negative and 39 (16.3%) HER2-positive cases.
Figure 1.
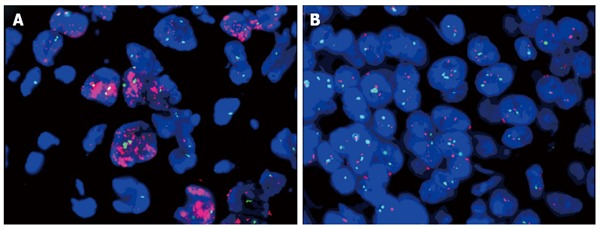
Representative gene amplification results of fluorescence in situ hybridization. A: Cases with HER2:CEP17 signal ratio > 2.0; B: Cases with HER2: CEP17 signal ratio < 2.0.
Predictive performance of serum HER2 ECD for tissue HER2 status
The median serum HER2 ECD concentration among the enrolled patients was 10.5 ng/mL (range: 4.2-190.2 ng/mL). Patients with HER2-positive gastric cancer had significantly higher serum HER2 ECD levels than those with HER2-negative gastric cancer (P < 0.001) (Figure 2A). The median serum HER2 ECD concentrations in HER2-positive and HER2-negative patients were 25.2 ng/mL (range: 7.3-190.2 ng/mL) and 9.9 ng/mL (range: 4.2-77.7 ng/mL), respectively.
Figure 2.
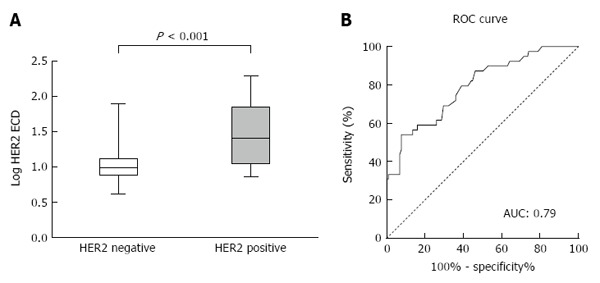
Serum HER2 ECD concentrations in patients with tissue HER2-positive and HER2-negative gastric cancer (A) and receiver operating characteristic curve plot of serum HER2 ECD for tissue HER2 status (B).
To investigate the predictive potential of serum HER2 ECD for tissue HER2 status, we initially choose a cut-off value of 15.0 ng/mL, as recommended for breast cancer by the Food and Drug Administration (FDA). The sensitivity and specificity were 0.59 (95%CI: 0.42-0.74) and 0.83 (95%CI: 0.77-0.88), respectively (Table 2). The ROC plot showed an area under the curve value of 0.79 (95%CI: 0.71-0.87, P < 0.001) (Figure 2B). Youden’s index yielded an optimal cut-off value of 24.75 ng/mL, with a sensitivity and specificity of 0.54 (95%CI: 0.37-0.70) and 0.93 (95%CI: 0.88-0.96), respectively.
Table 2.
Diagnostic performance of serum HER2 extracellular domain for tissue HER2 status at different cut-off values
| Cut-off values (ng/mL) | True positive | False positive | True negative | False negative | Sensitivity (95%CI) | Specificity (95%CI) |
| 15.001 | 23 | 35 | 165 | 16 | 0.59 (0.42-0.74) | 0.83 (0.77-0.88) |
| 24.752 | 21 | 15 | 185 | 18 | 0.54 (0.37-0.70) | 0.93 (0.88-0.96) |
Value recommended by FDA for breast cancer;
Value determined by Youden’s index in ROC plot.
Survival analysis
There were 93 deaths after a median follow-up time of 33 mo (range: 19-42 mo). The Kaplan-Meier curves are shown in Figure 3. Survival analysis demonstrated no significant difference in survival time between patients with HER2-positive and those with HER2-negative gastric cancer. The median OS in patients with HER2-positive gastric cancer was 37 mo, compared with 38 months in patients with HER2-negative gastric cancer (HR: 1.46 (0.92-2.73), P = 0.11) (Figure 3A). There was also no difference in survival time between patients with serum HER2 ECD levels ≥ 15.0 ng/mL and those with levels < 15.0 ng/mL [median time: 37 mo vs 38 mo, HR: 1.37 (0.87-2.36), P = 0.17] (Figure 3B). However, using a cut-off point of 24.75 ng/mL (determined by Youden’s index in the ROC plot), patients with higher serum HER2 ECD levels had significantly shorter survival compared with patients with lower serum HER2 ECD [median time: 36 mo vs 38 mo, HR: 1.93 (1.32-4.38), P = 0.006] (Figure 3C).
Figure 3.
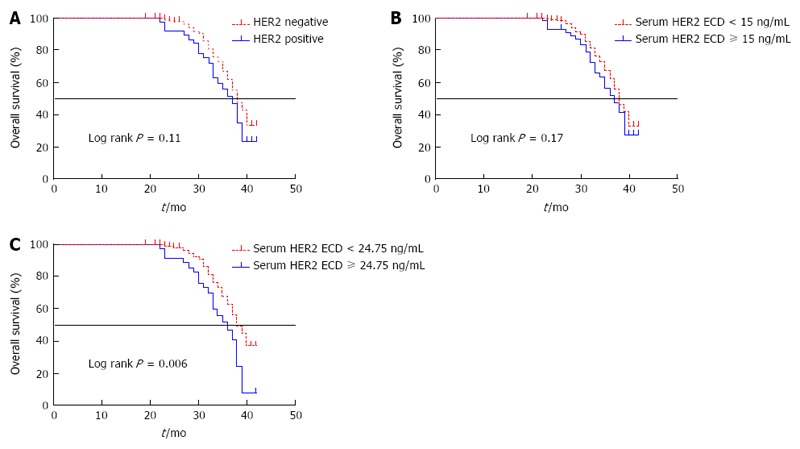
Kaplan-Meier curves for overall survival. A: Overall survival in patients with HER2-positive and HER2-negative gastric cancer; B: Serum HER2 extracellular domain (ECD) level < 15 ng/mL and serum HER2 ECD ≥ 15.0 ng/mL; and C: Serum HER2 ECD < 24.75 ng/mL and serum HER2 ECD ≥ 24.75 ng/mL.
Correlations between serum HER2 ECD levels and clinicopathological variables
We divided the enrolled patients into high-and low-serum HER2 ECD groups based on the cut-off value of 24.75 ng/mL. High serum HER2 ECD concentrations were significantly correlated with large tumor size (P < 0.001), intestinal type (P = 0.021), and tissue HER2 status (P < 0.001) (Table 3).
Table 3.
Association between clinicopathological variables and serum HER2 extracellular domain concentrations n (%)
| Variables |
Serum HER2 ECD concentrations |
P value | |
| > 24.75 ng/mL (n = 36) | ≤ 24.75 ng/mL (n = 203) | ||
| Age (yr) | 0.474 | ||
| ≥ 60 | 17 (47.2) | 109 (53.7) | |
| < 60 | 19 (52.8) | 94 (46.3) | |
| Gender | 0.396 | ||
| Male | 23 (63.9) | 144 (70.9) | |
| Female | 13 (36.1) | 59 (29.1) | |
| Smoking status | 0.452 | ||
| Never | 11 (30.6) | 50 (24.6) | |
| Ever | 25 (69.4) | 153 (75.4) | |
| Tumor location | 0.573 | ||
| GEJ | 5 (13.9) | 36 (17.7) | |
| Non-GEJ | 31 (86.1) | 167 (82.3) | |
| Tumor size | < 0.001 | ||
| ≥ 5 cm | 27 (75.0) | 75 (36.9) | |
| < 5 cm | 9 (25.0) | 128 (63.1) | |
| Type of gastrectomy | 0.266 | ||
| Total | 21 (58.3) | 98 (48.3) | |
| Subtotal | 15 (41.7) | 105 (51.7) | |
| Type of surgery | 0.730 | ||
| Open | 25 (69.4) | 135 (66.5) | |
| MIS | 11 (30.6) | 68 (33.5) | |
| Lymph node involvement | 0.419 | ||
| Positive | 22 (61.1) | 138 (68.0) | |
| Negative | 14 (38.9) | 65 (32.0) | |
| pTNM stage | 0.576 | ||
| I | 6 (16.7) | 33 (16.3) | |
| II | 12 (33.3) | 66 (32.5) | |
| III | 13 (36.1) | 89 (43.8) | |
| IV | 5 (13.9) | 15 (7.4) | |
| Lauren classification | 0.021 | ||
| Intestinal type | 22 (61.1) | 82 (40.4) | |
| Diffuse type | 14 (38.9) | 121 (59.6) | |
| Tissue HER2 status (IHC/FISH) | < 0.001 | ||
| Positive | 21 (58.3) | 18 (8.9) | |
| Negative | 15 (41.7) | 185 (91.1) | |
GEJ: Gastroesophageal junction; HER2: Human epidermal growth factor receptor-2; ECD: Extracellular domain; MIS: Minimally invasive surgery; IHC: Immunohistochemistry; FISH: Fluorescence in situ hybridization.
DISCUSSION
The clinical application of serum HER2 ECD has been extensively investigated in breast cancer, and has shown promising diagnostic and prognostic potentials[5]. However, investigations of its role in gastric cancer remain in the initial stages, and inconsistent results related to the use of different assays and cut-off values[16] highlight the need for further studies in this field[17].
The current study enrolled 239 patients, which, to the best of our knowledge, comprises the largest cohort to date in studies using the chemiluminescence immunoassay method to determine serum HER2 ECD concentrations. Our results showed that patients with HER2-positive gastric cancer had higher serum HER2 ECD concentrations than patients with HER2-negative gastric cancer. Furthermore, there was a significant association between tissue HER2 status and serum HER2 ECD levels. Moreover, high serum HER2 ECD concentrations were correlated with tumor size, suggesting that circulating HER2 ECD might be released from tumor cells. The ROC plot yielded an optimal sensitivity and specificity of 0.54 and 0.93, respectively, indicating that the serum HER2 ECD assay had high value for identifying patients with HER2-negative expression. Furthermore, our study also showed that serum HER2 ECD concentrations ≥ 24.75 ng/mL had a negative impact on OS in patients with gastric cancer, and could thus be a useful prognostic indicator.
Using the cut-off value of 15.0 ng/mL recommended for breast cancer by the FDA, we observed no survival difference between patients with high and low serum HER2 ECD concentrations. However, our ROC analysis identified the higher level of 24.75 ng/mL as the optimal cut-off value. A previous study reported similar results, and calculated an optimal cut-off value of > 15.0 ng/mL from an ROC plot[19]. These results suggest that the value of 15.0 ng/mL recommended by the FDA for breast cancer might not be suitable for gastric cancer. There were also different HER2 gene amplification criteria for breast cancer and gastric cancer; a HER2:CEP17 signal ratio > 2.2 was considered to indicate as HER2-positive tumor eligible for trastuzumab therapy in breast cancer patients[20], whereas a HER2:CEP17 signal ratio > 2.0 was considered to indicate HER2-positive gastric cancer in the ToGA trial[1]. The cut-off value of 24.75 ng/mL identified in the current study may therefore be more appropriate for gastric cancer, but more studies in clinical settings are needed to confirm the optimal cut-off value.
In addition to its diagnostic and prognostic potentials, serum HER2 ECD levels have other promising clinical values. Because serum HER2 ECD can be measured rapidly and repeatedly, monitoring HER2 ECD levels at different times might yield clinically meaningful data. Oyama et al[15] found that changes in serum HER2 ECD levels during chemotherapy were significantly correlated with response to chemotherapy in patients with HER2-positive tumor tissue. More recently, patients with higher baseline serum HER2 ECD were shown to have a better response rate before initiation of trastuzumab treatment[21]. These results demonstrated that serum HER2 ECD could be a predictor of response to chemotherapy. The results of our study showed a significant association between high serum HER2 ECD level and tumor size. It is therefore reasonable to speculate that serum HER2 ECD level might correlate with tumor recurrence. However, no studies have yet reported on this relationship in gastric cancer patients, and we plan to conduct such experiments in the future.
In conclusion, this study showed that serum HER2 ECD could be a highly specific surrogate biomarker for tissue HER2 status in patients with gastric cancer. Furthermore, serum HER2 ECD levels ≥ 24.75 ng/mL may be associated with poorer OS. Further prospective studies in clinical settings are needed to validate the eligibility of serum HER2 ECD for predicting response to chemotherapy and tumor recurrence in gastric cancer.
COMMENTS
Background
The ToGA trial demonstrated that patients with advanced gastric cancer, positive for human epidermal growth factor receptor 2 (HER2) expression or gene amplification, could benefit from HER2-targeted therapy. However, there are some limitations for current methods to accurately determine HER2 status. More convenient and reproducible detection methods to identify HER2-positive gastric cancer are needed.
Research frontiers
This study investigated the association between serum HER2 extracellular domain (ECD) and tissue HER2 status as well as the prognostic value of serum HER2 ECD in a large cohort of patients.
Innovations and breakthroughs
There were different levels of serum HER2 ECD between patients with HER2-positive tissue expression and those with HER2-negative tissue expression. High serum HER2 ECD had negative impact on patient overall survival [hazard ratio: 1.93 (95%CI: 1.32-4.38), P = 0.006].
Applications
Serum HER2 ECD could be a surrogate biomarker for tissue HER2 status with high specificity.
Terminology
The HER2 ECD, the extracellular fragment of HER2 protein, locates on the surface of tumor cells and could be released into the circulation by shedding.
Peer-review
The manuscript is very interesting, and it has several important new findings on gastric cancer therapy. It is of particular notice the use of serum HER2 as a predictive biomarker for tissue HER2 status and prognosis, which seems to be a potential new tool to improve the treatment of gastric cancer.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was approved by the Research Ethics Committee of the Chinese People’s Liberation Army General Hospital.
Informed consent statement: All study participants provided written consent prior to study enrollment.
Conflict-of-interest statement: All the authors have no conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: October 25, 2016
First decision: December 28, 2016
Article in press: January 17, 2017
P- Reviewer: Christodoulidis G, Fiorentini G S- Editor: Yu J L- Editor: Ma JY E- Editor: Zhang FF
References
- 1.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:1286–1312. doi: 10.6004/jnccn.2016.0137. [DOI] [PubMed] [Google Scholar]
- 3.Yano T, Doi T, Ohtsu A, Boku N, Hashizume K, Nakanishi M, Ochiai A. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15:65–71. [PubMed] [Google Scholar]
- 4.Pietrantonio F, Caporale M, Morano F, Scartozzi M, Gloghini A, De Vita F, Giommoni E, Fornaro L, Aprile G, Melisi D, et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. Int J Cancer. 2016;139:2859–2864. doi: 10.1002/ijc.30408. [DOI] [PubMed] [Google Scholar]
- 5.Lam L, McAndrew N, Yee M, Fu T, Tchou JC, Zhang H. Challenges in the clinical utility of the serum test for HER2 ECD. Biochim Biophys Acta. 2012;1826:199–208. doi: 10.1016/j.bbcan.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross JS. Update on HER2 testing for breast and upper gastrointestinal tract cancers. Biomark Med. 2011;5:307–318. doi: 10.2217/bmm.11.31. [DOI] [PubMed] [Google Scholar]
- 7.Carlson RW, Moench SJ, Hammond ME, Perez EA, Burstein HJ, Allred DC, Vogel CL, Goldstein LJ, Somlo G, Gradishar WJ, et al. HER2 testing in breast cancer: NCCN Task Force report and recommendations. J Natl Compr Canc Netw. 2006;4 Suppl 3:S1–S22; quiz S23-S24. [PubMed] [Google Scholar]
- 8.Colomer R, Montero S, Lluch A, Ojeda B, Barnadas A, Casado A, Massutí B, Cortés-Funes H, Lloveras B. Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin Cancer Res. 2000;6:2356–2362. [PubMed] [Google Scholar]
- 9.Ludovini V, Gori S, Colozza M, Pistola L, Rulli E, Floriani I, Pacifico E, Tofanetti FR, Sidoni A, Basurto C, et al. Evaluation of serum HER2 extracellular domain in early breast cancer patients: correlation with clinicopathological parameters and survival. Ann Oncol. 2008;19:883–890. doi: 10.1093/annonc/mdm585. [DOI] [PubMed] [Google Scholar]
- 10.Farzadnia M, Meibodi NT, Shandiz FH, Mahmoudi M, Bahar MM, Memar B, Amoian S, Maroozi F, Moheghi N. Evaluation of HER2/neu oncoprotein in serum and tissue samples of women with breast cancer: correlation with clinicopathological parameters. Breast. 2010;19:489–492. doi: 10.1016/j.breast.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res. 2003;9:4423–4434. [PubMed] [Google Scholar]
- 12.Imoto S, Wada N, Hasebe T, Ochiai A, Kitoh T. Serum c-erbB-2 protein is a useful marker for monitoring tumor recurrence of the breast. Int J Cancer. 2007;120:357–361. doi: 10.1002/ijc.22166. [DOI] [PubMed] [Google Scholar]
- 13.Lipton A, Ali SM, Leitzel K, Demers L, Harvey HA, Chaudri-Ross HA, Brady C, Wyld P, Carney W. Serum HER-2/neu and response to the aromatase inhibitor letrozole versus tamoxifen. J Clin Oncol. 2003;21:1967–1972. doi: 10.1200/JCO.2003.09.098. [DOI] [PubMed] [Google Scholar]
- 14.Peng Z, Liu Y, Li Y, Zhang X, Zhou J, Lu M, Li Q, Shen L. Serum HER2 extracellular domain as a potential alternative for tissue HER2 status in metastatic gastric cancer patients. Biomark Med. 2014;8:663–670. doi: 10.2217/bmm.14.10. [DOI] [PubMed] [Google Scholar]
- 15.Oyama K, Fushida S, Tsukada T, Kinoshita J, Watanabe T, Shoji M, Nakanuma S, Okamoto K, Sakai S, Makino I, et al. Evaluation of serum HER2-ECD levels in patients with gastric cancer. J Gastroenterol. 2015;50:41–45. doi: 10.1007/s00535-014-0941-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang K, Cui J, Xi H, Bian S, Ma L, Shen W, Li J, Wang N, Wei B, Chen L. Serum HER2 Is a Potential Surrogate for Tissue HER2 Status in Gastric Cancer: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0136322. doi: 10.1371/journal.pone.0136322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimada H. Is “liquid biopsy” useful for assessing HER2 status in gastric cancer? J Gastroenterol. 2015;50:119–120. doi: 10.1007/s00535-014-0967-6. [DOI] [PubMed] [Google Scholar]
- 18.Xi HQ, Cui JX, Shen WS, Wu XS, Bian SB, Li JY, Song Z, Wei B, Chen L. Increased expression of Lgr5 is associated with chemotherapy resistance in human gastric cancer. Oncol Rep. 2014;32:181–188. doi: 10.3892/or.2014.3207. [DOI] [PubMed] [Google Scholar]
- 19.Dai SQ, An X, Wang F, Shao Q, Chen YC, Kong YN, Chen C, Li C, Luo HY, Liang Y, et al. Serum HER 2 extracellular domain level is correlated with tissue HER 2 status in metastatic gastric or gastro-oesophageal junction adenocarcinoma. PLoS One. 2013;8:e63458. doi: 10.1371/journal.pone.0063458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Peng Z, Liu Y, Gong J, Zhang X, Lu M, Gao J, Li Y, Li Y, Shen L. Predictive value of serum HER2 ECD in patients with HER2-positive advanced gastric cancer treated with trastuzumab plus chemotherapy. J Gastroenterol. 2015;50:955–961. doi: 10.1007/s00535-015-1046-3. [DOI] [PubMed] [Google Scholar]


