Abstract
AIM
To describe the relationships between non-alcoholic fatty-liver disease (NAFLD) patient’s disease consequences and treatment perceptions, self-efficacy, and healthy lifestyle maintenance.
METHODS
A cross-sectional study among 146 ultrasound diagnosed NAFLD patients who visited the fatty liver clinic at the Tel-Aviv Medical Center. Eighty-seven of these individuals, participated in a clinical trial of physical activity and underwent fasting blood tests, analyzed at the same lab. Exclusion criteria included positivity for serum HBsAg or anti-HCV antibodies; fatty liver suspected to be secondary to hepatotoxic drugs; excessive alcohol consumption (≥ 30 g/d in men or ≥ 20 g/d in women) and positive markers of genetic or immune-mediated liver diseases. Patients were asked to complete a self-report structured questionnaire, assembled by the Israeli Center for Disease Control. Nutrition habits were measured using six yes/no questions (0 = no, 1 = yes) adopted from the national survey questionnaire. Participants in the clinical trial completed a detailed semi-quantitative food frequency questionnaire (FFQ) reporting their habitual nutritional intake during the past year. Self-efficacy was assessed by the Self-Efficacy Scale questionnaire, emotional representation, degree of illness understanding, timeline perception, treatment perception and symptoms were measured by the Brief Illness Perception questionnaire. Illness consequences were measured by the Personal Models of Diabetes Interview questionnaire. A path analysis was performed to describe the interrelationships between the patients’ illness perceptions, and assess the extent to which the data fit a prediction of nutritional habits.
RESULTS
The study sample included 54.1% men, with a mean age of 47.76 ± 11.68 years (range: 20-60) and mean body mass index of 31.56 ± 4.6. The average perceived nutrition habits score was 4.73 ± 1.45 on a scale between 0-6, where 6 represents the healthiest eating habits. Most of the study participants (57.2%) did not feel they fully understood what NAFLD is. Better nutritional habits were positively predicted by the degree of illness understanding (β = 0.26; P = 0.002) and self-efficacy (β = 0.25; P = 0.003). Perceptions of more severe illness consequences were related with higher emotional representation (β = 0.55; P < 0.001), which was related with lower self-efficacy (β = -0.17; P = 0.034). The perception of treatment effectiveness was positively related with self-efficacy (β = 0.32; P < 0.001). In accordance with the correlation between self-efficacy and the perceived nutrition habits score, self-efficacy was also correlated with nutrient intake evaluated by the FFQ; negatively with saturated fat (percent of saturated fat calories from total calories) (r = -0.28, P = 0.010) and positively with fiber (r = 0.22, P = 0.047) and vitamin C intake (r = 0.34, P = 0.002). In a sub analysis of the clinical trial participants, objectively measured compliance to physical activity regimen was positively correlated with the self-efficacy level (r = 0.34, P = 0.046).
CONCLUSION
Self-efficacy and illness understanding are major determinants of lifestyle-modification among NAFLD patients. This information can assist clinicians in improving compliance with lifestyle changes among these patients.
Keywords: Non-alcoholic fatty-liver disease, Physical activity, Diet, Illness perception, Self-efficacy
Core tip: Dietary modification is a main route of treatment in non-alcoholic fatty liver disease (NAFLD), however it is difficult to maintain in the long term and better ways for implementation are needed. Higher perceptions of understanding the illness and a higher self-efficacy are positively related to better nutritional habits, and therefore its enhancement should be part of the behavioral treatment. Emphasizing to patients that although NAFLD is a chronic condition, it is effectively treatable by diet, increases their self-efficacy. “Scaring” the patients and leading them to believe that NAFLD has severe consequences may lead to the undesirable outcome of reduced self-efficacy and worse dietary habits.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is emerging globally as the most prevalent liver disease. Its prevalence is strongly related to growing obesity rates, adoption of a sedentary lifestyles and the globalization of a Western diet[1]. NAFLD is a leading cause for chronic liver disease, cirrhosis and hepatocellular carcinoma[2], and is an independent risk factor for diabetes and cardiovascular disease[3].
Poor dietary habits represent a main modifiable target for the primary prevention and treatment of NAFLD and the more advanced form of non-alcoholic steatohepatitis (NASH)[4]. Weight reduction is the most established treatment for both NAFLD and NASH[5], but changing dietary composition without reducing caloric intake may offer a more feasible alternative to treat NAFLD patients. Maintaining or striving towards healthier dietary composition is crucial regardless of body fatness, as indicated by several epidemiological studies[6,7] demonstrating that normal weight NAFLD patients are more likely to consume an unhealthy diet compared to controls. Thus, efficient and sustainable lifestyle modification programs are needed for NAFLD patients. However, building and implementing such programs may be difficult without adequate knowledge about disease and treatment perceptions among this patient group. This is particularly important in the context of an asymptomatic disease such as NAFLD, which has no accepted pharmacological treatment, although under intensive investigation including antihypertensive and antidiabetic medications[4,8]. Our group recently showed that NAFLD patients do not think of themselves as sick and accordingly do not utilize more health services[9]. Not surprisingly, NAFLD patients were found to have limited readiness to lifestyle changes[10].
It is generally accepted that self-efficacy, defined as “beliefs in one’s capabilities to organize and execute the courses of action required for producing given attainments”, is an important component in health promotion including lifestyle modification[11,12]. Importantly, self-efficacy was demonstrated to be low among NAFLD patients as compared to patients with other liver diseases[13]. However, the factors associated with self-efficacy among NAFLD patients have never been tested. Furthermore, the association between self-efficacy, along with disease and treatment perceptions, and keeping a healthy lifestyle among NAFLD patients has not been studied. Understanding NAFLD patients’ cognitive representations of their disease, which may underlie individual differences in illness-related behaviors[14], and the relationship of these representations to diet maintenance may help in developing NAFLD-tailored lifestyle interventions.
The current study aim was to describe, for the first time, the relationships between NAFLD patient’s perceptions regarding disease consequences and treatment, self-efficacy, and lifestyle habits maintenance.
MATERIALS AND METHODS
Study population
A cross-sectional survey was conducted among ultrasound diagnosed adult (above the age of 18) NAFLD patients who visited the fatty liver clinic at the Tel Aviv Medical Center during 2011 and 2012. Eighty-seven of these individuals participated in a clinical trial testing the effect of resistance training (RT) vs stretching on NAFLD[15]. Exclusion criteria included positivity for serum HBsAg or anti-HCV antibodies; fatty liver suspected to be secondary to hepatotoxic drugs; known diabetes treated by medications other than Metformin; cancer; renal failure; heart disease; non-compensated cirrhosis; inflammatory bowel disease; excessive alcohol consumption (≥ 30 g/d in men or ≥ 20 g/d in women)[2]; positive markers of genetic or immune-mediated liver diseases.
The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the Tel-Aviv Medical Center ethics committee. All patients signed an informed consent. The clinical trial was pre-registered in the NIH registration website (TRIAL no. NCT01264198).
Data collection
Patients were asked to complete a self-report structured questionnaire. The questionnaire was assembled by the Israeli Center for Disease Control and was used in the first Israeli National Health Survey (MABAT)[16]. Following a 12-h fast, each participant of the clinical trial underwent blood tests, analyzed at the same lab.
Nutrition habits were measured using six yes/no questions (0 = no, 1 = yes) adopted from the national survey questionnaire tailored for the Israeli population[17]. Individuals’ nutritional habits score was calculated based on the patient’s answers (0-6, where 0 represents poor nutritional habits and 6 represents excellent nutritional habits). Participants in the clinical trial completed a detailed semi-quantitative food frequency questionnaire (FFQ) reporting their habitual nutritional intake during the past year. The FFQ was assembled by the Food and Nutrition Administration, Ministry of Health as previously described[18].
The main independent variables were measured by questions adopted from validated questionnaires as follows (the questions included are specified in Table 1): Self-efficacy was measured by adopting the Self-Efficacy Scale questionnaire[11,19], which was modified for the aims of the current study, referring to both physical activity and healthy dietary habits and keeping a food diary. The questions included “how confident you are that you could do things like these consistently, for at least six months. Ratings were made on a 5-point Likert-type scale ranging from “Sure I could not do it” (1) to “Sure I can do it” (5). The patient’s self-efficacy rating was calculated as the mean of 7 questions on a scale from 1 to 5 (α = 0.785, 5 represents high level of self-efficacy).
Table 1.
Description of disease perceptions, illness emotional representation, perceived illness consequences, self-efficacy and reported nutritional habits among non-alcoholic fatty liver disease patients
| Variable (n = 146) | Items | % of patients who responded 4 and above (range 1-5) |
| Disease perceptions | ||
| Time line perception | 1. How long do you think your NAFLD will continue? | 53.6% |
| Treatment perception | 2. How much do you believe that there is a treatment that can help in reducing or healing NAFLD | 72.6% |
| Symptoms | 3. How much do you experience symptoms due to NAFLD? | 9.0% |
| Illness understanding degree | 4. How well do you feel that you understand what NAFLD is? | 42.8% |
| Emotional representation | 5. How concerned are you about having NAFLD? | 60.0% |
| 6. How much does your illness (NAFLD) affect you emotionally? (e.g., does it make you angry, scared, upset or depressed?) | 20.0% | |
| Illness consequences | 7. To what extent having a NAFLD affects your everyday life? | 13.7% |
| 8. To what extent do you believe that NAFLD is a severe health problem? | 59.6% | |
| 9. To what extent do you believe that NAFLD is a disease? | 50.0% | |
| 10. To what extent do you anticipate having medical complications due to NAFLD? | 46.6% | |
| Self-efficacy | 11. I'm sure that I can make the time and persist in performing physical activity even if I'm very busy at work or at home | 63.0% |
| 12. I'm sure that I can persist keeping low fat diet | 71.9% | |
| 13. I'm sure that I can persist in keeping low sugar diet | 67.1% | |
| 14. I'm sure that I can persist in keeping low sodium diet | 72.6% | |
| 15. I'm sure that I can persist in eating smaller portions during my meals | 64.1% | |
| 16. I'm sure that I can avoid buying snacks and candies | 61.0% | |
| 17. I'm sure that I can persist in keeping a food diary | 51.7% | |
| Nutrition habits1 | 18. I make an effort to reduce the amount of fat in my diet | 88.8% |
| 19. I make an effort to reduce the amount of calories in my diet | 67.5% | |
| 20. I make an effort to reduce the amount of sugar and sweats in my diet | 83.3% | |
| 21. I make an effort to reduce soft drinks consumption (with sugar not diet) | 89.5% | |
| 22. I make an effort to reduce red meat and sausages consumption | 65.1% | |
| 23. I make an effort to increase vegetables consumption | 79.4% |
Percent of respondents that answered YES to each item. NAFLD: Non-alcoholic fatty liver disease.
The questions regarding disease and treatment perceptions were adopted from the brief illness perception questionnaire[20], including: emotional representation (mean of 2 questions number 5, 6), illness understanding degree (a single question number 4), timeline perception (a single statement number 1), treatment perception (a single statement number 2) and symptoms (a single question number 3).
In addition, Illness consequences were measured based on questions adopted from the Personal Models of Diabetes Interview (PMDI) questionnaire[14], which is based on the Health Belief Model (HBM)[21,22] and the Self-Regulation Model of illness (SRM)[22,23] (mean of 4 questions number 7-10, α = 0.722).
All questions were measured using a scale from 1 to 5 (e.g., 5 represents: high level of illness emotional representation, high level of perceived understanding, perceiving NAFLD as a long-term disease, a high level of symptoms experienced severe perceived consequences and high belief in treatment). In addition, the questionnaire included an open-ended question[20], “Please list in rank-order the three most important factors that you believe caused your NAFLD”. Respondents’ answers were categorized into six main reasons (see Figure 1).
Figure 1.
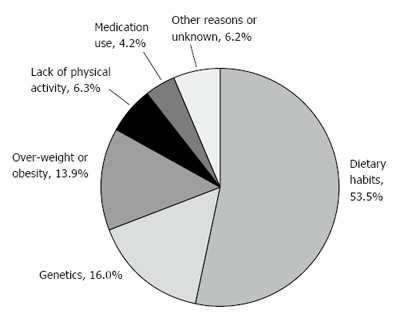
Patients’ perceptions regarding the main reasons for non-alcoholic fatty liver disease.
Statistical analysis
Statistical analyses were performed using SPSS version 22 (SPSS Inc., Chicago, IL, United States). Continuous variables are presented as mean ± SD. Independent samples t-test were performed to test differences in continuous variables between the two groups. The Mann-Whitney test was used if non-parametric tests were required based on data distribution. Pearson’s χ2 tests were performed to examine associations between nominal variables. Pearson or Spearmen correlations were used to test correlations between the diet score, self-efficacy and disease perception.
A path analysis was performed, using AMOS version 22 software (IBM Corp., SPSS, 2013), to describe the interrelationships between the study variables and assess the extent to which the data fit a predicted model associated with nutritional habits. This is a useful methodological and statistical tool to assess the fit between a set of variables and a theory. This method estimates the unidirectional relationship between a set of independent predictors and a set of dependent criteria. A model-modification approach is used to estimate existing relationships and to evaluate and improve them. The final result is a model that, on the one hand, is theoretically based and, on the other, fits the specific set of data.
Model fit was assessed with NFI, NNFI, CFI, and RMSEA. Usually, two statistical criteria are applied: a measure of the model fit (represented by χ2 and its derivates) and significant estimates of the interrelationship of the model variables (represented by Bs) (Arbuckle, 2013). The most common measures of model fit, χ2, should be non-significant in order to represent a non-significant departure from the best model possible; the Fit Indexes (NFI - normal fit index, NNFI - non-normal fit index, CFI - comparative fit index) should be close to a value of 1. RMSEA (Root mean standard error of approximation) should be lower than 0.05. Estimates given by the model (β) should be significant[24]. P < 0.05 was considered statistically significant for all analyses.
RESULTS
Description of the study population
The study sample included 146 NAFLD patients, 54.1% men, with a mean age of 47.76 ± 11.68 years (range 19-72), mean body mass index (BMI) of 31.56 ± 4.62, 25 patients (28.7% out of the 87 subjects with blood tests) had the metabolic syndrome according to the accepted criteria[25] and 13 patients (8.9%) had type-2 diabetes, all were treated solely by Metformin. The average nutrition habits score was 4.73 ± 1.45 on a scale between 0-6, where 6 represents the healthiest eating habits. Table 2 describes the study sample characteristics.
Table 2.
Description of the study sample (mean ± SD unless otherwise stated)
| Variable (units; normal range) | Total (n = 146) |
| Gender (%; males) | 54.1 |
| Nutrition habits (score) | 4.73 ± 1.45 |
| BMI (kg/m2; 20-25) | 31.56 ± 4.62 |
| Age (yr) | 47.76 ± 11.68 |
| Education (%; high school and above) | 66.7 |
| Smoking (%; current smoker) | 12.3 |
| Time since diagnosis (%; one year or less) | 36.0 |
| HOMA-IR (score) | 6.10 ± 2.79 |
| HbA1C (%; 3.9-6) | 5.65 ± 0.46 |
| AST (U/L; 5-40) | 32.66 ± 14.95 |
| Glucose (mg/dL; 70-110) | 85.62 ± 10.63 |
| ALT (U/L; 5-39) | 50.16 ± 34.12 |
| GGT (U/L; 6-28) | 48.29 ± 50.60 |
| Albumin (g/L; 35-50) | 45.05 ± 2.75 |
| Total Cholesterol (mg/dL; 150-200) | 187.97 ± 38.08 |
| Ferritin (ng/mL; 7.1-151) | 148.77 ± 129.31 |
| Metabolic syndrome (%) | 28.7 |
| Lipid-lowering medications (%) | 28.8 |
| Antihypertensive medications (%) | 20.5 |
Blood tests were available to 87 subjects.
Description of disease perceptions, illness emotional representation, perceived illness consequences, self-efficacy and reported nutritional habits among NAFLD patients
Table 1 presents the patients’ perceptions regarding different aspects of having NAFLD. Most of the study participants (57.2%) did not feel they fully understood what having a NAFLD really meant. Only a little more than half of the patients (53.6%) perceived NAFLD as a long-term condition. The majority (72.6%) believed there was some form of treatment that could help healing or reducing the severity of NAFLD.
With regard to emotional representation, 60% of the patients reported high levels of concern regarding having a NAFLD, but only 20% reported that having a NAFLD greatly affected them emotionally. In addition, with regard to illness consequences, almost 60% believed that NAFLD is a severe health problem, but only half of the patients believed NAFLD is a disease. Furthermore, only a little more than half of the patients (53.4%) anticipated having medical complications due to NAFLD. Only 13.7% felt that having NAFLD severely affected their everyday life.
The majority of the patients reported relatively high self-efficacy regarding their ability to maintain a healthy diet. For instance, 72.6% of participants reported high levels of self-efficacy to maintain a low sodium diet, 71.9% reported high levels of self-efficacy to maintain a low fat diet, and 67.1% reported high levels of self-efficacy to maintain a low sugar diet. The overall self-efficacy mean rating of the patients was 3.76 ± 0.72 on a scale of 1-5, which indicates medium-high self-efficacy rating.
The majority of the patients reported maintaining healthy eating habits. Most patients reported making efforts to reduce dietary intake of fat (88.8%), calories (67.5%), sugar (83.3%), soft drinks (89.5%), and red meat (65.1%), and to increase intake of vegetables (79.4%).
Perceptions regarding the main reasons for NAFLD among patients
Figure 1 presents the distribution of the perceived main reasons for having NAFLD. Most patients (73.7%) believed that behavioral factors were the most important reasons for NAFLD; approximately 54% reporting dietary habits as the main reason for fatty liver, followed by 13.9% who reported overweight or obesity and 6.3% who reported lack of physical activity. The second most prevalent perceived reason was genetics at 16%. Medication use was cited as the main reason for fatty liver by 4.2% of respondents.
Bivariate correlations between illness perception, emotional representation, self-efficacy and perceived nutritional habits
Table 3 presents bivariate correlations between the study variables. Maintaining healthy nutritional habits was significantly positively correlated with the patients’ perceptions regarding their illness consequences (rs = 0.19; P = 0.035), the degree of understanding their illness (rs = 0.27; P = 0.003) and their self-efficacy (rs = 0.20; P = 0.028). Self-efficacy was significantly negatively correlated with timeline perceptions (rs = -0.27; P = 0.001).
Table 3.
Bivariate correlations between variables of illness perception, emotional representation, self-efficacy and perceived nutritional habits (n = 146)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| 1 | Nutrition habits | - | ||||||
| 2 | Illness consequences perception | 0.19a | - | |||||
| 3 | Emotional representation | -0.05 | 0.58b | - | ||||
| 4 | Self efficacy | 0.20a | -0.02 | -0.09 | - | |||
| 5 | Treatment perception | -0.02 | 0.06 | 0.26b | 0.27b | - | ||
| 6 | Symptoms | 0.13 | 0.45b | 0.24b | -0.14a | -0.10 | - | |
| 7 | Time line perception | 0.12 | 0.21a | 0.09 | -0.27b | -0.32b | 0.19a | - |
| 8 | Illness understanding degree | 0.27b | 0.38b | 0.22b | 0.03 | 0.00 | 0.04 | 0.05 |
| mean | 4.73 | 3.19 | 3.03 | 3.76 | 3.97 | 1.80 | 3.14 | |
| SD | 1.45 | 0.73 | 1.06 | 0.72 | 0.99 | 1.08 | 1.14 |
P < 0.05;
P < 0.01.
There was a significant, strong positive correlation between the perception of illness consequences and high emotional representation (reflecting the extent by which the patients were afraid or concerned about having NAFLD; rs = 0.58; P ≤ 0.001), timeline perceptions (reflecting the extent by which the patients perceived NAFLD as a long term illness, rs = 0.21; P = 0.011), symptoms perception (rs = 0.45; P ≤ 0.001) and perceived Illness understanding degree (rs = 0.38; P ≤ 0.001).
Path analysis model for the prediction of nutritional habits
A path model was developed to describe the interrelationships between the study variables and assess the extent by which nutritional habits can be predicted by them.
The model was found to fit the data: NFI = 0.870, NNFI = 0.985, CFI = 0.992, RMSEA = 0.018, and χ2(29) = 30.413, P = 0.394, as shown in Figure 2 and in Supplementary Table.
Figure 2.
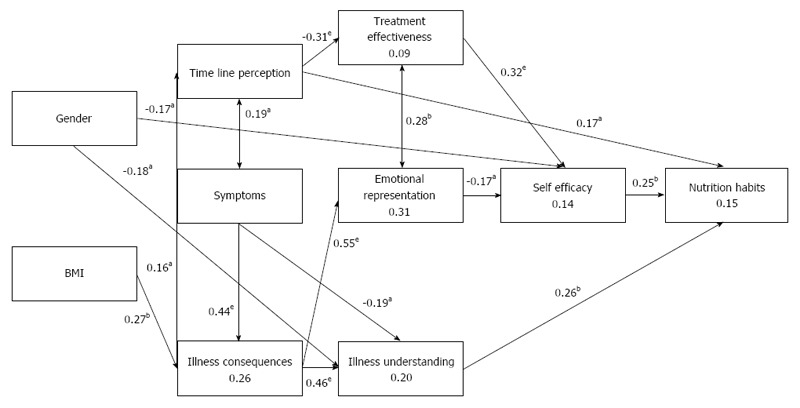
Path model for the study variables predicting nutritional habits. Values attached to the arrows represent regression standardized coefficients (β values), with significance levels in asterisks. Values within the rectangles represent the explained variance by the variable (R2). NFI = 0.870, NNFI = 0.985, CFI = 0.992, RMSEA = 0.018, χ2(29) = 30.413, P = 0.394. aP < 0.05, bP < 0.01, eP < 0.001 NFI: Normal fit index; NNFI: Non-normal fit index; CFI: Comparative fit index; RMSEA: Root mean standard error of approximation.
Better nutritional habits were positively and directly predicted by illness understanding degree (β = 0.26; P = 0.002), self-efficacy (β = 0.25; P = 0.003) and timeline perception (longer-term illness) (β = 0.17; P = 0.036).
Several indirect significant associations were observed. First, male gender was negatively related with illness understanding (β = -0.46; P = 0.014) and self-efficacy (β = -0.24; P = 0.031). Meaning that, women perceived higher illness understanding and higher self-efficacy. Second, BMI was positively related with a perception of more severe illness consequences (β = 0.27; P = 0.002). The latter was related with a higher perception of illness understanding and a perception of a longer term illness, both of which were positively predictive of better nutrition habits. However, perceptions of more severe illness consequences were strongly related with higher emotional representation (β = 0.55; P < 0.001), which in turn was related with lower self-efficacy (β = -0.17; P = 0.034), predicting worse nutritional habits. Third, although the perceptions of a longer term illness were directly related with better nutritional habits, these perceptions were also negatively related with the perception of effective treatment (β = -0.31; P < 0.001). The perception of treatment effectiveness was positively related with self-efficacy (β = 0.32; P < 0.001), which predicts better nutritional habits. Fourth, experiencing many symptoms was related with lower perceptions of illness understanding (β = -0.19; P = 0.026) and may lead indirectly to a higher illness emotional representation. However, only 9% of the sample experienced significant NAFLD-related symptoms.
The accordance between perceived nutritional habits and reported nutritional intake
A sub-sample of the study population (n = 84) completed FFQ. There was a positive correlation between healthy eating habits scores and fiber intake (r = 0.26, P = 0.027), and there was a negative correlation between healthy eating habits scores and saturated fat intake (percent of saturated fat calories from total calories) (r = -0.43, P < 0.001).
The association between self-efficacy and reported nutritional intake and actual compliance with physical activity regimen
In accordance with the correlation between self-efficacy and the perceived diet score, self-efficacy was also correlated with nutrient intake evaluated by the FFQ; negatively with saturated fat (percent of saturated fat calories from total calories) (r = -0.28, P = 0.010) and positively with fiber (r = 0.22, P = 0.047) and vitamin C intake (r = 0.34, P = 0.002).
In a sub analysis of RCT participants who in the intervention arm (resistance training) who had regular training sessions at the gym (n = 34). The number of training sessions at the gym was automatically recorded every time a patient entered the gym with his/her personal chip. Compliance was defined as the number of recorded training sessions divided by the recommended number of sessions (i.e., 3 times a week during the 12-wk trial). This objective compliance measure was positively correlated with the self-efficacy level (r = 0.34, P = 0.046).
DISCUSSION
In the present study, we demonstrated that nutritional habits and lifestyle modification among NAFLD patients may be associated with the patients’ perceptions concerning their illness, and with their self-efficacy regarding their ability to change their lifestyle. Our results highlight the impotence and the complexity of achieving NAFLD patient’s adherence to healthy nutritional habits. The model suggests ways by which clinicians can improve NAFLD patient’s eating habits, or, on the other hand, may unintentionally discourage them from trying. The main conclusions that can be drawn are that higher perceptions of understanding the illness and a higher self-efficacy are positively related to better nutritional habits, and therefore its enhancement should be part of the behavioral treatment. Conversely, “scaring” the patients and leading them to believe that NAFLD has severe consequences may lead to the undesirable outcome of greater disease-related emotional stress, which, in turn, reduces self-efficacy and thus may lead to worse nutritional habits. In addition, although the patients should know that NAFLD is a chronic disease, a perception of a longer-term illness was related with a perception of a less effective treatment and lower self-efficacy. Therefore, it may be important to emphasize to patients that although NAFLD is a chronic condition, it is still treatable and even reversible if diet is maintained[5]. Our results emphasize the importance of explaining patients the effectiveness of dietary treatment in NAFLD, a step that may increase their self-efficacy and compliance.
In support of our results, the Health Belief Model (HBM)[22], indicates that perceived risk, severity, or threat of disease among patients as well as their confidence to make lifestyle changes may be critical to motivate patients into chancing their eating and physical activity habits. The HBM was never tested in the context of improving eating habits among NAFLD patients. However, several studies have tested patients’ perceptions according to the HBM among diabetic patients, and the similarity between diabetes and NAFLD may help in the interpretation of the current study results. In a recent study among type-2 diabetes patients, both self-efficacy and perceived medication benefits were significant HBM predictors for medication adherence[26].
The current study results shows that perceptions of more severe illness consequences were related with higher emotional representation, which in turn was related with lower self-efficacy, predicting poor nutritional habits. We believe that this is a very crucial finding for care-givers treating NAFLD patients. Understanding of the emotional aspects of having a NAFLD may be a key element in a successful treatment and a better modification of healthy lifestyle. The impotence of the illness emotional representation and the patients’ emotional status among chronic patients is well documented. For instance, higher emotional representation was negatively correlated with well-being among patients with chronic kidney disease[27]. Among type-2 diabetes patients, it was suggested that patients construct their own individual self-management from an emotional base. Therefore, balanced emotional status can contribute to a better self-management[28].
The current study suggests that the mechanism by which emotional representation affect the health outcomes, may be through the patients’ self- efficacy. Patients with a better balanced emotional status may be less emotionally distressed, and therefore may have higher self-efficacy related to their ability to make the changes needed in their life. A previous study showed that lower illness-associated emotional representation is associated with a better sense of control among haemodialysis patients[29].
The positive association between perceived understanding of NAFLD and maintaining healthy nutritional habits is another important finding. This may indicate that patients who feel they understand their illness will make more efforts or will have better self-efficacy to maintain healthy eating habits[30-32]. In patients with alcohol-related liver disease, illness understanding made a significant contribution to their self-management confidence[33].
Our study highlights the major role of self-efficacy as a determinant of lifestyle modification maintenance. This is consistent with past research supporting the role of self-efficacy in lifestyle modification[34,35]. Furthermore, we demonstrated a positive association between self-efficacy and actual compliance with a physical activity regimen, as measured by an objective tool of automatically recorded number of training sessions at the gym. This finding further supports the importance of self-efficacy in lifestyle-modification demonstrated in our study as well as in previous study which demonstrated a particularly low self-efficacy to perform physical activity among NAFLD patients[13]. Interestingly, low parental self-efficacy for lifestyle modification was demonstrated among parents to obese children with NAFLD[36]. Indeed, similarly to the treatment of obesity and other metabolic disorders, the promotion of self-efficacy as part of behavioral therapy should be adopted in the treatment of NAFLD as well[12].
This study provides critical information that can be used and disseminated among interventionists, clinicians, and other key stakeholders. This study may also serve as a model to other studies seeking to identify targets for lifestyle interventions among this relatively understudied group.
Our study has several limitations to consider. First, nutritional habits relied on self-report, which may lead to report bias. This bias is most likely non-differential and thus can only weaken the observed associations. To provide construct validity to the eating habits score, we correlated it with another type of nutritional assessment, based on a detailed FFQ, indicating correlations between reported nutritional habits and calculated nutrients consumption, as expected. Furthermore, self-efficacy was positively correlated with the FFQ-estimated fiber and vitamin C intake, which are indicators for fruit and vegetable intake, and negatively with saturated fat.
Second, generalization to other populations needs to be validated, especially given the potential for cultural differences in health beliefs[37]. Lastly, the cross-sectional design of the study prevents the determination of the directions of associations, and is insufficient to determine causality.
In conclusion, complex relationships exist between disease perception, knowledge, emotional stress, self-efficacy and nutritional habits in NAFLD patients. The identification of these relationships may help tailor behavioral interventions delivered by clinicians treating NAFLD patients. Self-efficacy enhancement seems to be a key-factor, along with believing in treatment effectiveness and improving illness understanding. Focusing on these parameters is likely to enhance effective lifestyle interventions achieving long term engagement of NAFLD patients.
COMMENTS
Background
Non-alcoholic fatty liver disease (NAFLD) is emerging globally as the most prevalent liver disease. Poor dietary habits represent a main modifiable target for the primary prevention and treatment of NAFLD. Thus, efficient and sustainable lifestyle modification programs are needed for NAFLD patients. However, building and implementing such programs may be difficult without adequate knowledge about disease and treatment perceptions of NAFLD patients and their association with self-efficacy to execute lifestyle changes.
Research frontiers
The factors associated with self-efficacy among NAFLD patients have never been tested. Furthermore, the association between self-efficacy, along with disease and treatment perceptions, and keeping a healthy lifestyle among NAFLD patients has not been studied.
Innovations and breakthroughs
In the present study, the authors demonstrated that nutritional habits and lifestyle modification among NAFLD patients may be associated with the patients’ perceptions concerning their illness, and with their self-efficacy regarding their ability to change their lifestyle. Current results highlight the importance and the complexity of achieving NAFLD patient’s adherence to healthy nutritional habits.
Applications
Understanding NAFLD patients’ cognitive representations of their disease can help in developing NAFLD-tailored lifestyle interventions. The model suggests ways by which clinicians can improve NAFLD patient’s eating habits, or, on the other hand, may unintentionally discourage them from trying. The main conclusions that can be drawn are that higher perceptions of understanding the illness and a higher self-efficacy are positively related to better nutritional habits, and therefore its enhancement should be part of the behavioral treatment. Conversely, “scaring” the patients and leading them to believe that NAFLD has severe consequences may lead to the undesirable outcome of lower self-efficacy. In addition, it may be important to emphasize to patients that although NAFLD is a chronic condition, it is still treatable and even reversible if diet is maintained.
Terminology
Self-efficacy, defined as beliefs in one’s capabilities to organize and execute the courses of action required for producing given attainments. The health Belief Model (HBM), indicates that perceived risk, severity, or threat of disease among patients as well as their confidence to make lifestyle changes may be critical to motivate patients into chancing their eating and physical activity habits.
Peer-review
The topic is quite interesting and the paper is very well written, with clear language and discussion consistent with the results obtained. The study by Zelber-Sagi et al has investigated the relation between illness perception and dietary modification among 146 patients with NAFLD. The study has shown significant correlations between disease perception and dietary habits among NAFLD patients, suggesting self-efficacy enhancement as an effective tool for improving illness understanding, thus treatment effectiveness. Aim of the study has been clearly stated. Data relevant to the topic have been precisely presented and discussed in detail. Statistical methods have been meticulously mentioned in the text.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was approved by the institutional review board of Tel Aviv medical center.
Informed consent statement: All participants signed an informed consent.
Conflict-of-interest statement: We declare that in this manuscript there was no Conflict of interest and no financial support.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at zelbersagi@bezeqint.net.
Peer-review started: December 6, 2016
First decision: December 28, 2016
Article in press: February 8, 2017
P- Reviewer: Emre A, Gregorio BM S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 3.Lonardo A, Sookoian S, Pirola CJ, Targher G. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism. 2016;65:1136–1150. doi: 10.1016/j.metabol.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:3673–3678.e5; quiz e14-15. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Fagà E, Silli B, Pagano G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 7.Yasutake K, Nakamuta M, Shima Y, Ohyama A, Masuda K, Haruta N, Fujino T, Aoyagi Y, Fukuizumi K, Yoshimoto T, et al. Nutritional investigation of non-obese patients with non-alcoholic fatty liver disease: the significance of dietary cholesterol. Scand J Gastroenterol. 2009;44:471–477. doi: 10.1080/00365520802588133. [DOI] [PubMed] [Google Scholar]
- 8.Souza-Mello V, Gregório BM, Cardoso-de-Lemos FS, de Carvalho L, Aguila MB, Mandarim-de-Lacerda CA. Comparative effects of telmisartan, sitagliptin and metformin alone or in combination on obesity, insulin resistance, and liver and pancreas remodelling in C57BL/6 mice fed on a very high-fat diet. Clin Sci (Lond) 2010;119:239–250. doi: 10.1042/CS20100061. [DOI] [PubMed] [Google Scholar]
- 9.Mlynarsky L, Schlesinger D, Lotan R, Webb M, Halpern Z, Santo E, Shibolet O, Zelber-Sagi S. Non-alcoholic fatty liver disease is not associated with a lower health perception. World J Gastroenterol. 2016;22:4362–4372. doi: 10.3748/wjg.v22.i17.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centis E, Moscatiello S, Bugianesi E, Bellentani S, Fracanzani AL, Calugi S, Petta S, Dalle Grave R, Marchesini G. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J Hepatol. 2013;58:771–777. doi: 10.1016/j.jhep.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Kroll T, Kehn M, Ho PS, Groah S. The SCI Exercise Self-Efficacy Scale (ESES): development and psychometric properties. Int J Behav Nutr Phys Act. 2007;4:34. doi: 10.1186/1479-5868-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellentani S, Dalle Grave R, Suppini A, Marchesini G. Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology. 2008;47:746–754. doi: 10.1002/hep.22009. [DOI] [PubMed] [Google Scholar]
- 13.Frith J, Day CP, Robinson L, Elliott C, Jones DE, Newton JL. Potential strategies to improve uptake of exercise interventions in non-alcoholic fatty liver disease. J Hepatol. 2010;52:112–116. doi: 10.1016/j.jhep.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Hampson SE, Glasgow RE, Toobert DJ. Personal models of diabetes and their relations to self-care activities. Health Psychol. 1990;9:632–646. doi: 10.1037//0278-6133.9.5.632. [DOI] [PubMed] [Google Scholar]
- 15.Zelber-Sagi S, Buch A, Yeshua H, Vaisman N, Webb M, Harari G, Kis O, Fliss-Isakov N, Izkhakov E, Halpern Z, et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol. 2014;20:4382–4392. doi: 10.3748/wjg.v20.i15.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaluski DN, Goldsmith R, Arie OM, Mayer C, Green M. The first Israeli national health and nutrition survey (MABAT) as a policy maker. Public Health Rev. 2000;28:23–26. [PubMed] [Google Scholar]
- 17.Niskar A, Baron-Epel O, Garty-Sandalon N, Keinan-Boker L. Body weight dissatisfaction among Israeli Jewish and Arab women with normal or overweight-obese body mass index, Israeli INHIS-1, 2003-2004. Prev Chronic Dis. 2009;6:A51. [PMC free article] [PubMed] [Google Scholar]
- 18.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, Oren R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol. 2007;47:711–717. doi: 10.1016/j.jhep.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Hofstetter CR, Sallis JF, Hovell MF. Some health dimensions of self-efficacy: analysis of theoretical specificity. Soc Sci Med. 1990;31:1051–1056. doi: 10.1016/0277-9536(90)90118-c. [DOI] [PubMed] [Google Scholar]
- 20.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60:631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Mirotznik J, Ginzler E, Zagon G, Baptiste A. Using the health belief model to explain clinic appointment-keeping for the management of a chronic disease condition. J Community Health. 1998;23:195–210. doi: 10.1023/a:1018768431574. [DOI] [PubMed] [Google Scholar]
- 22.Harvey JN, Lawson VL. The importance of health belief models in determining self-care behaviour in diabetes. Diabet Med. 2009;26:5–13. doi: 10.1111/j.1464-5491.2008.02628.x. [DOI] [PubMed] [Google Scholar]
- 23.Heijmans M, de Ridder D. Assessing illness representations of chronic illness: explorations of their disease-specific nature. J Behav Med. 1998;21:485–503. doi: 10.1023/a:1018788427100. [DOI] [PubMed] [Google Scholar]
- 24.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 25.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 26.Alatawi YM, Kavookjian J, Ekong G, Alrayees MM. The association between health beliefs and medication adherence among patients with type 2 diabetes. Res Social Adm Pharm. 2015;12:914–925. doi: 10.1016/j.sapharm.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Fowler C, Baas LS. Illness representations in patients with chronic kidney disease on maintenance hemodialysis. Nephrol Nurs J. 2006;33:173–174, 179-186. [PubMed] [Google Scholar]
- 28.Furler J, Walker C, Blackberry I, Dunning T, Sulaiman N, Dunbar J, Best J, Young D. The emotional context of self-management in chronic illness: A qualitative study of the role of health professional support in the self-management of type 2 diabetes. BMC Health Serv Res. 2008;8:214. doi: 10.1186/1472-6963-8-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Covic A, Seica A, Gusbeth-Tatomir P, Gavrilovici O, Goldsmith DJ. Illness representations and quality of life scores in haemodialysis patients. Nephrol Dial Transplant. 2004;19:2078–2083. doi: 10.1093/ndt/gfh254. [DOI] [PubMed] [Google Scholar]
- 30.AbuSabha R, Achterberg C. Review of self-efficacy and locus of control for nutrition- and health-related behavior. J Am Diet Assoc. 1997;97:1122–1132. doi: 10.1016/S0002-8223(97)00273-3. [DOI] [PubMed] [Google Scholar]
- 31.Kelly RB, Zyzanski SJ, Alemagno SA. Prediction of motivation and behavior change following health promotion: role of health beliefs, social support, and self-efficacy. Soc Sci Med. 1991;32:311–320. doi: 10.1016/0277-9536(91)90109-p. [DOI] [PubMed] [Google Scholar]
- 32.Adams RJ. Improving health outcomes with better patient understanding and education. Risk Manag Healthc Policy. 2010;3:61–72. doi: 10.2147/RMHP.S7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau-Walker M, Presky J, Webzell I, Murrells T, Heaton N. Patients with alcohol-related liver disease--beliefs about their illness and factors that influence their self-management. J Adv Nurs. 2016;72:173–185. doi: 10.1111/jan.12826. [DOI] [PubMed] [Google Scholar]
- 34.Dalle Grave R, Calugi S, Centis E, Marzocchi R, El Ghoch M, Marchesini G. Lifestyle modification in the management of the metabolic syndrome: achievements and challenges. Diabetes Metab Syndr Obes. 2010;3:373–385. doi: 10.2147/DMSOTT.S13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 36.Iñiguez IR, Yap J, Mager DR. Parental perceptions regarding lifestyle interventions for obese children and adolescents with nonalcoholic fatty liver disease. Paediatr Child Health. 2014;19:e24–e29. doi: 10.1093/pch/19.5.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. 2003;118:293–302. doi: 10.1016/S0033-3549(04)50253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


