Abstract
Endophthalmitis following intravitreal dexamethasone (DEX) implant has been rarely reported. This report describes the case of a 70-year-old male who underwent intravitreal DEX implant injection under aseptic conditions, for diabetic macular edema. He developed a clinical picture suggestive of endophthalmitis within 2 weeks of the injection, and vitreous culture grew coagulase negative Staphylococcus. He was treated with intravitreal antibiotics followed by pars plana vitrectomy and removal of the implant. This was followed by resolution of the infection with a favorable final visual outcome. The challenges faced during surgical management of this case are discussed.
Keywords: Acute bacterial endophthalmitis, Intravitreal dexamethasone implant, Pars plana vitrectomy, Dexamethasone implant removal
Introduction
Sustained release intravitreal dexamethasone (DEX) implant, available as Ozurdex (Allergan, Inc, Irvine, CA), is approved for the treatment of diabetic macular edema (DME) following a randomized, masked, sham-controlled phase III clinical trial that demonstrated its efficacy and safety.1 Endophthalmitis following intravitreal injections is not uncommon owing to the increasing number of injections;2 however, there are very few reports of endophthalmitis after an intravitreal DEX implant.1, 3, 4, 5 This case adds to the literature on complications following administration of an intravitreal DEX implant and discusses the challenges faced while managing this scenario.
Case report
A 70-year-old male presented with decreased vision in his left eye since one month. He was a diabetic and hypertensive since 10 years, controlled on medications. He had history of both eyes panretinal photocoagulation performed 6 years back. Best corrected visual acuity (BCVA) was 20/30 in the right eye and 20/120 in the left eye. Anterior segment examination showed nuclear sclerosis grade 2 in both eyes with intraocular pressure (IOP) of 16 mmHg bilaterally. Fundus examination revealed lasered diabetic retinopathy in both eyes with clinically significant macular edema in the left eye. Optical coherence tomography (OCT) of the left macula was consistent with DME, with a central macular thickness of 437 μm. After written informed consent, he underwent intravitreal DEX implant injection in this eye. The injection was performed in the operation theater with topical anesthesia under aseptic conditions. The physician and all other involved medical staff wore caps and surgical masks. A sterile injection set was used and the instruments were prepared on a sterile tray. A periocular scrub using 10% povidone iodine was performed, and 5% povidone iodine was instilled in the conjunctival cul-de-sac before the injection. A fenestrated self-adhesive surgical drape that covered the patient’s nose and mouth was applied, and a sterile lid speculum was used. Following the injection, the eye was patched with sterile compresses. Moxifloxacin 0.5% drops were instilled prior to the injection as well as at the end of the procedure and prescribed four times daily for a week.
15 days after the injection, the patient presented with a history of sudden loss of vision in his left eye since 3 days. There were no complains of pain, redness or discharge. BCVA was counting fingers close to face with accurate projection of rays. Anterior segment examination revealed minimal circumcorneal congestion, the presence of keratic precipitates, 3 + cells in the anterior chamber and an IOP of 16 mmHg. Fundus examination showed a poor red reflex, grade 3 vitreous opacity and a hazy view of the disc. The implant was seen inferiorly and appeared to be fragmented in two pieces. An ultrasound B-scan confirmed the presence of numerous highly reflective echoes and membranes in the vitreous cavity with an attached retina.
A clinical diagnosis of acute endophthalmitis was made and the patient underwent a vitreous tap and intravitreal injection of 1 mg in 0.1 ml of vancomycin and 2.25 mg in 0.1 ml of ceftazidime the same day. Oral ciprofloxacin 750 mg twice daily was started along with topical fortified vancomycin and ceftazidime drops instilled hourly. Within 48 h, cultures grew out coagulase negative Staphylococcus which was sensitive to vancomycin by Kirby-Bauer disk diffusion method. The patient’s visual acuity and clinical picture showed no improvement and a decision to perform vitrectomy with removal of the DEX implant was taken. After written, informed consent 23 gauge pars plana vitrectomy was carried out. The vitreous was opacified and the fractured implant was present inferiorly (Fig.1a). Removal of the implant was attempted using the 23 gauge vitreous cutter; however, it was unable to do so. Following complete vitrectomy, the implant was lifted using the suction mode of the vitreous cutter, grasped with a 23 gauge forceps (Fig.1b) and removed. The remaining fragment was embedded in the vitreous inferotemporally and could not be grasped by the 23 gauge forceps as it was larger than the mouth of the forceps. After dissection in this area, it was removed using a 20 gauge forceps (Fig.1c). Two smaller fragments were seen on indentation (Fig.1d) and carefully dissected and removed. The implant was very friable and underwent fragmentation when held by forceps. Remnant particles were aspirated using a flute needle and the intravitreal antibiotics were repeated. Post operatively, the patient received a tapering regimen of topical antibiotics and prednisolone acetate 1% with oral ciprofloxacin and systemic corticosteroids 1 mg/kg/day for 10 days.
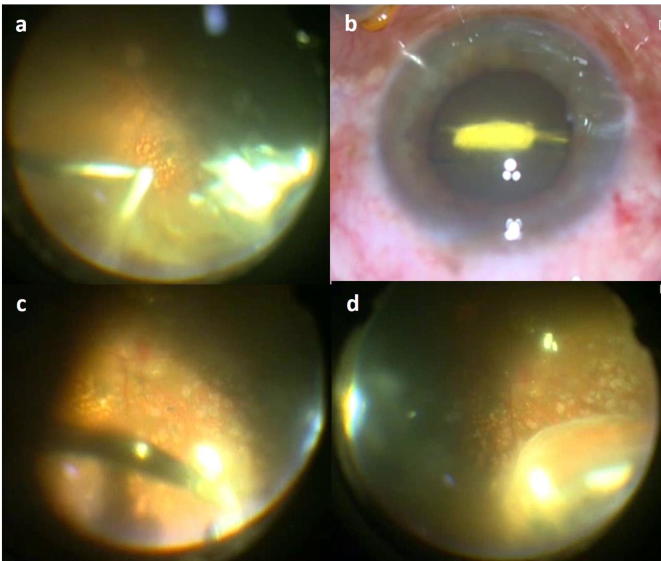
Figure 1.
(a) Intra-operative photograph showing opacified vitreous and the 23-gauge vitreous cutter engaging the dexamethasone (DEX) implant. The cutter was unable to remove the implant. (b) The implant was lifted using the cutter and removed using a 23-gauge intravitreal forceps. (c) The remaining fragment did not fit into a 23-gauge intravitreal forceps, and could be removed only with a 20-gauge intravitreal forceps. (d) Two more fractured pieces of the DEX implant were found on indentation. These were removed using intravitreal forceps after meticulous vitreous dissection.
Within 72 hours of the procedure, BCVA improved to 20/200 with a significant decrease in the anterior and posterior segment inflammation. Complete resolution was noted at six weeks post operatively. BCVA, however, was limited by progression of cataract and the presence of an epiretinal membrane (ERM). A month later, the patient underwent phacoemulsification with implantation of a posterior chamber intraocular lens with ERM peeling. At the final follow-up of 3 months, BCVA was 20/60 with no evidence of any inflammation (Fig.2a), or ERM on OCT (Fig.2b).
Figure 2.
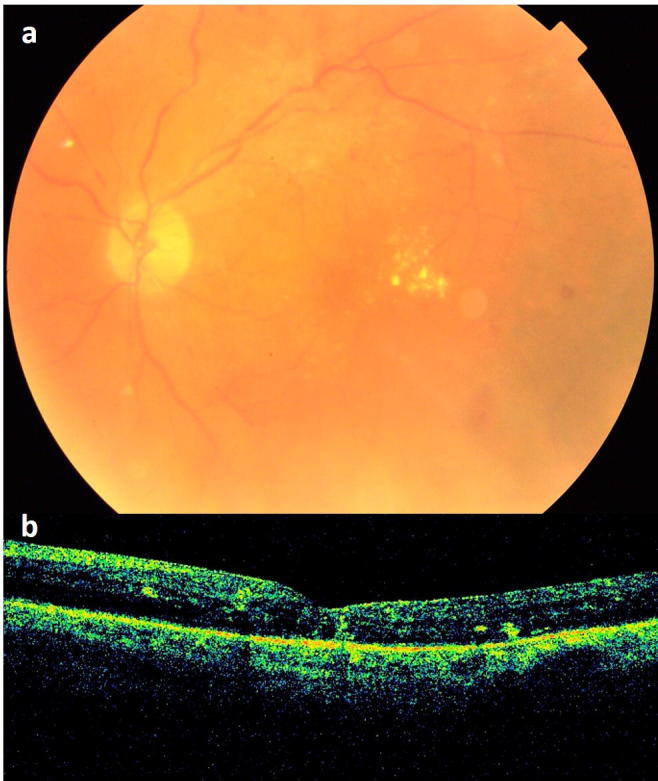
(a) Fundus photograph at the final follow-up of 3 months showing clear media and hard exudates at the macula. (b) Spectral domain optical coherence tomography demonstrating mild macular edema with hard exudates.
Discussion
Endophthalmitis is considered one of the most devastating complications following any intraocular procedure. The rates of endophthalmitis after intravitreal injections have been reported to vary from 0.038% to 0.065% as per large scale meta-analyses.2 Endophthalmitis following an intravitreal DEX implant is extremely uncommon. No case of endophthalmitis was noted after a total of 1830 intravitreal DEX implants administered in the GENEVA study.6 The MEAD study reported a single of acute endophthalmitis following a DEX implant, out of 2928 injections given during the study for DME, with no further details on presentation, management or outcome.1 A recent nationwide case series from France reported 4 cases of endophthalmitis in patients who had received the DEX implant; however, no information was provided regarding the clinical course or outcome.7 A review of literature revealed only four published cases of acute endophthalmitis following an intravitreal DEX implant administered for retinal vascular disorders with details of presentation and management (Table 1).3, 4, 5 All four cases presented within 2 to 3 days of receiving the injection with painful loss of vision. The current case, however, developed symptoms of painless decreased vision at 12 days post injection. While the first two cases were noted to have a hypopyon at presentation, the latter two as well as the current case did not. All cases had varying degrees of vitreous inflammation.
Table 1.
Reported cases of endophthalmitis following intravitreal dexamethasone implant injection.
| Authors, Year published | Age (years), Sex, Indication | Time of presentation | Clinical features | Management | Organism isolated | Final outcome |
|---|---|---|---|---|---|---|
| Marchino T, et al., 20133 | 74, F, CRVO | 2 days | Pain, redness, DOV, PL+, hypopyon, grade 4 vitreous opacity | IVAB, followed by PPV + implant removal (“Vitreotome tip”) | Alloiococcus otitidis | CF 30 cm, macular fibrosis |
| Arıkan Yorgun M, et al., 20144 | 58, M, BRVO | 2 days | Painful DOV, HM, hypopyon, no red reflex | PPV + implant removal (forceps) + IVAB, repeat PPV after 2 days | Negative | 3/60 |
| Esen E, et al., 2015 Case 15 | 68, F, DME | 3 days | Pain, redness, DOV, HM, 4 + AC cells, poor red reflex | IVAB, repeated after 3 days | Negative | 6/60 |
| Esen E, et al., 2015 Case 25 | 63, F, BRVO | 3 days | Pain, redness, DOV, HM, 4 + AC cells, vitreous inflammation | IVAB, repeated after 3 days | Negative | 6/36 |
| Current case | 70, M, DME | 12 days | DOV, no pain, CF close to face, no hypopyon, grade 3 vitreous opacity, fragmented implant | IVAB, followed by PPV + implant removal (forceps/cutter) | Coagulase negative Staphylococcus | 6/18 |
M - male, F - female, CRVO - central retinal vein occlusion, BRVO - branch retinal vein occlusion, DME - diabetic macular edema, DOV - diminution of vision, PL - perception of light, HM - hand motions, CF - counting fingers, AC - anterior chamber, IVAB - intravitreal antibiotics, PPV - pars plana vitrectomy.
The Endophthalmitis Vitrectomy Study (EVS) outlined the recommendations for management of acute endophthalmitis following cataract surgery;8 however, the same cannot be extrapolated to endophthalmitis following intravitreal injections, due to differences in clinical aspects.9 Also, endophthalmitis after an intravitreal DEX implant should be delineated from that associated with anti-vascular endothelial growth factor (VEGF) agents,2 because of its pharmacological properties. It has been reported that the use of intravitreal steroids is associated with significantly increased odds of approximately 7 times higher than that of anti-VEGF agents for post injection endophthalmitis.9 This may be attributed to the higher gauge of needle employed while giving intravitreal steroids (27- or 25-gauge for triamcinolone, and 22-gauge for the DEX implant as compared to 30- or 32-gauge for anti-VEGF agents) resulting in a larger wound tract and hence easier bacterial penetration into the vitreous. Also, the immunosuppressive nature of steroids may contribute to this difference.10
In the absence of current, evidence-based guidelines for management of post-injection endophthalmitis, varied treatment approaches have been carried out for endophthalmitis following DEX implant as well.3, 4, 5 Pars plana vitrectomy with removal of the implant has been recommended as it has been hypothesized that the infectious agent may be stationed inside the device and the steroid itself may weaken the host’s defense mechanisms.3, 4 On the other hand, two cases have also been successfully managed by administration of intravitreal antibiotics, without vitrectomy or implant removal.5 These cases had better presenting visual acuity and less severe clinical features at presentation and were culture-negative. The current case showed minimal response to intravitreal antibiotics and was a culture-positive case, and hence was subjected to vitrectomy and implant removal, despite having a relatively less severe initial presentation. This obviates the risk of the implant acting as a permanent reservoir of organisms leading to recurrences. Coagulase negative Staphylococcus is the commonest bacterial isolate in post-injection endophthalmitis.9 During DEX implant injection, the 22-gauge needle tip may drag along, transport, and inoculate the microbes of the conjunctival flora into the vitreous cavity.5
Removal of the DEX implant from the vitreous cavity can be a challenging procedure. A case report of intractable glaucoma following intravitreal DEX implant described its removal with a 23-gauge vitreous cutter two months after injection;11 however, this was not possible in the current case. Differing times of removal could have accounted for this. Removal of the DEX implant has been reported mainly following its anterior migration in aphakic eyes. Several authors have encountered difficulty in repositioning or removing the implant with forceps owing to its brittle and friable nature, especially when it has been present in the eye for some time.12 A similar disintegration of the implant into smaller pieces was faced in this case, while grasping it with intravitreal forceps.
The DEX implant was noted to be fractured into two pieces at presentation in this case. Intra-operatively, two additional fragments were discovered. Desegmentation of the DEX implant immediately following injection is a fairly common event, though now recognized as being innocuous, without any alteration in efficacy or complications.13 None of the previously reported cases of endophthalmitis after intravitreal DEX implant had a fractured implant.
Endophthalmitis following intravitreal DEX implant represents an uncommon but serious complication of a procedure that is frequent in medical retina practice. Evidence based guidelines for the management of endophthalmitis following intravitreal injections, including slow-release devices are lacking. Based on the favorable visual and anatomical outcome achieved in our patient, pars plana vitrectomy with removal of the implant and intravitreal antibiotics can be recommended as a therapeutic option for endophthalmitis occurring after an intravitreal DEX implant. However, removal of the implant can be a challenging procedure.
Conflict of interest
The author declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Boyer D.S., Yoon Y.H., Belfort R., Jr., Bandello F., Maturi R.K., Augustin A.J. Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 2.McCannel C.A. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31:654–661. doi: 10.1097/IAE.0b013e31820a67e4. [DOI] [PubMed] [Google Scholar]
- 3.Marchino T., Vela J.I., Bassaganyas F., Sánchez S., Buil J.A. Acute-onset endophthalmitis caused by alloiococcus otitidis following a dexamethasone intravitreal implant. Case Rep Ophthalmol. 2013;4:37–41. doi: 10.1159/000348809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ar.ıkan Yorgun M., Mutlu M., Toklu Y., Cakmak HB., Cağıl N. Suspected bacterial endophthalmitis following sustained-release dexamethasone intravitreal implant: a case report. Korean J Ophthalmol. 2014;28:275–277. doi: 10.3341/kjo.2014.28.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esen E., Sizmaz S., Demircan N. Two cases of acute endophthalmitis after intravitreal dexamethasone implant injection. Retin Cases Brief Rep. 2015 doi: 10.1097/ICB.0000000000000213. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Haller J.A., Bandello F., Belfort R., Jr, Blumenkranz M.S., Gillies M., Heier J. Ozurdex GENEVA Study Group; Li J: Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Dossarps D., Bron A.M., Koehrer P., Aho-Glélé L.S., Creuzot-Garcher C. FRCR net (FRenCh Retina specialists net). Endophthalmitis after Intravitreal Injections: Incidence, Presentation, Management, and Visual Outcome. Am J Ophthalmol. 2015;160(17–25):e1. doi: 10.1016/j.ajo.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Endophthalmitis Vitrectomy Study Group Results of the endophthalmitis vitrectomy study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–1496. [PubMed] [Google Scholar]
- 9.Yu C.Q., Ta C.N. Prevention and treatment of injection-related endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2014;252:1027–1031. doi: 10.1007/s00417-014-2644-0. [DOI] [PubMed] [Google Scholar]
- 10.VanderBeek B.L., Bonaffini S.G., Ma L. The association between intravitreal steroids and post-injection endophthalmitis rates. Ophthalmology. 2015;122(2311–2315):e1. doi: 10.1016/j.ophtha.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumari N., Parchand S., Kaushik S., Singh R. Intractable glaucoma necessitating dexamethasone implant (Ozurdex) removal and glaucoma surgery in a child with uveitis. BMJ Case Rep. 2013 Dec;5:2013. doi: 10.1136/bcr-2013-201293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khurana R.N., Appa S.N., McCannel C.A., Elman M.J., Wittenberg S.E., Parks D.J. Dexamethasone implant anterior chamber migration: risk factors, complications, and management strategies. Ophthalmology. 2014;121:67–71. doi: 10.1016/j.ophtha.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Bhagat R., Zhang J., Farooq S., Li X.Y. Comparison of the release profile and pharmacokinetics of intact and fragmented dexamethasone intravitreal implants in rabbit eyes. J Ocul Pharmacol Ther. 2014;30:854–858. doi: 10.1089/jop.2014.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]