Abstract
Purpose
To evaluate the safety and effectiveness of accelerated corneal collagen crosslinking (CXL) in pediatric keratoconus.
Design
Prospective non-randomized observational study.
Methods
33 eyes of 25 children with keratoconus were included. The corneal epithelium was mechanically removed. Next, riboflavin/hydroxypropyl methylcellulose solution) was applied for 10 min. Accelerated CXL (10 mW/cm2 for 9 min), was accomplished. Visual acuity, slit lamp examination, refraction, keratometry readings, pachymetry, anterior and posterior elevations, average progression indices, and Q values were recorded. The follow-up visits were scheduled on one day, 3 days, 7 days, one month and then on 3, 6, 12 months after the procedure.
Results
It was statistically significant improvement of the mean UAVA, AVA, and the mean corneal astigmatism (P < .0001). The mean corneal thickness showed a significant reduction. The preoperative mean K max reading was reduced from 49.12 ± 3.7 D preoperatively to 47.9 ± 3.7 D at 12 months. The mean max anterior elevation, average progression index and Q value showed statistically significant improvement. No significant impact on posterior elevation was recorded. Serious complications were not encountered in this study.
Conclusion
Accelerated CXL shows a stabilization and beneficial clinical outcomes in pediatric keratoconus. It seems an effective and safe procedure in this age group. Effects of accelerated CXL on the posterior corneal surface will need further evaluation.
Keywords: Keratoconus, Accelerated, Cross-linking, Pediatric ophthalmology, Posterior corneal elevation
Introduction
Visual impairment in children has a broad negative impact on their quality of life and of course will affect their social and educational development.1
Keratoconus is a degenerative disorder characterized by ectasia and thinning of the axial or para-axial region of the cornea resulting in irregular astigmatism, myopia and scarring with mild to marked impairment in the quality of vision.2 Pediatric keratoconus displays a higher ratio of keratoconus eyes being about 88%. It is also diagnosed in more advanced stage (stage IV) comparing to adult patients (27.8% versus 7.8%).3, 4 Hence, keratoconus in children progresses aggressively with a higher rate of acute hydrops as compared to the adult group.1, 3, 5
Early treatment to stop the progression and to avoid future keratoplasty is of greater benefit in long run in those patients. For this reason, corneal cross-linking (CXL) has been widely utilized and evaluated in children after its success in adult keratoconus patients.6, 7, 8
The only treatment that is believed to be able to stop or decrease the keratoconus progression is collagen cross-linking.9 The overall treatment time of CXL is still a drawback of this process, so a reduction in the operation time and shorter UVA exposure time (accelerated UVA exposure) to a few minutes are currently being investigated in the pediatric group for better cooperation and comfort.10, 11
Since there are few published researches in this topic, this study is designed to evaluate this new modification of standard corneal collagen cross-linking (accelerated cross-linking) in treating the pediatric keratoconus and to estimate its clinical outcomes during one year follow-up.
Subjects and methods
Children with confirmed keratoconus were enrolled in this prospective study during the period from July 2013 to January 2015 at Mansoura Ophthalmic Center and Al-Mostakbel Ophthalmic Center, Mansoura, Egypt.
Inclusion criteria
Completely clear cornea with maximum keratometry (K max) reading less than 60 D, corneal thickness more than 400 microns at the thinnest location, and children (less than 18 years and older than 6 years) with the absence of any other ocular or systemic diseases.
Preoperative examination
Slit lamp examination, unaided visual acuity (UAVA), aided visual acuity (AVA) measurement, corneal tomography by Scheimpflug camera (Pentacam (Oculus, Wetzlar, Germany)), corneal thickness measurement by Scheimpflug camera and confirmed by a non-contact specular microscope (Tomy EM-3000). All patients underwent the above tests at baseline and at all follow-up visits. Dilated fundus examination was done also using indirect ophthalmoscope and/or non-contact lens (Volk 90). Topical steroid and anti-allergic eyedrops were used in any eye with any signs of allergic-related surface inflammation till became preoperatively quiet.
Collagen cross-linking technique
The procedure was conducted under sterile conditions in the operating room of Al-Mostakbel ophthalmic center –Mansoura, Egypt. All the procedure steps were conducted with topical anesthesia (benoxinate hydrochloride 0.4% -Benox 0.4%; Eipico Inc., Cairo, Egypt), while additional sedation with monitored anesthesia care was needed for only 7 young and uncooperative children. After installation of the topical anesthetic eye drops every 5 min for 30 min, the epithelium was mechanically scraped within the central 8 mm diameter area using a Beaver blade. Next, riboflavin ophthalmic solution (0.1% riboflavin, Saline with hydroxypropyl methylcellulose (HPMC) solution) (VibeX Rapid™, Avedro, USA) was applied every 2 min for 10 min until the stroma was completely saturated. Ultraviolet A irradiation, was accomplished at an irradiance of 10 mW/cm2 for 9 min and 5 cm distance from the cornea using a commercially available UVA system CBM Vega 10 mW X-Linker (UV Emitter Mod. VEGA C.S.O. srlViadegliStagnacci, 12/E 50018 Scandicci, Firenze, Italy). During irradiation, Vibex Rapid solution was applied every 2 min to secure saturation.
Vibex Rapid with its content of HPMC provides faster diffusion into the corneal stroma (twice the traditional riboflavin) and so decreasing the pre-irradiation saturation time under 10 min. In addition, Vega CBM X-Linker with an irradiation power of 10 mW (accelerated CXL) and an irradiation phase that lasts 9 min participate to shorten the total operative time. This will be convenient for children and young patients. The proper fixation of patients was in mind to avoid peripheral irradiation of limbal stem cells.
Postoperative care
Patients received Vigamox 0.5% eyedrops (moxifloxacin hydrochloride 0.5%) five times per day for one week. A soft contact lens was used till complete re-epithelialization. Then, combined topical steroid-antibiotic eyedrops (Dexamethasone and Tobramycin) were prescribed 4 times a day for another week and then tapered over the next three weeks. Carboxymethylcellulose - Sodium (CMC) (0.5%) eye lubricant also was given 6 times daily for one month.
Follow-up
Follow-up was first done one day postoperatively, 3 days for assurance of completion epithelization and contact lens removal, then after one week for prescription of steroid-antibiotic drops (Tobramycin and Dexamethasone) and one month for assessment of corneal haze. Unaided visual acuity, aided visual acuity, refractive changes, tomographic changes, and pachymetry were recorded at 3, 6, and 12 months. At least three measurements were performed to improve intersession repeatability of pentacam.
This study was registered and reviewed by the Ethics Committee, Faculty of Medicine, Mansoura University. An approval from the Ethical Committee was taken and adhered to the Declaration of Helsinki (R/16.03.44). Written informed consent was obtained from all patients' parents after the nature of the procedure and its possible consequences were clearly explained.
Data analysis
The statistical analysis was carried out using the SPSS (Statistical Package for Social Science) program, version 16. Test for normal distribution of data was performed. Quantitative continuous data were summarized in mean and standard deviation. Preoperative and postoperative means were compared using the paired t-test. All values are declared as a mean ± standard deviation in tables. P value < 0.05 was deemed statistically valuable.
Results
Thirty-three eyes of 25 children (8 bilateral and 17 unilateral) with confirmed keratoconus were included in this prospective study. The mean age was 12 ± 2.02 years (range: 8–15 years). Eighty percent of the study population was boys, and 20% was girls. Of these, 29 eyes showed progression, as defined by an increase in anterior surface (K max) readings of at least 1.00 diopter (D) in serial corneal topographies over a maximum of one year.
Unaided visual acuity (UAVA): Visual acuity was measured using the Landolt C or Tumbling E metric charts and transformed into logarithm of the Minimum Angle of Resolution (Log MAR) for further statistical analysis as recommended by Holladay. 12. Table 1 summarizes the UAVA and AVA data, expressed in Log MAR and covering 12-month follow-up period. There was a statistically significant improvement from the preoperative values (P < 0.001). The preoperative mean UAVA was 0.54 ± 0.2 which changed to 0.46 ± 0.2 at 3 months, 0.35 ± 0.23 at 6 months and 0.34 ± 0.22 at one year. Eight eyes (24.2%) maintained the preoperative UAVA; twelve eyes (36.4%) gained one line, eight eyes (24.2%) gained two lines and five (15.2%) eyes gained three to four lines. None of the eyes lost lines of the preoperative UAVA.
Table 1.
The mean UAVA &AVA and its P values.
| Visual acuity | Preoperative Mean ± SD Min-max |
3 months Mean ± SD Min-max |
P1 value | 6 months Mean ± SD Min-max |
P2 value | 12 months Mean ± SD Min-max |
P3 value |
|---|---|---|---|---|---|---|---|
| Unaided | 0.54 ± 0.2 (0.2–1.00) |
0.46 ± 0.20AC (0.2–1.00) |
0.029* | 0.35 ± 0.23A (0–0.8) |
<0.001* | 0.34 ± 0.22C) 0–0.8) | <0.001* |
| Aided | 0.36 ± 0.20 | 0.31 ± 0.17AC | 0.06 | 0.19 ± 0.15A | <0.001* | 0.17 ± 0.13C | <0.001* |
| (0–0.8) | (0–0.6) | (0–0.6) | (0–0.5) |
Paired samples t test. ABC: Similar letters indicate statistically significant differences (p < 0.05).
P1: difference between preoperative and after 3 month.
P2: difference between preoperative and 6 month.
P3: difference between preoperative and 12 months.
SD: Standard deviation; UAVA: unaided visual acuity; AVA: Aided visual acuity.
Aided visual acuity (AVA): The preoperative AVA, at 3 and 6 months, and one-year postoperative examinations is shown in Table 1. There was a statistically significant (P < 0.001) improvement in AVA between the preoperative and 1-year evaluations. Out of the 33 eyes were evaluated at one year, 17 eyes (51.5%) experienced gained one line of AVA and 10 eyes (30.3%) gained 2–4 lines. Five eyes (15.2%) experienced no change in AVA. There was no statistically significant change from the 6-months examination values and one-year values (P > 0.05). The preoperative mean AVA was 0.36 ± 0.2 and changed to 0.31 ± 0.17 at 3 months, 0.19 ± 0.13 at 6 months and 0.17 ± 0.15 at one year.
Corneal Astigmatism: Corneal astigmatism values at the end of one-year showed a significant change. The values at 1-year examination (mean 2.01 ± 0.8 D) were statistically significantly less than the preoperative values (mean 2.4 ± 1.01 D) (P < 0.001). The changes in refractive errors at 1-year compared with the preoperative baseline are presented in Table 2.
Table 2.
The mean corneal astigmatism and its P values.
| Corneal astigmatism | Preoperative Mean ± SD Min-max |
3 months Mean ± SD Min-max |
P1 value | 6 months Mean ± SD Min-max |
P2 value | 12 months Mean ± SD Min-max |
P3 value |
|---|---|---|---|---|---|---|---|
| 2.4 ± 1.01 | 2.6 ± 1.00AC | <0.001* | 2.17 ± 0.8AB | <0.001* | 2.01 ± 0.8BC | <0.001* | |
| 1.10–5.4 | 1.3–5.6 | 1.1–4.9 | 0.9–4.4 |
Paired samples t test. ABC: Similar letters indicate statistically significant differences (p < 0.05).
P1: difference between preoperative and after 3 months.
P2: difference between preoperative and 6 months.
P3: difference between preoperative and 12 months.
SD: Standard deviation.
Keratometry: There was a statistically significant decrease in the mean anterior surface keratometry in steep and flat axes with flattening of about 0.76 D in the mean K1, 1.1 D in mean K2 and 1.2 D in the mean K max at the end of the one-year follow-up. The preoperative mean K1 value was (44.38 ± 2.07 D) and changed to (43.6 ± 2.09 D) at one year (P < 0.001). The mean K2 value decreased from (46.5 ± 2.4 D) preoperatively to (45.4 ± 2.00D) and the mean K max changed from (49.12 ± 3.7D) to (47.9 ± 3.7 D) (P < 0.001). Table 3 shows the topographic changes with a reduction in K readings from pre-operative value to one year.
Table 3.
The mean K readings and its P values.
| Preoperative Mean ± SD Min-max |
3 months Mean ± SD Min-max |
P1 value | 6 months Mean ± SD Min-max |
P2 value | 12 months Mean ± SD Min-max |
P3 value | |
|---|---|---|---|---|---|---|---|
| K1 | 44.38 ± 2.07 | 44.66 ± 2.01AC | <0.001* | 43.8 ± 1.98AB | <0.001* | 43.6 ± 2.09CB | <0.001* |
| (41.0–49.3) | (41.7–50.10) | (40.6–49.0) | (40.5–49.8) | ||||
| K2 | 46.5 ± 2.4 | 46.76 ± 2.4AC | 0.002* | 45.57 ± 1.97AB | <0.001* | 45.4 ± 2.00BC | <0.001* |
| (43.4–55.4) | (43.9–55.1) | (42.2–50.5) | (42.2–50.5) | ||||
| K max | 49.12 ± 3.7 | 49.3 ± 3.9AC | 0.007* | 48.12 ± 3.7AB | <0.001* | 47.9 ± 3.7BC | <0.001* |
| (44.5–59.5) | (44.5–60.0) | (42.6–59.4) | (42.6–59.2) |
Paired samples t test. ABC: Similar letters indicate statistically significant differences (p < 0.05).
P1: difference between preoperative and after 3 months.
P2: difference between preoperative and 6 months.
P3: difference between preoperative and 12 months.
SD: Standard deviation; K max: maximum keratometric value.
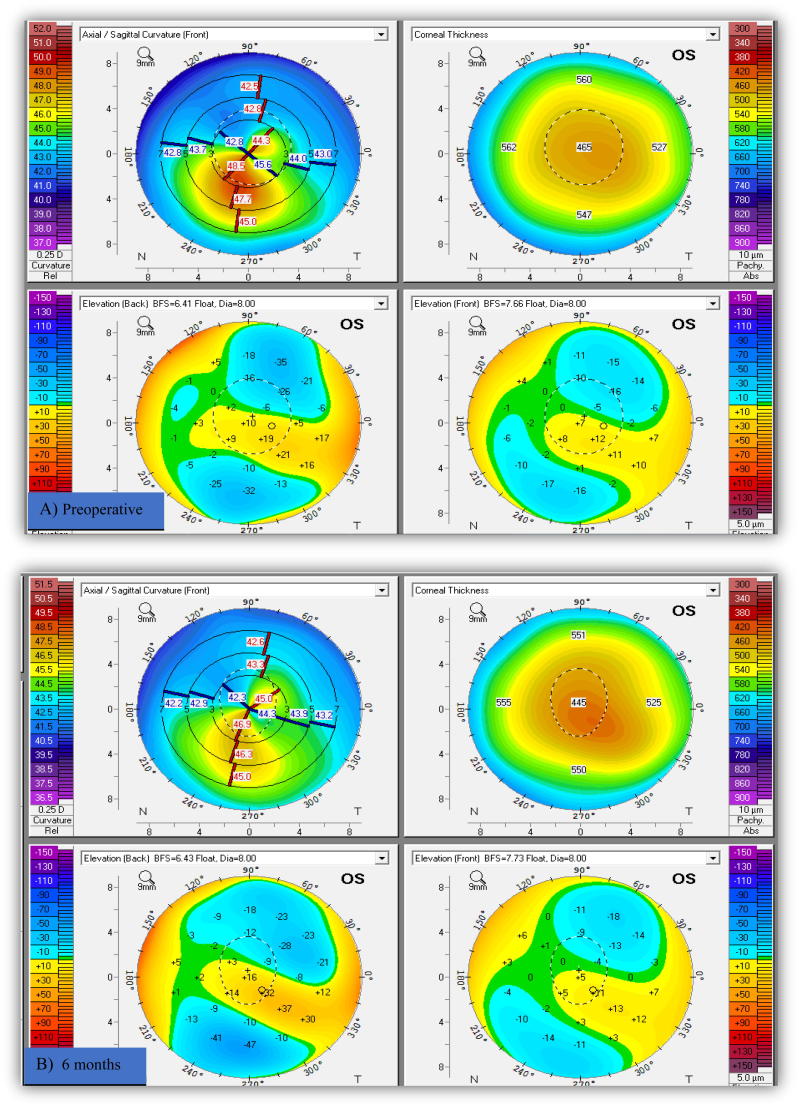
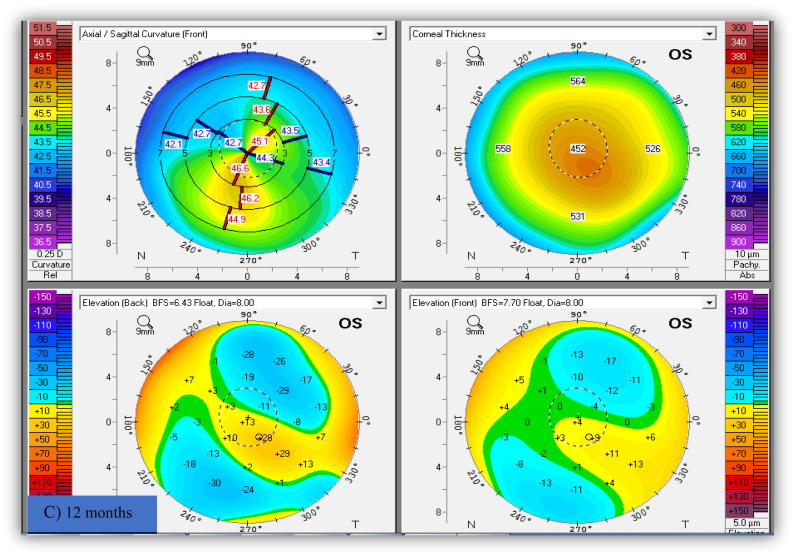
Preoperative K max ranged from 44.5 to 59.5 D (keratoconus grade I to III according to Amsler-Krumeich). Ten eyes (30.3%) maintained the preoperative or showed minimal change (<0.5 D) in K max front values. Reduction in K max front values was (0.5 D-1.4D) in eleven eyes (33.3%) and from 1.5 D to 3 D in twelve eyes (36.4%). These statistically significant changes in K readings denoted the short term stability of the topographic patterns. Progression of corneal steepening from the preoperative value was not observed in any of the study eyes. Fig. 1.
Fig. 1.
Pentacam image of the left keratoconus eye in a boy (11 years old) shows improvement of keratometric indices and regularization of corneal shape without disease progression. (A) Pre-accelerated crosslinking, (B) 6 months after, and (C) 12 months after crosslinking.
Corneal thickness: Central corneal thickness values were measured preoperatively, 3-months, 6-months and one-year postoperatively Table 4. At 3-months postoperative examination, there was a significant reduction in pachymetry (P < 0.001). Central pachymetry reduced from 486.9 ± 26.5 μm preoperatively to 474.8 ± 28.6 μm at 3 months. The one-year evaluation showed a decrease in the pachymetric values to 467.6 ± 27.9 μm and still there was a significant reduction in pachymetry than preoperative values (P < 0.001). As regards thinnest location values, there was a significant reduction in pachymetry (P < 0.001). The mean thinnest location values reduced from 472.15 ± 31.3 μm preoperatively to 456.09 ± 33.2 μm at 3 months, 449.5 ± 32.0 μm at 6 months and to 448.2 ± 32.2 μm at 12 months. Maximum anterior surface elevation (within 6 mm) decreased significantly after 6 and 12 months (P < 0.001). The mean values changed from 17.38 ± 6.7 μm preoperatively to 15.3 ± 6.5 μm postoperatively. This result cannot be confirmed in posterior elevation values. Also, both average progression indices and Q values showed significant changes at one-year postoperatively, Table 5, Table 6.
Table 4.
The mean corneal thickness and its P values.
| Corneal thickness | Preoperative Mean ± SD Min-max |
3 months Mean ± SD Min-max |
P value | 6 months Mean ± SD Min-max |
P value | 12 months Mean ± SD Min-max |
P value |
|---|---|---|---|---|---|---|---|
| Central corneal thickness | 486.9 ± 26.5 (411–534) |
474.8 ± 28.6AC (393–525) |
<0.001* | 470.2 ± 28.3AB (390–521) |
<0.001* | 467.6 ± 27.9CB (390–520) |
<0.001* |
| Thinnest location | 472.15 ± 31.3 | 456.09 ± 33.2AC | <0.001* | 449.5 ± 32.0AB | <0.001* | 448.2 ± 32.2 CB | <0.001* |
| (405–529) | (386–516) | (385–511) | (380–511) |
Paired samples t test. ABC: Similar letters indicate statistically significant differences (p < 0.05).
P1: difference between preoperative and after 3 months.
P2: difference between preoperative and 6 months.
P3: difference between preoperative and 12 months.
SD: Standard deviation.
Table 5.
The mean anterior & posterior elevations and its P values.
| Preoperative Mean ± SD Min-max | 3 months Mean ± SD Min-max | P1 value | 6 months Mean ± SD Min-max | P2 value | 12 months Mean ± SD Min-max | P3 value | |
|---|---|---|---|---|---|---|---|
| Anterior elevation | 17.38 ± 6.7 | 18.2 ± 6.5AC | 0.003* | 15.6 ± 6.5AB | <0.001* | 15.3 ± 6.5CB | <0.001* |
| (6.0–43.0) | (8.0–42.0) | (6.0–39.0) | (6.0–38.0) | ||||
| Posterior elevation | 30.09 ± 14.0 | 32.5 ± 15.7AC | 0.01* | 30.5 ± 15.3A | 0.6 | 30.12 ± 15.5C | 0.9 |
| (13.0–72.0) | (13.0–78.0) | (11.0–75.0) | (11.0–75.0) |
Paired samples t test. ABC: Similar letters indicate statistically significant differences (p < 0.05).
P1: difference between preoperative and after 3 months.
P2: difference between preoperative and 6 months.
P3: difference between preoperative and 12 months.
SD: Standard deviation.
Table 6.
The mean average progression index & Q value and its P values.
| Preoperative Mean ± SD Min-max |
3 months Mean ± SD Min-max |
P1 value | 6 months Mean ± SD Min-max |
P2 value | 12 months Mean ± SD Min-max |
P3 value | |
|---|---|---|---|---|---|---|---|
| Average progression index | 1.7 ± 0.5 | 1.6 ± 0.5AC | <0.001* | 1.4 ± 0.46AB | <0.001* | 1.37 ± 0.46CB | <0.001* |
| 1.11–2.98 | 1.0–2.90 | 0.9–2.6 | 0.9–2.5 | ||||
| Q value | 0.72 ± 0.14 | 0.75 ± 0.13AC | 0.001* | 0.69 ± 0.13AB | <0.001* | 0.68 ± 0.13BC | <0.001* |
| 0.5–1.07 | 0.52–1.07 | 0.49–0.98 | 0.46–0.97 |
Paired samples t test. ABC: Similar letters indicate statistically significant differences (p < 0.05).
P1: difference between preoperative and after 3 months.
P2: difference between preoperative and 6 months.
P3: difference between preoperative and 12 months.
SD: Standard deviation.
Regarding complications, visual threatening complications were not found in this study denoting the safety of this procedure. None of the cases lost lines of AVA. Transient subepithelial haze was noted in 93.9% of the study eyes which cleared within 4–6 weeks in most cases. Two eyes only developed marked anterior stromal haze (grade 4) that decreased gradually with topical steroids. Complete recovery occurred at 4 months in one case and the other at 6-month postoperative, without clinically significant opacity. It has a maximum intensity at 1 month, and it significantly decreases after 3 months till complete disappearance. The incidence of corneal haze or its course did not correlate with VA, K max values, or corneal thickness.
Discussion
Keratoconus progression in children is aggressive and may not stop on its own,1, 13 and treating patients at an earlier age could be of greater benefit than waiting until patients are older and have to do corneal transplantation.14 The timing of CXL remains a topic of controversy, and in the current study, all eyes with diagnosed keratoconus were operated even progressive or not. This is for many reasons. Firstly, if we compare the significant complications of CXL with the problems resulting from the disease progression and the risk of the keratoplasty in young age, we will find the comparison in favor of early CXL without waiting for the disease progression. Secondly, the problem is more complex in developing countries like Egypt as most of the patients belong to low socioeconomic environments and thus don’t care about the periodic follow-up as well as the high cost of keratoplasty operation.
Corneal cross-linking is one of the interesting topics in corneal surgery, with several recent modifications of the original standard Dresden protocol still under investigation. Accelerated CXL is one of the exciting modifications of the original technique but with few published results.15, 16, 17 Many international studies recommended that cross-linking should be the primary choice in young patients with keratoconus,18, 19 but there are few published reports on the results of accelerated CXL in the pediatric group.10, 11
In the current study, there was a statistically significant improvement in the mean UAVA, AVA, and corneal astigmatism one year after the surgery. However, there was some fluctuation of the visual acuity in the first month; I think that this was probably due to epithelial removal, associate pain, photophobia, and corneal edema. Then gradual continuous improvement occurred mainly from 3 to 6 month followed by slow mild improvement from 6 to 12 years. In spite of this, there was a decrease in corneal astigmatism, but it was not correlated with the improvement of VA. Recently, published results in pediatric CXL were shown similar significant improvement in both UCVA and BCVA which were considered parameters of procedure success.6, 7, 11, 20 On the other hand, Waszczykowska and Jurowski had different results in 2-year follow-up study after the accelerated CXL. They did not note any significant improvement in visual acuity, and also they did not report any statistically significant differences in pre- and postoperative astigmatism.17 The mechanism by which CLX improves the vision is not known completely. It might be due to decrease in corneal steepness, astigmatism, and also an improvement in different topographic indices.21 However, BCVA is not an accurate parameter of keratoconus progression as it changes markedly according to pupil size and which part of the cornea is evaluated.22
It was believed that K-max a remarkable indicator of CXL success and decreased significantly after the CXL procedure (up to 2.01 D).23. Also, it is the most regularly used parameter to document disease progression.24 The K values achieved clinically significant decrease at the end of one year in the current study. The mean K max value was reduced from 49.12 D to 47.9 D. This significant flatting was recorded in 69.7% of all study cases which was in contrast to Waszczykowska and Jurowski results of accelerated CXL study, where they found a significant decrease in mean K max only in 18.7% of patients.
The current study recorded reduction of 1.2 D in mean K max. These results were comparable to other studies involving the standard CXL procedure6, 8, 25, 26 and studies utilizing accelerated CXL.10, 11
In a different state, K max has been recognized as a poor parameter for both progression and cross-linking efficacy.27 This is because, K max represents the steepest anterior corneal curvature taken from a small area and it fails to reflect the degree of ectasia, and so marked progression can occur with no change in K-max.28
According to some reports, changes of corneal thickness were recorded after the standard CXL procedure. The reasons for the corneal thickness changes after the CXL procedure should be due to the keratocyte restoration process, the rearrangement of the corneal lamellae, anatomic and structural changes of the collagen fibers, and changes in corneal stroma lasting from one year up to 3 years in some cases.29, 30 Corneal ischemia and changes in the arrangement of the new epithelium were also reported possible causes.31 In our study, the corneal pachymetry showed significant thinning in the 12th month after the procedure. These findings are consistent with other studies involving the standard CXL procedure6, 8, 32 and accelerated CXL procedure10, 11. The early thinning may be explained by the compression of collagen fibrils or keratocyte apoptosis, but the cause of the later changes between 6 and 12 months is still unclear.33 On the other hand, a different behavior was recorded by a previous report of non-statistically significant changes.34. The current results also showed significant changes in both central corneal thickness and thinnest points which were paralleled by a decrease in the average progression index after 12 months.
It was reported that pachymetry changes one of the best parameters for documenting the progression of keratoconus.22 But after cross-linking corneal thickness measurements are regularly thinned, thus limiting its value to document early progression.35 Therefore it is difficult to judge this thinning is either due to cross-linking efficacy or due to early disease progression. Anterior corneal surface elevations showed significant deviations (decreased) at the end of one year, but the posterior elevation values didn’t show significant changes (minimally increased). This increase in posterior elevation may explain the visual improvement through reducing corneal dioptric power in patients. Using Pentacam before and after CXL within one year, Grewal et al. didn’t find a significant change in anterior or posterior corneal elevation values.36 In contrast to the current findings, Mirzaei et al. found significant changes in posterior elevation points rather than anterior elevation ones.37 Actually, the elevation data obtained by optical devices such as Pentacam may not be reliable and accurate due to corneal haze artifacts and also the demarcation line may disturb data acquisition by the instrument.38, 39 Since the posterior surface is the least affected by outside forces and changes on the posterior cornea may occur without concurrent anterior changes, posterior elevation maps are so effective for evaluating ectatic change.22 One of the interesting issues in the current results is failure to document posterior elevation improvement and contrariwise increased in spite of anterior parameters improvement. Moreover tomographic-derived pachymetry progression index and corneal asymmetry have been suggested to be more additional valuable methods to document ectatic disease and follow progression.40, 41 In the existing study, there was a significant improvement in average pachymetric progression indices and Q values after CXL as an indicator of improving corneal regularity. Unfortunately, there are few evidenced reports in the literature explaining the changes in anterior, posterior surfaces elevation and other parameters after CXL.42, 43 However - according to our results- I think these changes are in favor that cross-linking treatment can improve most of the corneal parameters not only refraction or keratometryic values. As mentioned above, the artifact effect of the Pentacam in acquiring these types of data should be considered and extra care is needed when analyzing them and for sure we need more studies to evaluate such changes. There was not visual threatening or major complications in the current study. This finding is consistent with other previous reports.10, 11
In conclusion, this study demonstrated significant, rapid and beneficial clinical outcomes of the accelerated CXL in pediatric group. But, it could not prove the accelerated CXL impact on the posterior surface of the cornea despite the improvement and stability of anterior surface parameters. This controversy in the results may partially be explained by the artifacts of the device used for analyzing corneal elevation.
What this study adds
-
•
This study supports the previously reported findings of the safety and efficiency of accelerated CXL in children as a new modification of standard protocol.
-
•
This study fails to demonstrate the positive impact of accelerated CXL on posterior surface elevations within one year. This point needs for further evaluation in future to detect this due to either actual disease progression or just device artifacts.
Conflict of interests
The authors declared that there is no conflict of interest.
Financial disclosure
No financial or proprietary interest in any material or method mentioned.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Ertan A., Muftuoglu O. Keratoconus clinical findings according to different age and gender groups. Cornea. 2008;27:1109–1113. doi: 10.1097/ICO.0b013e31817f815a. [DOI] [PubMed] [Google Scholar]
- 2.Rabinowitz Y.S. Keratoconus. SurvOphthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 3.Al Suhaibani A.H., Al-Rajhi A.A., Al-Motowa S., Wagoner M.D. Inverse relationship between age and severity and sequelae of acute corneal hydrops associated with keratoconus. Br. J. Ophthalmol. 2007;91:984. doi: 10.1136/bjo.2005.085878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Léoni-Mesplié S., Mortemousque B., Touboul D., Malet F., Praud D., Mesplié N. Scalability and severity of keratoconus in children. Am J Ophthalmol. 2012;154:56–62. doi: 10.1016/j.ajo.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Li X., Yang H., Rabinowitz Y.S. Longitudinal study of keratoconus progression. Exp Eye Res. 2007;85:502–507. doi: 10.1016/j.exer.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinciguerra P., Albé E., Frueh B.E., Trazza S., Epstein D. Two-year corneal cross-linking results in patients younger than 18 years with documented progressive keratoconus. Am J Ophthalmol. 2012;154:520–526. doi: 10.1016/j.ajo.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Caporossi A., Mazzotta C., Baiocchi S., Caporossi T., Denaro R., Balestrazzi A. Riboflavin-UVA-induced corneal collagen cross-linking in pediatric patients. Cornea. 2012;31:227–231. doi: 10.1097/ico.0b013e31822159f6. [DOI] [PubMed] [Google Scholar]
- 8.Zotta P.G., Moschou K.A., Diakonis V.F., Kymionis G.D., Almaliotis D.D., Karamitsos A.P. Corneal collagen cross-linking for progressive keratoconus in pediatric patients: a feasibility study. J Refract Surg. 2012;28:793–799. doi: 10.3928/1081597X-20121011-08. [DOI] [PubMed] [Google Scholar]
- 9.Asri D., Touboul D., Fournié P., Malet F., Garra C., Gallois A. Corneal collagen crosslinking in progressive keratoconus: multicenter results from the French National Reference Center for Keratoconus. J Cataract Refract Surg. 2011;37(12):2137–2143. doi: 10.1016/j.jcrs.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Ozgurhan E.B., Kara N., Cankaya K.I., Kurt T., Demirok A. Accelerated corneal cross-linking in pediatric patients with keratoconus: 24-month outcomes. J Refract Surg. 2014 Dec;30(12):843–849. doi: 10.3928/1081597X-20141120-01. [DOI] [PubMed] [Google Scholar]
- 11.Shetty R, Nagaraja H, Jayadev C, Pahuja NK, Kurian Kummelil M, Nuijts RM. Accelerated corneal collagen cross-linking in pediatric patients: two-year follow-up results. Biomed Res Int 2014; 2014:894095. [DOI] [PMC free article] [PubMed]
- 12.Holladay J.T. Visual acuity measurements. J Cataract Refract Surg. 2004;30(2):287–290. doi: 10.1016/j.jcrs.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Reeves S.W., Stinnett S., Adelman R.A., Afshari N.A. Risk factors for progression to penetrating keratoplasty in patients with keratoconus. Am J Ophthalmol. 2005;140:607–611. doi: 10.1016/j.ajo.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Vanathi M., Panda A., Vengayil S., Chaudhuri Z., Dada T. Pediatric keratoplasty. SurvOphthalmol. 2009;54:245–271. doi: 10.1016/j.survophthal.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Touboul D., Efron N., Smadja D., Praud D., Malet F., Colin J. Corneal confocal microscopy following conventional, transepithelial, and accelerated corneal collagen cross-linking procedures for keratoconus. J Refract Surg. 2012;28(11):769–776. doi: 10.3928/1081597X-20121016-01. [DOI] [PubMed] [Google Scholar]
- 16.Mrochen M. Current status of accelerated corneal cross-linking. Ind J Ophthalmol. 2013;61(8):428–429. doi: 10.4103/0301-4738.116075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waszczykowska A., Jurowski P. Two-year accelerated corneal cross-linking outcome in patients with progressive keratoconus. Biomed Res Int. 2015;2015:325157. doi: 10.1155/2015/325157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caporossi A., Mazzotta C., Baiocchi S., Caporossi T. Long-term results of riboflavin ultraviolet A corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149(4):585–593. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Wittig-Silva C1., Whiting M., Lamoureux E., Lindsay RG., Sullivan LJ., Snibson GR. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus preliminary results. J Refract Surg. 2008;24(7):S720–S725. doi: 10.3928/1081597X-20080901-15. [DOI] [PubMed] [Google Scholar]
- 20.Arora R., Gupta D., Goyal J.L., Jain P. Results of corneal collagen cross-linking in pediatric patients. J Refract Surg. 2012;28:759–762. doi: 10.3928/1081597X-20121011-02. [DOI] [PubMed] [Google Scholar]
- 21.Greenstein S.A., Fry K.L., Hersh P.S. Corneal topography indices after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011 Jul;37(7):1282–1290. doi: 10.1016/j.jcrs.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Belin M. Parameters to Document Progression of Keratoconus. The ability to measure how a patient’s disease changes is key. Cataract & Refractive Surgery Today Europe. July/August 2014:20–22.
- 23.Wollensak G., Spoerl E., Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 24.Wittig-silva C., Chan E., Islam F.M., Wu T., Whiting M., Snibson G.R. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;121(4):812–821. doi: 10.1016/j.ophtha.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 25.Chatzis N., Hafezi F. Progression of keratoconus and efficacy of pediatric [corrected] corneal collagen cross-linking in children and adolescents. J Refract Surg. 2012;28(11):753–758. doi: 10.3928/1081597X-20121011-01. [DOI] [PubMed] [Google Scholar]
- 26.Bakshi E, Barkana Y, Goldich Y, Avni I, Zadok D. Corneal cross-linking for progressive keratoconus in children:our experience. Int J Keratoconus Ectatic Corneal Diseases 2012(1):53–55.
- 27.Barbara R., Castillo J.H., Hanna R., Berkowitz E., Tiosano B., Barbara A. Keratoconus expert meeting, London, 2014. J Kerat Ect Cor Dis. 2014;3(3):141–158. [Google Scholar]
- 28.Mahmoud A.M., Nuñez M.X., Blanco C., Koch D.D., Wang L., Weikert M.P. Expanding the cone location and magnitude index to include corneal thickness and posterior surface information for the detection of keratoconus. Am J Ophthalmol. 2013;156(6):1102–1111. doi: 10.1016/j.ajo.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Mazzotta C., Traversi C., Baiocchi S., Caporossi O., Bovone C., Sparano M.C. Corneal healing after riboflavin ultraviolet-A collagen cross-linking determined by confocal laser scanning microscopy in vivo: early and late modifications. Am J Ophthalmol. 2008;146(4):527–533. doi: 10.1016/j.ajo.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 30.Croxatto J.O., Tytiun A.E., Argento C.J. Sequential in vivo confocal microscopy study of corneal wound healing after cross-linking in patients with keratoconus. J Refract Surg. 2010;26(9):638–645. doi: 10.3928/1081597X-20091111-01. [DOI] [PubMed] [Google Scholar]
- 31.Holopainen J.M., Krootila K. Transient corneal thinning in eyes undergoing corneal cross-linking. Am J Ophthalmol. 2011;152(4):533–536. doi: 10.1016/j.ajo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Soeters N., van der Valk R., Tahzib N.G. Corneal cross-linking for treatment of progressive keratoconus in various age groups. J Refract Surg. 2014;30(7):454–460. doi: 10.3928/1081597X-20140527-03. [DOI] [PubMed] [Google Scholar]
- 33.Greenstein S.A., Shah V.P., Fry K.L., Hersh P.S. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37(4):691–700. doi: 10.1016/j.jcrs.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 34.Buzzonetti L., Petrocelli G. Transepithelial corneal cross-linking in pediatric patients: early results. J Refract Surg. 2012;28(11):763–767. doi: 10.3928/1081597X-20121011-03. [DOI] [PubMed] [Google Scholar]
- 35.Sefic Kasumovic S, Racic-Sakovic A, Kasumovic A, Pavljasevic S, Duric-Colic B, Cabric E, Mavija M, Lepara O, Jankov M. Assessment of the tomographic values in keratoconic eyes after collagen crosslinking procedure. Med Arch. 2015; 69(2):91–94. [DOI] [PMC free article] [PubMed]
- 36.Grewal D.S., Brar G.S., Jain R., Sood V., Singla M., Grewal S.P. Corneal collagen crosslinking using riboflavin and ultraviolet-A light for keratoconus: one-year analysis using Scheimpflug imaging. J cataract Refract Surg. 2009;35(3):425–432. doi: 10.1016/j.jcrs.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 37.Mirzaei M., Mortazavi S.Z., Taheri N., LotfiSadigh A., Najafi A. Effects of collagen cross-linking on the corneal optical and topographic characteristics in progressive keratoconus. AdvOphthalmol Vis Syst. 2015;2(3):00043. [Google Scholar]
- 38.Koller T., Iseli H.P., Hafezi F., Vinciguerra P., Seiler T. Scheimpflug imaging of cornea after crosslinking. Cornea. 2009;28(5):510–515. doi: 10.1097/ICO.0b013e3181915943. [DOI] [PubMed] [Google Scholar]
- 39.Koller T., Pajic B., Vinciguerra P., Seiler T. Flattening of the cornea after collagen crosslinking for kertoconus. J Cataract Refract Surg. 2011;37(8):1488–1492. doi: 10.1016/j.jcrs.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 40.Ambrósio R., Jr, Caiado A.L., Guerra F.P., Louzada R., Roy A.S., Luz A. Novel pachymetric parameters based on corneal tomography for diagnosing keratoconus. J Refract Surg. 2011;27(10):753–758. doi: 10.3928/1081597X-20110721-01. [DOI] [PubMed] [Google Scholar]
- 41.Kanellopoulos A.J., Asimellis G. Revisiting keratoconus diagnosis and progression classification based on evaluation of corneal asymmetry indices, derived from Scheimpflug imaging in keratoconic and suspect cases. Clin Ophthalmol. 2013;7:1539–1548. doi: 10.2147/OPTH.S44741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinberg J., Ahmadiyar M., Rost A., Frings A., Filev F., Katz T. Anterior and posterior corneal changes after crosslinking for keratoconus. Optom Vis Sci. 2014;91(2):178–186. doi: 10.1097/OPX.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 43.Hassan Z., Modis L., Szalai E., Berta A., Nemeth G. Scheimpflug imaged corneal changes on anterior and posterior surfaces after collagen cross-linking. Int J Ophthalmol. 2014;7(2):313–316. doi: 10.3980/j.issn.2222-3959.2014.02.21. [DOI] [PMC free article] [PubMed] [Google Scholar]