Abstract
Cataract is a principal cause of blindness in the world and is characterized by clouding of eye’s natural lens. Surgery is the major therapeutic step taken to cure cataract; however, it is having its own limitations and complications such as iris prolapse, raised IOP, infection, cystoid macular edema and posterior capsular opacification (PCO). So world is looking toward more robust and natural ways to prevent cataract. One of the important factors that can play a role in prevention of any and many diseases is diet of the people. The inclusion of certain naturally occurring food and nutraceuticals is coming up as a best alternative for curing cataract because of their presumed safety, potential nutritional and therapeutic effects. Some nutraceuticals can act as an anticataract agent through some or the other molecular mechanism if consumed by normal population deliberately or inadvertently.
Keywords: Cataract, Nutraceutical, Age, Antioxidant, Diabetes
Introduction
Cataract is a principal cause of blindness in the world and occurs due to the clouding of the eye’s natural lens. The proteins in the lens aggregate resulting in clouding of the lens and formation of cataract. As the light cannot pass clearly through the lens, there is some loss of vision. Since new cells cover the outside of lens, the other cells are compacted into the center of the lens resulting in the cataract. Cataract ultimately results in the loss of vision in people over the age of 40 years. The most recent estimates from World Health Organization (WHO) reveal that 47.8% of global blindness is due to cataract. In India cataract is the principal cause of blindness accounting for 62.6% cases of blindness and 77.5% cases of avoidable blindness.1 India is one of the signatories in a program Vision 2020 for elimination of avoidable blindness. It can occur due to aging, infection in newborn babies, injury or poor development prior to birth or during childhood, complications of various diseases and exposure to toxic substances such as UV radiations, corticosteroids and diuretics.
In the early stages of the disease, optimal refractive management and advice on glare reduction can lessen the impact of cataract formation. Surgery is undertaken only in case other measures are no longer adequate for the patient’s visual needs because of its known limitations. Significant intraoperative complications of phacoemulsification in experienced hands are rare. Early postoperative complications include iris prolapse, raised IOP and infection. Cystoid macular edema (CMO) and posterior capsular opacification (PCO) are the most common late complications. So world is looking toward more robust and natural ways to prevent cataract. One of the important factors that can play a role in prevention of any and many diseases is the diet of people.
The inclusion of certain naturally occurring food and nutraceuticals is coming up as a best alternative that reminds words of Hippocrates 2500 years ago “Let thy food be medicine and medicine be thy food”. A nutraceutical is the opposite of “junk food and according to the World Health Organization, over 80% of the world’s population (4.3 billion people) rely upon such traditional plant-based systems of medicine as phytochemicals, nutritional constituents or as functional food”.2 The term “Nutraceutical” was coined in 1979 by Stephen De Felice and is defined “as a food or part of diet with medical or health benefits, including the prevention and treatment of disease”. Nutraceuticals may be isolated nutrients, dietary supplements, genetically engineered “designer” food, traditional herbal product and processed products such as cereals, soups, and beverages. Plant derived nutraceuticals/functional foods have received considerable attention because of their presumed safety, potential nutritional and therapeutic effects. This renewed interest in nutraceuticals reflects the fact that consumers are aware about epidemiological studies which indicate the role of a specific diet or component of the diet in association with a lower risk of certain diseases. This review is about the hypothesis behind the mechanism of action of various nutraceuticals in prevention of cataract. Authors have compiled a list of commonly used vegetables, fruits, nuts and grains that have a probable mechanism of action against cataract formation. This compilation is intended to provide information to scientists working in this particular field to create more evidences for the mechanism of action and to disseminate the idea of use of nutraceuticals for prevention of cataract.
Pathogenesis of age related cataract
An eye lens consists of crystallins, cytoskeletal and membrane proteins. Crystallins make up to 90% of lens proteins and have high refractive index. It exists in the cytoplasm of lens fibers in the form of complex protein solution. The majority of proteins are in a soluble phase, and this soluble form accounts for transparency. With increase in age a wide range of proteins leave the soluble phase and form high molecular weight aggregates. The primary mechanism that lies behind protein aggregation is posttranslational modification associated disulfide bond formation and non-enzymatic glycation. These changes occur in the nucleus that contains the long-lived proteins.3, 4 Reactive oxygen species (ROS) such as peroxide, superoxide and hydroxyl radicals are causes of protein modification. Normally the healthy lens contains antioxidants such as glutathione, ascorbate and catalase that protect lens proteins against ROS. Glutathione is one of the most important antioxidants found in eye lens.5 Reduced glutathione (GSH) reacts with ROS and is converted to its oxidized form (GSSG). GSH is restored through the action of the enzyme glutathione reductase (GR). Hydrogen peroxide (H2O2) has been considered as the major oxidant in the pathogenesis of cataract. Normally, H2O2 is eliminated by GSH, or through the action of the enzymes glutathione peroxidase and catalase. However, with age there is decrease in activity of these protective mechanisms that result into elevation of H2O2 levels in the lens.3 This acts on the lens epithelium and inhibits membrane lipids as well as transporter proteins such as Na+K+ATPase ultimately leading to epithelial cell death and loss of lens transparency. Although individuals may have a genetic susceptibility to ROS, yet exposure to environmental factors such as smoking and UV exposure, the presence of certain diseases such as diabetes and the intake of systemic drugs are also important variables.
Pathogenesis of diabetic cataract
In diabetes, there is high concentration of glucose in the aqueous humor that is passively transported into the lens. The enzyme Aldose Reductase (AR) catalyzes the conversion of glucose to sorbitol through the polyol pathway and results in intracellular accumulation of sorbitol that further leads to osmotic changes resulting in degeneration of hydropic lens fibers and formation of cataract.6, 7 In addition, the intracellular sorbitol cannot be removed through diffusion because of its polar character. The intracellular accumulation of sorbitol leads to a collapse and liquefaction of lens fibers that causes opacities in lens.6, 8 Further studies have shown that osmotic stress in the lens caused by sorbitol accumulation9 induces apoptosis in Lens Epithelial Cells (LEC)10 leading to the development of cataract.11 Moreover, increased glucose levels in the aqueous humor may cause glycation of lens proteins, a process resulting in the generation of superoxide radicals (O2−) and in the formation of Advanced Glycation End products (AGE).12 As the AGE interacts with cell surface receptors in the epithelium of the lens, there is generation of O2− and H2O2.
Prevention of cataract
Cataract is a major global cause of blindness, and large section of the world’s population cannot assess cataract surgery. It has been found that mechanisms related to glucose toxicity, namely oxidative stress, processes of non-enzymatic glycation and enhanced polyol pathway are significantly involved in the development of eye lens opacity. There is an urgent need for inexpensive, non-surgical approaches to prevent cataract. The following types of dietary phytochemicals could be implied to obtain the desired therapeutic action:
-
1.
Antioxidants or ROS scavengers
-
2.
Aldose Reductase inhibitors
-
3.
Antiglycating agents
-
4.
Inhibitors of Lens Epithelial Cell apoptosis.
Antioxidants
Various classes of antioxidants that can be used to prevent cataract are flavonoids, carotenoids, ascorbic acid, tocopherol, caffeine, and pyruvate.
Flavonoids: Flavonoids are C6-C3-C6 compounds with fifteen carbon atoms. Flavonoids exert antioxidant effects due to their ability to scavenge free radicals, donate hydrogen as hydrogen donating compounds, and act as singlet oxygen quenchers and metal ion chelators. Examples of few flavonoids acting as antioxidants are myrcetin, quercetin, rhamnetin, morin, diosmetin, naringenin, apigenin, catechin, kaempferol and flavones. These flavonoids can be obtained from fruits such as apple, grapes, bananas, cherries, and berries and from green leafy vegetables.
Vitamins: Vitamin C and vitamin E are the main sources of antioxidants. Corn oil and wheat germ oil are major sources of vitamin E, whereas vitamin C i.e. ascorbic acid is mainly found in amla and other citrus fruits.
Carotenoids: Carotenoids are a family of 700 compounds found in fruits, vegetables and green plants. Out of these 700 compounds, about 20 have been detected in human plasma and tissues. Lutein and zeaxanthin are two dietary carotenoids that are in the human eye lens. It has been reported that these two carotenoids can be beneficial in prevention of cataract. These compounds have the potential to filter harmful short wave blue light, to reduce H2O2 mediated damage of lens protein, lipid and DNA,13 to function as antioxidants and to stabilize membrane integrity. These biological functions are believed to play a crucial role in helping to reduce light-induced oxidative damage caused by ROS, which is major contributing factor in the pathogenesis of cataract.14 Table 1 depicts sources of lutein and zeaxanthin.
Table 1.
Aldose reductase inhibitors
The accumulation of polyol sorbitol in the lens results in the formation of diabetic cataract.19, 20, 21 The enzyme aldose reductase within the lens converts glucose to sorbitol and is responsible for the accumulation of sorbitol in eye lens. Hence Aldose Reductase inhibitors can be used as potential therapeutic agents to prevent the onset or progression of diabetic cataract.22, 23, 24 A large hydrophobic pocket forms the inhibitor-binding site of Aldose Reductase and acts as a target for pharmacophore.25 Inhibitor binding is therefore a repercussion of polar and non-polar interactions between the inhibitor and the complementary residues that match the enzyme-binding pocket. It has been proposed that the specificity for the inhibitor was mainly due to inhibitor-enzyme interactions at the non-polar domain.26 There are some dietary phytochemicals, illustrated in Table 2, Table 3, Table 4, Table 5, that act as ARI (Aldose Reductase Inhibitors).
Table 2.
| S. no. | Source | Active constituent |
|---|---|---|
| 1. | Belamcanda chinensis (blackberry) | Tectoridin, tectorigenin |
| 2. | Myrciaria dubia (Rumberry) | Ellagic acid |
| 3. | Syzygium cumin (jamun) | Ellagic acid |
| 4. | Litchi chinensis (lychee) | Delphinidin 3-O-β-galactopyranoside-3′-O-β-glucopyranoside |
| 5. | Citrus limon (lemon) | Rutin |
| 6. | Citrus aurantium (orange) | Rutin |
| 7. | Psidium guajava (guava) | Quercetin derivatives |
| 8. | Malus pumila (apple) | Quercetin, epicatechin, procyanidin |
| 9. | Vitis vinifera (grapes) | Citronellol |
Table 3.
| S. no. | Source | Active constituent |
|---|---|---|
| 1. | Curcuma longa (turmeric) | Curcumin |
| 2. | Zingiber officinalis (Ginger) | 2-(4-hydroxy-3-methoxyphenyl) ethanol |
| 3. | Glycyrrhiza glabra (liquorice) | Semilicoisoflavone B |
| 4. | Ocimum sanctum (tulsi) | Ursolic acid |
| 5. | Cinnamomum cassia (cinnamon) | Trans-cinnamaldehyde |
| 6. | Cuminum cyminum (cumin) | Cuminaldehyde |
| 7. | Foeniculum vulgare (fennel) | Trans-anethole |
| 8. | Piper nigrum (Black pepper) | Piperine |
| 9. | Allium sativum (garlic) | Allicin |
| 10 | Coriandrum sativum (coriander) | Linalool, alpha-pinene |
Table 4.
| S. no. | Source | Active constituent |
|---|---|---|
| 1. | Ganoderma lucidum (polypore mushroom) | Ganoderic acid |
| 2. | Spinaceae oleracea (spinach) | Apigenin-7-glucoside |
| 3. | Trigonella foenumgraceum (fenugreek) | 4-hydroxyleucine |
| 4. | Momordica charantia (bitter gourd) | Momordin, charantin |
| 5. | Murraya koenigii (Curry leaves) | Mahanine, koenine |
| 6. | Allium sepa (onion) | Alliin |
Table 5.
| S. no. | Source | Active constituent |
|---|---|---|
| 1. | Camellia sinensis (Tea leaves) | Catechol |
| 2. | Nelumbo nucifera (lotus) | Rutin, Quercetin |
| 3. | Oryza sativa (rice) | Cyanidin-3-O-β-glucoside, Peonidin-3-O-β-glucoside |
| 4. | Eleusine coracana (finger millet) | Quercetin derivatives |
Antiglycating agents
The process of non-enzymatic glycation is one of the well-known mechanisms involved in diabetic cataract.43, 44, 45, 46, 47 With the age, there is accumulation of advanced glycation end products, which may contribute to lens opacity.48 So clinically used antiglycating agents are also reasonable option as anticataract agents. Some of these agents are given below:
Polyphenols: Polyphenols are the most abundant dietary antioxidants, which are common constituents of fruits, vegetables, cereals, seeds, nuts, chocolate and beverages such as coffee, tea, and wine. These dietary constituents have shown strong antiglycating activity. Based on their chemical structure, these are further classified as phenolic acids and flavonoids.
Phenolic acids: These are the most important non-vitamin antioxidant phytochemicals naturally present in almost all vegetables and fruits. Caffeic acid is a naturally occurring cinnamic acid (type of phenolic acid), found in various plants such as coffee, pear, basil, oregano and apple.49 Caffeic acid present in Ilex paraguariensis, Chrysanthemum morifolium and Chrysanthemum indicum has the ability to inhibit the formation of AGEs.50, 51 Ferulic acid is another naturally occurring cinnamic acid reported in drinks and foods such as rice, wheat, and oats, some fruits and vegetables.52 It has been reported that ferulic acid being an antioxidant prevents AGE formation. It binds to the amino groups and inhibits the sugar autoxidation as well as early Maillard Reaction Products (MRP) degradation.53 However the exact mechanism of anti-glycation by ferulic acid needs to be investigated further. The leaves and stems of Erigeron annuus contain quinic acid derivative: 3,5-di-O-caffeoyl-epi-quinic acid, a potent inhibitor of AGEs formation and thus prevents opacification of eye lenses.54 The potent inhibitory effect of rosmarinic acid isolated from Salvia miltiorrhiza Bge has been reported against the formation of AGEs.55 Protocatechuic acid obtained from Rhus verniciflua extracts has been shown to inhibit aldose reductase and accumulation of AGEs.56 Various phenolic compounds such as gallic acid, p-coumaric acid (a typical cinnamic acid) and epicatechin (flavanol) from Cyperus rotundus, have been reported to show potent inhibitory activity on AGEs formation and protein oxidation.57
Flavonoids: A number of naturally occurring flavonoids show inhibitory effects on advanced glycation end product formation. Cuminum cyminum commonly known as Jeera, contains approximately 51.87% w/w flavonoids and acts as antiglycating agent. Quercetin, eriodictyol, 5,6,4′-trihydroxy-7,8,3′-trimethoxyflavone and cirsilineol isolated from the methanol extract of Thymus vulgaris have been reported to reduce the levels of advanced glycation end products under in vitro conditions.58 Chalcones are also considered as members of the flavonoid family.59 One of the chalcones named butein isolated from R. verniciflua has been reported to inhibit the formation of AGEs. Phloridzin, sieboldin and trilobatin are three dihydrochalcones found in Malus domestica. Out of these three dihydrochalcones, sieboldin is more potent antiglycating agent than others.60 Vaccinium vitis-idaea berry extract flavonoids (luteolin, quercetin, and rutin) have been shown as potent antiglycating agents.61, 62 Both the fluorescent and non-fluorescent AGEs formation is inhibited by rutin and its metabolites.63 Besides this, the flavonoids such as engeletin and astilbin from extract of the leaves of Stelechocarpus cauliflorus are potentially useful for therapeutic prevention of diabetic complications resulting from AGEs accumulation.64 It has been studied that components of green tea epigallocatechin (EGC), epicatechin (EC), epigallocatechin-3-gallate (EGCG) and epicatechin-3-gallate (ECG) decrease the accumulation of AGEs.65
Terpenes, carotenoids and polyunsaturated fatty acids: A terpene 8 (17), 12-Labdadiene-15,16-dial (labdadiene) and 5,6-dehydrokawain (DK) isolated from the rhizome of Alpinia zerumbet, have the potential to inhibit glycation-induced protein oxidation. Number of antioxidants such as carotenoids, polyunsaturated fatty acids and polysaccharides can be produced in microalgae.66 Strong antiglycating capacities of lutein (carotenoid) present in Chlorella and linoleic acid, arachidonic acid, and eicosapentaenoic acid (unsaturated fatty acids) in Nitzschia laevis have also been revealed.67 The green microalgae Chlorella zofingiensis contains primary carotenoids such as lutein and β-carotene and protects the cells from oxidative damage.68 The green microalgae C. zofingiensis is considered as a natural source of astaxanthin (a red ketocarotenoid) which is a potent antioxidant and is the major carotenoid having role against excessive oxidative damage.68 Astaxanthin has stronger antioxidant activity than other carotenoids such as zeaxanthin, lutein, canthaxanthin and β-carotene and hundred times stronger antioxidant than that of α-tocopherol.69
Polysaccharides: Longan pericarp fruit (Dimocarpus longan) contains polysaccharide that acts as free radical scavenger and competes with glucose for binding to free amino group in proteins, and thus reduces the concentration of glycation targets in proteins.70 Similarly, Ganoderma lucidum polysaccharides have the ability to decrease lipid peroxidation and blood glucose levels in diabetes.71 Polysaccharides from pumpkin (Cucurbita moschata) have also shown antiglycating activity.72
Other antiglycating agents
Citrate a natural dietary constituent found in citrus fruits73 when administered orally has the potential to delay the development of cataracts and inhibit the accumulation of AGEs in lens proteins. Fermentation by-products are also capable to inhibit glycation.74 Recycled distilled residues of rice and barley spirit along with their vinegars have shown inhibitory effect on one of the major AGEs such as carboxymethyl lysine. Pyridoxamine as well as α-lipoic acid has also shown inhibitory effect on formation of glycation end products.75, 76
Inhibitors of lens epithelial cell apoptosis: Apoptosis is a physiological process of cell death that provides an important molecular basis for both the initiation and progression of cataracts.77, 78 Depending upon the different apoptotic stimuli, there are several mechanisms involved in apoptosis classified as intrinsic pathway and extrinsic pathway. Mitochondria-dependent pathway is associated with lens opacification. Certain stimuli such as radiations, drugs, toxins and free radicals cause mitochondrial damage and dysfunction. All this results in the release of pro-apoptotic proteins (including cytochrome c and SMAC) from the inner mitochondrial surface into cytosol,79 which contributes to programmed cell death. Oxidative stress has been recognized as an important mediator of apoptosis in lens epithelial cells and plays an important role in the pathogenesis of cataracts.
Epigallo catechin gallate (EGCG), the most abundant component in green tea (Camellia sinensis), has potent antioxidant activity. It has been shown that EGCG reduces the H2O2-induced generation of reactive oxygen species (ROS), and prevents the loss of mitochondrial membrane potential (Δψm), and the release of cytochrome c from the mitochondria into the cytosol. Epigallocatechin gallate inhibits the activities of caspase-9 and caspase-3 and thus prevents intrinsic apoptosis.80 There are many other polyphenols such as flavonoids, phenolic acids, phenolic alcohols, stilbenes and lignans which act as dietary antioxidants and are thus effective in apoptosis inhibition. Polyphenols are major constituents of fruits, vegetables, grains, roots, chocolate, coffee, tea, and wine.81, 82
Grape seed extract (GSE) is a dietary supplement that acts as potent antioxidant and free radical scavenger by influencing various signaling pathways and therefore beneficial in preventing cataracts. GSE contains 70–95% standardized proanthocyanidins (class of phenolic compounds). The seeds of the grape are particularly rich source of proanthocyanidins. NF-кB is transcription factor that regulates various genes including apoptosis, cell adhesion, proliferation, inflammation, and cellular-stress response. In un-stimulated or normal cells, NF-кB remains in the cytoplasm as an inactive complex with inhibitor kappa B. Pathogenic stimuli like free radicals activate NF-кB and causes its phosphorylation. After phosphorylation there is subsequent release of inhibitor kappa B, resulting in translocation of NF-кB to the nucleus followed by binding to DNA control elements that influence the transcription of certain specific genes.83, 84 ultimately resulting in cell apoptosis. However, it has been shown that grape seed extract reduces the generation of ROS induced by H2O2 as well as translocation of NF-кB in lens epithelial cells ultimately inhibiting apoptosis.85
Resveratrol (RES) is a naturally occurring polyphenol that decreases production of ROS and increases protection against oxidative stress. RES has been shown to suppress apoptosis of lens epithelial cells and hence prevents cataract formation.86 Table 6 cites some dietary sources of resveratrol.
Table 6.
| S. no. | Common name | Scientific name |
|---|---|---|
| 1. | Grapes | Vitis vinifera |
| 2. | White hellobore | Veratrum grandiflorum |
| 3. | Peanut | Arachis hypogea |
| 4. | Blueberry | Vaccinium myritillus |
| 5. | Ko-jo-kon | Polygonum cuspidatum |
| 6. | Mulberry | Morus rubra |
Coenzyme Q10 (ubiquinone) is a vitamin-like benzoquinone compound that acts as free radical scavenger.94 It prevents light induced apoptosis in human lens epithelial cells.95, 96, 97 Sources of coenzyme Q10 are mentioned in Table 7. Common nutraceuticals used in market and their common mechanism of actions are listed in Table 8 and Fig. 1.
Table 7.
Sources of coenzyme Q10 from various foods.98
| Vegetables | Fruits | Oils | Nuts |
|---|---|---|---|
| Spinach | Apple | Soya bean | Peanuts |
| Chinese cabbage | Strawberry | Olive | Walnuts |
| Cauliflower | Grapes | Sunflower | Almonds |
| Parsley | Avocado | Hazelnuts | |
| Broccoli | Orange | Sea same seeds |
Table 8.
Common nutraceuticals and their common mechanism of actions.
| S. no. | Dietary source | Antioxidants | Aldose reductase inhibitors | Antiglycating agents | Inhibitors of lens epithelial cell apoptosis |
|---|---|---|---|---|---|
| 1. | Lemon | ✓ | ✓ | ✓ | |
| 2. | Orange | ✓ | ✓ | ✓ | ✓ |
| 3. | Guava | ✓ | ✓ | ✓ | |
| 4. | Grapes | ✓ | ✓ | ✓ | ✓ |
| 5. | Apple | ✓ | ✓ | ✓ | ✓ |
| 6. | Turmeric | ✓ | ✓ | ✓ | |
| 7. | Ginger | ✓ | ✓ | ✓ | |
| 8. | Liquorice | ✓ | ✓ | ✓ | |
| 9. | Tulsi | ✓ | ✓ | ✓ | |
| 10. | Fennel | ✓ | ✓ | ||
| 11. | Black pepper | ✓ | ✓ | ✓ | |
| 12. | Garlic | ✓ | ✓ | ✓ | |
| 13. | Coriander | ✓ | ✓ | ✓ | |
| 14. | Onion | ✓ | ✓ | ✓ | |
| 15. | Rice | ✓ | ✓ | ✓ | |
| 16. | Wheat | ✓ | ✓ | ✓ | |
| 17. | Green tea | ✓ | ✓ | ✓ | ✓ |
| 18. | Pumpkin | ✓ | ✓ | ||
| 19. | Oats | ✓ | ✓ | ||
| 20. | Black berries | ✓ | ✓ | ✓ | |
| 21. | Mango | ✓ | ✓ | ✓ | |
| 22. | Pomegranate | ✓ | ✓ |
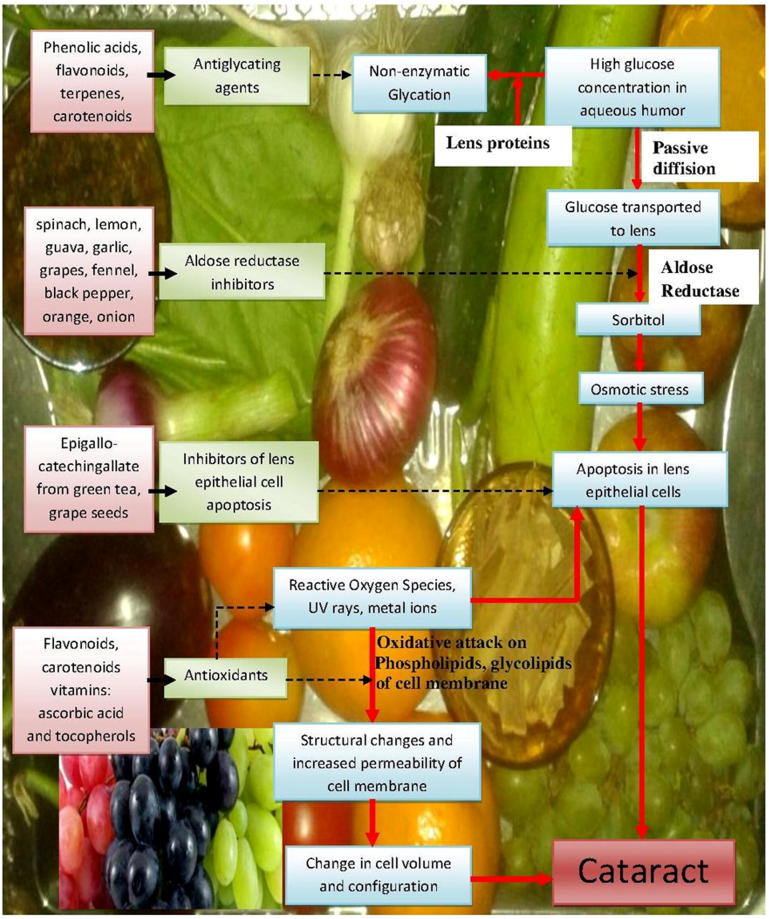
Figure 1.
A diagrammatic representation of mechanism of action of various nutraceuticals in prevention of cataract.
Conclusion
In the era of evidence-based medicine, it is pertinent to find alternative ways of treating common ocular morbidities such as cataract. This manuscript is about the scientific evidences in favor of some nutraceuticals consumed by normal population knowingly or inadvertently that act as anticataract agent through some or the other molecular mechanism. From meta-analysis of data in the literature, it can be concluded that there is a plethora of commonly used nutraceuticals that if consumed daily can prevent or revert changes responsible for cataract pathogenesis. These nutraceuticals play their role by adopting one or more of mechanisms singly or simultaneously and work against development of cataract. Most common mechanism followed by nutraceuticals seems to be antioxidant activity and antiglycating activity.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgment
The authors fully acknowledge the support by university authorities for preparation of this manuscript.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
Contributor Information
Manzoor Ahmad Malik, Email: maliksgpgi@gmail.com.
Parveen Bansal, Email: bansal66@yahoo.com, aman11091991@gmail.com.
References
- 1.John N., Rachel J., Vashist P. Rapid assessment of avoidable blindness in India. PLoS One. 2008;3:e2867. doi: 10.1371/journal.pone.0002867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasbia G.S. Functional foods and nutraceuticals in the management of obesity. Nutr Food Sci. 2005;35:344–352. [Google Scholar]
- 3.Spector A. Oxidative stress-induced cataract: mechanisms of action. FASEB. 1995;9:1173–1182. [PubMed] [Google Scholar]
- 4.Ottonello S., Foroni C., Carta A. Oxidative stress and age-related cataract. Ophthalmologica. 2000;214:78–85. doi: 10.1159/000027474. [DOI] [PubMed] [Google Scholar]
- 5.Truscott R.J.W. Age-related nuclear cataract: a lens transport problem. Ophthalmic Res. 2000;32:185–194. doi: 10.1159/000055612. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita J.H. Mechanisms initiating cataract formation. Proctor lecture. Invest Ophthalmol Vis Sci. 1974;13:713–724. [PubMed] [Google Scholar]
- 7.Kinoshita J.H., Fukushi S., Kador P. Aldose reductase in diabetic complications of the eye. Metabolism. 1979;28:462–469. doi: 10.1016/0026-0495(79)90057-x. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita J.H. Cataracts in galactosemia. The Jonas S. Friedenwald memorial lecture. Invest Ophthalmol Vis Sci. 1965;4:786–799. [PubMed] [Google Scholar]
- 9.Srivastava S.K., Ramana K.V., Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 10.Takamura Y., Sugimoto Y., Kubo E. Immunohistochemical study of apoptosis of lens epithelial cells in human and diabetic rat cataracts. Jpn J Ophthalmol. 2001;45:559–563. doi: 10.1016/s0021-5155(01)00418-x. [DOI] [PubMed] [Google Scholar]
- 11.Li W.C., Kuszak J.R., Dunn K. Lens epithelial cell apoptosis appears to be a common cellular basis for noncongenital cataract development in humans and animals. J Cell Biol. 1995;130:169–181. doi: 10.1083/jcb.130.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stitt A.W. The Maillard reaction in eye diseases. Ann N Y Acad Sci. 2006;1043:582–597. doi: 10.1196/annals.1338.066. [DOI] [PubMed] [Google Scholar]
- 13.Gao S., Qin T., Liu Z. Lutein and zeaxanthin supplementation reduces H2O2 induced oxidative damage in human lens epithelial cells. Mol. Vision. 2011;17:3180–3190. [PMC free article] [PubMed] [Google Scholar]
- 14.Krinsky N.I., Landrum J.T., Bone R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 15.Maiani G., Caston M.J.P., Catasta G. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. 2009;53:S194–S218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- 16.Abdel Aal S.M., Young J.C., Akhtar H. Stability of lutein in wholegrain bakery products naturally high in lutein or fortified with free lutein. J Agric Food Chem. 2010;58:10109–10117. doi: 10.1021/jf102400t. [DOI] [PubMed] [Google Scholar]
- 17.De La Parra C., Saldivar S.O., Lui R.H. Effect of processing on the phytochemical profiles and antioxidant activity of corn for production of masa, tortillas and tortilla chips. J Agric Food Chem. 2007;55:4177–4183. doi: 10.1021/jf063487p. [DOI] [PubMed] [Google Scholar]
- 18.Abdel Aal S.M., Young J.C., Rabalski I. Identification and quantification of seed carotenoids in selected wheat species. J Agri Food Chem. 2007;55:787–794. doi: 10.1021/jf062764p. [DOI] [PubMed] [Google Scholar]
- 19.Chihiro Y.N. Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharmacol Rev. 1998;50:21–33. [PubMed] [Google Scholar]
- 20.Alexiou P., Pegklidou K., Chatzopoulou M. Aldose reductase enzyme and its implication to major health problems of the 21(st) century. Curr Med Chem. 2009;16:734–752. doi: 10.2174/092986709787458362. [DOI] [PubMed] [Google Scholar]
- 21.Del Corso A., Cappiello M., Mura U. From a dull enzyme to something else: facts and perspectives regarding aldose reductase. Curr Med Chem. 2008;15:1452–1461. doi: 10.2174/092986708784638870. [DOI] [PubMed] [Google Scholar]
- 22.Costantino L., Rastelli G., Gamberini M.C. Pharmacological approaches to the treatment of diabetic complications. Expert Opin Ther Patents. 2000;10:1245–1262. [Google Scholar]
- 23.Miyamoto S. Recent advances in aldose reductase inhibitors: potential agents for the treatment of diabetic complications. Expert Opin Ther Patents. 2002;12:621–631. [Google Scholar]
- 24.Suzen S., Buyukbingol E. Recent studies of aldose reductase enzyme inhibition for diabetic complications. Curr Med Chem. 2003;10:1329–1352. doi: 10.2174/0929867033457377. [DOI] [PubMed] [Google Scholar]
- 25.El-Kabbani O., Ruiz F., Darmanin C. Aldose reductase structures: implications for mechanism and inhibition. Cell Mol Life Sci. 2004;61:750–762. doi: 10.1007/s00018-003-3403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Kabbani O., Podjarny A. Selectivity determinants of the aldose and aldehyde reductase inhibitor-binding sites. Cell Mol Life Sci. 2007;64:1970–1978. doi: 10.1007/s00018-007-6514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung S.H., Lee Y.S., Lee S. Isoflavonoids from the rhizomes of Belamcanda chinensis and their effects on aldose reductase and sorbitol accumulation in streptozotocin induced diabetic rat tissues. Arch Pharm Res. 2002;25:306–312. doi: 10.1007/BF02976631. [DOI] [PubMed] [Google Scholar]
- 28.Ueda H., Kuroiwa E., Tachibana Y. Aldose reductase inhibitors from the leaves of Myrciaria dubia (H. B. & K.) McVaugh. Phytomedicine. 2004;11:652–656. doi: 10.1016/j.phymed.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Lee S.J., Park W.H., Park S.D. Aldose reductase inhibitors from Litchi chinensis Sonn. J Enzyme Inhib Med Chem. 2009;24:957–959. doi: 10.1080/14756360802560867. [DOI] [PubMed] [Google Scholar]
- 30.Saraswat M., Muthenna P., Suryanarayana P. Dietary sources of aldose reductase inhibitors: prospects for alleviating diabetic complications. Asia Pac J Clin Nutr. 2008;17:558–565. [PubMed] [Google Scholar]
- 31.Kandil F.E., El-Sayed N.H., Micheal H.N. Flavonoids from Psidium guajava. Asian J Chem. 1997;9:871–872. [Google Scholar]
- 32.Du Z.Y., Bao Y.D., Liu Z. Curcumin analogs as potent aldose reductase inhibitors. Arch Pharm. 2006;339:123–128. doi: 10.1002/ardp.200500205. [DOI] [PubMed] [Google Scholar]
- 33.Kato A., Higuchi Y., Gato H. Inhibitory effects of Zingiber officinale Roscoe derived components on aldose reductase activity in vitro and in vivo. J Agric Food Chem. 2006;54:6640–6644. doi: 10.1021/jf061599a. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y.S., Kim S.H., Jung S.H. Aldose reductase inhibitory compounds from Glycyrrhiza uralensis. Biol Phram Bull. 2010;33:917–921. doi: 10.1248/bpb.33.917. [DOI] [PubMed] [Google Scholar]
- 35.Halder N., Joshi S., Gupta S.K. Lens aldose reductase inhibiting potential of some indigenous plants. J Ethanopharmacol. 2003;86:113–116. doi: 10.1016/s0378-8741(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 36.Lee H.S. Inhibitory activity of Cinnamomum cassia bark derived component against rat lens aldose reductase. J Pharm Pharmaceut Sci. 2002;5:226–230. [PubMed] [Google Scholar]
- 37.Lee H.S. Cuminaldehyde: aldose reductase and a-glucosidase inhibitor derived from Cuminum cyminum L. seeds. J Agric Food Chem. 2005;53:2446–2450. doi: 10.1021/jf048451g. [DOI] [PubMed] [Google Scholar]
- 38.Fatmawati S., Shimizu K., Kondo R. Ganoderic acid Df, a new triterpenoid with aldose reductase inhibitory activity from the fruiting body of Ganoderma lucidum. Fitoterapia. 2010;81:1033–1036. doi: 10.1016/j.fitote.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Murata M., Irie J., Homma S. Aldose reductase inhibitors from green tea. LWT-Food Sci Technol. 1994;27:401–405. [Google Scholar]
- 40.Jung H.A., Jung Y.J., Yoon N.Y. Inhibitory effects of Nelumbo nucifera leaves on rat lens aldose reductase, advanced glycation endproducts formation, and oxidative stress. Food Chem Toxicol. 2008;46:3818–3826. doi: 10.1016/j.fct.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Yawadio R., Shinji T., Morita N. Identification of phenolic compounds isolated from pigmented rices and their aldose reductase inhibitory activities. Food Chem. 2007;101:1616–1625. [Google Scholar]
- 42.Chethan S., Dharmesh S.M., Malleshi N.G. Inhibition of aldose reductase from cataracted eye lenses by finger millet (Eleusine coracana) polyphenols. Bioorg Med Chem. 2008;16:10085–10090. doi: 10.1016/j.bmc.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Shamsi F.A., Sharkey E., Creighton D. Maillard reactions in lens proteins: methylglyoxal-mediated modifi cations in the rat lens. Exp Eye Res. 2000;70:369–380. doi: 10.1006/exer.1999.0800. [DOI] [PubMed] [Google Scholar]
- 44.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 45.Stitt A.W. The maillard reaction in eye diseases. Ann N Y Acad Sci. 2005;1043:582–597. doi: 10.1196/annals.1338.066. [DOI] [PubMed] [Google Scholar]
- 46.Monnier V.M., Sell D.R., Genuth S. Glycation products as markers and predictors of the progression of diabetic complications. Ann N Y Acad Sci. 2005;1043:567–581. doi: 10.1196/annals.1333.065. [DOI] [PubMed] [Google Scholar]
- 47.Nagaraj R.H., Linetsky M., Stitt A.W. The pathogenic role of Maillard reaction in the aging eye. Amino Acids. 2012;42:1205–1220. doi: 10.1007/s00726-010-0778-x. [DOI] [PubMed] [Google Scholar]
- 48.Monnier V.M., Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981;211:491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- 49.Clifford M.N. Chlorogenic acids and other cinnamates-nature, occurrence and dietary burden. J Sci Food Agric. 1999;79:362–372. [Google Scholar]
- 50.Tsuji-Naito K., Saeki H., Hamano M. Inhibitory effects of Chrysanthemum species extracts on formation of advanced glycation end products. Food Chem. 2009;116:854–859. [Google Scholar]
- 51.Gugliucci A., Bastos D.H., Schulze J. Caffeic and chlorogenic acids in Ilex paraguariensis extracts are the main inhibitors of AGE generation by methylglyoxal in model proteins. Fitoterapia. 2009;80:339–344. doi: 10.1016/j.fitote.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Wang J., Sun B., Cao Y. Protein glycation inhibitory activity of wheat bran feruoyl oligosaccharides. Food Chem. 2009;112:350–353. [Google Scholar]
- 53.Silván J.M., Assar S.H., Srey C. Control of the Maillard reaction by ferulic acid. Food Chem. 2011;128:208–213. doi: 10.1016/j.foodchem.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 54.Jang D.S., Yoo N.H., Kim N.H. 3,5-Di-caffeoyl-epi-quinic acid from the leaves and stems of Erigeron annuus inhibits protein glycation, aldose reductase, and cataractogenesis. Biol Pharm Bull. 2010;33:329–333. doi: 10.1248/bpb.33.329. [DOI] [PubMed] [Google Scholar]
- 55.Ma H.Y., Gao H.Y., Sun L. Constituents with α-glucosidase and advanced glycation end-product formation inhibitory activities from Salvia miltiorrhiza Bge. J Nat Med. 2011;65:37–42. doi: 10.1007/s11418-010-0453-2. [DOI] [PubMed] [Google Scholar]
- 56.Lee E.H., Song D.G., Lee J.Y. Inhibitory effect of the compounds isolated from rhus verniciflua on aldose reductase and advanced glycation endproduct. Biol Pharm Bull. 2008;31:1626–1630. doi: 10.1248/bpb.31.1626. [DOI] [PubMed] [Google Scholar]
- 57.Proestos C., Chorianopoulos N., Nychas G.J. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J Agric Food Chem. 2005;53:1190–1195. doi: 10.1021/jf040083t. [DOI] [PubMed] [Google Scholar]
- 58.Morimitsu Y., Yoshida K., Esaki S. Protein glycation inhibitors from thyme (Thymus vulgaris) Biosci Biotechnol Biochem. 1995;59:2018–2021. doi: 10.1271/bbb.59.2018. [DOI] [PubMed] [Google Scholar]
- 59.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dugé de Bernonville T., Guyot S., Paulin J.P. Dihydrochalcones: Implication in resistance to oxidative stress and bioactivities against advanced glycation end-products and vasoconstriction. Phytochemistry. 2010;71:443–452. doi: 10.1016/j.phytochem.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Beaulieu L.P., Harris C.S., Saleem A. Inhibitory effect of the Cree traditional medicine wiishichimanaanh (Vaccinium vitis-idaea) on advanced glycation endproduct formation: identification of active principles. Phytother Res. 2010;24:741–747. doi: 10.1002/ptr.3025. [DOI] [PubMed] [Google Scholar]
- 62.Wu C.H., Yen G.C. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J Agric Food Chem. 2005;53:3167–3173. doi: 10.1021/jf048550u. [DOI] [PubMed] [Google Scholar]
- 63.Cervantes-Laurean D., Schramm D.D., Jacobson E.L. Inhibition of advanced glycation end product formation on collagen by rutin and its metabolites. J Nutr Biochem. 2006;17:531–540. doi: 10.1016/j.jnutbio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Wirasathien L., Pengsuparp T., Suttisri R. Inhibitors of aldose reductase and advanced glycation end-products formation from the leaves of Stelechocarpus cauliflorus R.E.Fr. Phytomedicine. 2007;14:546–550. doi: 10.1016/j.phymed.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Babu P.V., Sabitha K.E., Shyamaladevi C.S. Effect of green tea extract on advanced glycation and cross-linking of tail tendon collagen in streptozotocin induced diabetic rats. Food Chem Toxicol. 2008;46:280–285. doi: 10.1016/j.fct.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Chen F. High cell density culture of microalgae in heterotrophic growth. Trends Biotechnol. 1996;14:421–426. [Google Scholar]
- 67.Sun Z., Peng X., Liu J. Inhibitory effect of microalgal extracts on the formation of advanced glycation endproducts (AGEs) Food Chem. 2010;120:261–267. [Google Scholar]
- 68.Bar E., Rise M., Vishkautsan M. Pigment and structural changes in Chlorella zofingiensis upon light and nitrogen stress. J Plant Pysiol. 1995;146:527–534. [Google Scholar]
- 69.Miki W. Biological functions and activities of animal carotenoids. Pure Appl Chem. 1991;63:141–146. [Google Scholar]
- 70.Yang B., Zhao M.M., Shi J. Variations in water-soluble saccharides and phenols in longan fruit pericarp after drying. J Food Process Eng. 2008;31:66–77. [Google Scholar]
- 71.Chen X.P., Chen Y., Li S.B. Free radical scavenging of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes and immunity activities in cervical carcinoma rats. Carbohydr Polym. 2009;77:389–393. [Google Scholar]
- 72.Wang X., Zhang L.S., Dong L.L. Inhibitory effect of polysaccharides from pumpkin on advanced glycation end-products formation and aldose reductase activity. Food Chem. 2010;120:261–267. [Google Scholar]
- 73.Penniston K.L., Nakada S.Y., Holmes R.P. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J Endourol. 2008;22:567–570. doi: 10.1089/end.2007.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye X.J., Ng T.B., Nagai R. Inhibitory effect of fermentation byproducts on formation of advanced glycation end-products. Food Chem. 2010;121:1039–1045. [Google Scholar]
- 75.Voziyan P.A., Hudson B.G. Pyridoxamine: the many virtues of a Maillard Reaction inhibitor. Ann N Y Acad Sci. 2005;1043:807–816. doi: 10.1196/annals.1333.093. [DOI] [PubMed] [Google Scholar]
- 76.Dicter N., Madar Z., Tirosh O. Alpha-lipoic acid inhibits glycogen synthesis in rat soleus muscle via its oxidative activity and the uncoupling of mitochondria. J Nutr. 2002;132:3001–3006. doi: 10.1093/jn/131.10.3001. [DOI] [PubMed] [Google Scholar]
- 77.Yan Q., Liu J.P., Li D.W. Apoptosis in lens development and pathology. Differentiation. 2006;74:195–211. doi: 10.1111/j.1432-0436.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 78.Yao K., Tan J., Gu W.Z. Reactive oxygen species mediates the apoptosis induced by transforming growth factor beta (2) in human lens epithelial cells. Biochem Biophys Res Commun. 2007;354:278–283. doi: 10.1016/j.bbrc.2006.12.198. [DOI] [PubMed] [Google Scholar]
- 79.Saelens X., Festjens N., Walle L.V. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 80.Yao K., Ye P.P., Zhang L. Epigallocatechin gallate protects against oxidative stress-induced mitochondria-dependent apoptosis in human lens epithelial cells. Mol Vision. 2008;14:217–223. [PMC free article] [PubMed] [Google Scholar]
- 81.Scalbert A., Manach C., Morand C. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 82.D’Archivio M., Filesi C., Di Benedett R. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita. 2007;43:348–361. [PubMed] [Google Scholar]
- 83.Schreck R., Albermann K., Baeuerle P.A. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic Res Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 84.Boileau T.W., Bray T.M., Bomser J.A. Ultraviolet radiation modulates nuclear factor kappa B activation in human lens epithelial cells. J Biochem Mol Toxicol. 2003;17:108–113. doi: 10.1002/jbt.10067. [DOI] [PubMed] [Google Scholar]
- 85.Jia Z., Song Z., Zhao Y. Grape seed proanthocyanidin extract protects human lens epithelial cells from oxidative stress via reducing NF-кB and MAPK protein expression. Mol Vision. 2011;17:210–217. [PMC free article] [PubMed] [Google Scholar]
- 86.Doganay S., Borazan M., Iraz M. The effect of resveratrol in experimental cataract model formed by sodium selenite. Curr Eye Res. 2006;31:147–153. doi: 10.1080/02713680500514685. [DOI] [PubMed] [Google Scholar]
- 87.Creasy L.L., Cofee M. Phytoalexin production potential of grape berries. J Am Soc Hort Sci. 1988;113:230–234. [Google Scholar]
- 88.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 89.Sobolev V.S., Cole R.J. Trans-resveratrol content in commercial peanuts and peanut products. J Agric Food Chem. 1999;47:1435–1439. doi: 10.1021/jf9809885. [DOI] [PubMed] [Google Scholar]
- 90.Rimando A.M., Kalt W., Magee J.B. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J Agric Food Chem. 2004;52:4713–4719. doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- 91.Sanders T.H., McMichael R.W., Hendrix W. Occurrence of resveratrol in edible peanuts. J Agric Food Chem. 2000;48:1243–1246. doi: 10.1021/jf990737b. [DOI] [PubMed] [Google Scholar]
- 92.Burns J., Yokota T., Ashihara H. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 93.Stewart J.R., Artime M.C., O’Brian C.A. Resveratrol: a candidate nutritional substance for prostate cancer prevention. J Nutr. 2003;133:2440S–2443S. doi: 10.1093/jn/133.7.2440S. [DOI] [PubMed] [Google Scholar]
- 94.Papucci L., Schiavone N., Witort E. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278:28220–28228. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- 95.Sandhu J.K., Pandey S., Monette R. Molecular mechanisms of glutamate neurotoxicity in mixed cultures of NT2-derived neurons and astrocytes: protective effects of coenzyme Q10. J Neurosci Res. 2003;72:691–703. doi: 10.1002/jnr.10579. [DOI] [PubMed] [Google Scholar]
- 96.McCarthy S., Somayajulu M., Sikorska M. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol Appl Pharmacol. 2004;201:21–31. doi: 10.1016/j.taap.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 97.Somayajulu M., McCarthy S., Hung M. Role of mitochondria in neuronal cell death induced by oxidative stress; neuroprotection by Coenzyme Q10. Neurobiol Dis. 2005;18:618–627. doi: 10.1016/j.nbd.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 98.Igor P., Katja Z., Janko Z. Coenzyme Q10 contents in foods and fortification strategies. Crit Rev Food Sci Nutr. 2010;50:269–280. doi: 10.1080/10408390902773037. [DOI] [PubMed] [Google Scholar]