Abstract
The human dopamine transporter gene (gene symbol: SLC6A3) is considered as a candidate risk factor for Parkinson’s disease because dopamine transporter accumulates cytotoxic dopamine or other toxins in the dopamine neurons. However, findings from numerous association studies in different populations have been inconsistent with each other. In this study, we performed a combined analysis of published case–control genetic association data between SLC6A3 and Parkinson’s disease. The results indicate that SLC6A3 confers a modest but significant risk for Parkinson’s disease in various populations. Allele 10-repeat of the 40-base pair variable number tandem repeat, a well studied polymorphism in the 3′ untranslated region of SLC6A3, confers neuroprotection in East Asian (OR: 0.78, 95% CI: 0.65, 0.94 and p = 0.009) but not in Caucasian populations. Genotype GG and allele G of the promoter single nucleotide polymorphism rs2652510 is associated with a risk in Caucasians (allelic G, OR: 1.26, 95% CI: 1.04–1.54, and p = 0.018; genotypic GG OR: 1.37, 95% CI: 1.03–1.84 and p = 0.032). Such information implies a population-dependent involvement of SLC6A3 in the etiology of Parkinson’s disease.
Keywords: Dopamine transporter, Parkinson’s disease, Genetic etiology, Polymorphism, Neurodegeneration
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder with incidence of 8–18 per 100,000 person-years and prevalence of 0.3% in the whole population of industrialized countries [4]. Epidemiology studies showed PD prevalence could be ethnicity-dependent. In particular, the prevalence seems slightly lower in Asian than in Western countries [4,6,32]. The etiology of PD is attributable to three main factors, aging, environment (dietary and pesticides, etc.) and genetics (e.g., DNA sequence polymorphisms). The estimated sibling risk ratio for Parkinson’s disease increases from 1.7 for all ages into around 12 for those younger than 66 years [6], implying a significant genetic contribution to disease risk.
Since loss of dopaminergic neurons (DA neurons) is the hallmark of PD, genes especially those expressed exclusively in these neurons are candidate contributors to the genetic etiology of PD [1,30]. In this regard, the dopamine transporter (DAT or DAT1, gene symbol: SLC6A3) plays a critical role in maintaining the integrity of DA neurons. First, DAT is expressed exclusively in DA neurons, at the highest levels in substantia nigra that happens to be the most vulnerable brain region in patients with PD. Second, DAT takes up dopamine from the synaptic cleft into the presynaptic neurons, terminating DA transmission as the principal function of DAT in the brain [11,43]. However, this uptake activity accumulates DA and confers vulnerability in the DA neurons because oxidation of DA causes neurotoxicity by, for instance, producing free radical species [10,15,37,41,42]. Third, DAT could also take up neurotoxins such as 1-methyl-4-phenylpyridinium [MPP+, a metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)] that may damage the DA neurons as well [21,44]. Accordingly, genetic variation in DAT expression levels is well positioned to explain the selective vulnerability of nigrostriatal DA neurons observed in patients with PD.
Based on its unique candidacy, SLC6A3 has been investigated extensively by association studies but the results have not reached a consensus for its genetic relevance to PD. To systematically evaluate those association results, a combined analysis, or meta-analysis [12], of published findings on SLC6A3 association with PD was carried out to assess whether lack of consistency was underlain by a small effect size in each study or by potential confounders among these studies.
2. Material and methods
2.1. Literature search
To find publications suitable for this analysis, we searched the PubMed and the Chinese National Knowledge Infrastructure Database (CNKI) from 1995 to October 2012 by using the keywords ‘dopamine transporter’, ‘DAT’, ‘SLC6A3’, combined with ‘Parkinson’s disease or PD’. From the references cited in these publications, additional original reports were identified.
2.2. Criteria for inclusion
Studies on at least one genetic marker in the SLC6A3 gene were included in this meta-analysis [2,3]. In addition, selected studies met all of the following criteria [47]: (1) from a peer-reviewed journal; (2) reporting original data on allele/genotype frequencies, and no genotype frequencies deviated from Hardy–Weinberg equilibrium (HWE) among control subjects (p > 0.01); (3) independence from other studies (without sharing of any data with another reported studies, all that included and re-analyzed a previously published data set were not regarded as independent and in such cases, only the study composed of the largest sample size and the most detailed information was included in the meta-analysis); and (4) case–control studies. From each study, we collected information on publication year and authors, sample location, demography including age-at-diagnosis and -onset, cohort size, and genetic (allele and genotype) composition.
2.3. Statistic analysis
Estimation of statistic power calculations was based on the G*Power program [9]. Meta-analysis was performed when the sample size used had >80% power to detect a positive signal (p < 0.05) for the given marker in allelic as well as genotypic analysis, given an effect size index of 0.1 corresponding to ‘weak’, for a low risk of a type II error under the predefined weak effect size.
The meta-analysis was carried out via Stata 11.0 (Stata Corporation, College Station, TX, USA). Every selected study was given a contingency table where subjects were categorized by case vs. control and allelic or genotypic frequency. A combined log-odds ratio (OR) and its 95% confidence interval (CI) were estimated by random effects method for each marker. We used the z-test to evaluate the significance of the combined OR. The heterogeneity among different cohorts used in these studies was estimated by χ2-based Q-testing. Sensitivity analysis of the effect of each study on the combined OR was completed by excluding the study, followed by re-estimation of the combined OR and 95% CI sequentially. The effect of publication bias was weighed by the Egger test [8]. Stratified meta-analyses were performed to examine possible moderating effects of ethnicity on combined OR. Two-tailed p values below 0.05 were recognized as statistical significance.
3. Results
The criteria-based search identified 29 studies. After estimation of statistic power, a variable number of tandem repeat located in 3′untranslated region (3′VNTR) and four SNPs (rs6347, rs3756450, rs2652510, rs2550956) were included in current combined-analysis, as listed in Table 1 [5,7,13,16,18–20,22–27,31,33–36,38–40,46,48,49]. There was lack of power for combined-analysis of other SLC6A3 polymorphisms.
Table 1.
Features of the studies included.
| SLC6A3 | Ethnicity | Study | Year | Area | Ratio of male | Sample size | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Marker | Case | Control | Case | Control | ||||
| 3′VNTR | ||||||||
| Caucasian | ||||||||
| Le Couteur et al. | 1997 | Australia | 0.6 | 0.61 | 100 | 200 | ||
| Plante-Bordeneuve et al. | 1997 | France and UK | 60 | 79 | ||||
| Mercier et al. | 1999 | France | 0.6 | 0.6 | 75 | 78 | ||
| Nicholl et al. | 1999 | UK | 0.59 | 0.59 | 201 | 203 | ||
| Goudreau et al. | 2002 | USA | 0.62 | 0.38 | 183 | 146 | ||
| Kelada et al. | 2005 | USA | 0.63 | 0.62 | 293 | 395 | ||
| Ritz et al. (c) | 2009 | USA | 264 | 268 | ||||
| Subtotal | n = 7 | 1195 | 1350 | |||||
| Asian | ||||||||
| Leighton et al. | 1997 | China | 0.52 | 0.5 | 203 | 230 | ||
| Kim et al. | 2000 | Korea | 0.39 | 0.37 | 116 | 128 | ||
| Wang et al. | 2000 | China | 171 | 180 | ||||
| Kimura et al. | 2001 | 204 | 300 | |||||
| Zhang et al. | 2001 | China | 128 | 85 | ||||
| Lin et al. | 2003 | Taiwan | 0.51 | 0.51 | 193 | 254 | ||
| Zhao et al. | 2004 | China | 138 | 184 | ||||
| Subtotal | n = 7 | 1153 | 1361 | |||||
| Other/mixed | ||||||||
| Juyal et al. | 2006 | India (north) | 0.71 | 0.72 | 333 | 129 | ||
| Juyal et al. | 2006 | India (south) | 0.65 | 0.65 | 147 | 122 | ||
| Lynch et al. | 2003 | USA | 0.71 | 0.45 | 100 | 63 | ||
| Ritz et al. (m) | 2009 | USA | 60 | 66 | ||||
| Subtotal | n = 4 | 640 | 380 | |||||
| Total | n = 18 | 2988 | 3091 | |||||
| rs6347 (1215A/G) | ||||||||
| Caucasian | ||||||||
| Dick et al. | 2007 | Europe | 0.56 | 0.53 | 741 | 1926 | ||
| Pankratz et al. | 2009 | USA (PROGENI, GenePD) | 0.59 | 0.4 | 880 | 867 | ||
| Simon-Sanchez et al. | 2009 | USA, Germany | 0.55 | 0.5 | 1712 | 3944 | ||
| Subtotal | n = 3 | 3333 | 6737 | |||||
| Asian | ||||||||
| Morino et al. | 2000 | Japan | 0.39 | 0.5 | 172 | 132 | ||
| Kimura et al. | 2001 | 204 | 300 | |||||
| Lin et al. | 2002 | Taiwan | 0.74 | 0.7 | 102 | 174 | ||
| Nishimura et al. | 2002 | Japan | 0.4 | 0.4 | 236 | 220 | ||
| Subtotal | n = 4 | 714 | 826 | |||||
| Other/mixed | ||||||||
| Singh et al. | 2008 | India | 0.73 | 0.73 | 70 | 100 | ||
| Total | n = 8 | 4117 | 7663 | |||||
| rs3756450 (T-C) | ||||||||
| Caucasian | ||||||||
| Kelada et al. | 2005 | USA | 0.63 | 0.62 | 293 | 395 | ||
| Pankratz et al. | 2009 | USA (PROGENI, GenePD) | 0.59 | 0.4 | 877 | 867 | ||
| Simon-Sanchez et al. | 2009 | USA, Germany | 0.55 | 0.5 | 1711 | 3977 | ||
| Total | n = 3 | 2881 | 5239 | |||||
| rs2652510 (A–G) | ||||||||
| Caucasian | ||||||||
| Kelada et al. | 2005 | USA | 0.63 | 0.62 | 293 | 395 | ||
| Ritz et al. (c) | 2009 | USA | 264 | 268 | ||||
| Subtotal | n = 2 | 557 | 663 | |||||
| Other/mixed | ||||||||
| Ritz et al. (m) | 2009 | USA | 60 | 66 | ||||
| Total | n = 3 | 617 | 729 | |||||
| rs2550956 (C–T) | ||||||||
| Caucasian | ||||||||
| Kelada et al. | 2005 | USA | 0.63 | 0.62 | 261 | 376 | ||
| Ritz et al. (c) | 2009 | USA | 264 | 268 | ||||
| Subtotal | n = 2 | 525 | 644 | |||||
| Other/mixed | ||||||||
| Ritz et al. (m) | 2009 | USA | 60 | 66 | ||||
| Total | n = 3 | 595 | 710 | |||||
With 3′VNTR, 18 studies have been performed, seven in Caucasians and another seven in East Asians (Table 1). The combined ORs and 95% CIs from the case–control studies were not statistically significant (for 9-repeat allele: OR = 1.10, 95% CI: 0.996–1.220, z = 1.885, p = 0.059, Fig. S1A; for 10-repeat allele: OR = 0.91, 95% CI: 0.82–1.01, z = −1.738, p = 0.082, Fig. S1B). No significant heterogeneity was observed during these analyses (for 9-repeat allele: heterogeneity Q = 17.512 (d.f. = 17), p = 0.420; for 10-repeat allele: heterogeneity Q = 21.94 (d.f. = 17), p = 0.187). Egger’s tests did not suggest publication bias among the studies (for 9-repeat allele: p = 0.346, Fig. S2A; for 10-repeat allele: p = 0.167, Fig. S2B). Results of the sensitivity analysis indicated certain extents of biasing (Table S1, shown in bold). Given potential influences of ethnicity on the combined results, the analyses were stratified by the two main populations, Caucasian and East Asian.
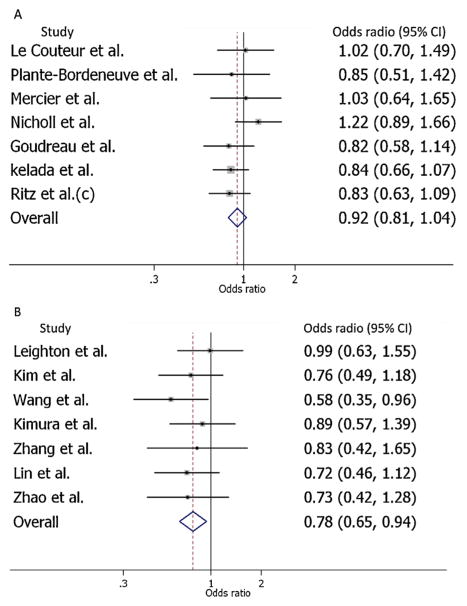
After stratification, positive signals are revealed with the 10-repeat allele only in 1158 East Asian cases vs. 1361 controls (Fig. 1A) but not in 1195 Caucasian cases vs. 1350 controls (Fig. 1B). The stratified analytic results are for 10-repeat allele: OR = 0.78, 95% CI: 0.65–0.94, z = −2.615, p = 0.009 in East Asians and OR = 0.92, 95% CI: 0.81–1.04, z = −1.337, p = 0.181 in Caucasians (Table 2). No publication bias was found among Caucasian and East Asian studies. Sensitivity analysis showed no individual study was significantly biasing the combined results in the Caucasian and East Asian studies (Table 3).
Fig. 1.
Forest plots of meta-analysis for the 3′ VNTR polymorphism in Caucasian (A) and East Asian (B) populations. Allele 10 vs. others.
Table 2.
Stratified meta-analysis of effect of 3′VNTR on risk for PD.
| Subgroup (n of studies included) | Allele 9 vs. others | Allele 10 vs. others | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Odds ratio | 95% CI | Significance test | Test for heterogeneity | Odds ratio | 95% CI | Significance test | Test for heterogeneity | |||||
|
|
|
|
|
|||||||||
| z | p | Q | p | z | p | Q | p | |||||
| Caucasian (7) | 1.07 | 0.93, 1.23 | 0.953 | 0.34 | 7.012 | 0.32 | 0.92 | 0.81, 1.04 | −1.337 | 0.181 | 5.347 | 0.500 |
| Asian (7) | 1.14 | 0.87, 1.48 | 0.947 | 0.344 | 7.131 | 0.309 | 0.78 | 0.65, 0.94 | −2.615 | 0.009 | 2.998 | 0.809 |
| All (18) | 1.1 | 1.00, 1.22 | 1.885 | 0.059 | 17.513 | 0.309 | 0.91 | 0.82, 1.01 | −1.738 | 0.082 | 21.937 | 0.187 |
All: all studies involved.
Table 3.
Sensitivity analysis of 3′VNTR effect on risk for PD in Caucasian and Asian populations.
| Ethnicity | Study omitted | Allele 9 vs. others | Allele 10 vs. others | ||
|---|---|---|---|---|---|
|
|
|
||||
| OR | (95% CI) | OR | (95% CI) | ||
| Caucasian | |||||
| Le Couteur et al. | 1.1 | (0.95, 1.27) | 0.91 | (0.80, 1.03) | |
| Plante-Bordeneuve et al. | 1.07 | (0.92, 1.25) | 0.92 | (0.81, 1.05) | |
| Mercier et al. | 1.08 | (0.92, 1.26) | 0.91 | (0.80, 1.04) | |
| Nicholl et al. | 1.13 | (0.99, 1.30) | 0.87 | (0.76, 1.00) | |
| Goudreau et al. | 1.04 | (0.90, 1.20) | 0.94 | (0.82, 1.07) | |
| kelada et al. | 1.03 | (0.87, 1.22) | 0.95 | (0.82, 1.10) | |
| Ritz et al. (c) | 1.03 | (0.88, 1.20) | 0.94 | (0.82, 1.08) | |
| Combined | 1.07 | (0.93, 1.23) | 0.92 | (0.81, 1.04) | |
| Asian | |||||
| Leighton et al. | 1.21 | (0.89, 1.64) | 0.75 | (0.61, 0.91) | |
| Kim et al. | 1.2 | (0.87, 1.64) | 0.79 | (0.64, 0.96) | |
| Wang et al. | 1.08 | (0.82, 1.42) | 0.82 | (0.67, 1.00) | |
| Kimura et al. | 1.17 | (0.86, 1.60) | 0.76 | (0.62, 0.93) | |
| Zhang et al. | 1.09 | (0.86, 1.38) | 0.78 | (0.64, 0.94) | |
| Lin et al. | 1.07 | (0.79, 1.45) | 0.8 | (0.65, 0.97) | |
| Zhao et al. | 1.18 | (0.88, 1.58) | 0.79 | (0.65, 0.96) | |
| Combined | 1.14 | (0.87, 1.48) | 0.78 | (0.65, 0.94) | |
We have also shown the combined effects of the four SNPs (rs6347, rs3756450, rs2652510 and rs2550956) ìn the SLC6A3 gene locus on risk for PD (Table 4). For rs2652510, we found positively combined results for genotype-wise recessive genetic model in both overall involved studies and two Caucasian studies, and allele-wise in two Caucasian studies (shown in bold). Genotype GG is associated with the higher PD risk in both overall involved studies and Caucasian studies, compared to genotype AG+AA, with G as the Caucasian risk allele. For rs6347, rs3756450 and rs2550956, the combined allele-wise and genotype-wise ORs lack any statistical significance in either overall involved studies or particular ethnicity (if applicable). For rs2652510 and rs2550956, there was significant heterogeneity (shown in italics), when the ethnicity is mixed, indicating that the effects of rs2652510 and rs2550956 on the risk of PD may be confounded by ethnicity. For rs6347, the sensitivity results are shown in Table S2. In general, no individual study was found to be significantly biased from the combined results. For rs3756450, rs2652510 and rs2550956, given the involved studies no more than three, the sensitivity analysis is not well applicable. There is no publication bias in each of those combined studies on the four SNPs.
Table 4.
Effects of SLC6A3 SNPs (rs6347, rs3756450, rs2550956 and rs2652510) on risk for PD.
| SNPs | Ethnicity (no. of studies included) | Sample size | Allele-wise 2 vs. 1 | 11 vs. (12 + 22) | 22 vs.(12 + 11) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||||||||||
| Case | Control | Odds ratio | 95% CI | Significance test | Test for heterogeneity | Odds ratio | 95% CI | Significance test | Test for heterogeneity | Odds ratio | 95% CI | Significance test | Test for heterogeneity | ||||||||
|
|
|
|
|
|
|
||||||||||||||||
| z | p | Q | p | z | p | Q | p | z | p | Q | p | ||||||||||
| rs6347 (1215A/G) | Mixed (8) | 4117 | 7663 | 0.99 | 0.93, 1.06 | −0.205 | 0.838 | 8.497 | 0.291 | 1.04 | 0.96, 1.12 | 0.868 | 0.386 | 10.747 | 0.15 | 1.10 | 0.93, 1.29 | 1.11 | 0.267 | 0.719 | 0.949 |
| Caucasian (3) | 3333 | 6737 | 1.01 | 0.94, 1.08 | 0.18 | 0.857 | 0.269 | 0.874 | 1.02 | 0.93, 1.11 | 0.352 | 0.725 | 0.452 | 0.798 | 1.10 | 0.93, 1.29 | 1.117 | 0.264 | 0.551 | 0.759 | |
| Asian (4) | 714 | 826 | 0.84 | 0.66, 1.08 | −1.376 | 0.169 | 6.331 | 0.097 | 1.32 | 0.86, 2.03 | 1.259 | 0.208 | 7.922 | 0.048 | 1.47 | 0.09, 23.68 | 0.273 | 0.785 | NA | NA | |
| rs3756450 (T/C) | Caucasian (3) | 2881 | 5239 | 1.02 | 0.93, 1.13 | 0.476 | 0.634 | 0.879 | 0.645 | 0.97 | 0.87, 1.08 | −0.515 | 0.607 | 0.299 | 0.861 | 0.93 | 0.41, 2.08 | −0.187 | 0.852 | 5.589 | 0.061 |
| rs2652510 (A/G) | Mixed (3) | 617 | 729 | 1.12 | 0.83, 1.51 | 0.721 | 0.471 | 6.346 | 0.042 | 1.01 | 0.55, 1.86 | 0.034 | 0.973 | 10.596 | 0.005 | 1.32 | 1.01, 1.73 | 1.995 | 0.046 | 0.764 | 0.683 |
| Caucasian (2) | 557 | 663 | 1.26 | 1.04, 1.54 | 2.364 | 0.018 | 1.427 | 0.232 | 0.74 | 0.52, 1.05 | −1.684 | 0.092 | 2.076 | 0.150 | 1.37 | 1.03, 1.84 | 2.143 | 0.032 | 0.153 | 0.696 | |
| rs2550956 (C/T) | Mixed (3) | 585 | 710 | 0.98 | 0.69, 1.40 | −0.119 | 0.905 | 6.222 | 0.045 | 1.07 | 0.78, 1.49 | 0.426 | 0.67 | 3.571 | 0.168 | 0.91 | 0.43, 1.95 | −0.234 | 0.815 | 4.785 | 0.091 |
| Caucasian (2) | 525 | 644 | 0.84 | 0.69, 1.01 | −1.864 | 0.062 | 0.377 | 0.539 | 1.20 | 0.95, 1.52 | 1.533 | 0.125 | 0.001 | 0.969 | 0.74 | 0.44, 1.22 | −1.197 | 0.231 | 1.384 | 0.239 | |
The allele before the “/” is predefined as allele 1, and the allele after the “/” is predefined as allele 2.
NA: not applicable.
4. Discussion
This meta-analysis reveals that the 10-repeat allele of 3′VNTR is protective against PD in East Asians but not in Caucasians. A number of factors may have contributed to this racial disparity. First, this allele has higher frequency in Asians than in Caucasians. Several studies have demonstrated ethnic differences in the SLC6A3 3′VNTR frequency distributions [17,29]. The 10-repeat allele is more common in East Asian populations (about 87%) and less common in Caucasian populations (about 70%). Linkage disequilibrium (LD) patterns and genetic structure of the SLC6A3 locus appear different between Caucasian and East Asian populations (Fig. S3). In Caucasian populations, the 3′VNTR is located in the first haplotype block; while in East Asian population, the surrounding region of the 3′VNTR is highly recombinant. Studies with 3′VNTR have revealed some associations with the risk for PD. However, the association results have been conflicted [13,16,19,20,22–24,26,27,33,36,38]. The confliction may underscore ethnic differences and poor power of detection in individual samples especially when the genetic effect is small. Second, functional studies have shown that this allele is associated with lower DAT availability [45]. These genetic and functional findings are consistent with the notion that low DAT levels may prevent DA from entering the DA neurons, resulting in low cytosolic concentration of toxic DA.
Third, the positive signals may be attributed to the environment in East Asia. In developing countries such as China, agricultural use of pesticides and herbicides, daily use of well water, air pollution and less drink of coffee, etc. all could contribute to the pathogenesis of PD [28]. A combination of these factors, high frequency of neuroprotective 10-repeat allele for low DAT availability and low cytosolic DA concentration, and the interaction of oxidative small molecules and heavy metals with DA could explain the 10-repeat allele as a protective factor against Asian PD. Positive signals with rs2652510 in Caucasians suggested that the SLC6A3 promoter confers risk as well, perhaps in an additive model for PD. Such information encourages replication of these findings in more samples and investigation of the functionality of this SNP.
Among the meta-analyses available on the PDGene website [http://www.pdgene.org/], our meta-analysis has the following advantages: (1) We checked with the corresponding authors, if important information was not mentioned in the original articles. (2) We searched multiple databases to cover as many available studies as possible in this analysis, whereas the PDGene meta-analyses rely on the PubMed. (3) We went through a rigorous analytical process that included repeating tests for HWE in genotype distributions among the controls of all included studies, evaluation of possible sources of heterogeneity, sensitivity analysis, stratified meta-analysis, and assessment of publication bias. For the above reasons, our results are reliable and robust. However, this study has certain limitations as well. For instance, there were not enough data available for stratifications with age of onset, gender and subtypes of PD. Other regions of SLC6A3 that appear to modulate transcriptional activity (e.g. intron 8 VNTR) [14] and other genes (COMT, MOA, VMAT, etc.) involved in dopaminergic system should be considered in future studies as well. Moreover, like many other chronic diseases, PD is multifactorial [4], with multi-genetic factors, epigenetic regulation, gene–gene interaction, environmental factors (such as environment pollution, pesticide exposure), life style (smoke, nutrition status and exercise activities), and environment–gene interaction playing integral roles. Hence, these multifactors should be considered in future studies as well.
Supplementary Material
HIGHLIGHTS.
We performed a combined analysis of published case–control genetic associations between SLC6A3 and Parkinson’s disease.
SLC6A3 confers a modest but significant risk for Parkinson’s disease in various populations.
Polymorphism in the 3′ untranslated region of SLC6A3 confers neuroprotection in East Asian but not in Caucasian populations.
Acknowledgments
This work was supported by National Nature Science Foundation of China 81100956 (to YZ), US National Institutes of Health grant R01DA021409 (to ZL), a Foundation of He’nan Educational Committee, China 13A310859 (to DZ), Foundation for University Key Teacher by He’nan Educational Committee, China, 2008 (to YZ), Graduate innovation project by Xinxiang Medicial University YJSCX201208Z (to SL).
Abbreviations
- DAT1 or DAT
dopamine transporter
- PD
Parkinson’s disease
- VNTR
variable number tandem repeat
- UTR
untranslated region
- SNP
single nucleotide polymorphism
- LD
linkage disequilibrium
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2013.10.060.
Footnotes
Competing interests
All authors declare that they have no competing interests.
References
- 1.Agid Y. Parkinson’s disease: pathophysiology. Lancet. 1991;337:1321–1324. doi: 10.1016/0140-6736(91)92989-f. [DOI] [PubMed] [Google Scholar]
- 2.Carlquist JF, Menlove RL, Murray MB, O’Connell JB, Anderson JL. HLA class II (DR and DQ) antigen associations in idiopathic dilated cardiomyopathy, Validation study and meta-analysis of published HLA association studies. Circulation. 1991;83:515–522. doi: 10.1161/01.cir.83.2.515. [DOI] [PubMed] [Google Scholar]
- 3.Brown AJ, Roberts DC. The effect of fasting triacylglyceride concentration and apolipoprotein E polymorphism on postprandial lipemia. Arterioscler Thromb Vasc Biol. 1991;11:1737–1744. doi: 10.1161/01.atv.11.6.1737. [DOI] [PubMed] [Google Scholar]
- 4.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 5.De Palma G, Dick FD, Calzetti S, Scott NW, Prescott GJ, Osborne A, Haites N, Mozzoni P, Negrotti A, Scaglioni A, Mutti A. A case–control study of Parkinson’s disease and tobacco use: gene–tobacco interactions. Mov Disord. 2010;25:912–919. doi: 10.1002/mds.22980. [DOI] [PubMed] [Google Scholar]
- 6.de Rijk MC, Breteler MM, Graveland GA, Ott A, Grobbee DE, van der Meche FG, Hofman A. Prevalence of Parkinson’s disease in the elderly: the Rotterdam Study. Neurology. 1995;45:2143–2146. doi: 10.1212/wnl.45.12.2143. [DOI] [PubMed] [Google Scholar]
- 7.Dick FD, De Palma G, Ahmadi A, Osborne A, Scott NW, Prescott GJ, Bennett J, Semple S, Dick S, Mozzoni P, Haites N, Wettinger SB, Mutti A, Otelea M, Seaton A, Soderkvist P, Felice A. Gene–environment interactions in parkinsonism and Parkinson’s disease: the Geoparkinson study. Occup Environ Med. 2007;64:673–680. doi: 10.1136/oem.2006.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 10.Fazeli G, Oli RG, Schupp N, Stopper H. The role of the dopamine transporter in dopamine-induced DNA damage. Brain Pathol. 2011;21:237–248. doi: 10.1111/j.1750-3639.2010.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 12.Glass GV. Primary, secondary, and meta-analysis of research. Educational Researcher. 1976;5:3–8. [Google Scholar]
- 13.Goudreau JL, Maraganore DM, Farrer MJ, Lesnick TG, Singleton AB, Bower JH, Hardy JA, Rocca WA. Case–control study of dopamine transporter-1, monoamine oxidase-B, and catechol-O-methyl transferase polymorphisms in Parkinson’s disease. Mov Disord. 2002;17:1305–1311. doi: 10.1002/mds.10268. [DOI] [PubMed] [Google Scholar]
- 14.Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Mol Psychiatry. 2006;11:125–133. 115. doi: 10.1038/sj.mp.4001764. [DOI] [PubMed] [Google Scholar]
- 15.Jeng W, Ramkissoon A, Wells PG. Reduced DNA oxidation in aged prostaglandin H synthase-1 knockout mice. Free Radic Biol Med. 2011;50:550–556. doi: 10.1016/j.freeradbiomed.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Juyal RC, Das M, Punia S, Behari M, Nainwal G, Singh S, Swaminath PV, Govindappa ST, Jayaram S, Muthane UB, Thelma BK. Genetic susceptibility to Parkinson’s disease among South and North Indians: I. Role of polymorphisms in dopamine receptor and transporter genes and association of DRD4 120-bp duplication marker. Neurogenetics. 2006;7:223–229. doi: 10.1007/s10048-006-0048-y. [DOI] [PubMed] [Google Scholar]
- 17.Kang AM, Palmatier MA, Kidd KK. Global variation of a 40-bp VNTR in the 3′-untranslated region of the dopamine transporter gene (SLC6A3) Biol Psychiatry. 1999;46:151–160. doi: 10.1016/s0006-3223(99)00101-8. [DOI] [PubMed] [Google Scholar]
- 18.Kelada SN, Costa-Mallen P, Checkoway H, Carlson CS, Weller TS, Swanson PD, Franklin GM, Longstreth WT, Jr, Afsharinejad Z, Costa LG. Dopamine transporter (SLC6A3) 5′ region haplotypes significantly affect transcriptional activity in vitro but are not associated with Parkinson’s disease. Pharmacogenet Genomics. 2005;15:659–668. doi: 10.1097/01.fpc.0000170917.04275.d6. [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Kim DH, Kim SH, Cha JK. Association of the dopamine transporter gene with Parkinson’s disease in Korean patients. J Korean Med Sci. 2000;15:449–451. doi: 10.3346/jkms.2000.15.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura M, Matsushita S, Arai H, Takeda A, Higuchi S. No evidence of association between a dopamine transporter gene polymorphism (1215A/G) and Parkinson’s disease. Ann Neurol. 2001;49:276–277. doi: 10.1002/1531-8249(20010201)49:2<276::aid-ana54>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 22.Le Couteur DG, Leighton PW, McCann SJ, Pond S. Association of a polymorphism in the dopamine-transporter gene with Parkinson’s disease. Mov Disord. 1997;12:760–763. doi: 10.1002/mds.870120523. [DOI] [PubMed] [Google Scholar]
- 23.Leighton PW, Le Couteur DG, Pang CC, McCann SJ, Chan D, Law LK, Kay R, Pond SM, Woo J. The dopamine transporter gene and Parkinson’s disease in a Chinese population. Neurology. 1997;49:1577–1579. doi: 10.1212/wnl.49.6.1577. [DOI] [PubMed] [Google Scholar]
- 24.Lin CN, Liu HC, Tsai SJ, Liu TY, Hong CJ. Association study for Parkinson’s disease and a dopamine transporter gene polymorphism (1215A/G) Eur Neurol. 2002;48:207–209. doi: 10.1159/000066162. [DOI] [PubMed] [Google Scholar]
- 25.Lin JJ, Yueh KC, Chang DC, Chang CY, Yeh YH, Lin SZ. The homozygote 10-copy genotype of variable number tandem repeat dopamine transporter gene may confer protection against Parkinson’s disease for male, but not to female patients. J Neurol Sci. 2003;209:87–92. doi: 10.1016/s0022-510x(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 26.Lynch DR, Mozley PD, Sokol S, Maas NM, Balcer LJ, Siderowf AD. Lack of effect of polymorphisms in dopamine metabolism related genes on imaging of TRODAT-1 in striatum of asymptomatic volunteers and patients with Parkinson’s disease. Mov Disord. 2003;18:804–812. doi: 10.1002/mds.10430. [DOI] [PubMed] [Google Scholar]
- 27.Mercier G, Turpin JC, Lucotte G. Variable number tandem repeat dopamine transporter gene polymorphism and Parkinson’s disease: no association found. J Neurol. 1999;246:45–47. doi: 10.1007/s004150050304. [DOI] [PubMed] [Google Scholar]
- 28.Migliore L, Coppede F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res. 2009;674:73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell RJ, Howlett S, Earl L, White NG, McComb J, Schanfield MS, Briceno I, Papiha SS, Osipova L, Livshits G, Leonard WR, Crawford MH. Distribution of the 3′VNTR polymorphism in the human dopamine transporter gene in world populations. Hum Biol. 2000;72:295–304. [PubMed] [Google Scholar]
- 30.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 31.Morino H, Kawarai T, Izumi Y, Kazuta T, Oda M, Komure O, Udaka F, Kameyama M, Nakamura S, Kawakami H. A single nucleotide polymorphism of dopamine transporter gene is associated with Parkinson’s disease. Ann Neurol. 2000;47:528–531. [PubMed] [Google Scholar]
- 32.Muangpaisan W, Hori H, Brayne C. Systematic review of the prevalence and incidence of Parkinson’s disease in Asia. J Epidemiol. 2009;19:281–293. doi: 10.2188/jea.JE20081034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholl DJ, Bennett P, Hiller L, Bonifati V, Vanacore N, Fabbrini G, Marconi R, Colosimo C, Lamberti P, Stocchi F, Bonuccelli U, Vieregge P, Ramsden DB, Meco G, Williams AC. A study of five candidate genes in Parkinson’s disease and related neurodegenerative disorders, European Stud Group on Atypical Parkinsonism. Neurology. 1999;53:1415–1421. doi: 10.1212/wnl.53.7.1415. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura M, Kaji R, Ohta M, Mizuta I, Kuno S. Association between dopamine transporter gene polymorphism and susceptibility to Parkinson’s disease in Japan. Mov Disord. 2002;17:831–832. doi: 10.1002/mds.10187. [DOI] [PubMed] [Google Scholar]
- 35.Pankratz N, Wilk JB, Latourelle JC, DeStefano AL, Halter C, Pugh EW, Doheny KF, Gusella JF, Nichols WC, Foroud T, Myers RH. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet. 2009;124:593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plante-Bordeneuve V, Taussig D, Thomas F, Said G, Wood NW, Marsden CD, Harding AE. Evaluation of four candidate genes encoding proteins of the dopamine pathway in familial and sporadic Parkinson’s disease: evidence for association of a DRD2 allele. Neurology. 1997;48:1589–1593. doi: 10.1212/wnl.48.6.1589. [DOI] [PubMed] [Google Scholar]
- 37.Ramkissoon A, Wells PG. Human prostaglandin H synthase (hPHS)-1- and hPHS-2-dependent bioactivation, oxidative macromolecular damage, and cytotoxicity of dopamine, its precursor, and its metabolites. Free Radic Biol Med. 2011;50:295–304. doi: 10.1016/j.freeradbiomed.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Ritz BR, Manthripragada AD, Costello S, Lincoln SJ, Farrer MJ, Cockburn M, Bronstein J. Dopamine transporter genetic variants and pesticides in Parkinson’s disease. Environ Health Perspect. 2009;117:964–969. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh M, Khan AJ, Shah PP, Shukla R, Khanna VK, Parmar D. Polymorphism in environment responsive genes and association with Parkinson disease. Mol Cell Biochem. 2008;312:131–138. doi: 10.1007/s11010-008-9728-2. [DOI] [PubMed] [Google Scholar]
- 41.Spencer WA, Jeyabalan J, Kichambre S, Gupta RC. Oxidatively generated DNA damage after Cu(II) catalysis of dopamine and related catecholamine neurotransmitters and neurotoxins: role of reactive oxygen species. Free Radic Biol Med. 2011;50:139–147. doi: 10.1016/j.freeradbiomed.2010.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 43.Uhl GR. Dopamine transporter: basic science and human variation of a key molecule for dopaminergic function, locomotion, and parkinsonism. Mov Disord. 2003;18(Suppl 7):S71–S80. doi: 10.1002/mds.10578. [DOI] [PubMed] [Google Scholar]
- 44.Uhl GR. Hypothesis: the role of dopaminergic transporters in selective vulnerability of cells in Parkinson’s disease. Ann Neurol. 1998;43:555–560. doi: 10.1002/ana.410430503. [DOI] [PubMed] [Google Scholar]
- 45.van de Giessen E, de Win MM, Tanck MW, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med. 2009;50:45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- 46.Wang Association between genetic polymorphism of dopamine transporter gene and susceptibility to Parkinson’s disease. Natl Med J China. 2000;80:346–348. [PubMed] [Google Scholar]
- 47.Xu M, Lin Z. Genetic influences of dopamine transport gene on alcohol dependence: a combined analysis of 13 studies with 2483 cases and 1753 controls. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1255–1260. doi: 10.1016/j.pnpbp.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Association between dopamine transporter gene polymorphism and Parkinson’s disease. Chin J Med Genet. 2001;18:431–434. [PubMed] [Google Scholar]
- 49.Zhao Parkinson’s disease sensitivity and the 402 bp VNTR polymorphism of DAT gene. Chin J Geriatr. 2004;23:457–459. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.