Abstract
Glaucoma is a multi-factorial neurodegenerative disorder. The common denominator in all types of glaucomas is retinal ganglion cell death through apoptosis. However, this cellular demise in glaucoma is detected late by structural or functional analyses. There can be a 10-year delay prior to the appearance of visual field defects and pre-perimetric glaucoma is an issue still being addressed. However, a new cutting-edge technology called detection of apoptosing retinal cells (DARC) is being developed. This technique is capable of non-invasive, real-time visualization of apoptotic changes at the cellular level. It can detect glaucomatous cell damage at a very early stage, at the moment apoptosis starts, and thus management can be initiated even prior to development of visual field changes. In future, this technique will also be able to provide conclusive evidence of the effectiveness of treatment protocol and the need for any modifications which may be required. This article aims to provide a concise review of DARC technology.
Keywords: Apoptosis, Retinal Ganglion Cells, Annexins, Propidium
Introduction
Glaucoma is a neuro-degenerative disorder characterized by wide-spread loss of retinal ganglion cells (RGC). This RGC demise occurs through apoptosis which is a cascade of cellular events leading to programmed cell death. Apoptosis of RGCs is an early and critical event in the evolution of glaucomatous visual field loss. However, it is well known that up to 20–40% of the RGCs might already be lost before the visual field defects become apparent. This leads to an unfortunate 10-year delay in the diagnosis of glaucoma.1, 2, 3
Recently, a promising new technology has been reported to detect RGC apoptosis in vivo. This technique is known as “Detection of Apoptosing Retinal Cells” (DARC). With the application of this method it will be possible to identify patients prior to the onset of irreversible vision loss. Apart from diagnosis of glaucoma suspects, it will also provide a rapid and objective assessment of the effectiveness of clinical intervention in the patient and shorten the period of clinical trials.1, 4
This review throws light on DARC technology and its utility in glaucoma management.
Apoptosis
RGC loss in glaucoma is an important early step. RGC demise is initiated due to a pathological event, such as ischemia, axonal injury or changes in the lamina cribrosa activating apoptosis. This is a type of programmed cell death characterized by a carefully coordinated collapse of cellular architecture associated with protein degradation and de-oxy ribonucleic acid (DNA) fragmentation. This process is seen in a large variety of tissues during the life cycle of the individual. It includes a genetically programmed destruction of specific cell lines during embryogenesis, hormone-dependent physiologic involution (such as the endometrium during the menstrual cycle), cell death of populations (like the intestinal epithelium and neutrophils), as well as following cellular injury due to non-specific factors such as drugs, cancer and infection.5
Apoptosis differs from necrosis which causes a potentially damaging inflammatory response. In necrosis the cell is a passive victim and follows an energy independent mode of death. Unlike apoptosis that can affect single-cells, necrosis usually involves large fields of cells. Apoptosis is a neat process characterized by pathognomonic features of cell shrinkage and chromatin condensation. This is followed by fragmentation of DNA, presumably by the activation of endonucleases. The cells shrink, form cytoplasmic buds and fragment into apoptotic bodies composed of membrane bound vesicles of cytosol and organelles. This is called membrane blebbing. Finally, the apoptotic bodies are extruded from the cell and phagocytosed or degraded by macrophages or neighboring cells.1, 3, 6, 7
Apoptosis consists of 2 phases:
-
(i)
Initiation Phase: During this period a group of enzymes called caspases become catalytically active.
-
(ii)
Execution Phase: This is characterized by activity of another set of caspases leading to cell death. Caspases are a family of proteases which have a cysteine at their active site and cleave their target proteins at specific aspartic acids (Cysteine requiring ASPartate proteASE activity). Caspases exist as inactive precursors or procaspases known as “initiator caspases”. Once activated, the caspases go on cleaving and activating other caspases resulting in an amplifying proteolytic cascade.8
Some of the activated caspases (“Effector caspases”) then cleave a number of key proteins in the cell, including cystolic proteins and nuclear lamins, leading to a controlled death of the cell. Procaspases can also be triggered by activation of “death receptors” on the cell surface. The adaptor proteins bind to the intracellular region of aggregated proteins causing further aggregation and subsequent cleavage of caspases. This initiates the caspase cascade.8, 9
An early and critical event during apoptosis is the acquisition of surface changes by the dying cells. Other changes also take place, including expression of thrombospondin binding sites, loss of sialic acid residues and exposure of the membrane phospholipid, including phosphotidylserine.10
In viable cells, phosphotidylserine is located in the inner leaflet of the plasma membrane. Phospholipid binding cytoskeletal proteins such as spectrin and aminophospholipid translocases are assumed to play a role for this asymmetric distribution of phosphotidylserine. During apoptosis, the equilibrium changes with an increasing appearance of phosphotidylserine on the outer leaflet of the plasma membrane. This activity is probably facilitated by unknown specific intrinsic membrane proteins. There is evidence for the transport (flip-flop) of phosphotidylserine from the internal to the external leaflet of the plasma membrane as a rapidly active process facilitated by an ATP-dependent membrane translocase-P.10, 11
Phosphotidylserine exposure precedes the nuclear changes which define apoptosis and also precedes the loss of plasma membrane integrity by several hours. Apparently, five separate membrane changes occur, which lead to recognition of apoptotic cells by phagocytes. The scavenging cells recognize the apoptotic cells due to the surface phosphotidylserine and other receptors, finally engulfing them. This is done without any inflammatory reaction.11
The DARC technique utilizes these activities going on in cells, specially the early exposure of phosphotidylserine to the outer leaflet of plasma membrane, in order to detect RGCs in the stage of apoptosis.
DARC technique
Annexin V is a Ca++ dependent phospholipid-binding protein. It has high affinity for phosphotidylserine localized on the outer leaflet of cell membranes. Thus, Annexin V bound to phosphotidylserine has been utilized as a sensitive probe for the identification of apoptosing cells.12
DARC technology has been tested on a number of animal models. In the Morrison rat model the intra-ocular pressure is elevated by injecting hypertonic saline into the episcleral veins. Dark Agouti rats have also been used as apoptosis models either by transecting the optic nerve 2–3 mm behind the eyeball (but sparing the vasculature) or by intravitreal injection of different doses of staurosporine. A similar staurosporine primate model has been developed in macaque monkeys. Annexin V is labeled with a fluorescent marker (such as fluorescein isothiocyante [FITC]). This Annexin V-FITC complex is then used as an intravitreal probe. The ideal dose of the fluoprobe is 2.5 μg in 5 ml of PBS for the rat and 25 μg in 5 ml of PBS for the primate model.4, 5, 13
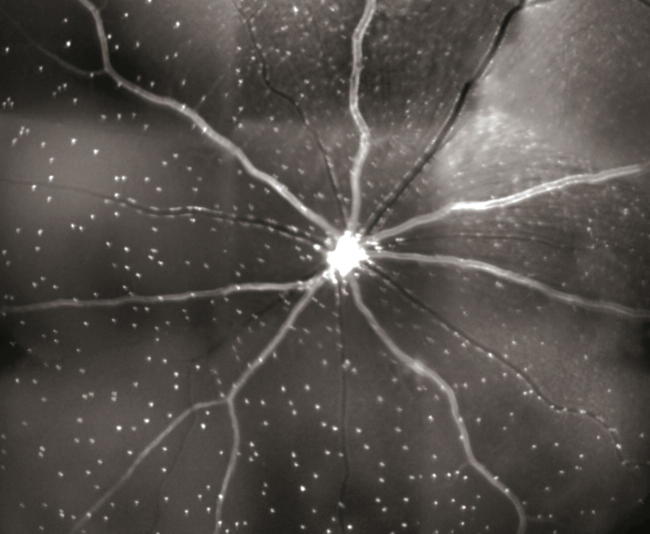
An argon laser of 488 nm wavelength is scanned across the retina to excite the fluoprobe. The emitted fluorescent rays are focused by a confocal aperture and pass through a pair of scanning mirrors. The fluorescence is detected by a solid-state photodetector, having a wide band-pass filter. A confocal scanning laser ophthalmoscope (cLSO) with image browser software is used to assess the retina. The software is able to compensate for any ocular movement and also improves the signal-to-noise ratio. The fluorescent white spots, representing apoptotic cells, are detected by a frame grabber and stored digitally (Fig. 1). The fluoprobe bound RGCs are then counted by manual, semi-automated or automated techniques.13, 14, 15, 16
Figure 1.
DARC image: Each white spot is an individual retinal ganglion cell labeled with fluorescent Annexin V undergoing apoptosis. (Published with permission from: Cordeiro MF, Guo L, Coxon KM, Duggan J, Nizari S, Normando E, et al. Real time imaging of retinal ganglion cell apoptosis. European Ophthalmic Review. 2010; 4: 88–91.)
Manual labeling of the DARC spots can be done by the ImageJ ‘multi-point selections’ tool. Manual counting is regarded as the gold standard but it requires experience, is time-consuming, monotonous, non-reproducible and subject to observer bias.17, 18
A semi-automated system has been developed by Cordeiro et al. In this procedure, the total number of apoptosing RGCs for each time point in vivo is calculated and an average density count per mm2 is generated. This DARC count is then utilized to assess disease activity in each eye, as well as the progression and response to treatment.14, 15, 16
Another automated cell labeling technique uses a Matlab script. The “cells” are labeled in green and “non-cellular” structures labeled in red. The script is designed to automatically count the total number of spots identified as cells.17
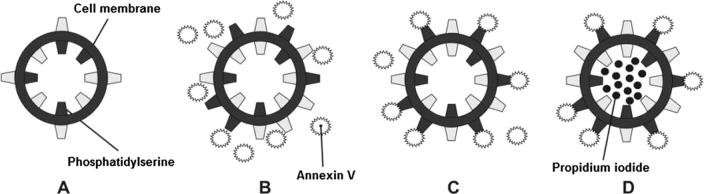
Annexin V binding is often done in conjunction with a vital dye such as propidium iodide (Fig. 2). Viable cells with an intact plasma membrane exclude propidium iodide. However, in dead or damaged cells, the plasma membrane is leaky, allowing propidium iodide to leak inside. Thus, cells can be tracked over time. They can progress from Annexin V and propidium iodide negative (viable cells), to Annexin V positive/propidium iodide negative (early apoptosis) and finally, Annexin V and propidium iodide positive (end stage apoptosis and death).19
Figure 2.
A: Healthy cell with phosphotidylserine on the inner leaflet of plasma membrane. B: Early apoptosing cell with externalization of phosphotidylserine. C: Binding of the fluoprobe to the exposed phosphotidylserine. D: Late apoptotic/necrotizing Propidium Iodide positive cell.
Advantages of DARC
There are other methods available for detection of apoptotic cells. These include loss of cell viability (failure to either exclude vital dyes or transform tetrazolium salts to colored products), DNA fragmentation (assayed by agarose gel electrophoresis or in situ terminal transferase labeling) and DNA condensation (detected by Hoechst dye staining of nuclear DNA). The methods providing quantitative data lack specificity, are time-consuming and usually require the destruction of cell integrity. These assays are also based on morphological changes and degradation of chromatin which occur late in apoptosis.5
Annexin V binding is non-enzymatic, does not require fixation and allows one to score apoptotic cells in living unfixed samples. A clear optical media permit direct visualization of disease processes as they occur; thus, images can be acquired repeatedly in a non-invasive manner. DNA fragmentation assays, mentioned above, are time consuming and take 3–4 h, while Annexin V binding requires only a few minutes.5, 17
Annexin V binding enables non-invasive real time visualization of single retinal cells undergoing apoptosis. It is also possible to monitor changes as they occur and longitudinally as they progress. The Annexin V assay also permits kinetic measurements of the number of apoptotic cells in relation to time after insult.2, 14, 15 In a study performed by Zhang et al., using a 32D cell line, the Annexin V positive apoptotic cells were scored over a period of time. A timeline of apoptotic spots showed the following relationship: 6.6% positive cells (At zero hours after initiation of apoptosis), 11% (at 6 h), 22% (at 8 h), 34% (at 15 h) and 40% (at 24 h).5
DARC has the potential to help elucidate the cellular mechanisms involved in the development of RGC degeneration and vision loss in glaucoma. It will also enable us to gauge when to start treatment to prevent visual field loss and monitor treatment effectiveness. It would provide a rapid and objective assessment of potential and effective therapies, providing a new and meaningful clinical endpoint in glaucomatous disease. DARC has shown to provide a test of efficacy for neuroprotective treatments in several models of glaucoma. This test may also serve as a biomarker providing rapid information which could dramatically reduce the duration of glaucoma clinical trials. These studies presently are dependent upon visual field status as a key endpoint and determinant of the outcome.1, 2, 4, 11, 13
Disadvantages of DARC
DARC is a fairly new and experimental technology. Thus, the parameters and procedures for this method have not yet been established. The intravitreal injection of fluoprobes should be replaced with oral or perhaps intravenous routes and operating on an office-based device to perform these tests in order to incorporate it in the total management of glaucoma patients. In situations where the DARC spots are small and of low luminance, it is difficult to differentiate between cellular and non-cellular structures (e.g. parts of blood vessels). It is not clear how or whether apoptotic non-RGCs may affect the DARC count. It is also not known whether DARC would be able to differentiate between cell deaths due to glaucoma from other causes (e.g. aging). Automatic cell counting is also affected by the problem of background noise being mislabeled as cells and the large variability in cell size limits the accuracy in identifying the cells.1, 16
Future
DARC technology can be further improved by the application of wide-angle lenses, selection of the best wavelength in order to enhance signal to noise ratios and improving the correlation between in vivo and histological counts. Better flow cytometry (FCM) techniques may allow dissemination between different cell subpopulations, which may or may not be involved in the apoptotic process.1, 10 These additional inputs can be incorporated into DARC studies.
Conclusion
DARC is a revolutionary new technology which is enabling us to perform in vivo resolution and real-time and non-invasive imaging of single cells undergoing apoptosis. This can provide us information rapidly and over a variable period of time. Thus, it will help us in diagnosis of RGC loss even before visual field defects develop. This technology will also permit an objective assessment of the efficacy of treatment and the need to modify management of glaucoma. It remains to be seen how this technology will be utilized in the clinical diagnosis of glaucoma in the future.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Cordeiro M.F., Migdale C., Bloom P., Fitzke F.W., Moss S.E. Imaging apoptosis in the eye. Eye (Lond) 2011;25:545–553. doi: 10.1038/eye.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cordeiro M.F., Guo L., Coxon K.M., Duggan J., Nizari S., Normando E. Real time imaging of retinal ganglion cell apoptosis. Eur Ophthal Rev. 2010;4:88–91. [Google Scholar]
- 3.Qu J., Wang D., Grosskreutz C.L. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp Eye Res. 2010;91:48–53. doi: 10.1016/j.exer.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordeiro M.F. DARC: a new method for detecting progressive neuronal death. Eye. 2007;21:S15–S17. [Google Scholar]
- 5.Zhang G., Gurtu V., Kain S.R., Yan G. Early detection of apoptosis using a fluorescent conjugate of Annexin V. BioTechniques. 1997;23:525–531. doi: 10.2144/97233pf01. [DOI] [PubMed] [Google Scholar]
- 6.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuehn M.H., Fingert J.H., Kwon Y.H. Retinal ganglion cell death in glaucoma: mechanisms and neuroprotective strategies. Ophthalmol Clin N Am. 2005;18:383–395. doi: 10.1016/j.ohc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad S.S., Ghani S.A., Rajagopal T.H. Current concepts in the biochemical basis of glaucomatous neurodegeneration. J Clin Glaucoma Pract. 2013;7:49–53. doi: 10.5005/jp-journals-10008-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H., Sun H., Liu C. Interference of the apoptotic signaling pathway in rgc stress response by sp600125 in moderate ocular hypertensive rats. Chin J Physiol. 2011;54:124–132. [PubMed] [Google Scholar]
- 10.Vermes I., Haanen C., Steffens-Nakken H., Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin C. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 11.Martin S.J., Reutelingsperger C.P., McGahon A.J., Rader J.A., van Schie R.C., LaFace D.M. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Normando E.M., Turner L.A., Cordiero M.F. The potential of annexin-labelling and follow-up of glaucoma. Cell Tissue Res. 2013;353:279–285. doi: 10.1007/s00441-013-1554-5. [DOI] [PubMed] [Google Scholar]
- 13.Cordeiro M.F., Guo L., Luong V., Harding G., Wang W., Jones H.E. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci USA. 2004;101:13352–13356. doi: 10.1073/pnas.0405479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz-Valckenberg S., Guo L., Maass A., Cheung W., Vugler A., Moss S.E. Real-time in vivo imaging of retinal cell apoptosis after laser exposure. Invest Ophthalmol Vis Sci. 2008;49:2773–2780. doi: 10.1167/iovs.07-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maass A., von Leithner P.L., Luong V., Guo L., Salt T.E., Fitzke F.W. Assessment of rat and mouse rgc apoptosis imaging in vivo with different scanning laser ophthalmoscopes. Curr Eye Res. 2007;32:851–861. doi: 10.1080/02713680701585872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordeiro M.F., Guo L., Coxon K.M., Duggan J., Nizari S., Normando E.M. Imaging multiple phases of neurodegeneration: a novel approach to assessing cell death in vivo. Cell Death Dis. 2010;1:e3. doi: 10.1038/cddis.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bizrah M., Dakin S.C., Guo L., Rahman F., Parnell M., Normando E. A semi-automated technique for labeling and counting of apoptosing retinal cells. BMC Bioinformatics. 2014;15:169. doi: 10.1186/1471-2105-15-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo L., Moss S.E., Alexander R.A., Ali R.R., Fitzke F.W., Cordeiro M.F. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthal Vis Sci. 2005;46:175–182. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Engeland M., Nieland L.J., Ramaekers F.C., Bert Schutte B., Reutelingsperger C.P. Annexin V-Affinity Assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]