Abstract
Inhalation of carbon dioxide (CO2) is frequently employed as a biological challenge to evoke intense fear and anxiety. In individuals with panic disorder, CO2 reliably evokes panic attacks. Sensitivity to CO2 is highly heterogeneous among individuals, and although a genetic component is implicated, underlying mechanisms are not clear. Preclinical models that can simulate differential responsivity to CO2 are therefore relevant. In the current study we investigated CO2-evoked behavioral responses in four different rat strains: Sprague-Dawley (SD), Wistar (W), Long Evans (LE) and Wistar-Kyoto, (WK) rats. We also assessed tryptophan hydroxylase 2 (TPH-2)-positive serotonergic neurons in anxiety/panic regulatory subdivisions of the dorsal raphe nucleus (DR), as well as dopamine β hydroxylase (DβH)-positive noradrenergic neurons in the locus coeruleus, implicated in central CO2-chemosensitivity. Behavioral responsivity to CO2 inhalation varied between strains. CO2-evoked immobility was significantly higher in LE and WK rats as compared with W and SD cohorts. Differences were also observed in CO2-evoked rearing and grooming behaviors. Exposure to CO2 did not produce conditioned behavioral responses upon re-exposure to CO2 context in any strain. Reduced TPH-2 positive cell counts were observed specifically in the panic-regulatory dorsal raphe ventrolateral (DRVL)-ventrolateral periaqueductal grey (VLPAG) subdivision in CO2-sensitive strains. Conversely, DβH positive cell counts within the LC were significantly higher in CO2-sensitive strains. Collectively, our data provide evidence for strain dependent, differential CO2-sensitivity and potential differences in monoaminergic systems regulating panic and anxiety. Comparative studies between CO2-vulnerable and resistant strains may facilitate the mechanistic understanding of differential CO2-sensitivity in the development of panic and anxiety disorders.
Keywords: panic, CO2 sensitivity, serotonergic, noradrenergic, dorsal raphe, locus coeruleus
Introduction
Inhalation of CO2-enriched air produces psychological and physiological responses that can promote anxiety and fear-like behavior. In humans CO2-sensitivity lies on a continuum (Colasanti et al., 2008), as the composition, frequency, and severity of CO2-evoked phenotypes have been found to be quite heterogeneous within the population. First described in 1951 by Cohen and White (Cohen and White, 1951), CO2 inhalation is an established biological challenge, as individuals with a heightened risk for panic disorder (PD) elicit CO2-hypersensitivity indexed by exaggerated emotional and respiratory responses (Papp et al., 1993; Rassovsky and Kushner, 2003; Leibold et al., 2013). In the extracellular fluid, CO2 is hydrolyzed to carbonic acid (H2CO3) by carbonic anhydrase which readily dissociates into bicarbonate (HCO3−) and H+ (Huckstepp and Dale 2011). The resulting acidosis is thought to be the trigger for the panic and fear symptoms, and neuroimaging studies on PD patients support a role of homeostatic pH disturbances in panic physiology (Maddock et al, 2009).
Evidence from genetically informed studies support risk factors that influence liability to heightened sensitivity to CO2, an endophenotype to PD (Battaglia et al., 2014). Heightened sensitivity to CO2 has been associated with both childhood separation anxiety and adult panic disorder (PD) with predispositions to either disorder founded largely on genetic factors (Battaglia et al., 2009). Additionally, twin studies have shown significant association with shared genetic components to CO2-sensitivity (Bellodi et al., 1998; Battaglia et al., 2007), and the degree of familial relationships to panic disorder patients has been shown to be associated with CO2 sensitivity (Perna et al., 1996; Coryell et al., 2001). Collectively, these observations strongly support CO2-hypersensitivity as a valid biological risk and trait marker for panic and anxiety disorders. Currently, biological underpinnings of individual variance in CO2 sensitivity are not well understood. Rodent and human studies provide evidence for a role of gene x environment interactions towards heightened CO2 reactivity (D’Amato et al, 2011; Spatola et al, 2011). Although a strong genetic predisposition and gene x environment contributions to CO2 hypersensitivity has been proposed, contributory mechanisms are not clear. Development of preclinical models that can simulate differential responsivity to CO2 inhalation is relevant and can facilitate mechanistic understanding of this phenomenon.
Rodent models of CO2 inhalation-evoked behavior and physiological responses have been studied previously (see Battaglia et al, 2014; Johnson et al, 2014; Vollmer et al, 2015 and references therein), however, studies on variation in CO2 responsivity have been limited. Previously, strain-dependent variation in CO2-evoked ventilation was reported in mice (Tankersley et al, 1994). The current study assessed differential behavioral sensitivity to CO2 inhalation in four rat strains with distinct genetic backgrounds. Our primary objective was to determine whether CO2 inhalation evoked distinct strain-dependent responses in rats with the purpose to develop a rodent model of susceptibility/resistance to CO2 inhalation. We selected three commonly used outbred strains (Sprague-Dawley (SD), Wistar (W) and Long Evans (LE) rats) and one inbred (Wistar-Kyoto, WK) strain. In this initial study, our objective was to capture the wide genetic diversity and phenotypic variation between these commonly used rat strains as there are no studies available comparing CO2-inhalation responses between these strains. We anticipated greater CO2 response variation within outbred strains compared with the inbred WK animals where genetic homogeneity and lower variability may be useful for mechanistic studies. In addition to behavioral measurements, we also investigated tryptophan hydroxylase 2 (TPH-2)-positive serotonergic neurons in anxiety and panic regulatory subdivisions of the dorsal raphe nucleus (DR), as well as dopamine β hydroxylase (DβH)-positive noradrenergic neurons in the locus coeruleus (LC). The DR houses topographically organized subsets of serotonergic neurons. These include subpopulations in the dorsal region (DRD) that project to forebrain circuits modulating anxiety-related behaviors, while neurons within the dorsal raphe ventrolateral (DRVL)-ventrolateral periaqueductal grey (VLPAG) division provide inhibitory input to the dorsal PAG to attenuate panic-relevant responses (Hale and Lowry, 2011). TPH-2 positive serotonergic neurons in the dorsal raphe are CO2-chemosensitive and therefore have the potential to impact CO2 evoked behavior and physiology (Severson et al., 2003). Serotonergic neurons within the DRVL are significantly activated by CO2 inhalation (Johnson et al., 2011) and may represent a ‘sympathomotor control system’ that normally limits autonomic/behavioral responses to interoceptive threats.
The LC is an established central CO2-chemosensitive site (Gargaglioni et al., 2010) and contains the major group of noradrenaline (NA) synthesizing neurons, the A6 cell group. NA cell bodies are well connected to brain regions regulating arousal, anxiety, autonomic responses, and memory. LC activation may regulate CO2-sensitivity, as lesioning of rat LC noradrenergic neurons has been associated with attenuated physiological responses to CO2 inhalation (Biancardi et al., 2008). Neuroimaging studies also reveal increased blood oxygen level dependent (BOLD) signal in brain stem areas including the LC following CO2 inhalation in humans (Pattinson et al., 2009). In addition to their well characterized regulatory role in panic and anxiety, the LC and the DR have reciprocal interactions, with the DR exerting an inhibitory effect on LC while the locus is reported to exert excitatory action on the DR (Szabo et al, 2001; Vandermaelen and Aghajanian 1983).
We hypothesized a strain-dependent variance in CO2 responsivity, accompanied by reduced TPH-2 and enhanced DβH immunoreactivity in the DR and LC respectively in CO2-sensitive strains.
Experimental Procedures
Animals
All experiments reported here were performed on adult rats (300–350g) purchased from Harlan. All rat strains (WI, WK, LE and SD) were housed under constant temperature (23–28°C) with a 12hr light, 12hr dark cycle (lights on at 06:00h). Food and water were provided ad libitum. All behavioral experiments were performed between 8am–1pm during the 12h light cycle. Study protocols were approved by the Institutional Animal Care and Use Committee of University of Cincinnati in a vivarium accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). A total of 48 animals were used for the study (n=6 rats/group).
CO2 inhalation
Animals were exposed to a three day paradigm (see Fig 1) consisting of habituation (day 1), air or CO2 exposure (day 2) and CO2-context exposure (day 3) in the absence of CO2 as described in previous studies from our group (Vollmer et al, 2016), with modifications. This enabled an assessment of unconditioned (day 2) and conditioned (day 3) behavioral responses to CO2 inhalation. Briefly, rats were habituated to a Plexiglas CO2 chamber (8″x 8″ x 6.5″) for 10 min one day prior to the CO2 challenge. On the following day, animals were exposed to infused breathing air or 10% CO2 (in 21% O2, balance with N2, Wright Brothers Inc., Cincinnati, OH) for 10 min in a dual, top-bottom chamber during which behavior was videotaped. Air/CO2 was infused into the top chamber and controlled through a valve in the ceiling of the bottom chamber (8″x8″x 6.5″) to avoid direct blowing, which is aversive to rodents. Prior to placing each animal the bottom chamber was pre-saturated with CO2, the animal was placed and the infusion continued for the rest of the experiment. This created a steady-state of infused air or 10% CO2 concentration, controlled by a flow meter to ensure a constant infusion rate of 10L/min for all animals. This CO2 concentration range is translationally relevant to challenge studies in humans (Rassovsky et al, 2003). Animals were returned to their home cage after the 10 min CO2 exposure. 24 hr following air/CO2 inhalation, rats were placed in the CO2 chamber (context) for 5 min in the absence of infused breathing air or CO2 and videotaped. Videos were scored by a trained observer blinded to genotype and treatment for total time spent immobile, as well as grooming and rearing frequency. Immobility, defined as the absence of all movement except for respiration, was our primary behavioral measure based on CO2-evoked fear behavior observed in previous studies from our group and others (Mongeluzi et al., 2003; Esquivel et al., 2010). Grooming included all bouts of facial and body grooming time. Rearing was scored when both front legs were lifted off the ground.
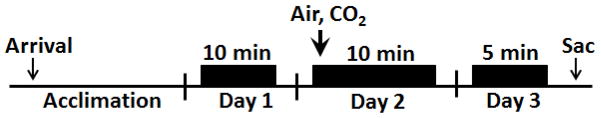
Figure 1.
Schematic showing the experimental layout for measuring CO2-evoked behavior and conditioned responses to context exposure. Following arrival animals were acclimated to the facility for a week prior to behavioral measurement. The CO2 inhalation paradigm (Day 1–3) consisted of habituation (Day 1) where animals were exposed to the CO2 chamber for 10 minutes. On Day 2, animals were exposed to either air or CO2 (10%) for 10 minutes while being videotaped. 24hr post inhalation animals were returned to the CO2 context for 5 minutes in the absence of gas inhalation and were videotaped. Following behavior, animals were sacrificed and brain tissue collected for further analyses.
Histology and Immunofluorescence
For histological assessments, air and CO2-exposed animals were sacrificed on Day 3, 1 hr following CO2-context exposure (Day 3). Rats were deeply anesthetized via i.p. administration of Fatal Plus (Vortech, USA) and perfused transcardially with ice cold 4% paraformaldehyde (in 0.1M Na2HPO4 / NaH2PO4 buffer, pH 7.4). Brains were removed, postfixed overnight, and then transferred to 30% sucrose (in KPBS) until ready for sectioning. Tissue was cut at 30 μm on a sliding microtome. The resulting sections were stored in cryoprotectant (0.1 M phosphate buffer, 30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylene glycol) at −20°C until processed for immunofluorescence. The following primary antibodies were used for immunofluorescence: tryptophan hydroxylase (TPH) 1:500 (MAB308, Millipore) and dopamine beta hydroxylase (DβH) 1:2500 (T0678, Sigma). Sections were transferred from cryoprotectant to 50 mM potassium PBS (KPBS; pH 7.4; 40 mM potassium phosphate dibasic, 10 mM potassium phosphate monobasic, and 0.9% sodium chloride) at RT. Cryoprotectant was rinsed (five times for 5 min) in KPBS, and the sections were transferred to KPBS plus 0.3% H2O2 and incubated for 10 min at RT. Sections were then washed (five times for 5 min) in KPBS at RT and placed in blocking solution [50 mM KPBS, 0.5% bovine serum albumin (BSA), and 0.2% Triton X-100] for 1 hr at RT. Sections were incubated overnight at 4°C in the specified primary antibodies diluted in blocking solution. The following day, sections were rinsed in KPBS (five times for 5 min) and incubated in CY3-conjugated mouse antibody (Jackson ImmunoResearch) diluted 1:500 in KPBS plus 0.5% BSA for 1 hr at RT. Sections were rinsed five times for 5 min in KPB at RT, mounted onto Superfrost Plus slides, and coverslipped with Gelvatol (Fluka).
Imaging and Cell Counting
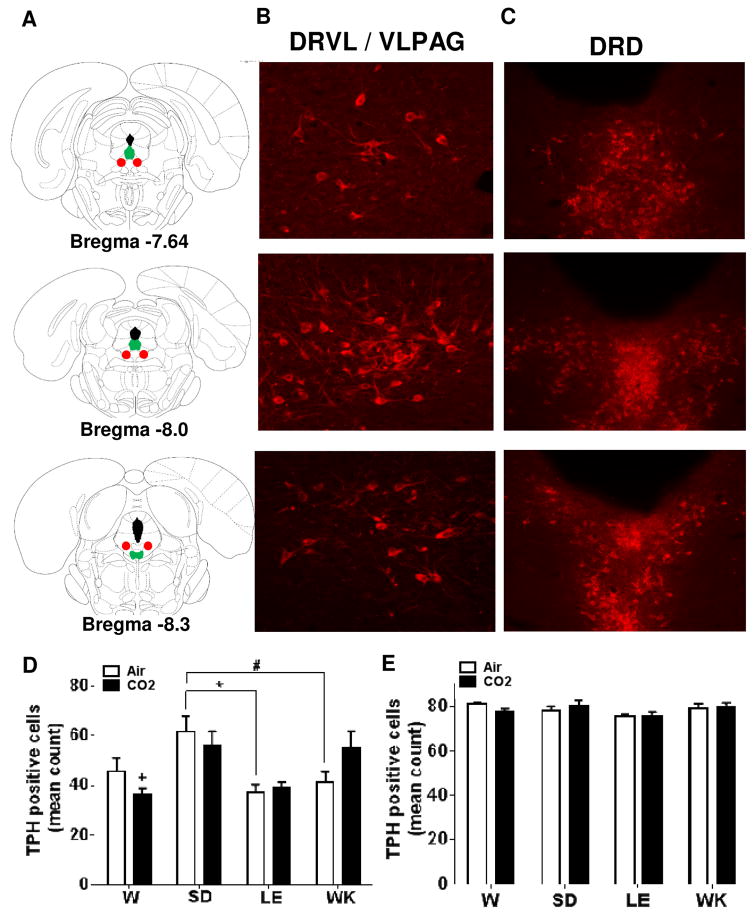
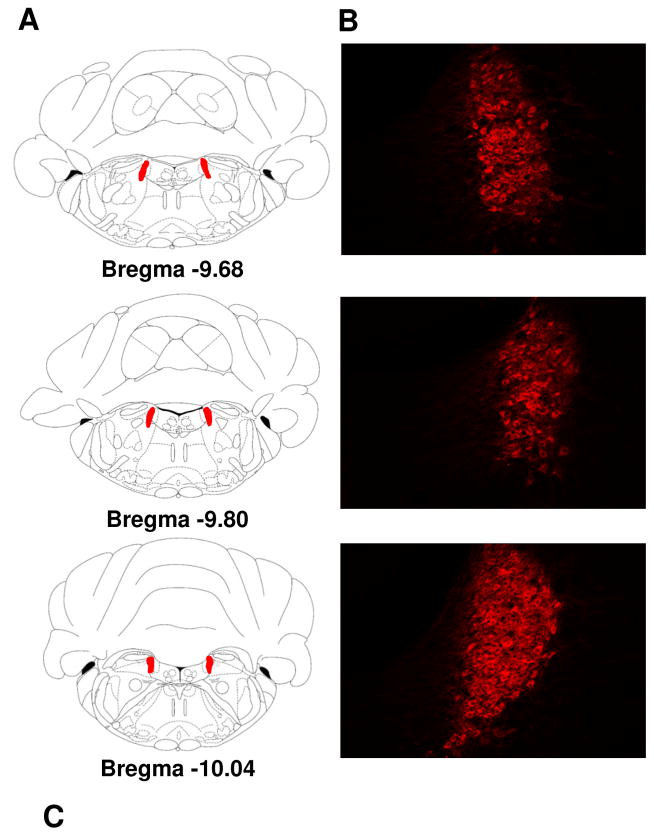
Brain sections were imaged using an AxioImager Z1 microscope (Zeiss), utilizing apotome imaging capability to create z-stacks (Axiocam MRm camera and AxioVision Release 4.8 software; Zeiss). Cy3 was excited using the 568 nm wavelength. Images were acquired for TPH-2 positive cells from the DRVL/VLPAG and the DRD between rostro-caudal levels (−7.64, −8.0, −8.3) and DβH-positive cells within the locus coeruleus at (−9.68, −9.80, −10.04) levels as Z-stacks (see Fig 4 a–c and 5 a–b). Cell counts were accumulated across 0.8μm z-stacks for each image using the interactive measurement and event tool (AxioVision 4.8) by an observer blinded to experimental groups. Cell counts from rostro-caudal coordinates specified above were averaged for each animal for statistical analysis.
Figure 4.
Alterations in tryptophan hydroxylase-2 (TPH-2) immunoreactivity within dorsal raphe subdivisions in air and CO2 exposed Wistar (W), Sprague Dawley (SD), Long Evans (LE) and Wistar Kyoto (WK) rats. (A) Panels on the left show stereotaxic illustrations from the atlas of Paxinos & Watson depicting rostral (bregma −7.64) to caudal (bregma −8.3) extent from which cells were quantified. Color represent areas where cells were distributed [red (dorsal raphe ventrolateral -ventrolateral periaqueductal grey; DRVL/VLPAG) and green (dorsal raphe dorsal; DRD)]. Representative images showing (TPH-2) immunopositive cells within the DRVL/VLPAG (panel B) and DRD (panel C). Panel (D) shows TPH-2 cell counts within the DRVL/VLPAG in air and CO2 exposed animals. Significant strain but no CO2-dependent differences were observed. (* p<0.05; # p=0.053; + p<0.05 versus SD and WK CO2 groups) (E) No significant differences were observed in TPH-2 positive cells within the DRD subdivision of the raphe. All data are mean ± s.e.m (n=6 animals/air or CO2 groups). Images were acquired at 10X magnification for the DRD and included core and shell areas; and 20X for the DRVL/VLPAG.
Figure 5.
Alterations in dopamine β hydroxylase (DβH)-positive noradrenergic neurons in the locus coeruleus in air and CO2 exposed Wistar (W), Sprague Dawley (SD), Long Evans (LE) and Wistar Kyoto (WK) rats. (A) Panels on the left show stereotaxic illustrations from the atlas of Paxinos & Watson depicting rostral (bregma −9.68) to caudal (bregma −10.04) from which cells were quantified (red shows LC area). Panel (B) shows representative images showing (DβH) immunopositive cells within the coordinates shown in (A). Panel (C) shows DβH cell counts in air and CO2 exposed animals (* p<0.05 LE and WK air versus SD group; # p= 0.06 LE and WK versus W group; + p<0.05 LE versus WK group). All data are mean ± s.e.m (n=6 animals/air or CO2 groups).
Data Analysis
Behavior scored manually by individuals blinded to groups was used for analysis. Normality was formally tested for all experiments. Data met assumptions of the statistical tests being used. Where appropriate, variance was tested between data sets using the F test or Bartlett’s test for equal variances. Variance was found to be similar between most data groups. Data were analyzed by two-way ANOVAs using strain x inhalation as variables. Data was also analyzed using a two-way between strains multivariate analysis of variance for analyzing strain x treatment effects using freezing, rearing and grooming as combined dependent variables. For immunofluorescence, two- way ANOVA was used for analyzing mean cell counts with strain x inhalation as variables. Where main effects were significant, post hoc comparisons were performed using Tukey’s analysis. Data are presented as mean ± sem and were considered significant at p <0.05. Prism software was used for two-way ANOVA statistical analysis (GraphPad Software version 6, Inc., La Jolla, CA) and Statistica (version 13 Dell, Round Rock, TX) was used for the multivariate analysis on combined variables.
Results
Behavioral responses to CO2 are strain-dependent
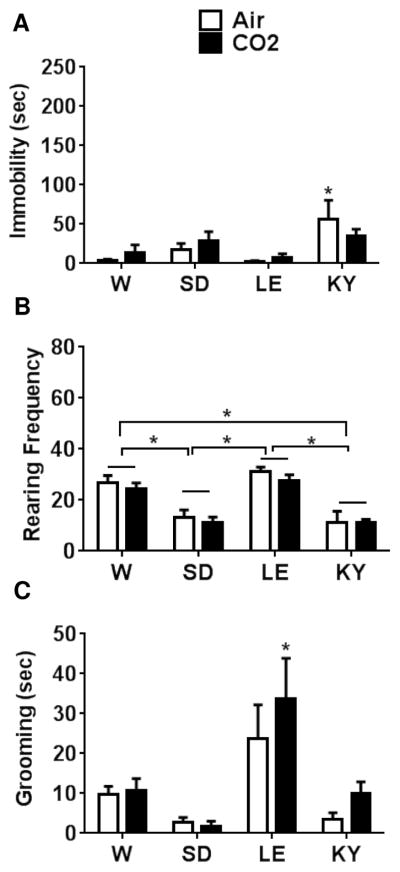
Inhalation of CO2 induced an increase in immobility, and reduced rearing and grooming behaviors in rats that was dependent on strain. Significantly higher immobility was observed in WK and LE rats compared with the W and SD strains (Fig 2a). CO2 exposed WK and LE rats showed significantly higher immobility versus air inhalation suggesting higher responsivity to CO2 in these strains compared with W and SD rats that showed no significant differences between air and CO2 exposed groups. A two-way ANOVA revealed significant effect of strain [F(3,40)=19.75; p<0.05], treatment [F(1,40)=28.67; p<0.05] and a strain x treatment interaction [F(3,40)= 4.59; p<0.05]. Post hoc analysis revealed a significantly higher immobility in WKs and LE strains compared with W and SD strains (p<0.05). Air inhalation by itself evoked some immobility in WK rats that was significantly higher than W SD and LE rats (p<0.05), suggesting this strain was also sensitive to the aversive effects of blowing gas in addition to CO2. We also assessed rearing frequency in air and CO2 exposed animals as a measure for exploration (Fig 2b). A significant effect of strain [F(3,40)= 34.18; p<0.05] as well as treatment [F(1,40)=34.41; p<0.05] but no strain x treatment interaction [F(3,40)=1.508; p>0.05] was observed.
Figure 2.
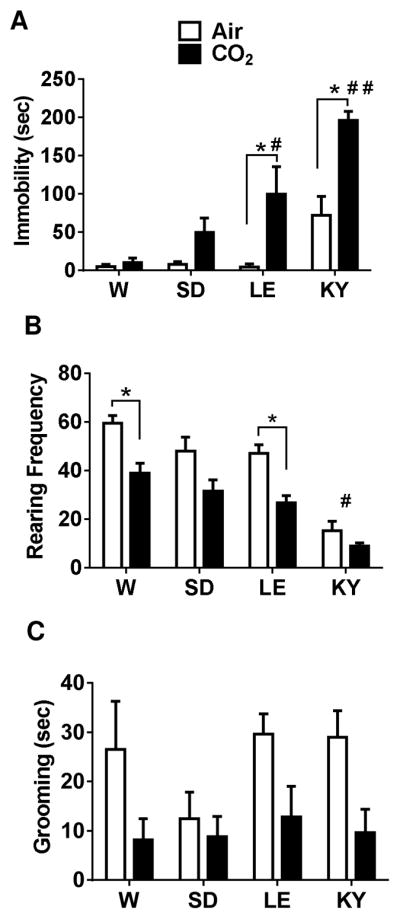
CO2 inhalation evokes differential behavioral responses in Wistar (W), Sprague Dawley (SD), Long Evans (LE) and Wistar Kyoto (WK) rats. See schematic (Fig 1) for experimental layout; data from Day 1 during air/CO2 inhalation is shown here. (A) CO2-evoked significantly different immobility behavior in strains. CO2-exposed LE and WK strains elicited significantly higher immobility compared with air inhalation groups within strain (*p<0.05 ) and significant between strain differences for LE rats (# p<0.05 versus W and SD air and CO2 groups) and WK rats (# # p<0.05 versus all other groups). (B) CO2-evoked a significant reduction in rearing behavior in W and LE rats (p<0.05 versus air groups within strains). Rearing in air and CO2-exposed WK rats was significantly lower than all other strains (# p<0.05 versus W, SD (air) and LE (air) groups). (C) CO2-evoked a significant reduction in grooming behavior across strains (significant main effect of treatment but no strain differences). No significant differences were revealed by posthoc analysis. All data are mean ± s.e.m (n=6 animals/air or CO2 groups)
Post hoc analysis revealed a significant reduction in rearing frequency in W and LE rats exposed to CO2 compared to the air group. Frequency of rearing was significantly lower in both air and CO2 exposed WK rats compared to all other strains suggestive of low exploratory behavior in this strain. Grooming, a measure of self-directed behavior was also assessed in air and CO2 exposed animals (Fig 2c). All strains except for SD rats showed reduced grooming time in the presence of CO2 as compared to air inhalation. Two-way ANOVA revealed a significant effect of treatment [F(1,40)=12.25; p<0.05] but no strain-dependent [F(3,40)=1.226; p>0.05] or strain x treatment interaction [F(3,40)=0.79; p>0.05]. No significant within or between differences were obtained following post hoc analysis.
Multivariate test statistic (Wilks’ Lambda) for two-way between strains analysis using freezing, rearing and grooming as combined dependent variables verified independent ANOVA results and showed significant effects of strain (F(9, 92.6) = 2.39); p < 0.05, Wilks’ lambda = 0.60), treatment (F(3, 38) = 20.32); p < 0.05, Wilks’ lambda = 0.38) and a significant strain x treatment interaction (F(9, 92.6) = 2.39); p < 0.05, Wilks’ lambda = 0.60).
To test whether 10% CO2 acts as an unconditioned stimulus to produce conditioned, associative responses, behavior was assessed in the CO2 context 24 hr post inhalation in the absence of infused air or CO2. As shown in Fig 3a–c, previous CO2 exposure did not impact immobility, rearing, or grooming as compared with the air exposed cohorts. However, significant strain dependent differences independent of CO2 inhalation were observed between groups. Although immobility scores were low, a two-way ANOVA analysis revealed a significant strain effect [F(3,40)=5.486; p<0.05] but no treatment [F(1,40)=0.03; p>0.05] or strain x treatment interaction [F(3,40)=0.97; p>0.05]. Post hoc analysis revealed significantly higher immobility in air exposed Wistar Kyoto rats as compared with air–exposed Wistar and LE rats (p<0.05). Rearing behavior also showed a significant strain-dependent difference but no effect of inhalation or strain x treatment interaction [strain: F(3,40)=25.39, p<0.05; treatment: F(1,40)=1.28, p>0.05; interaction: F(3,40)=0.12, p>0.05]. Post hoc analysis revealed significantly higher rearing in W and LE rats as compared to SD and WK rats (p<0.05). Exposure to context also led to strain-dependent differences in grooming behavior [F(3,40)=10.33, p<0.05], but no treatment [F(1,40)=1.279, p>0.05] or strain x treatment interaction F(3,40)=0.46, p>0.05]. CO2-exposed LE rats showed significantly higher grooming compared with SD, WK and W (air) groups.
Figure 3.
Prior inhalation of 10% CO2 does not evoke context conditioned responses in Wistar (W), Sprague Dawley (SD), Long Evans (LE) and Wistar Kyoto (WK) rats. (A) On exposure to context alone, no differences were observed between air and CO2 groups in immobility in any strain. Air exposed WK rats elicited significantly higher immobility (* p<0.05 versus air exposed W and LE rats). (B) Rearing behavior on day 2 showed significant strain but no CO2-evoked differences. Air and CO2-exposed SD and WK rats showed significantly lower rearing as compared with W and LE rats (* p<0.05). (C) Grooming behavior showed significant differences dependent on strain but not inhalation. CO2-exposed LE rats showed higher grooming than other strains but were not significantly different from air-exposed LE rats (*p< 0.05 versus SD, WK and W groups).
Strain dependent differences in TPH positive neurons within the dorsal raphe (DR)
Given the relevance of DR serotonergic neurons in the regulation of panic, anxiety, and central CO2 chemosensitivity, we quantified TPH-like immunoreactivity within two subdivisions of the raphe nucleus: the DRVL/VLPAG division where 5-HT neurons are implicated in the inhibition of panic-associated responses (Johnson et al., 2004), and the DRD division that is reported to facilitate anxiety-related responses (Hale et al., 2012). Figure panels 4a–c show representative photomicrographs of TPH-2 positive neurons and the rostro-caudal extent of quantified neurons within these areas. As seen in Fig 4d, the number of TPH2-positive cells within the DRVL/VLPAG showed significant strain-dependent differences, however no significant effect of CO2 inhalation was observed. Two- way ANOVA revealed a significant effect of strain [F(3, 38) =7.177; p<0.05] but no significant effect of inhalation [F (1, 38) = 0.003656; p>0.05] or a strain x inhalation interaction [F(3, 38) =1.920; p>0.05]. Post hoc analysis revealed a significant reduction in TPH-2 positive cells in LE rats (p<0.05) and a trending decrease (p=0.053) in WK rats as compared with SD rats. Although no significant differences existed within strains between air and CO2 inhalation groups there were significant between strain differences in CO2 exposed cohorts. Post hoc analysis revealed significantly lower number of TPH-2- positive cells in CO2-exposed Wistar rats compared with SD and WK rats. In contrast to changes within the DRVL/VLPAG, no significant strain- or CO2-dependent differences were observed in TPH-2 positive cells within the DRD (Fig 4e). Two-way ANOVA revealed no effect of strain [F (3, 38) = 2.50; p>0.05], inhalation [F (1, 38) = 0.015; p>0.05] or strain x inhalation interaction [F (3, 38) = 1.207; p>0.05].
Strain dependent differences in DβH positive noradrenergic neurons within the LC
Figure 5a–b shows representative images illustrating the rostro-caudal extent of DβH immunopositive cells that were quantified for the analysis. As seen in Fig 5c, significant differences in cell counts for DβH positive cells were observed between strains. Two-way ANOVA revealed a significant effect of strain [F(3, 40) =4.389; p<0.05] and a strain x inhalation interaction [F(3, 40) =4.872; p<0.05], but no significant effect of inhalation [F (1, 40) = 0.00126; p>0.05]. Post hoc analysis revealed a significantly higher number of DβH-positive cells in LE and WK rats as compared to SD rats. DβH positive cell counts in LE and WK groups also showed a trending (p=0.06) increase in comparison with W rats. Although no effect of CO2 inhalation was observed compared to air groups, a significant reduction in DβH immunoreactive cells was observed in CO2 exposed LE rats as compared with CO2 exposed WK rats.
Discussion
The current study investigated variability in behavioral responsivity to CO2 inhalation in rats to explore its utility as a potential rodent model of CO2 sensitivity, a pathological marker for panic and anxiety in humans. Our data revealed differential CO2-evoked behavior between strains, as well as strain-dependent differences in serotonergic and noradrenergic immunoreactivity in brain areas regulating panic and anxiety related behaviors.
The use of CO2-enriched air to provoke anxiety or panic responses, in both patient and healthy volunteers, has a long history in psychiatric research (for review see Esquivel et al., 2009; Vollmer et al., 2015). CO2 challenge studies in healthy volunteers have been used reliably to evoke acute anxiety while similar doses in panic disorder patients can evoke robust panic attacks, making CO2 a biological marker for trait anxiety and panic vulnerability. CO2 hypersensitivity, as indexed by exaggerated emotional and respiratory responses to CO2-enriched air mixture, is a useful investigational tool for identifying individuals at risk for later pathophysiology. Studies have used the CO2 challenge task for the validation of candidate genes for panic disorder (Savage et al., 2015), and treatment efficacy in panic patients (Perna et al., 2002). Recent work has established the CO2 inhalation paradigm as a translational cross-species model for panic (Leibold et al, 2016).
Exposure to CO2 produced a different magnitude of behavioral responses between strains. For this study, we chose a concentration of CO2 (10%) that elicits panic attacks in individuals with panic disorder while producing anxiety and panic in some healthy volunteers (Rassovsky and Kushner, 2003). A previous study reported strain-dependent variation in CO2-evoked ventilation in mice (Tankersley et al, 1994), however, behavioral responses have not been studied. The most prominent behavioral response evoked by CO2 was immobility (freezing-like behavior), that was markedly different between strains. Previous reports of CO2 inhalation in rats have reported freezing behavior using a similar concentration (Mongeluzi et al., 2003). 10% CO2 also evokes freezing in mice as reported by our group (Vollmer et al, 2016), and others (Ziemann et al., 2009; Taugher et al., 2014, Liebold et al, 2016). Thus CO2-evoked immobility/freezing behavior appears to be consistent across species and strains and likely represents a fear-associated defensive behavior. The inbred WK strain elicited the highest sensitivity to CO2-evoked immobility. To our knowledge, this is the first observation of CO2 inhalation-evoked behavior in this strain and suggests that, in addition to their well-recognized role as an animal model for depression (in comparison with Wistars as controls) (Overstreet, 2012), this strain could also be useful for investigating panic pathology or comorbid panic-depressive phenotypes. Outbred strains used in the study showed a large variation in CO2 reactivity with LE rats showing significantly higher immobility compared to W and SD rats. The Wistar cohort elicited negligible immobility suggesting resistance to this behavioral effect of CO2 inhalation. The Wistar strain has been reported to elicit lower anxiety-like behavior and active coping responses in the elevated plus maze in previous studies (Casarrubea et al., 2013; Keeley et al., 2015). Immobility to CO2 likely represents an acute defensive response to a homeostatic threat that appears to be strikingly different between LE/WK and SD/W strains. No comparison studies exist that have reported strain differences in CO2-evoked behavior. Previous studies have used SD or W rats in CO2 inhalation paradigms (Mongeluzi et al., 2003; Dumont et al., 2010; Johnson et al., 2012; Schimitel et al., 2012). In agreement with our data, CO2 inhalation (13%) did not affect immobility in Wistar rats (Schimitel et al., 2012). Significant anxiogenic, autonomic, and respiratory responses were reported in Wistar rats at high CO2 concentrations (20%) (Johnson et al., 2012). It is possible that higher CO2 concentrations are required to evoke CO2 effects in resistant strains as observed for CO2-sensitivity studies in humans (Colasanti et al., 2008).
Rearing frequency, representative of vertical exploration, was significantly reduced following CO2 inhalation in most strains, suggesting attenuation of explorative tendencies of animals. CO2, an interoceptive threat to survival is expected to reduce context awareness and elicit anxiogenic-like behaviors. Rearing behavior of WK rats was markedly reduced compared to other groups irrespective of treatment in agreement with previous studies reporting high trait anxiety and passive coping style of this strain (Pare, 1993). In this strain, CO2 elicited no further reduction in rearing possibly due to floor effects. Strains such as WK with significantly high trait anxiety may not elicit CO2-evoked anxiogenic behavior. As reported previously CO2-evoked anxiety and trait anxiety may have distinct genetic underpinnings (Roberson-Nay et al, 2013). Rearing frequency has also been reported to represent escape motivation which is reported to be reduced and replaced by immobility when the threat becomes more severe (Lever et al., 2006). Attenuated rearing and increased immobility evoked by CO2 in our model likely represents a tendency for passive defense responses rather than active escape behavior.
We also observed a CO2-evoked reduction in grooming behavior independent of strain. Rodent grooming represents complex repetitive and sequentially patterned behaviors that has aided in simulating phenotypes representing anxiety disorders (Kalueff et al., 2016). In our paradigm, exposure to CO2 inhalation may have led to a decrease in self-directed behaviors, as opposed to increased grooming, generally reported following exteroceptive stressors (Van Erp et al., 1994). Our data is in agreement with a previous study that reported reduced grooming in rats exposed to CO2 inhalation (Schimitel et al., 2012).
Exposure to 10% CO2 did not evoke context-conditioned immobility or conditioned anxiety-like behavior in any strain 24h post inhalation. This is in agreement with a previous study that reported absence of CO2-associated context conditioned freezing in mice (Ziemann et al., 2009). On the contrary, Mongeluzi et al reported significant freezing and conditioned fear at concentrations ranging from 5 to 100% CO2 (Mongeluzi et al., 2003). Differences in CO2 setups may have led to discrepant data between studies. As discussed in the previous study, the potential interaction of the sound of gas delivery, combined with CO2 itself, may have been a confounding factor that resulted in a more aversive contextual experience. In the current study, a dual chamber saturated with CO2 was used to avoid direct blowing and sound interaction that is highly aversive to rodents. It is possible that additional CO2 exposures are required to evoke context conditioned responses as reported in an earlier study in mice (D’Amato et al, 2011).
To study potential contributory transmitter systems that may underlie differential CO2 sensitivity between strains, we focused on serotonergic and noradrenergic expressing neurons in key panic and anxiety regulatory areas. Multiple lines of evidence support the potential role of midbrain serotonergic neurons in modulating CO2-evoked behaviors. Serotonin neurons in the raphe are CO2-chemosensors (Severson et al., 2003). Furthermore, serotonergic neurons in the DRVL/VLPAG provide inhibitory input to the dorsal PAG to modulate panic-associated responses (Johnson et al., 2004). Interestingly, association of polymorphisms within the TPH-2 gene and CO2 responses is observed, further supporting a role of the serotonergic system in the effects of CO2 (Abe et al., 2012). We hypothesized that strains that elicit enhanced reactivity to CO2 would have reduced serotonergic immunoreactivity (represented by TPH-2 positive cell counts) in panic-relevant subdivisions of the DR. Consistent with this, we observed significant reduction in TPH-2 immunopositive cells specifically within the DRVL/VLPAG in strains that elicited higher CO2-evoked immobility. No differences were observed in the DR subdivision known to modulate anxiogenic behaviors. In support of a functional distinction between these nuclei, homeostatic, panicogenic stimuli such as CO2 and lactate infusion recruit neurons within the DRVL/VLPAG while inescapable stress, anxiogenic drugs, and avoidance tasks on the elevated T-maze, considered as a test for generalized anxiety (not panic), activate serotonergic neurons within the DRD (Johnson et al., 2004; Lowry et al., 2008; Spiacci et al., 2012).
CO2-sensitive LE and WK rats had lower number of TPH-2 positive cells in the DRVL/VLPAG as compared to the more resistant SD rats. While this is not representative of actual transmitter levels, a relative reduction in serotonergic tone within the DRVL/VLPAG may contribute to compromised suppression of panic-relevant circuits that may potentially contribute to differential CO2-sensitivity observed between these strains. Inhalation of CO2 did not have a significant within strain effect on TPH-2 positive cells as compared to air groups, which is not surprising as low dose CO2 may not impact serotoninergic immunoreactivity. Although W rats did not elicit differences from other groups in control air groups, significantly lower TPH-2 positive cell counts as compared to other strains were observed in CO2-exposed W cohorts. Although the exact explanation for this change is not evident, it may represent altered activity and turnover of serotonin or possible recruitment in CO2 effects. Notably, this strain was most resistant to CO2-evoked immobility. A subset of 5-HT neurons within this area have been shown to increase in activity after exposure to a heightened CO2 gas mixture, which is implicated in the suppression of behavior and sympathetic drive (Johnson et al., 2011). In agreement with our observations, previous studies have also reported selective contribution of the DRVL/VLPAG subdivision in vulnerability to sodium lactate and CO2 (Johnson et al., 2008, 2011; Hale and Lowry, 2011). In a rat model of panic-like responses to sodium lactate, serotonergic neurons in the DRVL are highly activated in control rats, but fail to activate in panic-prone rats (Johnson et al., 2008). Decreased serotonergic neurotransmission in targets of the DRVL/VLPAG, as would be predicted by decreased TPH-2 expression under baseline conditions, would be expected to result in vulnerability to panicogen responsivity, such as for CO2. We did not observe active escape representative of “panic-like” behavior in our animals. Examining strain differences in physiological responses such as autonomic activation and ventilation would be important in future studies. Social isolation during adolescence downregulates the TPH-2 gene expression, suggesting regulatory effects of development x environment on this gene (Lukkes et al., 2013). It would be relevant to investigate gene x environment interactions between strains for mechanisms underlying individual variability to CO2.
In addition to serotonergic neurons in the raphe, we also investigated differences in DβH− positive noradrenergic neurons in the locus coeruleus (LC). It is estimated that ~50% of all the noradrenergic projections in the central nervous system originate in the LC, which are directed toward the forebrain, cerebellum, brainstem and spinal cord (Aston-Jones G et al, 1995). The LC is a key chemosensory site as focal acidosis, such as during CO2 inhalation, induces neuronal activation leading to increased ventilation and arousal (Gargaglioni, et al., 2010). Increased noradrenergic activity and turnover has been reported in patients with anxiety and panic disorders, implicating the relevance of the LC noradrenergic system (Coplan et al., 1997; Sullivan et al, 1999). In agreement with our hypothesis, we observed significantly higher DβH-positive immunoreactive cell counts in LE and WK rats as compared with the SD and W strains. Consistent with our observations, an augmented basal firing rate and burst activity of LC neurons has been reported in WK as compared to W and SD rats, suggestive of strain-dependent alterations in LC noradrenergic transmission (Bruzos-Cidón et al., 2015). Interestingly, in the same study, burst activity and sensitivity of serotonergic neurons in the dorsal raphe nucleus was significantly lower in the WK rats as compared with W and SD strains, also consistent with our observations. Interestingly, in addition to higher DβH-positive counts in air exposed LE rats, a significant decrease in DβH immunoreactivity was specifically noted in CO2 exposed LE rats, possibly suggesting recruitment of this system in CO2 effects or CO2- associated alterations in NE activity or turnover. Although not directly assessed in this study, strain-dependent differences in LC noradrenergic tone and activity may result in altered CO2-chemosensitivity given the key role of this area in central chemosensitivity and CO2-evoked respiratory drive (Gargaglioni, et al., 2010). Ventilatory responses were not measured in this study, however, alterations in respiration may have contributed to behavioral differences between strains. Collectively, our immunohistochemistry data on diminished serotonergic and increased noradrenergic immunoreactive cell counts is interesting in light of previous studies reporting reciprocal interaction between these two areas (Szabo et al, 2001; Vandermaelen and Aghajanian 1983). However, we used TPH-2 and DβH immunoreactivity as representative readouts for these systems. Our neurochemical cell count data does not reflect functionality per se, but suggests that potential differences in serotonergic and noradrenergic systems may be relevant to differential CO2 responses.
Comparing strains provides an avenue to investigate how polygenetic vulnerability may interact with acute insults to produce deficits relevant to pathophysiology. It may also aid in the investigation of mechanisms underlying individual variability and facilitate identification of novel targets and development of therapeutic testing paradigms. The rationale for strain selection for our study was based on several criteria. Inbred strains are often preferred over outbred stocks due to their genetic stability and homozygous traits. Rat strains such as the Sprague Dawley and Long Evans are cost-effective and have been widely used for behavioral assessments associated with stress and emotional reactivity. On the other hand, the WK rats, an inbred strain, has been used as a model for depressive-like behavior (Overstreet, 2012). It was interesting to observe robust behavioral differences in CO2-responsivity between strains and low variability within outbred strains. Significant, strain-dependent differences in serotonergic and noradrenergic tone observed within key CO2-chemosensitive sites regulating panic-relevant responses is also noteworthy.
Our studies open up several lines of investigation. Although robust strain-dependent differences in behavioral responses were observed, it remains to be investigated whether physiological reactivity to CO2 inhalation was different. Measures such as cardiovascular activation and ventilatory measurements in different strains would be required to further validate this model and its association with observed morphological changes. Our observations will also facilitate the investigation of gene x environment interaction effects on variable CO2-sensitivity. Recent studies have shown impact of early adversity and epigenetic modifications in regulating CO2 hypersensitivity (Cittaro et al., 2016). It will be interesting to investigate these manipulations in our model. In the current study we focused on altered serotonergic and noradrenergic tone between strains, however, it is possible that other mechanisms contribute to differential CO2 sensitivity between strains. Acid sensing ion channels (ASICs) in the amygdala and the bed nucleus of stria terminalis (BNST) have been reported to contribute to CO2 evoked freezing and anxiety (Ziemann et al., 2009; Taugher et al., 2014). Additionally, orexin neurons in the lateral hypothalamic area are CO2-senstive and are relevant to the behavioral and physiological effects of CO2 inhalation (Johnson et al, 2012). In recent studies, our lab reported regulation of CO2-evoked fear by microglial acid sensing mechanisms (Vollmer et al, 2016). It will be important to tease out contributions of these targets to differential CO2 sensitivity. It is likely that more than one mechanism(s) may determine CO2 -sensitivity. Lastly, correlations between cell counts and behavioral measures, as well as interventions in a larger “n” study are required to directly associate these systems to observed strain differences.
In conclusion, we report differential, strain-dependent behavioral responsivity to carbon dioxide inhalation in rats. Comparative studies between CO2-vulnerable and resistant rats may lead to mechanistic understanding and pathophysiological basis of CO2-sensitivity in humans, as well the role of gene x environment interactions and contributions of early life adversity on the development of panic and anxiety disorders.
Highlights.
CO2 inhalation, a biological challenge for panic and fear elicits differential behavioral sensitivity in rat strains
Long Evans and Wistar-Kyoto rats show significantly higher CO2-evoked immobility than Wistar and Sprague Dawley rats.
CO2-sensitive strains have decreased TPH2 –positive serotonergic neurons in panic regulatory raphe subnuclei, DRVL-VLPAG
CO2-sensitive strains have increased DβH-positive noradrenergic neurons in the locus coeruleus, a CO2-chemosensitive site
Rodent models of CO2-sensitivity will facilitate understanding of CO2-hypersensitivity in panic and anxiety disorders
Acknowledgments
This research was supported by National Institute of Mental Health (NIMH) Grant R01-MH093362 and VA Merit award 2I01BX001075 to Renu Sah.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe R, Watanabe Y, Tachibana A, Nunokawa A, Shindo M, Hasegawa N, Someya T. Exploration of a possible association between the tryptophan hydroxylase 2 (TPH2) gene and panic symptoms induced by carbon dioxide in healthy individuals. Psychiatry Res. 2012;197:358–359. doi: 10.1016/j.psychres.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Annerbrink K, Olsson M, Melchior LK, Hedner J, Eriksson E. Serotonin depletion increases respiratory variability in freely moving rats: implications for panic disorder. Int J Neuropsychopharmacol. 2003;6(1):51–6. doi: 10.1017/S1461145703003237. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus A5 and A7 noradrenergic cell groups. In: Paxinos George., editor. The Rat Nervous System. San Diego, CA: Academic Press; 1995. pp. 183–213. [Google Scholar]
- Battaglia M, Ogliari A, D’Amato F, Kinkead R. Early-life risk factors for panic and separation anxiety disorder: Insights and outstanding questions arising from human and animal studies of CO2 sensitivity. Neurosci Biobehav Rev. 2014;46(pt 3):455–64. doi: 10.1016/j.neubiorev.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Ogliari A, Harris J, Spatola CA, Pesenti-Gritti P, Reichborn-Kjennerud T, Torgersen S, Kringlen E, Tambs K. A genetic study of the acute anxious response to carbon dioxide stimulation in man. J Psychiatr Res. 2007;41:906–917. doi: 10.1016/j.jpsychires.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Pesenti-Gritti P, Medland SE, Ogliari A, Tambs K, Spatola CA. A genetically informed study of the association between childhood separation anxiety, sensitivity to CO2, panic disorder, and the effect of childhood parental loss. Arch Gen Psychiatry. 2009;66:64–71. doi: 10.1001/archgenpsychiatry.2008.513. [DOI] [PubMed] [Google Scholar]
- Bellodi L, Perna G, Caldirola D, Arancio C, Bertani A, Di Bella D. CO2-induced panic attacks: a twin study. Am J Psychiatry. 1998;155:1184–1188. doi: 10.1176/ajp.155.9.1184. [DOI] [PubMed] [Google Scholar]
- Biancardi V, Bícego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflügers Arch Eur J Physiol. 2008;455:1119–1128. doi: 10.1007/s00424-007-0338-8. [DOI] [PubMed] [Google Scholar]
- Bruzos-Cidón C, Llamosas N, Ugedo L, Torrecilla M. Dysfunctional Inhibitory Mechanisms in Locus Coeruleus Neurons of the Wistar Kyoto Rat. Int J Neuropsychopharmacol. 2015:1–11. doi: 10.1093/ijnp/pyu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarrubea M, Roy V, Sorbera F, Magnusson MS, Santangelo A, Arabo A, Crescimanno G. Significant divergences between the temporal structure of the behavior in Wistar and in the spontaneously more anxious DA/Han strain of rats tested in elevated plus maze. Behav Brain Res. 2013;250:166–173. doi: 10.1016/j.bbr.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Cittaro D, Lampis V, Luchetti A, Coccurello R, Guffanti A, Felsani A, Moles A, Stupka E, D’Amato FR, Battaglia M. Histone Modifications in a Mouse Model of Early Adversities and Panic Disorder: Role for Asic1 and Neurodevelopmental Genes. Sci Rep. 2016;6:25131. doi: 10.1038/srep25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ME, White PD. Life situations, emotions, and neurocirculatory asthenia (anxiety neurosis, neurasthenia, effort syndrome) Psychosom Med. 1951;13:335–357. doi: 10.1097/00006842-195111000-00001. [DOI] [PubMed] [Google Scholar]
- Colasanti A, Salamon E, Schruers K, van RD, van EJ, DMG Carbon dioxide induced emotion and respiratory symptoms in healthy volunteers. Neuropsychopharmacology. 2008a;33:3103–3110. doi: 10.1038/npp.2008.31. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Papp LA, Pine D, Martinez J, Cooper T, Rosenblum LA, Klein DF, Gorman JM. Clinical improvement with fluoxetine therapy and noradrenergic function in patients with panic disorder. Arch Gen Psychiatry. 1997;54:643–648. doi: 10.1001/archpsyc.1997.01830190069007. [DOI] [PubMed] [Google Scholar]
- Coryell W, Fyer A, Pine D, Martinez J, Arndt S. Aberrant Respiratory Sensitivity to CO2 as a Trait of Familial Panic Disorder. Biol Psychiatry. 2001;49:582–587. doi: 10.1016/s0006-3223(00)01089-1. [DOI] [PubMed] [Google Scholar]
- D’Amato FR, Zanettini C, Lampis V, Coccurello R, Pascucci T, Ventura R, Puglisi-Allegra S, Spatola CA, Pesenti-Gritti P, Oddi D, Moles A, Battaglia M. Unstable maternal environment, separation anxiety, and heightened CO2 sensitivity induced by gene-by-environment interplay. PLoS One. 2011;6(4):e18637. doi: 10.1371/journal.pone.0018637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont FS, Biancardi V, Kinkead R. Hypercapnic ventilatory response of anesthetized female rats subjected to neonatal maternal separation: Insight into the origins of panic attacks? Respir Physiol Neurobiol. 2010;175:288–295. doi: 10.1016/j.resp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Esquivel G, Schruers KRS, Moddock RJ, Colasanti A, Griez EJ. Acids in the brain: a factor in panic? J Psychopharmacol. 2010;24:639–647. doi: 10.1177/0269881109104847. [DOI] [PubMed] [Google Scholar]
- Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2011;213:243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- Hale MW, Shekhar A, Lowry CA. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol. 2012;32:695–708. doi: 10.1007/s10571-012-9827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RTR, Dale N. Redefining the components of central CO2 chemosensitivity--towards a better understanding of mechanism. J Physiol. 2011;589:5561–79. doi: 10.1113/jphysiol.2011.214759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P, Lowry C, Truitt W, Shekhar A. Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. J Psychopharmacol. 2008;22:642–652. doi: 10.1177/0269881107082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Fitz SD, Hollis JH, Moratalla R, Lightman SL, Shekhar A, Lowry CA. Induction of c-Fos in “panic/defence”-related brain circuits following brief hypercarbic gas exposure. J Psychopharmacol. 2011;25:26–36. doi: 10.1177/0269881109353464. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Lightman SL, Lowry CA. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann N Y Acad Sci. 2004;1018:58–64. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Lightman SL, Lowry CA, Shekhar A. Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology. 2012;37:1911–1922. doi: 10.1038/npp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Federici LM, Shekhar A. Etiology, triggers and neurochemical circuits associated with unexpected, expected, and laboratory-induced panic attacks. Neurosci Biobehav Rev. 2014;46:429–54. doi: 10.1016/j.neubiorev.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci. 2016;17:45–59. doi: 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley RJ, Bye C, Trow J, Mcdonald RJ. Strain and sex differences in brain and behaviour of adult rats: Learning and memory, anxiety and volumetric estimates. Behav Brain Res. 2015;288:118–131. doi: 10.1016/j.bbr.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Leibold NK, Viechtbauer W, Goossens L, De Cort K, Griez EJ, Myin-Germeys I, Steinbusch HW, van den Hove DL, Schruers KR. Carbon dioxide inhalation as a human experimental model of panic: The relationship between emotions and cardiovascular physiology. Biol Psychol. 2013;94:331–340. doi: 10.1016/j.biopsycho.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Leibold NK, van den Hove DLA, Viechtbauer W, Buchanan GF, Goossens L, Lange I, Knuts I, Lesch KP, Steinbusch HWM, Schruers KR. CO2 exposure as translational cross-species experimental model for panic. Trans. Psychiatry. 2016;6:e885. doi: 10.1038/tp.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever C, Burton S, O’Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 2006;17:111–133. doi: 10.1515/revneuro.2006.17.1-2.111. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Gargaglioni LH, Hartzler LK, Putnam RW. The locus coeruleus and central chemosensitivity. Respir Physiol Neurobiol. 2010;173:264–273. doi: 10.1016/j.resp.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Kopelman JM, Donner NC, Hale MW, Lowry CA. Development x environment interactions control tph2 mRNA expression. Neuroscience. 2013;237:139–50. doi: 10.1016/j.neuroscience.2013.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH, Copeland LE, Richards AL. Elevated brain lactate responses to neural activation in panic disorder: A dynamic 1H-MRS study. Mol Psychiatry. 2008;14:537–45. doi: 10.1038/sj.mp.4002137. [DOI] [PubMed] [Google Scholar]
- Mongeluzi DL, Rosellini RA, Ley R, Caldarone BJ, Stock HS. The conditioning of dyspneic suffocation fear. Effects of carbon dioxide concentration on behavioral freezing and analgesia. Behav Modif. 2003;27:620–636. doi: 10.1177/0145445503256316. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. Modeling depression in animal models. Methods Mol Biol. 2012;829:125–144. doi: 10.1007/978-1-61779-458-2_7. [DOI] [PubMed] [Google Scholar]
- Papp LA, Klein DF, Gorman JM. Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. Am J Psychiatry. 1993;150:1149–1157. doi: 10.1176/ajp.150.8.1149. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, Mason PA, Morilak DA. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115(1):229–42. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Pare WP. Passive-avoidance behavior in Wistar-Kyoto (WKY), Wistar, and Fischer-344 rats. Physiol Behav. 1993;54:845–852. doi: 10.1016/0031-9384(93)90291-m. [DOI] [PubMed] [Google Scholar]
- Pattinson KT, Mitsis GD, Harvey AK, Jbabdi S, Dirckx S, Mayhew SD, Rogers R, Tracey I, Wise RG. Determination of the human brainstem respiratory control network and its cortical connections in vivo using functional and structural imaging. Neuroimage. 2009;44:295–305. doi: 10.1016/j.neuroimage.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Perna G, Bertani A, Caldirola D, Bellodi L. Family history of panic disorder and hypersensitivity to CO2 in patients with panic disorder. Am J Psychiatry. 1996;153:1060–1064. doi: 10.1176/ajp.153.8.1060. [DOI] [PubMed] [Google Scholar]
- Perna G, Bertani A, Caldirola D, Gabriele A, Cocchi S, Bellodi L. Antipanic drug modulation of 35% CO2 hyperreactivity and short-term treatment outcome. J Clin Psychopharmacol. 2002;22:300–308. doi: 10.1097/00004714-200206000-00011. [DOI] [PubMed] [Google Scholar]
- Rassovsky Y, Kushner MG. Carbon dioxide in the study of panic disorder: issues of definition, methodology, and outcome. J Anxiety Disord. 2003;17:1–32. doi: 10.1016/s0887-6185(02)00181-0. [DOI] [PubMed] [Google Scholar]
- Savage JE, Mcmichael O, Gorlin EI, Beadel JR, Teachman B, Vladimirov VI, Hettema JM, Roberson-Nay R. Validation of candidate anxiety disorder genes using a carbon dioxide challenge task. Biol Psychol. 2015;109:61–66. doi: 10.1016/j.biopsycho.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimitel FG, de Almeida GM, Pitol DN, Armini RS, Tufik S, Schenberg LC. Evidence of a suffocation alarm system within the periaqueductal gray matter of the rat. Neuroscience. 2012;200:59–73. doi: 10.1016/j.neuroscience.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Spatola CA, Scaini S, Pesenti-Gritti P, Medland SE, Moruzzi S, Ogliari A, Tambs K, Battaglia M. Gene-environment interactions in panic disorder and CO2 sensitivity: Effects of events occurring early in life. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:79–88. doi: 10.1002/ajmg.b.31144. [DOI] [PubMed] [Google Scholar]
- Spiacci A, Coimbra NC, Zangrossi H. Differential involvement of dorsal raphe subnuclei in the regulation of anxiety- and panic-related defensive behaviors. Neuroscience. 2012;227:350–360. doi: 10.1016/j.neuroscience.2012.09.061. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biol Psychiatry. 1999;46:1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Functional and pharmacological characterization of the modulatory role of serotonin on the firing activity of locus coeruleus norepinephrine neurons. Brain Res. 2001;922:9–20. doi: 10.1016/s0006-8993(01)03121-3. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Fitzgerald RS, Kleeberger SR. Differential control of ventilation among inbred strains of mice. Am J Physiol. 1994;267:R1371–7. doi: 10.1152/ajpregu.1994.267.5.R1371. [DOI] [PubMed] [Google Scholar]
- Taugher RJ, Lu Y, Wang Y, Kreple CJ, Ghobbeh A, Fan R, Sowers LP, Wemmie JA. The Bed Nucleus of the Stria Terminalis Is Critical for Anxiety-Related Behavior Evoked by CO2 and Acidosis. J Neurosci. 2014;34:10247–10255. doi: 10.1523/JNEUROSCI.1680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Erp AM, Kruk MR, Meelis W, Willekens-Bramer DC. Effect of environmental stressors on time course, variability and form of self-grooming in the rat: handling, social contact, defeat, novelty, restraint and fur moistening. Behav Brain Res. 1994;65:47–55. doi: 10.1016/0166-4328(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–19. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Vollmer LL, Strawn JR, Sah R. Acid-base dysregulation and chemosensory mechanisms in panic disorder: a translational update. Transl Psychiatry. 2015;5:e572. doi: 10.1038/tp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer LL, Ghosal S, McGuire JL, Li K-Y, Ratliff CA, Lewkowich IP, Herman JP, Putnam RW, Sah R. Microglial acid sensing regulates carbon dioxide evoked fear. Biol Psychiatry. 2016;80(7):541–551. doi: 10.1016/j.biopsych.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]