Abstract
To determine the magnitude and mediators of the association between cigarette smoking and bone mass in the epidemiologic literature we reviewed articles, published abstracts, and conference proceedings, identified through MEDLINE, psychological abstracts, conference proceedings, and article bibliographies. We studied cross-sectional and prospective human studies that provided a quantitative measure of bone mass (X-ray, absorptiometry, or computed tomography) as a function of cigarette smoking exposure. Effects were expressed as pooled standardized mean differences for categorical comparisons (e.g., bone mass in current versus nonsmokers), and as pooled correlation coefficients for continuous comparisons (e.g., correlation of bone mass and pack-years of smoking). Effects were derived for combined bone sites (all bone sites pooled within each study) and four specific sites (hip, lumbar spine, forearm, and os calcis), and were examined overall and as a function of subject and methodologic characteristics (gender, age, body weight, menopausal status, health status). Data were pooled across 86 studies, enrolling 40,753 subjects. Smokers had significantly reduced bone mass compared with nonsmokers (never and former smokers) at all bone sites, averaging a one-tenth standard deviation (SD) deficit for combined sites. Deficits were especially pronounced at the hip, where the bone mass of current smokers was one-third of a SD less than that of never smokers. Overall, effects were greatest in men and in the elderly, and were dose-dependent. In prospective studies, smokers had greater rates of bone loss over time compared with nonsmokers. Bone mass differences remained significant after controlling for age and body weight differences between the two groups. Absolute effect sizes at most bone sites were greatest for current smokers compared with never smokers, intermediate for current smokers compared with former smokers, and lowest for former smokers compared with never smokers, suggesting that smoking cessation may have a positive influence on bone mass. Based on these data, it is estimated that smoking increases the lifetime risk of developing a vertebral fracture by 13% in women and 32% in men. At the hip, smoking is estimated to increase lifetime fracture risk by 31% in women and 40% in men. It appears that smoking has an independent, dose-dependent effect on bone loss, which increases fracture risk, and may be partially reversed by smoking cessation. Given the public health implications of smoking on bone health, it is important that this information be incorporated into smoking prevention and cessation efforts.
Keywords: Cigarette smoking, Bone mineral density, Osteoporosis, Body weight
There is a growing body of evidence that cigarette smoking is a risk factor for osteoporosis, but the nature and magnitude of this relationship remains uncertain. Numerous studies have documented inverse relationships between smoking and both bone mass and fracture risk [1, 2]. These relationships have been found at several bone sites, with smoking exposure measured in several ways (e.g., current smoking status and lifetime exposure), and in diverse populations. However, many studies have found no evidence of a relationship between smoking and bone mass [3–5]. It is likely that these inconsistencies are related to differences among studies in terms of design (e.g., cross-sectional vs. prospective assessment), statistical power for detecting significant effects, the specific bone sites investigated, participant characteristics (e.g., age, gender, body weight, health status, menopausal status), and the technique used to assess bone mass. In particular, it has been suggested that lower bone mass in smokers is attributable to lower body weight rather than any direct effect of tobacco [1].
Another important issue that has received little attention is whether quitting smoking reduces the risk of bone loss. No prospective studies have examined bone mass changes as a function of change in smoking status (i.e., initiation or cessation of smoking). Several cross-sectional studies, however, have reported that former smokers have bone mass that is intermediate of current smokers and never smokers, or that the bone mass of former smokers is similar to that of never smokers [e.g., 6]. These results suggest that smoking cessation may have a beneficial effect on bone mass, although the mechanism of such an effect is unclear.
For both clinical and public health purposes, it is important to determine whether smoking is a risk factor for low bone mass, the magnitude of this effect, what populations are most at risk for smoking-related bone loss, and what bone sites are most affected. Recently, the first meta-analysis to examine the smoking/bone mass relationship was published [2]. This review found that bone mass in male and postmenopausal female smokers was approximately one-third of a standard deviation lower than that of nonsmokers. Although an important contribution, this review was not comprehensive in several respects: (1) only three bone sites were evaluated (femoral neck, radius, and os calcis) which excluded a large portion of the published smoking/bone mass literature, (2) prospective studies examining rates of bone change were not evaluated, (3) the review did not evaluate the influence of several potentially important mediators and moderators, including body weight, physical activity level, health status, and bone characteristics (e.g., trabecular content).
The purpose of the present research was to conduct a comprehensive meta-analytic review of the epidemiologic literature on the association of smoking and bone mass. Several hypotheses regarding this association were tested. (1) An overall significant inverse association between smoking exposure and bone mass was expected. (2) This association was expected to be dose-dependent, such that bone mass would be negatively correlated with smoking exposure (e.g., pack-years of smoking). (3) Greater effects were expected in older individuals, including postmenopausal women. (4) Former smokers were expected to have bone mass that was intermediate between current smokers and never smokers, suggesting that smoking cessation has a positive effect on bone mass. (5) It was hypothesized that controlling for potential confounders of the smoking/bone mass relationship (e.g., age, physical activity, calcium intake), as well as body weight differences between smokers and nonsmokers, would reduce, but not entirely negate, the effects of smoking on bone mass.
Methods
Identification of Studies
Studies were identified in the Medline database using the words “bone or osteoporosis or fracture” and “smoking or tobacco or nicotine or lifestyle or behavior.” In addition, reference sections of all identified studies and review articles were searched, and published abstracts were identified by reviewing all convention abstracts published during the last 7 years in several relevant journals. Inclusion criteria for this meta-analysis were that the study be (1) either a published peer-reviewed article or published abstract, (2) published between 1966 and 1997, (3) provide a quantitative measure of bone mass (i.e., X-ray, absorptiometry, or computed tomography), and (4) report bone mass data as a function of cigarette smoking exposure.
Initially, 134 studies were identified that met these criteria. However, 40 of these were excluded because they included smoking exposure as a covariate in multivariate analyses predicting bone mass, but did not specifically analyze the relationship between smoking exposure and bone mass. Thus, 94 studies (83 peer-reviewed articles and 11 published abstracts) were included in the meta-analysis. Multiple publications were found for eight studies. In these cases, non redundant effect sizes were calculated from all publications and were coded as being from a single study, resulting in 86 independent studies being included in the meta-analysis.
Coding Procedures
Smoking exposure was represented in studies both categorically and continuously. Categorical effects compared bone mass in exposed and non-exposed (or less-exposed) individuals (e.g., current versus never smokers; former vs. never smokers). Continuous effects correlated bone mass with such exposure indicators as cigarettes smoked per day, number of years smoked, or pack-years of smoking. Both types of effects were analyzed in this meta-analysis. Because a variety of categorical comparisons were made across studies, these were collapsed into two partially overlapping groups which allowed all effect sizes to be analyzed. The first categorical comparison evaluated current smokers versus non-smokers, where nonsmokers included both former smokers and never smokers. The second comparison contrasted ever smokers (current or former) to never smokers. In addition, finer grain analyses were conducted to investigate the effects of smoking cessation on bone mass. These analyses compared effect sizes for current smokers vs. never smokers, current smokers vs. former smokers, and former smokers vs. never smokers.
Studies analyzed bone mass at a variety of sites, including the total body, lumbar spine, os calcis, metacarpal, humerus, forearm, and hip. Studies often reported results at multiple sites for both the hip (e.g., total hip, femoral neck, trochanter) and forearm (e.g., distal and midshaft ulna or radius). Analyses were conducted on “combined” bone sites (created by averaging all bone sites within each study), as well as the four most frequently reported sites (lumbar spine, os calcis, hip, and forearm). The hip and forearm categories were created by averaging all relevant sites within each study. It was deemed acceptable to combine ulna and radius measures into a single forearm site because the distributions of mineral mass and percentage of trabecular bone are similar in both bones [7]. For both combined and forearm bone sites, the relative percentage of trabecular bone was coded as <50% or ≤50% based on published estimates [7–9]1.
Analyses were conducted separately for effects that were assessed cross-sectionally (i.e., bone mass assessed only once) or prospectively (i.e., rate of change in bone mass, based on at least two assessments).
Several participant and methodologic characteristics were coded for each effect. Participant characteristics included age, gender, ethnicity, menopausal status, and smoking exposure (cigarettes/day, number of years smoked). Health status was coded to indicate whether participants had been selected based on the existence of a disorder affecting bone metabolism, including osteoporosis/osteopenia, alcoholism, anorexia nervosa, amenorrhea, diabetes, gastrointestinal disorders, rheumatoid arthritis, and endocrinopathies such as hyperparathyroidism or hypogonadism.
Bone mass assessment technique was coded dichotomously as dual energy X-ray absorptiometry (DXA) versus other techniques (X-ray, single photon absorptiometry, dual photon absorptiometry, or computed tomography). DXA has improved precision and accuracy, compared with these other techniques, which may influence the stability and magnitude of effect sizes [10, 11]. The vast majority of bone mass measures (81% of all coded effect sizes) were expressed as areal bone mineral density (BMD; g/cm2). Because areal BMD is highly correlated with other measures (e.g., linear BMD, expressed as g/cm), and all measures predict fracture risk [12], measures were pooled in analyses.
Dummy coding was used to indicate whether effects controlled for body weight differences in smokers and nonsmokers and several potential confounders. Effects were considered to control for age or body mass [weight or body mass index (kg/m2)] differences if participants were matched on these variables, or if statistical adjustment was performed. Statistical adjustment of several other potential confounders also was coded, including menopausal status, years since menopause, estrogen replacement status, use of oral contraceptives, calcium intake, height, use of medications that affect bone metabolism, physical activity, coffee intake, and alcohol intake. However, only calcium intake and physical activity were included as covariates in a sufficient number of studies to analyze.
Effect Size Calculation
Standardized mean differences (d) [13] were calculated for categorical comparisons (e.g., current- vs. nonsmoker) rather than absolute differences in bone mass, due to the diversity of bone sites and measurement techniques used. Each effect size was corrected for small sample bias and weighted by the inverse of its variance [14].
For comparisons involving continuous measures of smoking exposure (pack-years, number of cigarettes smoked per day, number of years smoked), Pearson product-moment correlations were calculated, converted to z-scores using Fisher’s variance stabilizing z-transform [15], and then weighted by the inverse of their variance. For interpretability, z’s were transformed back into estimates of weighted correlations. When findings were reported only as statistically nonsignificant, effect sizes were calculated conservatively as 0. Negative ds and rs indicate an adverse effect of smoking on bone mass.
Multiple effect sizes were coded within the majority of studies (mean = 8.9; range = 1–64) mainly because of assessment of several bone sites and smoking exposure comparisons (e.g., some studies reported comparisons for current smokers vs. never smokers, current vs. former smokers, and former vs. never smokers at several bone sites). Because significant positive intraclass correlations were observed for bone sites and smoking exposure categories within studies (ranging from 0.04 to 0.13, P values <0.006), which could bias effect size estimates, effect sizes were aggregated to the study level.
Statistical Analysis
Homogeneity testing was conducted for all analyses using the Q test [14] to determine whether the predictor(s) being modeled accounted for all systematic variance in effect size. Q is derived from weighted least squares (WLS) estimates of effect variability. In cases where Q was rejected (indicating the presence of non-random variability in effect sizes) homogeneity was accomplished by stratification of effect sizes according to relevant characteristics. Effect sizes were compared using WLS analysis of variance and multiple linear regression.
Results
Description of the Studies
Pooling of studies resulted in a combined sample size of 40,753 (30,293 women and 10,460 men) across 86 independent studies. A total of 18,988 participants were included in continuous-exposure comparisons, and 21,765 participants were included in categorical comparisons (which included 4,305 current smokers, and 17,460 never smokers or former smokers)2. The mean age of participants was 50.3 years (range = 16–80). Ethnicity of participants was not reported in a majority of studies (48, or 56%) and as such, was not examined in analyses. In terms of the health status of samples, 77 studies either excluded participants who had disorders affecting bone metabolism, or did not select participants based on disorders affecting bone metabolism (e.g., community-based cohorts). Nine studies selected participants for certain disorders known to influence bone metabolism, including osteoporosis [16], amenorrhea [17], anorexia nervosa [18], diabetes [19, 20], gastrointestinal disorders [21–23], and pustolosis palmaris et plantaris (a dermatologic condition associated with bone metabolic dysfunction) [24].
Homogeneity of Effects
Analyses initially were conducted with all relevant studies included, regardless of the health status of the samples. Effects sizes generally were more negative for the nine clinical samples compared with nonclinical samples, resulting in significant heterogeneity of effect sizes. For example, among studies comparing current smokers to nonsmokers at combined bone sites, homogeneity was rejected when clinical samples were included (P < 0.0001). In contrast, homogeneity was not rejected when clinical samples were excluded (P = 0.130). Accordingly, clinical samples were analyzed separately from nonclinical samples.
Overall Effects in Cross-Sectional Studies
Across bone sites, the bone mass of current smokers was one-tenth of a SD lower than that of nonsmokers (Table 1). This effect estimate was unchanged when adjusted for relative trabecular content of bone sites (<50% vs. ≥50%). Effect sizes at specific bone sites (i.e., lumbar spine, forearm, os calcis, and hip) ranged from −0.07 (95% confidence limits = −0.09, −0.05) for the forearm to −0.18 (−0.24, −0.12) for the hip. Effect sizes of similar magnitude were observed at these bone sites for comparisons of ever smokers versus never smokers, with the exception that no significant difference was found for the lumbar spine. Absolute effect sizes were larger when current smokers were compared with never smokers only. For these comparisons, ds were −0.13 [−0.17, −0.09] (combined bone sites), −0.08 [−0.14, −0.02] (forearm), −0.16 [−0.28, −0.04] (lumbar spine), −0.12 [−0.18, −0.06] (os calcis), and −0.29 [−0.44, −0.15] (hip).
Table 1.
Effect sizes comparing bone mass according to smoking statusa
| Bone site | Current vs. nonsmokersb
|
Ever vs. never smokers
|
Current vs. never smokers
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| d | 95% CL | n | d | 95% CL | n | d | 95% CL | n | |
| Combined | −0.10 | −0.12, −0.08 | 52 | −0.10 | −0.14, −0.06 | 21 | −0.13 | −0.18, −0.08 | 13 |
| Lumbar spine | −0.08 | −0.12, −0.04 | 27 | −0.03 | −0.07, 0.01 | 12 | −0.16 | −0.27, −0.05 | 5 |
| Forearm | −0.07 | −0.09, −0.05 | 25 | −0.07 | −0.11, −0.03 | 8 | −0.08 | −0.14, −0.02 | 5 |
| Os calcis | −0.13 | −0.19, −0.07 | 8 | −0.12 | −0.18, −0.06 | 4 | −0.12 | −0.18, −0.06 | 3 |
| Hip | −0.18 | −0.24, −0.12 | 21 | −0.13 | −0.21, −0.05 | 10 | −0.29 | −0.43, −0.15 | 5 |
Age, Gender, and Menopausal Status Effects
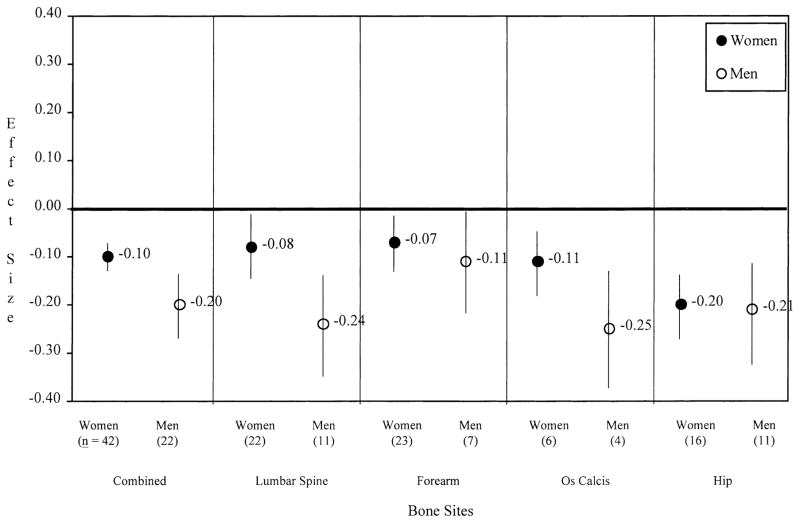
To assess age and gender effects, gender, age, and a gender by age interaction term were regressed on d for current smokers vs. nonsmokers. No significant interactions were observed at any bone site, indicating that age effects were similar for women and men. As shown in Figure 1, absolute effect sizes were greater in men than in women at combined bone sites (−0.20 [−0.26, −0.14] vs. −0.10 [−0.12, −0.08], respectively) and the lumbar spine (−0.24 [−0.34, −0.14] vs. −0.08 [−0.14, −0.02]). Similar (but statistically nonsignificant) trends were observed at the forearm and os calcis.
Fig. 1.
Effect sizes comparing current smokers to nonsmokers (d+, 95% CI) according to gender.
Effect sizes became more negative as age increased at combined bone sites (P = 0.0201), and a similar trend was observed at the forearm (P = 0.0543), but not at other bone sites. Gender and age were fairly strong predictors of bone mass differences in current and nonsmokers, accounting for 17.5% of the variance in d at combined bone sites (P = 0.0004).
To further evaluate age effects observed at combined bone sites, samples were stratified into three age groups (<40 years, 40–60 years, and >60 years). A significant difference among the three age groups was observed (P = 0.006). Post-hoc testing indicated that smoking had a more adverse effect on bone mass for individuals over 60 years of age (−0.17, [−0.21, −0.13]) compared with both individuals less than 40 years of age (−0.08 [−0.18, 0.02]) and 40–60 years of age (−0.08, [−0.12, −0.04]) (P-values <0.04).
To determine whether menopausal status influenced the smoking/bone mass association, effects were compared for female samples that consisted entirely of either premenopausal women [3, 4, 25–39] or postmenopausal women [5, 6, 27, 30, 32, 40–58]. Studies that combined pre- and post-menopausal participants in analyses were excluded, and one study that contained all perimenopausal women was included with postmenopausal samples. It was hypothesized that smoking would have a more negative effect on bone mass for postmenopausal women than premenopausal women due potentially to longer duration of smoking and adverse effects of smoking on estrogen status (e.g., precipitation of earlier menopause). This hypothesis was partially supported. No significant between-group differences comparing pre- and postmenopausal women were observed at any bone site. However, when effects were examined stratified by menopausal status, postmenopausal current smokers had significantly reduced bone mass compared with non-smokers, at combined bone sites (d = −0.13 [−0.17, −0.09]), the forearm (d = −0.07 [−0.13, −0.01]), and the hip (d = −0.22 [−0.28, −0.16]), but no significant effects were observed for premenopausal women.
Control of Potential Confounders
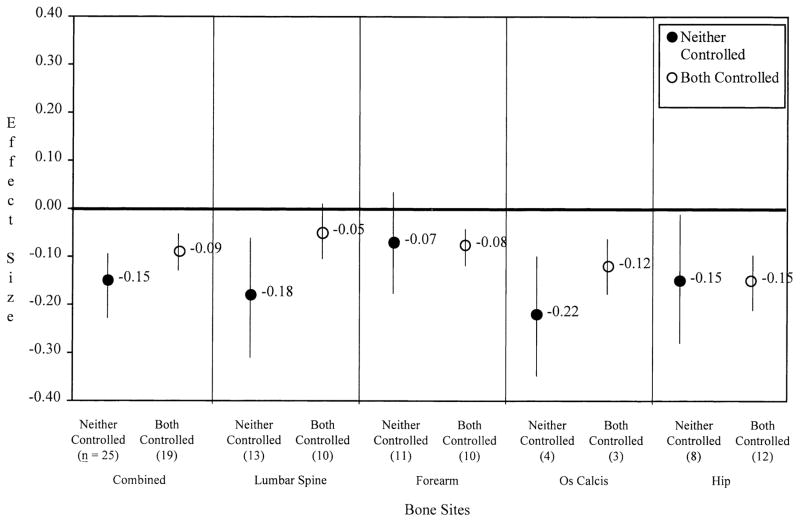
It was hypothesized that control of potential confounders (through matching or statistical adjustment) would reduce, but not entirely negate, the relationship between smoking and bone mass. Consistent with this hypothesis, the effects of current smoking or ever smoking did not differ significantly at any bone site according to whether age or body mass was controlled, either individually or simultaneously. Figure 2 illustrates that effects were generally similar for current smoking at all bone sites when both body mass and age were controlled simultaneously compared with when neither potential confounder was controlled. The only exception was a larger absolute effect at the lumbar spine when age and body mass were not controlled compared with when controlled (ds = −0.18 [−0.30, −0.06] and −0.05 [−0.11, 0.01], respectively; P = 0.084). No significant differences were observed between effects that were adjusted for physical activity and calcium intake in addition to age and body mass, compared with effects that did not control for these variables.
Fig. 2.
Effects of current smokers vs. nonsmokers (d+, 95% CI) according to control of body mass and age differences.
Effect sizes for current vs. never smokers and ever vs. never smokers did not differ according to bone mass assessment technique used (DXA vs. other techniques), or form of publication (peer-reviewed vs. published abstract). In addition, effects for combined and forearm bone sites did not differ significantly according to relative trabecular content of the bone.
Effects in Clinical Samples
As noted above, clinical samples (i.e., composed of individuals with diseases affecting bone metabolism) were examined separately from nonclinical samples because of heterogeneity in effect sizes in the former studies. Too few clinical samples were available to conduct analyses for all specific bone sites and smoking exposure comparisons. However, a sufficient number of clinical samples (n = 5) were available to compare effect sizes with nonclinical samples for current-smoking vs. nonsmoking at combined bone sites. These five studies included patients with osteoporosis, amenorrhea, Crohn’s disease, and diabetes [16, 17, 19–21]. Homogeneity was rejected for these clinical samples (P < 0.0001). Examination of the studies indicated that the d of one study [16] was more than 5 SDs lower than the mean d. After removing this outlier, homogeneity among the four remaining clinical studies was not rejected (P = 0.483). The mean effect of current smoking was stronger for clinical samples than nonclinical samples after removing this outlier (d = −0.34 [−0.54, −0.13] vs. d = −0.10 [−0.13, −0.07], respectively; P = 0.024). Most clinical studies did not provide smoking history data, so it was not possible to determine whether the stronger effects observed for these studies was dose-related.
Dose Effects
Correlation coefficients were calculated and combined from among 33 cross-sectional studies [5, 6, 34, 35, 38, 39, 40, 41, 42, 44, 46, 51, 52, 57, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82][5, 6, 34, 35, 38–42, 44, 46, 51, 52, 57, 59–82] that reported the degree of association between bone mass and smoking exposure expressed as a continuous variable (pack-years, number of years as a smoker, or cigarettes per day). Significant correlations were observed at combined bone sites, lumbar spine, hip, and os calcis, with r ranging from −0.04 [−0.06, −0.01] to −0.06 [−0.08, −0.03]. Effects were stronger when adjusted for age and body mass at both combined bone sites (r = −0.07 [−0.10, −0.05]) and the hip (r = −0.08 [−0.11, −0.04]). No significant dose effect was observed at the forearm.
Twin Studies
Dose effects also were examined in four twin studies that provided within-twin-pair correlations of differences in lumbar spine bone mass and differences in pack-years of smoking [74, 83–85]. This type of analysis controls for age, gender, and genetic composition, all of which are major determinants of bone mass. A significant negative correlation was observed (r = −0.10; [−0.21, −0.01], indicating that greater exposure to smoking was associated with lower bone mass. This analysis was re-run after excluding one study [85] which included children (mean age of sample = 16.6 years; range 10–26), since the magnitude of smoking exposure was likely to have been very low in this sample. A stronger negative correlation was observed for the three adult samples (r = −0.28 [−0.44, −0.11]).
Smoking Cessation Effects
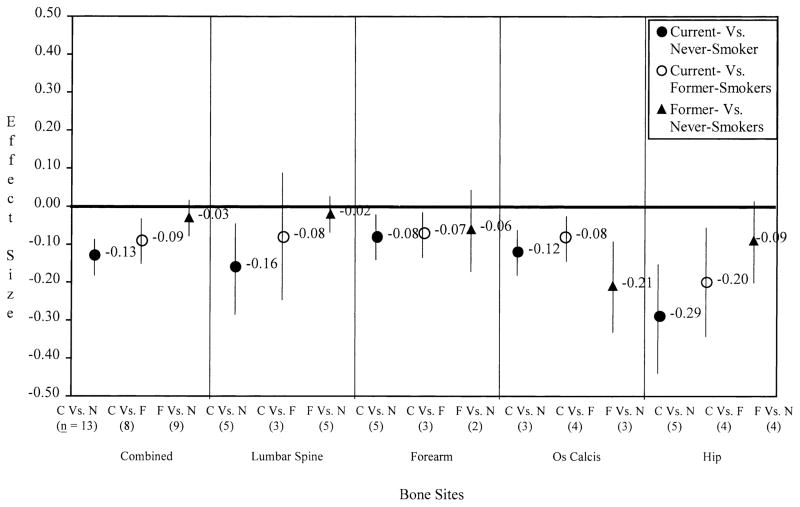
To evaluate whether smoking cessation might have a positive effect on bone mass, effect sizes were compared for current smokers vs. never smokers, current smokers vs. former smokers, and former smokers vs. never smokers (Fig. 3). Significant differences among smoking exposure comparisons were observed at combined bone sites (P = 0.002) and the lumbar spine (P = 0.048). Post-hoc testing revealed that the effect size for current vs. never smokers was lower than that for former vs. never smokers at both sites (P-values <0.02). No significant differences among the three smoking exposure comparisons were found at the forearm, hip, or os calcis, possibly because of low power (sample sizes were small, ranging from 2 to 5 samples per comparison). Nevertheless, similar trends were observed at all bone sites except the os calcis, such that current smokers had significantly lower bone mass compared with never smokers, whereas former smokers and never smokers did not differ significantly. At the os calcis, both current and former smokers had lower BMD than never smokers.
Fig. 3.
Effects (d+, 95% CI) as a function of smoking exposure.
Analyses on Rate of Change in Bone Mass
Thirteen prospective studies provided data on the rate of change in bone mass as a function of smoking exposure [33, 45, 49, 51–54, 57, 58, 75, 76, 78, 86–88]. Because of the small number of studies, analyses were conducted only for combined bone sites. Mean follow-up time was 5.3 years (range = 1–16 years). Length of follow-up was not significantly related to effect sizes, thus, effect sizes were combined for analysis. Both current smokers (compared with nonsmokers) and ever smokers (compared with never smokers) had greater rates of bone loss (d = −0.13 [−0.21, −0.05] and −0.10 [−0.20, −0.01], respectively). In stratified analyses, d did not differ as a function of gender, trabecular content, age, measurement technique, publication status, or control of age and body mass. Analyses could not be conducted stratified for menopausal status because only one study was conducted on premenopausal women.
Smoking-Related Fracture Risk
Table 2 presents estimates of the increase in age-adjusted fracture risk at various bone sites that is attributable to cigarette smoking for both women and men. These estimates are derived from standardized bone mass differences between current smokers and never smokers in this meta-analysis, and known changes in fracture risk as a function of age- adjusted bone mass differences. Predicted risk of fracture tended to be greater for men than women at all bone sites except the forearm. Across all bone sites, smoking is estimated to increase fracture risk by 5% in women and 11% in men. Smoking produced the greatest increases in fracture risk at the lumbar spine (13% for women and 32% for men) and at the hip (31% for women and 40% for men). For women, the estimated proportion of all fractures that is attributable to cigarette smoking is 2.8% for the lumbar spine and 6.4% for the hip. Among men, 8.1% of lumbar spine fractures and 9.9% of hip fractures are attributable to cigarette smoking.
Table 2.
Estimates of site-specific increase in fracture risk for current smokers compared with never smokers
| Bone site | Risk of fracture based on a 1 SD, decrease in age-adjusted bone massa | Women
|
Men
|
||||
|---|---|---|---|---|---|---|---|
| Standardized mean difference (SE) in bone mass (d+) | Predicted risk of fracture (95% CI)b | Proportion (%) of all fractures attributable to smokingc | Standardized mean difference (SE) in bone mass (d+) | Predicted risk of fracture (95% CI)b | Proportion (%) of all fractures attributable to smokingc | ||
| Any Site | 1.5 | −.13 (.03) | 1.05 (1.03 – 1.07) | 1.1 | −.25 (.05) | 1.11 (1.06 – 1.15) | 2.9 |
| Lumbar spine | 2.3 | −.15 (.06) | 1.13 (1.02 – 1.25) | 2.8 | −.33 (.08) | 1.32 (1.15 – 1.50) | 8.1 |
| Forearm | 1.7 | −.09 (.03) | 1.05 (1.02 – 1.08) | 1.1 | −.11 (.07) | 1.06 (0.98 – 1.02) | 1.6 |
| Os calcis | 1.5 | −.10 (.03) | 1.04 (1.02 – 1.06) | 0.9 | −.30 (.07) | 1.13 (1.07 – 1.20) | 3.5 |
| Hip | 2.6 | −.28 (.07) | 1.31 (1.14 – 1.49) | 6.4 | −.35 (.07) | 1.40 (1.22 – 1.60) | 9.9 |
Based on estimates from Marshall, Johnell, & Wedel, 1996. These estimates are derived from studies of women. However, the predictive ability of bone mass for fracture risk is similar in women and men [51, 110]
Calculated as antilog (standardized relative fracture risk based on smoking status × log of standardized relative facture risk based on bone mass change). For example, predicted risk of fracture at the lumbar spine for current vs. never smokers (female) is calculated as antilog (0.15 × log 2.3) = 1.13
Proportions reflect the population attributable risk, based on 1997 prevalence estimates for current smoking among U.S. adults (27.6% for men, 22.1% for women) [111]
Discussion
This meta-analysis provides evidence of an independent negative effect of cigarette smoking on bone mass at several major sites of osteoporotic fractures, including the hip, lumbar spine, and forearm. These results extend findings of another recent meta-analysis on this topic [2] by demonstrating that smoking is associated with a greater rate of bone loss, and that these effects are independent of body weight differences between smokers and nonsmokers. Averaging across bone sites, the bone mass of current smokers was one-tenth of a SD below that of nonsmokers. The deleterious effect of smoking was especially prominent at the hip, with the bone mass of current smokers being nearly one-third of a SD below that of never smokers.
The effects of smoking on bone mass appear to be dose-dependent. Across studies, bone mass was negatively correlated with measures of smoking dose (pack-years, cigarettes per day, number of years smoked) on the order of −0.04 to −0.06. Although the magnitude of these correlations is small, it is not surprising that a single variable would account for only a small proportion of the total variance in such a multiply-determined measure as BMD. It is noteworthy that a much stronger dose-response relationship was observed for twin studies, with a correlation of −0.28 observed for within-pair differences in lumbar spine bone mass and pack-years of smoking. These stronger relationships are likely due to the fact that within-twin-pair comparisons substantially reduce the “noise” in the smoking/ bone mass relationship by effectively controlling for age, gender, and genetic differences.
The literature has been unclear as to whether smoking may influence bone mass by hindering the achievement of peak bone mass during early adulthood, or by increasing bone loss later in life. We found evidence that the effects of smoking on bone are most pronounced in older individuals, including postmenopausal women. Effects were positively related to age for combined bone sites, with effects tending to be greatest for individuals past the age of 60. No significant effects were observed at any bone site for individuals younger than 40 years, arguing against a major influence of smoking on peak bone mass. It is likely that the effects of smoking on bone are cumulative, and that total smoking exposure in most young adult smokers is insufficient to produce observable decrements. A negative effect of smoking on bone mass among young adults would only be expected for individuals with greater tobacco intake. In support of this notion, several studies of young adults have demonstrated significant effects of smoking on bone mass when analyses are restricted to heavy smokers (>1 pack per day [38, 72, 89]. In the present meta-analysis, significant dose-response effects were observed among young adults (<40 years of age) for combined bone sites and the lumbar spine.
Smoking was shown to have a more deleterious effect on bone mass for men than for women. Effects were 50%–300% greater in men at combined bone sites, the lumbar spine, and the forearm, with similar, although statistically nonsignificant, trends at the os calcis and hip. Several factors may account for these gender differences. First, effects among women may be partially obscured by unmeasured confounders such as use of oral contraceptives or estrogen replacement therapy, which may protect against bone loss and thus increase error variability. In addition, gender differences are likely related to greater tobacco intake in men, as suggested by the dose-response relationships observed for both men and women. Unfortunately, too few of the studies reviewed in this meta-analysis reported detailed smoking exposure data to reliably determine whether tobacco exposure differences accounted for gender differences in smoking/bone mass effects.
One mechanism by which smoking may increase bone loss is through its effect on body weight [9, 48, 90]. By middle age, smokers weigh an average of 7–8 pounds less than nonsmokers [91]. Higher body weight among non-smokers compared with smokers could result in higher bone mass for a number of reasons, including increased mechanical load on weight-bearing bone [92], and greater conversion of androgens to estrogen in adipose tissue [93, 94]. Despite these plausible mechanisms, there was no evidence in this meta-analysis that the effects of smoking on bone mass were attributable to weight differences between smokers and nonsmokers. Effect sizes did not differ significantly at any bone site according to whether body mass was controlled. Further adjustment for differences in age, physical activity, or calcium intake—potentially important confounders of the smoking/bone mass association—did not modify these effects.
Smoking also may influence bone mass through several other mechanisms. Smoking is known to influence reproductive hormone functioning in women. Female smokers begin natural menopause an average of 1–2 years before nonsmokers [95, 96] and age at menopause is a strong predictor of subsequent osteoporosis [5, 77]. There is some evidence that smoking-related changes in reproductive hormonal functioning among men, as well, may be a contributor, but evidence is less consistent than in women [97–99].
Nicotine administration has been shown to reduce bone mass in both castrated and noncastrated mice, indicating a direct effect of nicotine on bone independent of its effect on androgens [100]. Smoking also adversely affects other hormones and enzymes involved in bone regulation, including parathyroid hormone [101] and alkaline phosphatase [102]. In addition, there is indirect evidence that smoking may damage the blood supply to bone. Cigarette smoking is a risk factor for ischemic osteonecrosis [103, 104], which may be related to nicotine’s peripheral vasoconstrictor effects [105]. More research is needed to determine whether any of these physiologic mechanisms underlie smoking’s effect on bone mass.
To date, no prospective studies have evaluated whether changes in smoking status (initiating or quitting smoking) are related to changes in bone mass. In this meta-analysis, absolute effects at most bone sites were greatest for current smokers compared with never smokers, intermediate for current smokers compared with former smokers, and smallest for former smokers compared with never smokers. At both combined bone sites and the lumbar spine, current smokers had lower bone mass than never smokers, whereas former and never smokers did not differ significantly. These differences in effect magnitude suggest that smoking cessation may slow, or partially reverse, the accelerated bone loss caused by years of smoking. Unfortunately, insufficient data were provided in most studies to evaluate whether the length of time since quitting smoking influenced effects. The only large, well-controlled study to evaluate time-since-quitting found significant negative linear relationships in hip bone mass for elderly men and women when comparing never smokers, short-term quitters (<16 years), long-term quitters (>16 years), and current smokers [6]. More work is needed to confirm these effects and to evaluate possible mechanisms, such as weight gain and change in reproductive hormonal balance.
Limitations of this research should be mentioned. The statistical association between smoking and bone mass observed in this meta-analysis does not necessarily imply a causal relationship. Furthermore, as with all observational research, assessing whether the observed association is valid is dependent on whether alternative explanations such as chance, bias, and confounding can be ruled out. The associations between smoking and bone mass are unlikely to be due to chance, given that estimates are derived from a large pooled sample size (more than 40,000 participants) drawn from studies that were heterogeneous in terms of several participant and methodologic characteristics. Likewise, selection bias is unlikely to be a significant problem in this research because participants typically were not selected specifically for either smoking exposure or bone mass. Rather, smoking status was usually one of numerous health risk behaviors assessed from community- or convenience-based samples. Bias in ascertainment of smoking exposure is possible since all studies relied on self-reports. However, self-reported smoking status in epidemiologic research is highly accurate [106].
The most serious threat to the validity of the associations found in this research is the likelihood of confounding. That is, there are several factors that influence bone mass and are unequally distributed across smoking status, including body weight, age, physical activity level, and intake of calcium, alcohol, and caffeine. Compared with nonsmokers, smokers often are found to have elevated risk status on several of these variables simultaneously (e.g., lower physical activity, lower intake of calcium, higher alcohol consumption) [107].
The role of potential confounders was explored analytically through the use of stratified and multivariate analyses. The results provide clear evidence that neither body weight nor age—important potential confounders that are precisely measured—significantly influence the observed smoking/ bone mass relationships. Although body weight might be on the causal pathway between smoking and bone mass rather than a confounder per se, our data suggest that its influence does not fully explain the smoking/bone mass association. The influence of other potential confounders cannot be ruled out, however. Self-reported variables such as dietary intake and physical activity level are notoriously difficult to measure precisely and in fact were not measured by the majority of studies included in the meta-analysis. These caveats notwithstanding, a causal role of smoking in bone density loss is suggested by the consistency of the observed associations, biologic plausibility, and the finding of a dose-response relationship.
The effects observed in this study are small, but translate into substantial increases in fracture risk. For example, based on these data, smokers are estimated to have a 32% greater risk of suffering a hip fracture compared with individuals who never smoked. We estimated that the proportion of hip fractures attributable to smoking is 6–10%. This is consistent with other estimates, and corresponds to approximately 34,000 additional hip fractures per year in the United States alone [2, 108].
Smoking-related osteoporosis is likely to take on even greater public health import in our aging society with recent trends for increased smoking prevalence among teenagers and young adults [109]. Given that smoking’s effect on bone is cumulative and dose-dependent, increased smoking in these young groups is likely to translate into a substantially increased future public health burden in osteoporosis. Smoking has a clear adverse effect on bone health, but stopping smoking may slow or partially reverse bone loss. Given the public health implications of smoking on bone health, it is important that this information be incorporated into smoking prevention and cessation efforts.
Acknowledgments
This research was supported by grant R29 AR 448909-01 from the National Institute of Arthritis and Musculo-skeletal and Skin Diseases. We thank Dr. Susan M. Zbikowski for her expert assistance. We thank the following investigators for supplying additional data from the referenced studies: Drs. C. Cooper [44], S. Franceschi [27], and S. Hagiwara [80].
Footnotes
It was not possible to classify trabecular bone content more precisely than <50% or ≥50% because some studies did not specify the exact anatomic locations of bone density measurements. Sites coded as <50% trabecular content included midshaft and proximal forearm, hip sites, humerus neck, metacarpal, lumbar spine, and total body. Sites coded as ≥50% trabecular content included distal and ultradistal forearm, and os calcis.
In many studies, sample sizes were not presented stratified by smoking status for effects involving correlations of bone mass with continuous exposure variables. As such, sample sizes according to smoking status are provided only for independent-groups comparisons.
References (asterisked articles in the bibliography were included in the meta-analysis)
- 1.Cooper C, Wickham C. Cigarette smoking and the risk of age-related fractures. In: Wald N, Baron J, editors. Smoking and hormone-related disorders. Oxford University Press; Oxford: 1990. pp. 93–100. [Google Scholar]
- 2.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315:841–846. doi: 10.1136/bmj.315.7112.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3(*).Cox ML, Khan SA, Gau DW, Cox SAL, Hodkinson HM. Determinants of forearm bone density in premenopausal women: a study in one general practice. Br J Gen Pract. 1991;41:194–196. [PMC free article] [PubMed] [Google Scholar]
- 4(*).Daniel M, Martin AD, Drinkwater DT. Cigarette smoking, steroid hormones, and bone mineral density in young women. Calcif Tissue Int. 1992;50:300–305. doi: 10.1007/BF00301626. [DOI] [PubMed] [Google Scholar]
- 5(*).Johnell O, Nilsson BE. Life-style and bone mineral mass in perimenopausal women. Calcif Tissue Int. 1984;36:354–356. doi: 10.1007/BF02405345. [DOI] [PubMed] [Google Scholar]
- 6(*).Hollenbach KA, Barrett-Connor E, Edelstein SL, Holbrook T. Cigarette smoking and bone mineral density in older men and women. Am J Public Health. 1993;83:1265–1270. doi: 10.2105/ajph.83.9.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlenker RA, VonSeggen WW. The distribution of cortical and trabecular bone mass along the lengths of the radius and ulna and the implications for in vivo bone mass measurements. Calcif Tissue Res. 1976;20:41–52. doi: 10.1007/BF02546396. [DOI] [PubMed] [Google Scholar]
- 8.Awbrey BJ, Jacobson PC, Grubb SA, McCartney WH, Vincent LM, Talmage RV. Bone density in women: a modified procedure for measurement of the distal radial density. J Orthop Res. 1984;2:314–321. doi: 10.1002/jor.1100020402. [DOI] [PubMed] [Google Scholar]
- 9.Nottestad SY, Baumel JJ, Kimmel DB, Recker RR, Heaney RP. The proportion of trabecular bone in human vertebrae. J Bone Miner Res. 1987;2:221–229. doi: 10.1002/jbmr.5650020309. [DOI] [PubMed] [Google Scholar]
- 10.Jebb SA, Elia M. Techniques for the measurement of body composition: a practical guide. Int J Obes. 1993;17:611–621. [PubMed] [Google Scholar]
- 11.Kellie SE. Measurement of bone density with dual-energy x-ray absorptiometry (DEXA) JAMA. 1992;267:286–294. [PubMed] [Google Scholar]
- 12.Cummings SR, Black DM. Bone mass measurements and risk of fracture in Caucasian women: a review of findings from prospective studies. Am J Med. 1995;98(suppl 2A):24S–27S. doi: 10.1016/s0002-9343(05)80041-5. [DOI] [PubMed] [Google Scholar]
- 13.Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Erlbaum Associates; New Jersey: 1988. [Google Scholar]
- 14.Hedges LV, Olkin I. Statistical methods for meta-analysis. Academic Press; Boston: 1985. [Google Scholar]
- 15.Shadish WR, Haddock CK. Combining estimates of effect size. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. Russell Sage Foundation; New York: 1994. pp. 262–280. [Google Scholar]
- 16(*).Broulik PD, Kapitola J. Interrelations between body weight, cigarette smoking and spine mineral density in osteoporotic Czech women. Endocr Regul. 1993;27:57–60. [PubMed] [Google Scholar]
- 17(*).Davies MC, Hall ML, Jacobs HS. Bone mineral loss in young women with amenorrhoea. BMJ. 1990;301:790–803. doi: 10.1136/bmj.301.6755.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18(*).Herzog W, Minne H, Deter C, Leidig G, Schellberg D, Wuster C, Gronwald R, Sarembe E, Kroger F, Bergmann G, Petzold E, Hahn P, Schepank H, Ziegler R. Outcome of bone mineral density in anorexia nervosa patients 11.7 years after first admission. J Bone Miner Res. 1993;8:597–605. doi: 10.1002/jbmr.5650080511. [DOI] [PubMed] [Google Scholar]
- 19(*).McNair P, Christensen MS, Madsbad S, Christiansen C, Binder C, Transbol I. Bone loss in patients with diabetes mellitus: effects of smoking. Miner Electrolyte Metab. 1980;3:94–97. [Google Scholar]
- 20(*).Munoz-Torres M, Jodar E, Escobar-Jimenez F, Lopez-Ibarra PJ, Lunda JD. Bone mineral density measured by dual x-ray absorptiometry in Spanish patients with insulin-dependent diabetes mellitus. Calcif Tissue Int. 1996;58:316–319. doi: 10.1007/BF02509378. [DOI] [PubMed] [Google Scholar]
- 21(*).Ghosh S, Cowen S, Hannan WJ, Ferguson A. Low bone mineral density in Crohn’s disease, but not in ulcerative colitis, at diagnosis. Gastroenterology. 1994;107:1031–1039. doi: 10.1016/0016-5085(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 22(*).Krogsgaard MR, Frolich A, Lund B, Lund B. Long-term changes in bone mass after partial gastrectomy in a well-defined population and its relation to tobacco and alcohol consumption. World J Surg. 1995;19:867–871. doi: 10.1007/BF00299788. [DOI] [PubMed] [Google Scholar]
- 23(*).Silvennoinen JA, Kehtola JK, Niemela SE. Smoking is a risk factor for osteoporosis in women with inflammatory bowel disease. Scand J Gastroenterol. 1996;31:367–371. doi: 10.3109/00365529609006412. [DOI] [PubMed] [Google Scholar]
- 24(*).Nymann P, Kollerup G, Jemec GBE, Grossmann E. Decreased bone mineral density in patients with pustulosis palmaris et plantaris. Dermatology. 1996;192:307–311. doi: 10.1159/000246400. [DOI] [PubMed] [Google Scholar]
- 25(*).Aloia JF, Vaswani AN, Yeh JK, Cohn SH. Pre-menopausal bone mass is related to physical activity. Arch Intern Med. 1988;148:121–123. [PubMed] [Google Scholar]
- 26(*).Fehily AM, Coles RJ, Evans WD, Elwood PC. Factors affecting bone density in young adults. Am J Clin Nutr. 1992;56:579–586. doi: 10.1093/ajcn/56.3.579. [DOI] [PubMed] [Google Scholar]
- 27(*).Franceschi S, Schinella D, Bidoli E, Dal Maso L, La Vecchia C, Parazzini F, Zecchin R. The influence of body size, smoking, and diet on bone density in pre- and postmenopausal women. Epidemiology. 1996;7:411–414. doi: 10.1097/00001648-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 28(*).Hansen MA. Assessment of age and risk factors on bone density and bone turnover in healthy premenopausal women. Osteoporos Int. 1994;4:123–128. doi: 10.1007/BF01623056. [DOI] [PubMed] [Google Scholar]
- 29(*).Hirota T, Nara M, Ohguri M, Manago E, Hirota K. Effect of diet and lifestyle on bone mass in Asian young women. Am J Clin Nutr. 1992;55:1168–1173. doi: 10.1093/ajcn/55.6.1168. [DOI] [PubMed] [Google Scholar]
- 30(*).Hu J-F, Zhao X-H, Fitzpatrick J, Parpia B, Campbell TC. Bone density and lifestyle characteristics in premenopausal and postmenopausal Chinese women. Osteoporos Int. 1994;4:288–297. doi: 10.1007/BF01622185. [DOI] [PubMed] [Google Scholar]
- 31(*).Lane N, Baptista J, Snow-Harter C. Bone mineral density of the lumbar spine in endometriosis subjects compared to an age-similar control population. J Clin Endocrinol Metab. 1991;72:510–514. doi: 10.1210/jcem-72-2-510. [DOI] [PubMed] [Google Scholar]
- 32(*).Lindquist O, Bengtsson C, Hanson T, Roos B. Bone mineral content in relation to age and menopause in middle-aged women: a study of bone density in lumbar vertebrae by dual photon absorptiometry in a population sample of women. Scand J Clin Lab Invest. 1981;41:215–223. doi: 10.3109/00365518109092037. [DOI] [PubMed] [Google Scholar]
- 33(*).Mazess RB, Barden HS. Bone density in premenopausal women: effects of age, dietary intake, physical activity, smoking, and birth control pills. Am J Clin Nutr. 1991;53:132–142. doi: 10.1093/ajcn/53.1.132. [DOI] [PubMed] [Google Scholar]
- 34(*).McCulloch RG, Bailey DA, Houston CS, Dodd BL. Effects of physical activity, dietary calcium intake and selected lifestyle factors on bone density in young women. Can Med Assoc J. 1990;142:221–227. [PMC free article] [PubMed] [Google Scholar]
- 35(*).McCulloch RG, Whiting SJ, Bailey DA, Houston CS. The effect of cigarette smoking on trabecular bone density in premenopausal women, aged 20–35 years. Can J Public Health. 1991;82:434–435. [PubMed] [Google Scholar]
- 36(*).Ortego-Ceneno N, Munoz-Torres M, Hernandez-Quero J, Jurado-Duce A, de la Higuera Torres-Puchol J. Bone mineral density, sex steroids, and mineral metabolism in premenopausal smokers. Calcif Tissue Int. 1994;55:403–407. doi: 10.1007/BF00298551. [DOI] [PubMed] [Google Scholar]
- 37(*).Turner JG, Gilchrist NL, Ayling EM, Hassall AJ, Hooke EA, Sadler WA. Factors affecting bone mineral density in high school girls. N Z Med J. 1992;105:95–96. [PubMed] [Google Scholar]
- 38(*).Valimaki MJ, Karkkainen M, Lamberg-Allardt C, Laitinen K, Alhava E, Heikkinen J, Impivaara O, Makela P, Palmgren J, Seppanen R, Vuori I. Exercise, smoking, and calcium intake during adolescence and early adulthood as determinants of peak bone mass. BMJ. 1994;309:230–235. doi: 10.1136/bmj.309.6949.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39(*).Cundy T, Cornish J, Roberts H, Reid IR. Is the reduction in bone mineral density in women using depot medroxyprogeserone contraception due to smoking? (abstract) J Bone Miner Res. 1997;12(suppl 1):S337. [Google Scholar]
- 40(*).Bauer DC, Browner WS, Cauley JA, Orwoll ES, Scott JC, Black DM, Tao JL, Cummings SR. Factors associated with appendicular bone mass in older women. Ann Intern Med. 1993;118:657–665. doi: 10.7326/0003-4819-118-9-199305010-00001. [DOI] [PubMed] [Google Scholar]
- 41(*).Orwoll ES, Bauer DC, Vogt TM, Fox KM. Axial bone mass in older women. Ann Intern Med. 1996;124:187–196. doi: 10.7326/0003-4819-124-2-199601150-00001. [DOI] [PubMed] [Google Scholar]
- 42(*).Cheng S, Suominen H, Heikkinen E. Bone mineral density in relation to anthropometric properties, physical activity and smoking in 75-year-old men and women. Aging (Milano) 1993;5:55–62. doi: 10.1007/BF03324127. [DOI] [PubMed] [Google Scholar]
- 43(*).Daniell HW. Osteoporosis of the slender smoker: vertebral compression fractures and loss of metacarpal cortex in relation to postmenopausal cigarette smoking and lack of obesity. Arch Intern Med. 1976;136:298–304. doi: 10.1001/archinte.136.3.298. [DOI] [PubMed] [Google Scholar]
- 44(*).Egger P, Duggleby S, Hobbs R, Fall C, Cooper C. Cigarette smoking and bone mineral density in the elderly. J Epidemiol Community Health. 1996;50:47–50. doi: 10.1136/jech.50.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45(*).Hansen MA, Overgaard K, Riis BJ, Christiansen C. Potential risk factors for development of postmenopausal osteoporosis—examined over a 12-year period. Osteoporos Int. 1991;1:95–102. doi: 10.1007/BF01880450. [DOI] [PubMed] [Google Scholar]
- 46(*).Hermann AP, Mosekilde L. Smoking and bone mineral density in 595 perimenopausal women (abstract) J Bone Miner Res. 1996;11(suppl 1):S222. doi: 10.1359/jbmr.2000.15.4.780. [DOI] [PubMed] [Google Scholar]
- 47(*).Hollo I, Gergely I, Boross M. Influence of heavy smoking upon the bone mineral content of the radius of the aged and effect of tobacco smoke on the sensitivity to calcitonin of rats. Aktuelle Gerontol. 1979;9:365–368. [PubMed] [Google Scholar]
- 48(*).Jensen GF. Osteoporosis of slender smoker revisited by epidemiologic approach. Eur J Clin Invest. 1986;16:239–242. doi: 10.1111/j.1365-2362.1986.tb01335.x. [DOI] [PubMed] [Google Scholar]
- 49.Jensen J, Christiansen C. Effects of smoking on serum lipoproteins and bone mineral content during postmenopausal hormone replacement therapy. Am J Obstet Gynecol. 1988;159:820–825. doi: 10.1016/s0002-9378(88)80144-3. [DOI] [PubMed] [Google Scholar]
- 50(*).Kiel DP, Zhang Y, Hannan MT, Anderson JJ, Baron JA, Felson DT. The effect of smoking at different life stages on bone mineral density in elderly men and women. Osteoporos Int. 1996;6:240–248. doi: 10.1007/BF01622741. [DOI] [PubMed] [Google Scholar]
- 51(*).Nguyen TV, Kelly PJ, Sambrook PN, Gilbert C, Pocock NA, Eisman JA. Lifestyle factors and bone density in the elderly: implications for osteoporosis prevention. J Bone Miner Res. 1994;9:1339–1346. doi: 10.1002/jbmr.5650090904. [DOI] [PubMed] [Google Scholar]
- 52(*).Jones G, Nguyen T, Sambrook P, Kelly PJ, Eisman JA. Progressive loss of bone in the femoral neck in elderly people: longitudinal findings from the Dubbo osteoporosis epidemiology study. BMJ. 1994;309:691–695. [PMC free article] [PubMed] [Google Scholar]
- 53(*).Krall EA, Dawson-Hughes B. Smoking and bone loss among postmenopausal women. J Bone Miner Res. 1991;6:331–337. doi: 10.1002/jbmr.5650060404. [DOI] [PubMed] [Google Scholar]
- 54(*).Lindsay R. The influence of cigarette smoking on bone mass and bone loss. In: DeLuca HF, Frost HM, Jee WSS, Johnston CC Jr, Parfitt AM, editors. Osteoporosis: recent advances in pathogenesis and treatment. University Park Press; Baltimore: 1981. [Google Scholar]
- 55(*).Rundgren A, Mellstrom D. The effect of tobacco smoking on the bone mineral content of the ageing skeleton. Mech Ageing Dev. 1984;28:273–277. doi: 10.1016/0047-6374(84)90027-7. [DOI] [PubMed] [Google Scholar]
- 56(*).Ooms ME, Lips P, Van Lingen A, Valkenburg HA. Determinants of bone mineral density and risk factors for osteoporosis in healthy elderly women. J Bone Miner Res. 1993;8:669–675. doi: 10.1002/jbmr.5650080604. [DOI] [PubMed] [Google Scholar]
- 57(*).Slemenda CW, Hui SL, Longcope C, Johnston CC., Jr Cigarette smoking, obesity, and bone mass. J Bone Miner Res. 1989;4:737–741. doi: 10.1002/jbmr.5650040513. [DOI] [PubMed] [Google Scholar]
- 58(*).Jensen J, Christiansen C, Rodbro P. Cigarette smoking, serum estrogens, and bone loss during hormone-replacement therapy early after menopause. N Engl J Med. 1985;313:973–975. doi: 10.1056/NEJM198510173131602. [DOI] [PubMed] [Google Scholar]
- 59(*).Erdtsieck RJ, Pols HAP, Algra D, Kooy PPM, Birkenhage JC. Bone mineral density in healthy Dutch women: spine and hip measurements using dual-energy x-ray absorptiometry. Neth J Med. 1994;45:198–205. [PubMed] [Google Scholar]
- 60(*).Glynn NW, Meilahn EN, Charron M, Anderson SJ, Kuller LH, Cauley JA. Determinants of bone mineral density in older men. J Bone Miner Res. 1995;10:1769–1777. doi: 10.1002/jbmr.5650101121. [DOI] [PubMed] [Google Scholar]
- 61(*).Guthrie JR, Ebeling PR, Hopper JL, Dennerstein L, Wark JD, Burger HG. Bone mineral density and hormone levels in menopausal Australian women. Gynecol Endocrinol. 1996;10:199–205. doi: 10.3109/09513599609027989. [DOI] [PubMed] [Google Scholar]
- 62(*).Johansson C, Mellstrom D, Lerner U, Osterberg T. Coffee drinking: a minor risk factor for bone loss and fractures. Age Ageing. 1992;21:20–26. doi: 10.1093/ageing/21.1.20. [DOI] [PubMed] [Google Scholar]
- 63(*).Johansson C, Mellstrom D, Milsom I. Reproductive factors as predictors of bone density and fractures in women at the age of 70. Maturitas. 1993;17:39–50. doi: 10.1016/0378-5122(93)90122-x. [DOI] [PubMed] [Google Scholar]
- 64(*).Kin K, Lee JHE, Kushida K, Sartoris DJ, Ohmura A, Clopton PL, Inoue T. Bone density and body composition on the pacific rim: a comparison between Japan-born and U.S.-born Japanese-American women. J Bone Miner Res. 1993;8:861–869. doi: 10.1002/jbmr.5650080712. [DOI] [PubMed] [Google Scholar]
- 65(*).Kroger H, Laitinen K. Bone mineral density measured by dual-energy x-ray absorptiometry in normal men. Eur J Clin Invest. 1992;22:454–460. doi: 10.1111/j.1365-2362.1992.tb01490.x. [DOI] [PubMed] [Google Scholar]
- 66(*).Laitinen K, Valimaki M, Keto P. Bone mineral density measured by dual-energy x-ray absorptiometry. Calcif Tissue Int. 1991;48:224–231. doi: 10.1007/BF02556372. [DOI] [PubMed] [Google Scholar]
- 67(*).Murphy S, Khaw K-T, May H, Compston JE. Cigarette smoking and bone mineral density in middle-aged and older women (abstract) Ir J Med Sci. 1994a;163:464. [Google Scholar]
- 68(*).Murphy S, Khaw K-T, May H, Compston JE. Parity and bone mineral density in middle-aged women. Osteoporos Int. 1994b;4:162–166. doi: 10.1007/BF01623063. [DOI] [PubMed] [Google Scholar]
- 69(*).May H, Murphy S, Khaw K-T. Cigarette smoking and bone mineral density in older men. Q J Med. 1994;8:625–630. [PubMed] [Google Scholar]
- 70(*).McKnight A, Steele K, Mills K, Gilchrist C, Taggart H. Bone mineral density in relation to medical and lifestyle risk factors for osteoporosis in premenopausal, menopausal and postmenopausal women in general practice. Br J Gen Pract. 1995;45:317–320. [PMC free article] [PubMed] [Google Scholar]
- 71(*).Nelson DA, Jacobsen G, Barondess DA, Parfitt AM. Ethnic differences in regional bone density, hip axis length, and lifestyle variables among healthy black and white men. J Bone Miner Res. 1995;10:782–787. doi: 10.1002/jbmr.5650100515. [DOI] [PubMed] [Google Scholar]
- 72(*).Ortego-Centeno N, Munoz-Torres M, Jodar E, Hernandez-Quero J, Jurado-Duce A, de la Higuera Torres-Puchol J. Effect of tobacco consumption on bone mineral density in healthy young males. Calcif Tissue Int. 1997;60:496–500. doi: 10.1007/s002239900270. [DOI] [PubMed] [Google Scholar]
- 73(*).Picard D, Ste-Marie LG, Coutu D, Carrier L, Chartrand R, Lepage R, Fugere P, D’Amour P. Premenopausal bone mineral content relates to height, weight and calcium intake during early adulthood. Bone Miner. 1988;4:299–309. [PubMed] [Google Scholar]
- 74(*).Pocock NA, Eisman JA, Kelly PJ, Sambrook PN, Yeates MG. Effects of tobacco use on axial and appendicular bone mineral density. Bone. 1989;10:329–331. doi: 10.1016/8756-3282(89)90128-2. [DOI] [PubMed] [Google Scholar]
- 75(*).Sowers MR, Clark MK, Jannausch ML, Wallace RB. Body size, estrogen use and thiazide diuretic use affect 5-year radial bone loss in postmenopausal women. Osteoporos Int. 1993;3:314–321. doi: 10.1007/BF01637317. [DOI] [PubMed] [Google Scholar]
- 76(*).Sowers M, Wallace RB, Lemke JH. Correlates of mid-radius bone density among postmenopausal women: a community study. Am J Clin Nutr. 1985;41:1045–1053. doi: 10.1093/ajcn/41.5.1045. [DOI] [PubMed] [Google Scholar]
- 77(*).Stevenson JC, Lees B, Davenport M, Cust MP, Ganger KF. Determinants of bone density in normal women: risk factors for future osteoporosis? BMJ. 1989;298:924–928. doi: 10.1136/bmj.298.6678.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78(*).Vogel JM, Davis JW, Nomura A, Wasnich RD, Ross PD. The effects of smoking on bone mass and the rates of bone loss among elderly Japanese-American men. J Bone Miner Res. 1997;12:1495–1501. doi: 10.1359/jbmr.1997.12.9.1495. [DOI] [PubMed] [Google Scholar]
- 79(*).Ward JA, Lord SR, Williams P, Anstey K, Zivanovic E. Physiologic, health and lifestyle factors associated with femoral neck bone density in older women. Bone. 1995;16:373S–378S. doi: 10.1016/8756-3282(95)00052-f. [DOI] [PubMed] [Google Scholar]
- 80(*).Hagiwara S, Izumotani-Sasao K, Kawakami H, Miki T, Morii H. Smoking is one of the risk factors for middle-aged male osteoporosis (abstract) J Bone Miner Res. 1996;11(suppl 1):S224. [Google Scholar]
- 81(*).Masaryk P, Lunt M, Dequeker J, Felsenberg D, Scheidt-Nave C, Poor G, Pols H, Falch J, Reid D, Benevolenskaya L, Weber K, Cannata J, Dodenhof C, O’Neill T, Silman A, Reeve J. Influence of historical and current lifestyle and dietary factors on BMD in men and women in the EVOS study (abstract) J Bone Miner Res. 1997;12(suppl 1):S485. doi: 10.1359/jbmr.1997.12.11.1883. [DOI] [PubMed] [Google Scholar]
- 82(*).Hagiwara S, Tanabe Y, Nitta K. Smoking influences male bone mineral density of the heel (abstract) J Bone Miner Res. 1997;12(suppl 1):S249. [Google Scholar]
- 83(*).Flicker L, Hopper JL, Rodgers L, Kaymakci B, Green RM, Wark JD. Bone density determinants in elderly women: a twin study. J Bone Miner Res. 1995;10:1607–1613. doi: 10.1002/jbmr.5650101102. [DOI] [PubMed] [Google Scholar]
- 84(*).Hopper JL, Seeman E. The bone density of female twins discordant for tobacco use. N Engl J Med. 1994;330:387–392. doi: 10.1056/NEJM199402103300603. [DOI] [PubMed] [Google Scholar]
- 85(*).Young D, Hopper JL, Nowson CA, Green RM, Shewin AJ, Kaymakci B, Smid M, Guest CS, Larkins RG, Wark JD. Determinants of bone mass in 10- to 26-year-old females: a twin study. J Bone Miner Res. 1995;10:558–567. doi: 10.1002/jbmr.5650100408. [DOI] [PubMed] [Google Scholar]
- 86(*).Slemenda CW, Christian JC, Reed T, Reister TK, Williams CJ, Johnston CC., Jr Long-term bone loss in men: effects of genetic and environmental factors. Ann Intern Med. 1992;117:286–291. doi: 10.7326/0003-4819-117-4-286. [DOI] [PubMed] [Google Scholar]
- 87(*).Sparrow D, Beausoleil NI, Rosner B, Garvey AJ, Silbert JE. The influence of cigarette smoking and age on bone loss in men. Arch Environ Health. 1982;37:246–249. doi: 10.1080/00039896.1982.10667572. [DOI] [PubMed] [Google Scholar]
- 88(*).Krall EA, Dawson-Hughes B. Smoking reduces fractional calcium absorption and increases bone loss (abstract) J Bone Miner Res. 1997;12(suppl 1):S225. doi: 10.1359/jbmr.1999.14.2.215. [DOI] [PubMed] [Google Scholar]
- 89(*).Whiting SJ, McCulloch RG, Bailey DA, Houston CS. The effect of cigarette smoking on trabecular bone density in premenopausal women, aged 20–35 years (abstract) J Bone Miner Res. 1990;5(suppl):S249. [PubMed] [Google Scholar]
- 90.Law M. Smoking and osteoporosis. In: Wald N, Baron J, editors. Smoking and hormone-related disorders. Oxford University Press; Oxford: 1990. pp. 83–92. [Google Scholar]
- 91.Klesges RC, Meyers AW, Klesges LM, La Vasque M. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol Bull. 1989;106:204–230. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- 92.Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37:411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- 93.Grodin JM, Siiteri PK, MacDonald PC. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab. 1973;36:207–214. doi: 10.1210/jcem-36-2-207. [DOI] [PubMed] [Google Scholar]
- 94.Longcope C, Pratt JW, Schneider SH, Fineberg SE. Aromatization of androgens by muscle and adipose tissue in vivo. J Clin Endocrinol Metab. 1977;46:146–152. doi: 10.1210/jcem-46-1-146. [DOI] [PubMed] [Google Scholar]
- 95.Jick H, Porter J. Relationship between smoking and age of natural menopause. Lancet. 1977;1:1354–1355. doi: 10.1016/s0140-6736(77)92562-4. [DOI] [PubMed] [Google Scholar]
- 96.Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, Cramer D, Hennekens CH. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 97.Barrett-Connor E, Khaw K-T. Cigarette smoking and increased endogenous estrogen levels in men. Am J Epidemiol. 1987;126:187–192. doi: 10.1093/aje/126.2.187. [DOI] [PubMed] [Google Scholar]
- 98.Foresta C, Ruzza G, Mioni R, Guarneri G, Gribaldo R, Meneghello A, Mastrogiacomo I. Osteoporosis and decline of gonadal function in the elderly male. Horm Res. 1984;19:18–22. doi: 10.1159/000179855. [DOI] [PubMed] [Google Scholar]
- 99.Murphy S, Khaw K-T, Cassidy A, Compston JE. Sex hormones and bone mineral density in elderly men. Bone Miner. 1993;20:133–140. doi: 10.1016/s0169-6009(08)80022-0. [DOI] [PubMed] [Google Scholar]
- 100.Broulik PD, Jarab J. The effect of chronic nicotine administration on bone mineral content in mice. Horm Metab Res. 1993;25:219–221. doi: 10.1055/s-2007-1002080. [DOI] [PubMed] [Google Scholar]
- 101.Landin-Wilhelmsen K, Wilhelmsen K, Lappas G, Rosen T, Linstedt G, Lundberg P-A, Wilske J, Bengtsson B-A. Serum intact parathyroid hormone in a random population sample of men and women: relationship to anthropometry, life-style factors, blood pressure, and vitamin D. Calcif Tissue Int. 1995;56:104–108. doi: 10.1007/BF00296339. [DOI] [PubMed] [Google Scholar]
- 102.Gordon T. Factors associated with serum alkaline phosphatase level. Arch Pathol Lab Med. 1993;117:187–190. [PubMed] [Google Scholar]
- 103.Hirota Y, Hirohata T, Fukuda K, Mori M, Yanagawa H, Ohno Y, Suiioka Y-I. Association of alcohol intake, cigarette smoking, and occupational status with the risk of idiopathic osteonecrosis of the femoral head. Am J Epidemiol. 1993;137:530–538. doi: 10.1093/oxfordjournals.aje.a116706. [DOI] [PubMed] [Google Scholar]
- 104.Matsuo K, Hirohata T, Sugioka Y, Ikeda M, Fukuda A. Influence of alcohol intake, cigarette smoking, and occupational status on idiopathic osteonecrosis of the femoral head. Clin Orthop. 1988;234:115–123. [PubMed] [Google Scholar]
- 105.Forrest CR, Pang CY, Lindsay WK. Dose and time effects of nicotine treatment on the capillary blood flow and viability of random pattern skin flaps in the rat. Br J Plast Surg. 1987;40:295–299. doi: 10.1016/0007-1226(87)90126-3. [DOI] [PubMed] [Google Scholar]
- 106.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84:1086–1093. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perkins KA, Rohay J, Meilahn EN, Wing RR, Matthews KA, Kuller LH. Diet, alcohol, and physical activity as a function of smoking status in middle-aged women. Health Psychol. 1993;12:410–415. doi: 10.1037//0278-6133.12.5.410. [DOI] [PubMed] [Google Scholar]
- 108.Lindsay R. The burden of osteoporosis. Am J Med. 1995;98(2A):9S–11S. doi: 10.1016/s0002-9343(05)80038-5. [DOI] [PubMed] [Google Scholar]
- 109.Centers for Disease Control and Prevention Youth risk behavior surveillance—United States. Morbidity and Mortality Weekly Reports. 1997;47:1–89. [PubMed] [Google Scholar]
- 110.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Centers for Disease Control and Prevention Cigarette smoking among adults—United States. MMWR. 1997;48:993–996. [Google Scholar]
Additional Studies Included in the Meta-Analysis
- (*).Bendavid EJ, Shan J, Barrett-Connor E. Factors associated with bone mineral density in middle-aged men. J Bone Miner Res. 1996;11:1185–1190. doi: 10.1002/jbmr.5650110818. [DOI] [PubMed] [Google Scholar]
- (*).Cheng S, Suominen H, Rantanen T, Parkatti T, Heikkinen E. Bone mineral density and physical activity in 50–60-year-old women. Bone Miner. 1991;12:123–132. doi: 10.1016/0169-6009(91)90041-w. [DOI] [PubMed] [Google Scholar]
- (*).Cooper C, Cawley M, Bhalla A, Egger P, Ring F, Morton L, Barker D. Childhood growth, physical activity, and peak bone mass in women. J Bone Miner Res. 1995;10:940–947. doi: 10.1002/jbmr.5650100615. [DOI] [PubMed] [Google Scholar]
- (*).Falch JA, Sandvik L, Van Beresteijn ECH. Development and evaluation of an index to predict early postmenopausal bone loss. Bone. 1992;13:337–341. doi: 10.1016/8756-3282(92)90080-g. [DOI] [PubMed] [Google Scholar]
- (*).Hall ML, Heavens J, Cullum ID, Ell PJ. The range of bone density in normal British women. Br J Radiol. 1990;63:266–269. doi: 10.1259/0007-1285-63-748-266. [DOI] [PubMed] [Google Scholar]
- (*).Hansson T, Sandstrom J, Roos B, Jonson R, Anderson GBJ. The bone mineral content of the lumbar spine in patients with chronic low-back pain. Spine. 1985;10:158–160. doi: 10.1097/00007632-198503000-00010. [DOI] [PubMed] [Google Scholar]
- (*).Kajita E, Iki M, Tobita Y, Mitamura S, Kusaka Y, Ogata A, Teramoto M, Tsuchida C, Yamamoto K, Ishii Y. Bone mineral density of the lumbar spine and its relation to biological and lifestyle factors in middle-aged and aged Japanese women (Part 3). Relationships of physical fitness and lifestyle factors to bone mineral density in premenopausal and post-menopausal women. Jpn J Hyg. 1995;50:893–900. doi: 10.1265/jjh.50.893. [DOI] [PubMed] [Google Scholar]
- (*).Krall EA, Dawson-Hughes B. Heritable and life-style determinants of bone mineral density. J Bone Miner Res. 1993;8:1–9. doi: 10.1002/jbmr.5650080102. [DOI] [PubMed] [Google Scholar]
- (*).Lethbridge-Cejku M, Hochberg MC, Roy TA, Plato CC, Tobin JD. Smoking is associated with lower femoral neck bone mineral density in men but not in women: data from the Baltimore Longitudinal Study of Aging (abstract) J Bone Miner Res. 1996;11(suppl 1):S222. [Google Scholar]
- (*).McDermott MT, Witte MC. Bone mineral content in smokers. South Med J. 1988;81:477–480. doi: 10.1097/00007611-198804000-00017. [DOI] [PubMed] [Google Scholar]
- (*).Mellstrom D, Gause-Nilsson I, Mattsson L-A, Oden A, Olsson J-O, Stenstrom M, Uvebrant M. Risk factors for osteoporosis in a screening study of 10,364 women aged 49–69 years (abstract) J Bone Miner Res. 1997;12(suppl 1):S483. [Google Scholar]
- (*).Rico H, Revilla M, Hernandez ER, Villa LF, del Buergo MA, Alonso AL. Age-and weight-related changes in total body bone mineral in men. Miner Electrolyte Metab. 1991;17:321–323. [PubMed] [Google Scholar]
- (*).Scott JC, Hochberg MC, Threets K, Sherwin R, Cummings SR, Bauer D, Vogt T, Nevitt MC, Genant H. Association of cigarette smoking with bone density (abstract) J Bone Miner Res. 1994;9:S273. [Google Scholar]
- (*).Seeman E, Melton LJ, III, O’Fallon WM, Riggs BL. Risk factors for spinal osteoporosis in men. Am J Med. 1983;75:977–983. doi: 10.1016/0002-9343(83)90878-1. [DOI] [PubMed] [Google Scholar]
- (*).Shaw C-K. An epidemiologic study of osteoporosis in Taiwan. Ann Epidemiol. 1993;3:264–271. doi: 10.1016/1047-2797(93)90029-4. [DOI] [PubMed] [Google Scholar]
- (*).Suominen H, Heikkinen E, Vainio P, Lahtinen T. Mineral density of calcaneus in men at different ages: a population study with special reference to life-style factors. Age Ageing. 1984;13:273–281. doi: 10.1093/ageing/13.5.273. [DOI] [PubMed] [Google Scholar]
- (*).Trichopoulou A, Georgiou E, Bassiakos Y, Lipworth L, Lagiou P, Proukakis C, Trichopoulos D. Energy intake and monounsaturated fat in relation to bone mineral density among women and men in Greece. Prev Med. 1997;26:395–400. doi: 10.1006/pmed.1997.0160. [DOI] [PubMed] [Google Scholar]