Abstract
Introduction
To evaluate the efficacy and adverse effects of repeated onabotulinumtoxinA (BoNT-A) treatment in patients suffering from Parkinson’s disease (PD) with sialorrhea.
Methods
A retrospective analysis of 16 patients with sialorrhea treated with BoNT-A at our movement disorders outpatient clinic was conducted from February 2009 to September 2013. A patient with adult cerebral palsy and a patient with PD who received only a single application were excluded. BoNT-A was injected into the parotid glands without ultrasound guidance. Pre-treatment sialorrhea severity was quantified according to the Drooling Frequency and Severity Scale (DFSS). The efficacy was evaluated four weeks after BoNT-A injections using DFSS and according to the subjective assessment of the patients and/or caregivers.
Results
The mean age of the patients was 70.00±9.82 years and the mean follow-up duration was 18.78±10.37 months. Totally, 37 applications were performed. The mean BoNT-A total dose was 34.35±6.41 units. The mean scores of DFSS before and after injections were 7.00±1.03 and 3.21±0.89, respectively (p<0.001). Efficacy was 100%, and the mean experienced sialorrhea improvement was 71.78±12.95%. We found a significant difference between the first and last application in the mean duration of efficacy (17.28±9.21 weeks and 18.03±9.02 weeks, respectively, p=0.001). We did not observe side effects in this study group.
Conclusion
Repeated injections of BoNT-A are safe and effective in treating sialorrhea in patients with PD. Based on our results, it seems that there is a maintenance of efficacy after a three-year period and an increase in the mean duration of efficacy with the number of injections. Further prospective clinical studies with larger number of patients and more longer duration of follow-up are needed to confirm our results.
Keywords: Parkinson’s disease, botulinum toxin, onabotulinumtoxinA, sialorrhea
INTRODUCTION
Sialorrhea or drooling, which is associated with social impediment, is a common problem in patients with Parkinson’s disease (PD) and can impact a patient’s health and quality of life (1,2,3). It is usually caused by oral motor or swallowing dysfunction leading to storage of saliva in the anterior part of the mouth and not by increased salivary secretion (4,5). The major salivary glands (two parotid, two submandibular, and two sublingual) account for 90% of the secretion of saliva (6). Secretory function to the salivary glands are controlled by the parasympathetic nervous system, and botulinum toxin (BT) has been shown to be effective to reduce the production of saliva (7). It has been suggested that BT injection is the most effective treatment option for Parkinsonian-related sialorrhea (2).
Several studies have reported the results of a single intraglandular BT injection in the treatment of sialorrhea in neurological disorders (1,8,9,10,11). Owing to the fact that BT treatment response is transient and repeated injections are generally required, it is important to evaluate effects of repeated BT treatment in sialorrhea. To the best of our knowledge, only two studies have reported the results of repeated BT injections to treat sialorrhea in neurological disorders (12,13). In this study, we aimed to evaluate the efficacy and adverse effect of repeated BT treatment in patients suffering from PD with sialorrhea.
METHODS
A retrospective analysis of 16 patients with sialorrhea treated with onabotulinumtoxinA (BoNT-A; Botox®, Allergan, USA) at our movement disorders outpatient clinic was conducted from February 2009 to September 2013. This analysis was conducted using the detailed medical records of these patients. All the patients were treated and evaluated by the same neurologist in our movement disorders outpatient clinic. Previous treatment options for sialorrhea, such as intraoral tropicamide or oral anticholinergic agents, were inefficient in these patients. All possible adverse effects and risks related to the applications were explained to the patients or their legal guardians, and written informed consent was received before treatment with BoNT-A. One hundred units of BoNT-A were reconstituted with 2 mL of 0.9% NaCl solution (5 units per 0.1 mL), and the toxin was used within 4 h after its reconstitution. All patients were injected into two separate targets in each parotid gland without ultrasound guidance with a 30-gauge insulin syringe. The pre-treatment sialorrhea severity of the patients was measured based on the Drooling Frequency and Severity Scale (DFSS) (Table 1) (14). The efficacy of each application was routinely evaluated four weeks after BoNT-A injections by a clinical interview with the patients and caregivers using DFSS and according to the subjective assessment of the patients and/or caregivers, which was termed to experienced improvement of sialorrhea. It was assessed using visual analog scale (VAS) as a percentage (0%=no effect–100%=marked improvement). Fifty percent or more indicated an improvement in sialorrhea, and more than a 2-point decrease in the DFSS score was accepted to be effective. In order to evaluate the duration of the efficacy and side effects, all the patients were evaluated with two months intervals. In addition, improvement diaries were also routinely recorded by the patients and/or caregivers. We used 0.5 unit/kg BoNT-A at the first application, and then, the doses of BoNT-A for each application were adjusted according to the severity of sialorrhea and the therapeutic response to the previous injections. The total BoNT-A dose per treatment, BoNT-A dose per target, response latency (days), peak improvement time (days), scores of DFSS before and four weeks after injections, subjective assessment of the patients and/or caregivers, adverse effects, duration of efficacy (weeks), and interval between each injection (weeks) were recorded for each application. The response latency was measured as the time between the injection and the beginning of a reduction in saliva. The peak improvement time was measured as the time between the injection and maximal improvement. The duration of efficacy was calculated as time between the peak improvement after injection and reappearance of sialorrhea symptoms. Re-injections were given to patients who or whose caregiver desired another injection. The injection intervals were not shorter than three months.
Table 1.
Drooling Severity and Frequency Scale (DSFS)
| Drooling | Points |
|---|---|
| Severity | |
| Dry-never drools | 1 |
| Mild-only lips wet | 2 |
| Moderate-drool reaches the lips and chin | 3 |
| Severe-drool drips off chin and onto clothing | 4 |
| Profuse-drooling off the body and onto objects (furniture, books) | 5 |
| Frequency | |
| Never drools | 1 |
| Occasionally drools | 2 |
| Frequently drools | 3 |
| Constantly drools | 4 |
The score of DSFS equals the sum of the severity and frequency sub-scores
This study was approved by the local ethics committee of Bakirkoy Research and Training Hospital for Psychiatry, Neurology and Neurosurgery (Istanbul-11.30.2012/51645).
Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences for Windows (Version 15.0; SPSS Inc. Chicago, IL, USA). Data are presented as mean±standard deviation. The mean sialorrhea severity was assessed before and four weeks after BoNT-A treatments and was compared using paired t-tests. The mean duration of efficacy was also determined for the first and last application and was compared using paired t-tests. p<0.05 was considered to be statistically significant.
RESULTS
Fourteen patients (11 males and three females) with the diagnosis of PD were included in this study. A patient with adult cerebral palsy and a patient with PD who was treated with only a single application were excluded. The mean age of the patients was 70.00±9.82 (range: 56–87) years, the mean duration of follow up after the start of BT treatment was 18.78±10.37 (range: 10–45) months, and the mean application interval was 5.82±2.75 (range: 4–13) months. The total number of applications was 37. Six patients received two, seven patients received three, and one patient received four consecutive treatments. The mean total dose of BoNT-A was 34.35±6.41 (range: 25–45) units. The mean response latency was 4.50±1.09 (range: 3–7) days, the mean peak improvement time was 12.92±2.16 (range: 7–15) days; 90% efficacy was assessed in two patients, 75% efficacy was assessed in nine, and 50% efficacy was assessed in three. BoNT-A was found to be effective in all patients (100%). The mean experienced improvement of sialorrhea was 71.78±12.95% according to the subjective assessment of the patients and/or caregivers. The characteristics of the patients are summarized in Table 2. DFSS showed a significant decrease at four weeks after the BoNT-A injections in comparison with the pre-treatment values; the mean scores of DFSS before and after injections were 7.00±1.03 and 3.21±0.89, respectively (p=0.001) (Figure 1). The mean duration of efficacy was 17.67±9.09 (range: 12.5–48) weeks. We found a significant difference between the first and last application in the mean duration of efficacy (17.28±9.21 and 18.03±9.02 weeks, respectively, p=0.001). The details of the results are outlined in Table 3. No side effects were observed in this study group.
Table 2.
Characteristics of patients suffering from PD with sialorrhea
| N | 14 |
| Gender (F/M) | 3/11 |
| Age (years) | 70.00±9.82 (56–87) |
| The duration of BoNT-A treatment (months) | 18.78±10.37 (10–45) |
| The dose of BoNT-A (units) | 34.35±6.41 (25–45) |
| Number of injections (n, %) | |
| 2 | 6 (42.8) |
| 3 | 7 (50.0) |
| 4 | 1 (7.2) |
| The response latency (days) | 4.50±1.09 (3–7) |
| The peak improvement time (days) | 12.92±2.16 (7–15) |
| The duration of efficacy (weeks) | 17.67±9.09 (12.5–48) |
| The application interval (months) | 5.82±2.75 (4–13) |
| The experienced improvement of sialorrhea (%) | 71.78±12.95 (50–90) |
Data are presented as mean±standard deviation (range), BoNT-A: Botulinum toxin type A; PD: Parkinson’s disease
Figure 1.
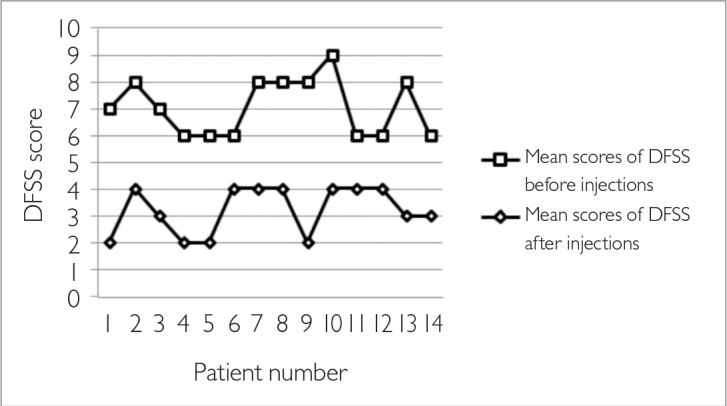
Mean scores of DFSS before and after injections
DFSS: Drooling Severity and Frequency Scale
Table 3.
Results of the treatments in patients suffering from PD with sialorrhea
| p value | ||
|---|---|---|
| The score of DFSS before injections | 7.00±1.03 | 0.000* |
| The score of DFSS after injections | 3.21±0.89 | |
| The duration of efficacy at first injections (weeks) | 17.28±9.21 | 0.001* |
| The duration of efficacy at last injections (weeks) | 18.03±9.02 |
Data are presented as mean±standard deviation,
indicates statistical significance
PD: Parkinson’s disease; DFSS: Drooling Severity and Frequency Scale
DISCUSSION
A review of the studies that evaluated the effects of BT treatment in sialorrhea showed that the most frequently treated diseases are infant cerebral palsy, PD, and amyotrophic lateral sclerosis (ALS) (12). Different glands can be selected for the injection; the parotid glands (10), submandibular glands (15,16), both the parotid and submandibular glands (13), and both the parotid and submaxillary glands (12), but generally, the parotid glands were selected in previous studies (12). Half the published studies were performed with ultrasound guidance, whereas the other half used anatomical detection of the gland based on palpation (12). We injected BoNT-A into the parotid glands without ultrasound guidance because the parotid glands lie superficially and are easily accessible. In addition, a study that compared these two methods showed that BT injections into the parotid glands do not require ultrasound guidance (10).
The total doses of toxins injected vary from 10 to 100 units of BoNT-A in previously published studies (12). The mean total dose of BoNT-A was 34.35±6.41 units in this study. It has been reported that the toxin becomes effective within the first two weeks after injection (13), and the duration of effect is approximately six weeks to seven months (12,17). The mean response latency was 4.50±1.09 days and the mean peak improvement time was 12.92±2.16 days in this study. There was interindividual variability for duration of BoNT-A effect; the mean duration of efficacy was 17.67±9.09 weeks; however, in a patient, the individual mean duration of effect was 48 weeks. The difference between the mean efficacy duration of the first and last applications was statistically significant (17.28±9.21 and 18.03±9.02 weeks, respectively, p=0.001). However, we assumed that this time difference is not enough for clinical practice. Studies with a larger sample size, more injections, and longer periods of follow-up may give more reliable results.
Recently, Breheret et al. (12) reported their results of BT treatment in sialorrhea. There were 25 patients with ALS, 14 with PD, 15 with cerebral palsy, and 16 with other diseases in their heterogeneous series. Some patients were treated several times in their study, which was similar to our study. While 5 of their 14 PD patients received a single injection, seven patients received two injections, and two patients received three injections. They proposed an injection of a total dose of 100 units BoNT-A into both the parotid and submaxillary glands in PD patients. They reported that the efficacy was 77% in patients with this disease, and they also added that the efficacy appeared to globally increase with the number of injections among the patients treated by several injection sessions (12). In another study, which has results of a single BT treatment for sialorrhea in patients with PD, the efficacy was found to be 66% (8). The efficacy was 100%, and the mean experienced improvement of sialorrhea was 71.78±12.95% in this study. The overall ratios of experienced improvement of sialorrhea were similar after repeated injections of BoNT-A even in a patient whose follow-up was nearly four years (45 months). Our success rate seems to be higher than the results of these studies. However, a patient treated with only a single BoNT-A application was excluded in our study. This patient was the one with inadequate clinical improvement. Therefore, we assumed that this success rate difference is related to the exclusion of this one-time treated patient.
It has been reported that BoNT-A doses greater than 50 units produce much stronger effects compared with doses less than or equal to 50 units (17). However, BoNT-A was found to be effective in all patients, and the mean total dose of BoNT-A was 34.35±6.41 units in this study. In addition, Breheret et al. (12) did not observe efficacy in 25.4% of their PD cases with a total dose of 100 units BoNT-A. A reason for this may be the interindividual variability for effective total dose of BoNT-A in sialorrhea treatment.
There was no adverse effect in this study, which was similar to other several studies (12,13). On the other hand, some adverse effects were reported with the use of BoNT-A for sialorrhea, such as dry mouth, deterioration of dysphagia, chewing difficulties, and recurrent mandibular luxation (5,18,19). It has been suggested that some of these side effects due to spread of the toxin to surrounding tissues (18). In addition, it was shown that secondary therapy failure after repeated injections of BT in cosmetic treatments and movement disorders treatments (20,21). It was also shown that antibody reaction, which is a cause of secondary resistance to BT (22,23), correlates with high doses and high frequency of BT treatments (24). We do not know the risk of developing antibodies against the neurotoxin in sialorrhea treatment. Therefore, it is important to keep the doses low to avoid potential side effects and to prevent antibody production against the neurotoxin.
It has been suggested that the evaluation of the efficacy of BT treatment cannot be based on objective parameters because an objective reduction of saliva flow rate is not necessarily correlated with an improvement of sialorrhea and patient comfort (12). Accordingly, we did not use an objective method for evaluating the efficacy. We evaluated the efficacy of each application using DFSS and experienced improvement of sialorrhea, which are simple and subjective methods. The number of patients was relatively small in this study. However, in both previous studies that we have mentioned, the results of repeated BT treatment for sialorrhea and the number of PD patients were small (one and nine PD patients) (12,13). In this study, the total number of applications was 37, and this increased the validity of our results.
In conclusion, this study showed that repeated injections of BoNT-A into the parotid glands is easy, safe, tolerable, and effective in treating sialorrhea in patients with PD. Based on the current results, it seems that there is a maintenance of efficacy after a three-year period and an increase in the mean duration of efficacy with the number of injections. Further prospective clinical studies with a larger number of patients and longer duration of follow-up are needed to confirm and extend our results.
Footnotes
Conflict of Interest: The authors declared no conflict of interest.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Kalf JG, Smit AM, Bloem BR, Zwarts MJ, Mulleners WM, Munneke M. Botulinum toxin A for drooling in Parkinson’s disease: a pilot study to compare submandibular to parotid gland injections. Parkinsonism Relat Disord. 2007;13:532–534. doi: 10.1016/j.parkreldis.2007.01.007. http://dx.doi.org/10.1016/j.parkreldis.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Molloy L. Treatment of sialorrhoea in patients with Parkinson’s disease: best current evidence. Curr Opin Neurol. 2007;20:493–498. doi: 10.1097/01.wco.0000280411.57836.aa. http://dx.doi.org/10.1097/01.wco.0000280411.57836.aa. [DOI] [PubMed] [Google Scholar]
- 3.Jongerius PH, Joosten F, Hoogen FJ, Gabreels FJ, Rotteveel JJ. The treatment of drooling by ultrasoundguided intraglandular injections of botulinum toxin type A into the salivary glands. Laryngoscope. 2003;113:107–111. doi: 10.1097/00005537-200301000-00020. http://dx.doi.org/10.1097/00005537-200301000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Sochaiwskyj AE, Koheil RM, Bablich K, Milner M, Kenny DJ. Oral motor functioning, frequency of swallowing and drooling in normal children and in children with cerebral palsy. Arch Phys Med Rehabil. 1986;12:866–874. [PubMed] [Google Scholar]
- 5.Naumann M, Jost W. Botulinum toxin treatment of secretory disorders. Mov Disord. 2004;19:137–141. doi: 10.1002/mds.20067. http://dx.doi.org/10.1002/mds.20067. [DOI] [PubMed] [Google Scholar]
- 6.Stuchell RN, Mandel ID. Salivary gland dysfunction and swallowing disorders. Otolaryngol Clin North Am. 1988;21:649–661. [PubMed] [Google Scholar]
- 7.Ellies M, Laskawi R, Gotz W, Arglebe C, Tormahlen G. Immunohistochemical and morphometric investigations of the influence of botulinum toxin on the submandibular gland of the rat. Eur Arch Otorhinolaryngol. 1999;256:148–152. doi: 10.1007/s004050050129. http://dx.doi.org/10.1007/s004050050129. [DOI] [PubMed] [Google Scholar]
- 8.Pal PK, Calne DB, Calne S, Tsui JK. Botulinum toxin A as treatment for drooling saliva in PD. Neurology. 2000;54:244–247. doi: 10.1212/wnl.54.1.244. http://dx.doi.org/10.1212/WNL.54.1.244. [DOI] [PubMed] [Google Scholar]
- 9.Jongerius PH, Rotteveel JJ, van Limbeek J, Gabreels FJ, van Hulst K, van den Hoogen FJ. Botulinum toxin effect on salivary flow rate in children with cerebral palsy. Neurology. 2004;63:1371–1375. doi: 10.1212/01.wnl.0000142040.57474.a6. http://dx.doi.org/10.1212/01.WNL.0000142040.57474.A6. [DOI] [PubMed] [Google Scholar]
- 10.Svetel M, Vasic M, Dragasevic N, Pekmezovic T, Petrovic I, Kostic V. Botulinum toxin in the treatment of sialorrhea. Vojnosanit Pregl. 2009;66:9–12. doi: 10.2298/vsp0901009s. http://dx.doi.org/10.2298/VSP0901009S. [DOI] [PubMed] [Google Scholar]
- 11.Giess R, Naumann M, Werner E, Riemann R, Beck M, Puls I, Reiners C, Toyka KV. Injections of botulinum toxin A into the salivary glands improve sialorrhoea in amyotrophic lateral sclerosis. J Neurol Neurochir Psychiatry. 2000;69:121–123. doi: 10.1136/jnnp.69.1.121. http://dx.doi.org/10.1136/jnnp.69.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breheret R, Bizon A, Jeufroy C, Laccourreye L. Ultrasound-guided botulinum toxin injections for treatment of drooling. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:224–229. doi: 10.1016/j.anorl.2010.12.010. http://dx.doi.org/10.1016/j.anorl.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Moller E, Karlsborg M, Bardow A, Lykkeaa J, Nissen FH, Bakke M. Treatment of severe drooling with botulinum toxin in amyotrophic lateral sclerosis and Parkinson’s disease: efficacy and possible mechanisms. Acta Odontol Scand. 2011;69:151–157. doi: 10.3109/00016357.2010.545035. http://dx.doi.org/10.3109/00016357.2010.545035. [DOI] [PubMed] [Google Scholar]
- 14.Thomas-Stonell N, Greenberg J. Three treatment approaches and clinical factors in the reduction of drooling. Dysphagia. 1988;3:73–78. doi: 10.1007/BF02412423. http://dx.doi.org/10.1007/BF02412423. [DOI] [PubMed] [Google Scholar]
- 15.Jongerius PH, Rotteveel JJ, van den Hoogen F, Joosten F, van Hulst K, Gabreels FJ. Botulinum toxin A: a new option for treatment of drooling in children with cerebral palsy. Presentation of a case series. Eur J Pediatr. 2001;160:509–512. doi: 10.1007/s004310100784. http://dx.doi.org/10.1007/s004310100784. [DOI] [PubMed] [Google Scholar]
- 16.Scheffer AR, Erasmus C, van Hulst K, van Limbeek J, Jongerius PH, van den Hoogen FJ. Efficacy and duration of botulinum toxin treatment for drooling in 131 children. Arch Otolaryngol Head Neck Surg. 2010;136:873–877. doi: 10.1001/archoto.2010.147. http://dx.doi.org/10.1001/archoto.2010.147. [DOI] [PubMed] [Google Scholar]
- 17.Vashishta R, Nguyen SA, White DR, Gillespie MB. Botulinum toxin for the treatment of sialorrhea: a meta-analysis. Otolaryngol Head Neck Surg. 2013;148:191–196. doi: 10.1177/0194599812465059. http://dx.doi.org/10.1177/0194599812465059. [DOI] [PubMed] [Google Scholar]
- 18.Bhatia KP, Munchau A, Brown P. Botulinum toxin is a useful treatment in excessive drooling in saliva. J Neurol Neurosurg Psychiatry. 1999;67:697. doi: 10.1136/jnnp.67.5.697. http://dx.doi.org/10.1136/jnnp.67.5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan EK, Lo YL, Seah A, Auchus AP. Recurrent jaw dislocation after botulinum toxin treatment for sialorrhoea in amyotrophic lateral sclerosis. J Neurol Sci. 2001;190:95–97. doi: 10.1016/s0022-510x(01)00565-2. http://dx.doi.org/10.1016/S0022-510X(01)00565-2. [DOI] [PubMed] [Google Scholar]
- 20.Dressler D, Wohlfahrt K, Meyer-Rogge E, Wiest L, Bigalke H. Antibody-induced failure of botulinum toxin a therapy in cosmetic indications. Dermatol Surg. 2010;36:2182–2187. doi: 10.1111/j.1524-4725.2010.01710.x. http://dx.doi.org/10.1111/j.1524-4725.2010.01710.x. [DOI] [PubMed] [Google Scholar]
- 21.Badarny S, Susel Z, Honigman S. Effectivity of Dysport in patients with blepharospasm and hemifacial spasm who experienced failure with Botox. Isr Med Assoc J. 2008;10:520–522. [PubMed] [Google Scholar]
- 22.Goschel H, Wohlfarth K, Frevert J, Dengler R, Bigalke H. Botulinum A toxin therapy: neutralizing and non-neutralizing Abs - therapeutic consequences. Exp Neurol. 1997;147:96–102. doi: 10.1006/exnr.1997.6580. http://dx.doi.org/10.1006/exnr.1997.6580. [DOI] [PubMed] [Google Scholar]
- 23.Smith LA. Development of recombinant vaccines for botulinum neurotoxin. Toxicon. 1998;36:1539–1548. doi: 10.1016/s0041-0101(98)00146-9. http://dx.doi.org/10.1016/S0041-0101(98)00146-9. [DOI] [PubMed] [Google Scholar]
- 24.Dressler D. Clinical features of secondary failure of botulinum toxin therapy. Eur Neurol. 2002;48:26–29. doi: 10.1159/000064953. http://dx.doi.org/10.1159/000064953. [DOI] [PubMed] [Google Scholar]


