Abstract
Introduction
Sleep deprivation has detrimental effects on cognitive processes, including decision making. The present study investigated how 72 h of sleep deprivation influenced individual neural performance in the Iowa gambling task using event-related potential technology.
Methods
Eleven healthy male adults who participated in our study were randomized to be either in group with 72 h of social isolation condition or 72 h of sleep deprivation condition.
Results
Results showed that, in the feedback stage, the N250–400 amplitude was smaller in post-test than in pre-test for the sleep deprivation condition, especially in the frontal cortex. No significant difference between the pre-test and post-test condition was found in the social isolation condition.
Conclusion
These results suggested that 72 h of sleep deprivation affected an individual’s response to feedback stimuli, causing the individual to evaluate the stimuli slowly.
Keywords: Sleep deprivation, Iowa gambling task, event-related potential
INTRODUCTION
Sleep deprivation is a state of sustained wakefulness and has become increasingly common in modern society. Research shows that sleep deprivation is associated with decrements in basic cognitive functions, including alertness, vigilance, and attention (1,2,3). Increasing interest, however, has been more specifically focused on the examination of sleep deprivation impaired performance on higher-order cognitive processing, such as cognitive control and decision making (4,5,6). The Iowa gambling task (IGT) simulates real-world decision making and investigates how the feedback of immediate reward and punishment after each election changes the tendency to choose cards (7). The present study focuses on how sleep deprivation affects IGT.
Some studies have investigated the effects of sleep deprivation on IGT. Killgore et al. (8) found that 49 h of sleep deprivation increased the likelihood of an individual to make risky choices in IGT (greater number of choices made from the short-term advantage decks). Similarly, Killgore et al. (9) reported that individuals chose more frequently from the disadvantageous high-risk deck after 75 h of sleep deprivation. Recently, Singh (10) repeated these results and found that 24 h of sleep deprivation already added individual risk-taking into IGT. Event-related potentials (ERPs) have very high temporal resolution (on the order of tenths of milliseconds) and can provide an on-line record of brain function at the level of large neuronal populations. However, few ERP studies have reported on how sleep deprivation influences the neural performance of IGT.
The present study investigated how 72 h of sleep deprivation in a social isolation condition influenced the neural performance of IGT using ERP technology. Combined with previous studies (11,12,13,14), this study assumed that long-term sleep deprivation may impair individual decision making and that sleep deprivation can reduce the ERP amplitude of the feedback stage of IGT.
METHODS
Participants
Twelve healthy college students (aged 20–32 years, M=24.83, SD=2.88; right-handed; all males) were recruited into the study. Exclusion criteria included a past or current history (even family history was included) of psychiatric, neurological disorder, and other medical conditions (such as epilepsy, neurasthenia, and insomnia), as well as serious addictions (such as alcoholism). All participants had normal sleep cycles and did not take sleeping pills, stimulants, or other psychoactive drugs. Informed consent was obtained from all students who volunteered for the study. The study was approved by the local ethics committee of the Beijing Normal University.
Experimental Procedure
The study was conducted in the sleep deprivation laboratory of the China Astronaut Research and Training Center. Participants were instructed to maintain a regular sleep schedule (10:00 pm–08:00 am) without alcohol consumption for 3 weeks before the experiment, during which smoking, drinking, and drinking coffee was prohibited. The 12 participants were divided into four groups; each group experienced two stages: social isolation and sleep deprivation. The time interval between social isolation and sleep deprivation conditions was more than 1 week. In addition, the social isolation and sleep deprivation conditions were counterbalanced between groups. One day before the beginning of the experiment, participants took a baseline IGT (pre-test), and after the 72 h of social isolation and sleep deprivation, they immediately took the post-test.
Electroencephalogram Data Acquisition
Electroencephalogram (EEG) were recorded using 40 Ag/AgCl electrodes mounted on an elastic cap according to the standard 10–20 system and continuously sampled at 1000 Hz using a DC-coupled amplifier (NuAmps) and recording software (Neuroscan 4.5; Compumedics USA Ltd, El Paso, TX, USA). A unilateral mastoid for the left ear was used as a reference electrode, and the average binaural mastoid was used as the reference during off-line analysis. During the recording, impedance of all electrodes was maintained <5 kΩ. EEG was recorded using a 0.1–100 Hz band-pass filter. Because electromagnetic interference from the environment could not be shielded, the averaged waveforms were digitally low-pass filtered at 50 Hz to reduce high-frequency noise. We used Neuroscan 4.5 software for off-line analysis. Amplitudes >±100 μv were automatically rejected. ERPs for correctly identified items were computed from 0 to 500 ms post-stimulus onset with a 100 ms pre-stimulus baseline for the feedback stage of IGT.
Statistical Analysis
One participant could not complete IGT in the sleep deprivation post-test condition. Thus, 11 participants were analyzed. Behavioral data: For the pre-test and post-test, a net score was calculated for each block by subtracting the number of cards chosen from the disadvantageous decks from the number of cards chosen from the advantageous decks. In order to test the behavioral effect of sleep deprivation on IGT, we conducted two-way repeated measures analysis of variance (ANOVA). Therefore, the two within-subject factors were time (pre-test or post-test) and condition (72 h of social isolation or 72 h of sleep deprivation).
ERP data
ERP results in the feedback stage were averaged for the winning feedback, neither winning nor losing feedback, and losing feedback. The results showed that the average number of artifact-free trials of the neither winning nor losing feedback was less than 30 times for three participants and that of the losing feedback was less than 30 times for four participants. Thus, ERP results were averaged for all the winning feedback, neither winning nor losing feedback, and losing feedback. Three scalp electrodes (Fz, Cz, and Pz) were used in the present study. The amplitudes of N250–400 (250–450 ms) components were computed for the two experimental conditions. A two repeated-measures ANOVA was conducted with time (pre-test or post-test) and electrodes (Fz, Cz, and Pz) as within-subject factors for the two experimental conditions. The p values in ANOVA were corrected using the Greenhouse Geisser method.
RESULTS
Behavioral Results
In order to test the behavioral effect of sleep deprivation on IGT, we conducted two-way repeated measures ANOVA. Therefore, the two within-subject factors were time (pre-test and post-test) and condition (72 h of social isolation and 72 h of sleep deprivation). The results showed that the main effect of time was not significant, F(1, 10)=4.033, p=0.072, ηp2=0.287; the main effect of condition was not significant, F(1, 10)=0.148, p=0.709, ηp2=0.015; and the interaction effect of time* condition was also not significant, F(1, 10)=1.177, p=0.303, ηp2=0.105.
ERP Results
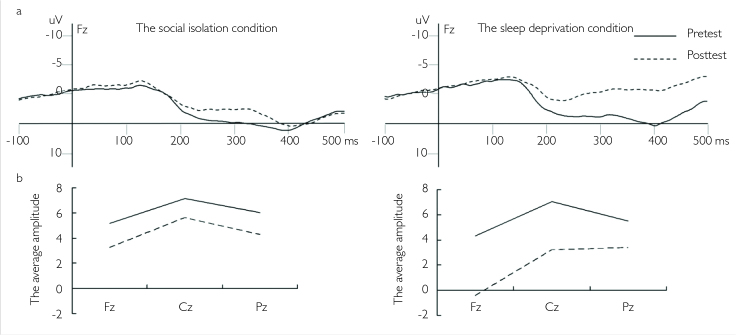
The N250–400 mean amplitudes in the 250–400 ms time window were as the index of analysis (Figure 1a). In the social isolation condition, repeated-measures ANOVA was conducted with time (pre-test and post-test) and electrodes (Fz, Cz, and Pz) as within-subject factors: the main effect of time was not significant, F(1, 10)=1.200, p=0.107, ηp2=0.107; the main effect of electrodes was not significant, F(2, 20)=1.834, p=0.204, ηp2=0.155; and the interaction effect was also not significant, F(2, 20)=0.081, p=0.902, ηp2=0.008. Similarly, in the sleep deprivation condition, repeated-measures ANOVA was conducted with time (pre-test and post-test) and electrodes (Fz, Cz, and Pz) as within-subject factors: the main effect of time was significant, F(1, 10)=6.094, p=0.033, ηp2=0.379, which suggested that the mean amplitude in the post-test was smaller than that in the pre-test; the main effect of electrodes was significant, F(2, 20)=8.518, p=0.002, ηp2=0.460; post hoc analysis showed that the amplitude in the Fz electrode was smaller than that in the Cz and Pz electrodes; and the interaction effect was not significant, F(2, 20)=0.923, p=0.414, ηp2=0.084.
Figure 1. a, b.
(a) The average ERP of the Fz electrode for the pre-test and post-test in the social isolation and sleep deprivation conditions. (b) The average amplitudes of the N250–400 component of Fz, Cz, and Pz electrodes for the pre-test and post-test in the sleep deprivation and social isolation conditions
In addition, a paired sample T-test was conducted between the pre-test and post-test in the sleep deprivation condition for the Fz, Cz, and Pz electrodes (Figure 1b). The results showed that there was marginal significance between the pre-test and post-test for Fz (t(10)=2.127, p=0.059) and Cz (t(10)=2.213, p=0.051), suggesting the amplitude in the post-test was smaller than that in the pre-test. There was no significant difference between the pre-test and post-test for Pz (p>0.1).
DISCUSSION
The present study examined how 72 h of sleep deprivation influenced IGT. The results of the ERP showed that the N250400 amplitude of the post-test was smaller than the pre-test in the sleep deprivation condition, especially in the frontal cortex; whereas there was no significance between the post-test and pre-test in the social isolation condition. These results suggested that 72 h of sleep deprivation impaired individual neural performance in IGT.
The ERP results showed that the social isolation condition did not affect individual neural performance. However, the N250–400 amplitude was reduced after sleep deprivation. This was in line with previous ERP results, which suggested that sleep deprivation could decrease the P300 amplitude in a vigilance test and reaction unit test (11), the N2/P3 amplitude in the go/no-go task (12), and the N200 and P600 amplitudes in the recognition memory task (13). Gehring and Willoughby (15) found that negativity was present 250–300 ms following the feedback of a win or a loss, and that the source was located in the medial frontal cortex. This medial frontal negativity was called FRN. Previous studies have indicated that FRN reflected the early appraisal of feedback on a binary classification basis of good vs. bad outcomes (15). In the present study, the feedback results were averaged for all the winning feedback, neither winning nor losing feedback, and the losing feedback. Thus, the FRN component was not obvious. But the time window of the N250–400 component was 250–400 ms, which was similar to the time window of the FRN component. Therefore, we classified the N250–400 component as the class FRN amplitude. The results of the present study suggested sleep deprivation reduced the FRN amplitude, indicating that sleep deprivation affected an individual’s response to the feedback stimuli, causing individuals to evaluate the stimuli more slowly.
Furthermore, the present study showed that sleep deprivation affected the N250–400 amplitudes of FZ and CZ, but not the N250–400 amplitude of PZ in the post-test. The FRN studies showed that the source was located in the medial frontal cortex, especially ACC (15, 16). The ERP research of IGT showed that the FRN amplitude of feedback stage was also reflected the activation of the prefrontal cortex (17). Thus, combined with the present study, this suggested sleep deprivation affected the activation of the prefrontal cortex. This was consistent with previous studies, which suggested that sleep deprivation affected risk-taking behavior by changing the activation of the prefrontal cortex (14).
The present study investigated how 72 h of sleep deprivation and social isolation condition influenced the neural performance of IGT. The results showed that 72 h of sleep deprivation reduced the amplitude of the N250–400 amplitude, especially in the prefrontal cortex. These results suggested that sleep deprivation caused individual mental fatigue and slowed an individual’s ability to evaluate stimuli.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Beijing University.
Informed Consent: Written informed consent was obtained from students who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – L.L., R.Z.; Design – L.L., R.Z.; Supervision – L.L., R.Z.; Resources – L.L., R.Z.; Materials – L.L., R.Z.; Data Collection and/or Processing – L.L., R.Z.; Analysis and/or Interpretation – L.L., R.Z.; Literature Search – L.L., R.Z.; Writing Manuscript – L.L., R.Z.; Critical Review – L.L., R.Z.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This work was funded by the Main Test Technique Research Program of China (2011CB711000). The authors would like to express their gratitude for the support of these projects.
REFERENCES
- 1.Horne JA, Anderson NR, Wilkinson RT. Effects of sleep deprivation on signal detection measures of vigilance: implications for sleep function. Sleep. 1983;6:347–358. doi: 10.1093/sleep/6.4.347. [DOI] [PubMed] [Google Scholar]
- 2.Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- 3.Wesensten NJ, Belenky G, Thorne DR, Kautz MA, Balkin TJ. Modafinil vs. caffeine: effects on fatigue during sleep deprivation. Aviat Space Environ Med. 2004;75:520–525. [PubMed] [Google Scholar]
- 4.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–249. doi: 10.1037//1076-898x.6.3.236. https://doi.org/10.1037/1076-898X.6.3.236. [DOI] [PubMed] [Google Scholar]
- 5.Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. https://doi.org/10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterpenich V, Albouy G, Darsaud A, Schmidt C, Vandewalle G, Dang Vu TT, Desseilles M, Phillips C, Degueldre C, Balteau E, Collette F, Luxen A, Maquet P. Sleep promotes the neural reorganization of remote emotional memory. J Neurosci. 2009;29:5143–5152. doi: 10.1523/JNEUROSCI.0561-09.2009. https://doi.org/10.1523/JNEUROSCI.0561-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. J Sleep Res. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. https://doi.org/10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- 9.Killgore W, Lipizzi E, Kamimori G, Balkin T. Caffeine effects on risky decision-making after 75 hours of sleep deprivation. Aviat Space Environ Med. 2007;78:957–962. doi: 10.3357/asem.2106.2007. https://doi.org/10.3357/ASEM.2106.2007. [DOI] [PubMed] [Google Scholar]
- 10.Singh V. Dual conception of risk in the Iowa Gambling Task: effects of sleep deprivation and test-retest gap. Front Psychol. 2013;4:628. doi: 10.3389/fpsyg.2013.00628. https://doi.org/10.3389/fpsyg.2013.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HJ, Kim L, Sun KY. Cognitive deterioration and changes of P300 during total sleep deprivation. Psychiatry Clin Neurosci. 2003;57:490–496. doi: 10.1046/j.1440-1819.2003.01153.x. https://doi.org/10.1046/j.1440-1819.2003.01153.x. [DOI] [PubMed] [Google Scholar]
- 12.Qi JL, Shao YC, Miao D, Fan M, Bi GH, Yang Z. The effects of 43 hours of sleep deprivation on executive control functions: event-related potentials in a visual go/no go task. SBP Journal. 2010;38:29–42. https://doi.org/10.2224/sbp.2010.38.1.29. [Google Scholar]
- 13.Mograss MA, Guillem F, Brazzini-Poisson V, Godbout R. The effects of total sleep deprivation on recognition memory processes: A study of event-related potential. Neurobiol Learn Mem. 2009;91:343–352. doi: 10.1016/j.nlm.2009.01.008. https://doi.org/10.1016/j.nlm.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Womack SD, Hook JN, Reyna SH, Ramos M. Sleep loss and risk-taking behavior: a review of the literature. Behav Sleep Med. 2013;11:343–359. doi: 10.1080/15402002.2012.703628. https://doi.org/10.1080/15402002.2012.703628. [DOI] [PubMed] [Google Scholar]
- 15.Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. https://doi.org/10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- 16.Miltner W, Braun C, Coles M. Event-related brain potentials following incorrect feedback in a time estimation task: Evidence for a “Generic” neural system for error detection. J Cogn Neurosci. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. https://doi.org/10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- 17.Cui JF, Chen YH, Wang Y, Shum DHK, Chan RCK. Neural correlates of uncertain decision making: ERP evidence from the Iowa Gambling Task. Front Hum Neurosci. 2013;7:776. doi: 10.3389/fnhum.2013.00776. https://doi.org/10.3389/fnhum.2013.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]