Abstract
Introduction
The receptor for advanced glycation end products (RAGE) is a pattern recognition receptor expressed in tissues and cells, which plays a role in immunity. The activation of RAGE results in the translocation of nuclear factor kappa B (NF-κB) to the nucleus for expression of proinflammatory molecules. The role of the RAGE pathway in the pathogenesis of diabetic complications is well determined. We aimed to investigate the role of the RAGE pathway in axonal and vasculitic neuropathy.
Methods
We immunoreacted nerve biopsy samples from 17 axonal neuropathy (AN), 11 vasculitic neuropathy (VN) and 12 hereditary neuropathy (as a control group) with liability to pressure palsy (HNPP) patients with antibodies to NF-κB and RAGE. Subsequently, we performed double staining with the antibodies to NF-κB or RAGE and T cells, macrophages and Schwann cells.
Results
RAGE and NF-κB immunoreactivities were higher in the perivascular cuff and in endoneurial cells in VN than in AN and HNPP. Although there is no significant difference, nerve biopsies with AN showed higher NFκB and RAGE immunoreactivities than HNPP. The colocalization study showed that most of the NFκB- and RAGE-positive cells were CD8 (+) T cells in VN. In AN, all NFκB- and RAGE-positive cells were macrophages, whereas all NFκB- and RAGE-positive cells were Schwann cells in HNPP.
Conclusion
The activation of the RAGE pathway predominant in CD8 (+) T cells underscores its role in VN. In AN patients, the immunoreactivity to NFκB and RAGE in macrophages may support their role in axonal degeneration without inflammatory milieu.
Keywords: Vasculitic neuropathy, axonal neuropathy, RAGE, NF-κB
INTRODUCTION
Advanced glycation end products (AGE) are nonenzymatic additions of glucose or other saccharides to proteins, lipids and nucleotides (1,2). The receptor for AGE, named receptor of advanced glycation end products (RAGE), is a pattern recognition receptor (PRR) such as Toll-like receptors (TLR), which is expressed in tissues and cells that are critical for immune surveillance such as lung, liver, endothelium, monocytes, dendritic cells and neurons (2). The binding of AGE to RAGE leads to the translocation of nuclear factor kappa B (NF-κB) to the nucleus. It regulates target genes such as cytokines (Tumor necrosis factor-α, interleukin-1β and 6), adhesion molecules (ICAM and VCAM-1), prothrombotic and vasoconstructive gene products and RAGE and its inhibitor IκBα (3).
The RAGE pathway plays a key role in diabetic complications including diabetic neuropathy (4,5). The activation of this pathway has been demonstrated in inflammatory neuropathies such as vasculitic neuropathy (6,7). AGE, RAGE and NF-κB have been detected in lymphocytes and macrophages, particularly near the vessels both in vasculitic and diabetic neuropathy. However, the role of this pathway in axonal degeneration and demyelination remains to be well determined.
In this immunohistochemical study, we aimed to investigate the role of the RAGE pathway in different neuropathies. We used nerve biopsy specimens of patients with axonal neuropathy (AN), systemic and nonsystemic vasculitic neuropathy (NSVN) and hereditary neuropathy with liability to pressure palsy (HNPP) (as a control group).
METHODS
Patients
Nerve biopsies performed in our university Neurology Department Neuromuscular Disease Laboratory between 1999 and 2010 were analyzed. We included 17 axonal neuropathy (AN) patients, 11 vasculitic neuropathy (VN) patients and 12 hereditary neuropathy with liability to pressure palsy (HNPP) who had undergone sural and superficial peroneal nerve biopsy as part of the diagnostic work-up of their neuropathy and whose nerve biopsy specimens were suitable for the immunohistochemical analysis.
Axonal neuropathy was diagnosed by electrophysiology and nerve biopsy findings with active axonal degeneration, mild fiber loss and without inflammation. Diabetes mellitus or other endocrinopathies, hereditary neuropathies (HSMN types II and IV), mitochondrial neuropathies, chronic alcohol abuse, vitamin deficiencies, neoplasia, bacterial or viral infections, drugs, intoxication, vasculitic neuropathies and metabolic causes such as a-beta lipoproteinemia, Fabry disease and uremic neuropathy were excluded. VN was diagnosed according to published criteria (8). Systemic vasculitis was diagnosed by the Chapel Hill consensus criteria (9). Patients with HNPP were diagnosed by clinical, electrophysiological and nerve biopsy findings.
Twelve sural nerve biopsies with hereditary neuropathy with liability to pressure palsy (HNPP) were included in the study.
Approval of the Hacettepe University Ethics Committee was granted and patients participated after providing their written informed consent (01.07.2010; B.30.2.HAC.020.05.04/352).
Nerve Biopsies
The biopsies were all performed for diagnostic purposes. Seven of 11 VN patients had superficial peroneal nerve biopsy with peroneus brevis muscle biopsy whereas four patients had sural nerve biopsy. All biopsies were snap-frozen within 5 min of surgical intervention and stored at −80°C until analysis. All frozen sections were stained with hematoxylin/eosin and modified Gomori trichrome stains. Semithin (1 μm) plastic sections from nerve tissue were also prepared. Frozen sections (10 μm) were taken from each tissue for immunohistochemistry.
Immunohistochemistry
Air-dried serial 10-μm frozen sections were blocked with Histostatin-plus kit (Zymed Laboratories Inc., San Francisco, CA, USA) for 20 min. Furthermore, the sections were incubated with rabbit polyclonal anti-NFκB (1:50; Santa Cruz Biotechnology Inc, Santa Cruz, CA, sc-109) or with rabbit polyclonal anti-RAGE (1:50; Santa Cruz Biotechnology Inc, Santa Cruz, CA, sc-5563) antibodies for 1 h at 37°C. Thereafter, a biotinylated secondary antibody against mouse IgG and an avidin-biotinylated peroxidase complex were used. The peroxidase reaction product was developed with diaminobenzidine H2O2 (6 mg diaminobenzidine, 10 cm3 PBS and 0.01 cm3 3% H2O2).
Semiquantitative Analysis
Semiquantitative analysis was used to evaluate RAGE and NFκB positive cells. The sections were independently analyzed by two authors. For each specimen, the mean value of the two authors’ assessment was calculated. The values of semiquantitative assessment were defined as follows: negative 0 (without cell), scattered positive cells + (1–2 positive cells), few positive cells ++ (3–5 positive cells), many positive cells +++ (6–10) and dense positive cells ++++ (more than 10 positive cells).
Colocalization Studies
To determine the immunoreactive cells, we performed double staining with the immunofluorescence methods. Three samples from each group were randomly selected. The samples were staining with rabbit polyclonal anti-NFκB or with rabbit polyclonal anti-RAGE and with mouse monoclonal anti-CD4 (1/50; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, Sc-65544) for CD4 (+) T cells, mouse monoclonal anti-CD8 (1/50; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, Sc-70794) for CD8 (+) T cells, mouse monoclonal anti-CD68 (1/50; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, Sc-20060) for macrophages, or mouse monoclonal anti-S100 (1/50; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, Sc-71993) for Schwann cells. The sections were analyzed with confocal microscope (Zeiss, Oberkochen, Germany). First T cells, macrophages and Schwann cells were counted, then NFκB and RAGE positive T cells, macrophages and Schwann cells were determined and the ratio was calculated for each section staining.
RESULTS
Patients
The group of AN consisted of 11 men and six women with a median age of 62 years (range 21–83). The group of VN consisted of four men and seven women with a median age of 56 years (range 7–78). The group of HNPP consisted of six men and six women with a median age of 26 years (range 17–40). The mean age was 56 (7–78) in patients with VN, 59 (21–83) in patients with AN and 26 (16–62) in patients with HNPP. The median age of HNPP patients was lower than VN and AN patients (p<0.05).
Six of the VN patients had NSVN, whereas five had systemic vasculitis. Among the systemic VN patients, two had polyarteritis nodosa (PAN), two had rheumatoid arthritis (RA) and one had Churg–Straus Syndrome (CSS).
Immunohistochemistry
The staining patterns of NFκB and RAGE were similar to each other.
NfκB and RAGE immunoreactivities were observed in the perivascular cuff in epineurial vessels in all nerve biopsies from patients with VN except one patient. There was no difference in staining patterns between systemic and nonsystemic vasculitic neuropathy patients. NFκB and RAGE immunoreactivities were higher in patients with VN than in those with AN and HNPP (p<0.05) (Table 1) (Figure 1).
Table 1.
NFκB and RAGE immunoreactivities in patients
| Localization | AN (n=17) | VN (n=11) | HNPP (n=12) | |
|---|---|---|---|---|
| Staining intensity | Staining intensity | Staining intensity | ||
| Epineural vessels | RAGE | ++ (n=3)* | + (n=1) ++ (n=1) +++ (n=9)* |
0* |
| NF-κB | + (n=1) ++ (n=4)‡ |
++ (n=2) +++ (n=8)‡ |
0‡ | |
| Endoneurial cells | RAGE | + (n=1) ++ (n=12) +++ (n=4)§ |
+++ (n=7) ++++ (n=4)§ |
+ (n=1), ++ (n=10), ++ +(n=1)§ |
| NF-κB | + (n=3) ++ (n=6) +++ (n=7) ++++ (n=1)** |
+ (n=1) +++ (n=5) ++++ (n=5)** |
+ (n=2), ++ (n=10)** |
RAGE immunoreactivity in epineurial vessels was higher in VN patients than with AN and HNPP patients (p<0.05).
NF-κB immunoreactivity in epineurial vessels was higher in VN patients than with AN and HNPP patients (p<0.05).
RAGE immunoreactivity in endoneurial cells was higher in VN patients than with AN and HNPP patients (p<0.05)
NF-κB immunoreactivity in endoneurial cells was higher in VN patients than with AN and HNPP patients (p<0.05)
AN: axonal neuropathy; HNPP: hereditary neuropathy with liability to pressure palsy; NF-κB: nuclear factor kappa B; RAGE: receptor advanced glycation end products; VN: vasculitic neuropathy
Figure 1. a, b.
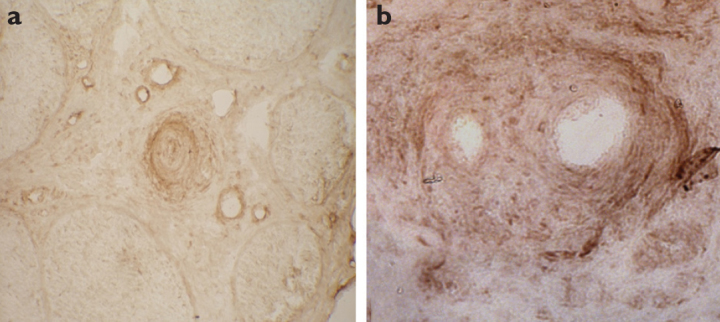
NFκB (a) (×20) and RAGE (b) (×40) immunoreactivities in epineurial vessels in vasculitic neuropathy patients
NFκB and RAGE immunoreactive endoneurial cells were more than 10 in each fascicule in 40% of patients with VN and endoneurial immunoreactivities to NFκB and RAGE were higher in VN patients than in AN and HNPP patients. Although there is no significant difference between the groups, nerve biopsies from patients with AN showed higher NFκB and RAGE immunoreactivities than those with HNPP (Table 1) (Figure 2).
Figure 2.
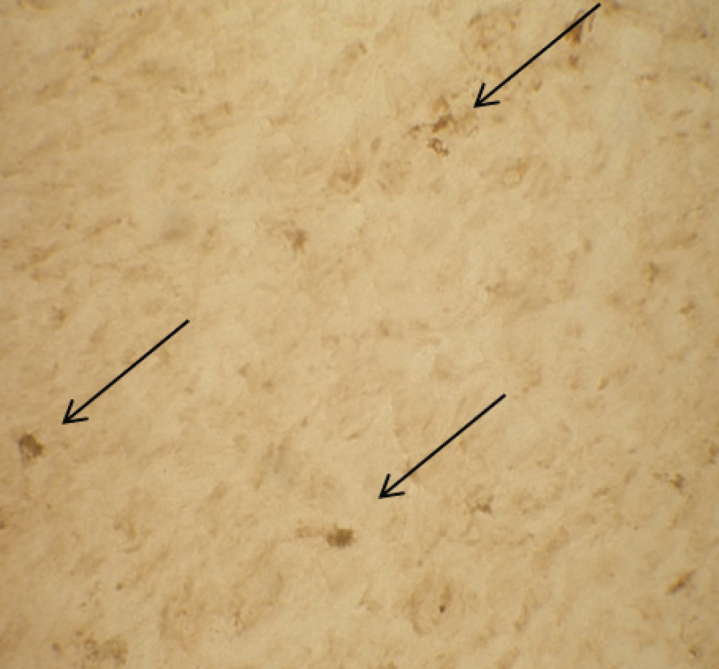
Endoneurial NFκB immunoreactive cells (arrows) in a patient with axonal neuropathy (×40)
Colocalization Studies
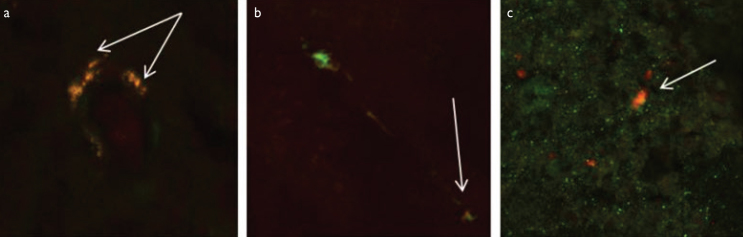
In VN patients, 70% of NFκB and RAGE positive cells were CD8 (+) T lymphocytes, whereas 30% of positive cells were macrophages (Figure 3a).
Figure 3. a–c.
Representative nerve biopsy photomicrographs: (a) Double staining of receptor of advanced glycation end products (RAGE) with CD8 (+) T cells in a patient with vasculitic neuropathy. Arrows indicate RAGE-positive T-cells. (b) Double staining of NFκB with CD 68 as a macrophage marker in a patient with axonal neuropathy. Arrows indicate NFκB-positive macrophages. (c) Double staining of RAGE with S100 as a Schwann cell marker in a patient with hereditary neuropathy with liability to pressure palsy. Arrow indicate RAGE-positive Schwann cell (–20)
In AN patients, all NFκB and RAGE positive cells were macrophages, whereas all NFκB and RAGE positive cells were Schwann cells in HNPP patients (Figure 3b, c).
DISCUSSION
The receptor of advanced glycation end products pathway has been extensively studied in the context of diabetic complications and has been shown to constitute a link between hyperglycemia and microvascular damage (5). Furthermore, RAGE serves as an important proinflammatory receptor in vasculitis (10). The RAGE pathway has been demonstrated as a mediator in the pathogenesis of a number of inflammatory neuropathies (3,4,6,7). Thus, RAGE may be a therapeutic target and its activation may have a prognostic value (11). Therefore, it is important to know more about the expression of NFκB and RAGE in different neuropathies.
In this study, we aimed to compare vasculitic neuropathy and axonal neuropathy without an identifiable cause because both groups show axonal degeneration in their pathology and a demyelinating hereditary neuropathy, HNPP as a control group.
We observed that NFκB and RAGE immunoreactivities were higher around epineurial vessels and endoneurial cells in nerve biopsies from patients with VN than from those with AN and HNPP and immunoreactive cells were usually CD8 (+) T cells. Previously, Kissel et al. (12) showed that 70% of epineurial inflammatory cells were T cells and 2/3 of them were CD8 (+) T cells in 22 VN patients. The increased NFκB and RAGE immunoreactivities in CD8 (+) T cells confirm the prominent role of CD8 (+) T cells. Similarly, we recently showed that AGE and RAGE were increased in dermal endothelial cells and T-cells of NSVN and DN patients compared with controls (13). Haslbeck et al. (7) reported increased NFκB and RAGE immunopositivity in 70–100% macrophages, whereas 40–70% in CD8 (+) T cells in VN patients. Although we observed NFκB and RAGE immunoreactive macrophages, they were fewer than CD8 (+) T cells. This difference could be related to the interaction of NFκB with several transcription factors, such as CBP300, which hide NFκB from immunohistochemical studies (14). The downregulation of RAGE because of high AGE concentration may cause decreased RAGE immunoreactivity in macrophages.
We also observed that although there is no significant difference between the groups, nerve biopsies from patients with AN showed higher endoneurial NFκB and RAGE immunoreactivities than HNPP patients and the immunoreactive cells were macrophages in AN patients. The hematoxylin–eosin sections of the nerve samples with AN did not show inflammation; however, macrophages, which are responsible for the axonal degeneration, showed NFκB and RAGE activity. This observation is not sufficient to speculate a prominent role of the RAGE pathway in axonal degeneration but supports the fact that the RAGE pathway is one of the activated pathway in macrophages without need of inflammatory milieu.
Previous studies on inflammatory neuropathies and RAGE pathway used the nerve samples of patients with Charcot-Marie-Tooth neuropathies (6,7). In our study, we selected the nerve samples of HNPP patients because of reversible clinical symptoms and distinct pathological findings with the focal enlargement of myelin sheath called “tomaculas” and scattered onion bulb structures and Schwann cells proliferation (15). We observed that NFκB and RAGE immunoreactivities were low in HNPP patients than in VN and AN patients and the immunoreactive cells were Schwann cells. The RAGE pathway activation could be related with remyelination and Schwann cell proliferation in HNPP patients. In Schwann cells, the increase of cAMP and phosphorylation of Ser 276 of p65 subunit of NFκB by protein kinase A start the myelin formation (16). Moreover, the suppression of peripheral nerve regeneration with sRAGE (an inhibitor of RAGE) has been shown in mice with sciatic nerve injury (17). Our study confirms these findings because the activation of RAGE pathway has been shown in Schwann cells of HNPP patients.
Our study has some limitations. The number of subjects was small and the immunoreactivity in patients could not be correlated with the treatment response. This is because the samples were obtained from our tissue archive and the clinical follow-up data of some patients are missing because they were only evaluated for biopsy.
Our findings underscore the role of the RAGE pathway in vasculitic neuropathy and show its activation in axonal degeneration and provide the idea for new treatment studies.
Acknowledgements
The authors thank to Nuhan Puralı for analysis with confocal microscope and to Bülent Çakır for his technical support. C.E Bekircan-Kurt was awarded Investigator Award in 2010 by European Federation of Neurological Societies with the preliminary findings of this study.
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Bucciarelli LG, Wendt T, Rong L, Lalla E, Hofmann MA, Goova MT, Taguchi A, Yan SF, Yan SD, Stern DM, Schmidt AM. RAGE is a multiligand receptor of the immunglobulin superfamily: implication for homeostasis and chronic disease. Cell Mol Life Sci. 2002;59:1117–1128. doi: 10.1007/s00018-002-8491-x. http://dx.doi.org/10.1007/s00018-002-8491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin L, Park S, Lakatta EG. RAGE signaling and arterial aging. Front Biosci. 2009;14:1403–1413. doi: 10.2741/3315. http://dx.doi.org/10.2741/3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andorfer B, Kieseier BC, Mathey E, Armati P, Pollard J, Oka N, Hartung HP. Expression and distribution of transcription factor NF-kappaB and inhibitor IkappaB in the inflamed peripheral nervous system. J Neuroimmunol. 2001;116:226–232. doi: 10.1016/s0165-5728(01)00306-x. http://dx.doi.org/10.1016/S0165-5728(01)00306-X. [DOI] [PubMed] [Google Scholar]
- 4.Lukic IK, Humpert PM, Nawroth PP, Bierhaus A. The RAGE pathway: activation and perpetuation in the pathogenesis of diabetic neuropathy. Ann N Y Acad Sci. 2008;1126:76–80. doi: 10.1196/annals.1433.059. http://dx.doi.org/10.1196/annals.1433.059. [DOI] [PubMed] [Google Scholar]
- 5.Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci. 2011;1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x. http://dx.doi.org/10.1111/j.1749-6632.2011.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haslbeck KM, Neundörfer B, Schlötzer-Schrehardtt U, Bierhaus A, Schleicher E, Pauli E, Haslbeck M, Hecht M, Nawroth P, Heuss D. Activation of the RAGE pathway: a general mechanism in the pathogenesis of polyneuropathies? Neurol Res. 2007;29:103–110. doi: 10.1179/174313206X152564. http://dx.doi.org/10.1179/174313206X152564. [DOI] [PubMed] [Google Scholar]
- 7.Haslbeck KM, Bierhaus A, Erwin S, Kirchner A, Nawroth P, Schlötzer U, Neundörfer B, Heuss D. Receptor for advanced glycation end product (RAGE)-mediated nuclear factor-kappaB activation in vasculitic neuropathy. Muscle Nerve. 2004;29:853–860. doi: 10.1002/mus.20039. http://dx.doi.org/10.1002/mus.20039. [DOI] [PubMed] [Google Scholar]
- 8.Collins MP, Dyck PJ, Gronseth GS, Guillevin L, Hadden RD, Heuss D, Léger JM, Notermans NC, Pollard JD, Said G, Sobue G, Vrancken AF, Kissel JT Peripheral Nerve Society. Peripheral Nerve Society Guideline on the classification, diagnosis, investigation, and immunosuppressive therapy of non-systemic vasculitic neuropathy: executive summary. J Peripher Nerv Syst. 2010;15:176–184. doi: 10.1111/j.1529-8027.2010.00281.x. http://dx.doi.org/10.1111/j.1529-8027.2010.00281.x. [DOI] [PubMed] [Google Scholar]
- 9.Jennette JC1, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. http://dx.doi.org/10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 10.Sun W, Jiao Y, Cui B, Gao X, Xia Y, Zhao Y. Immune complexes activate human endothelium involving the cell-signaling HMGB1-RAGE axis in the pathogenesis of lupus vasculitis. Lab Invest. 2013;93:626–638. doi: 10.1038/labinvest.2013.61. http://dx.doi.org/10.1038/labinvest.2013.61. [DOI] [PubMed] [Google Scholar]
- 11.Ramasamy R, Yan SF, Schmidt AM. RAGE: therapeutic target and biomarker of the inflammatory response-the evidence mounts. J Leukoc Biol. 2009;86:505–512. doi: 10.1189/jlb.0409230. http://dx.doi.org/10.1189/jlb.0409230. [DOI] [PubMed] [Google Scholar]
- 12.Kissel JT, Riethman JL, Omerza J, Rammohan KW, Mendell JR. Peripheral nerve vasculitis: immune characterization of the vascular lesions. Ann Neurol. 1989;25:291–297. doi: 10.1002/ana.410250314. http://dx.doi.org/10.1002/ana.410250314. [DOI] [PubMed] [Google Scholar]
- 13.Bekircan-Kurt CE, Uçeyler N, Sommer C. Cutaneous activation of rage in non-systemic vasculitic and diabetic neuropathy. Muscle Nerve. 2014;50:377–383. doi: 10.1002/mus.24164. http://dx.doi.org/10.1002/mus.24164. [DOI] [PubMed] [Google Scholar]
- 14.Hayden MS, Ghosh S. Shared Principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. http://dx.doi.org/10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Madrid R, Bradley WG. The pathology of neuropathies with thickening of the myelin sheath (Tomaculous neuropathy) J Neurol Sci. 1975;25:415–448. http://dx.doi.org/10.1016/0022-510X(75)90263-4. [Google Scholar]
- 16.Yoon C, Korade Z, Carter BD. Protein Kinase A-Induced Phosphorylation of the p65 Subunit of Nuclear Factor - B promotes schwann cell differentiation into a myelinating phenotype. J Neurosci. 2008;28:3738–3746. doi: 10.1523/JNEUROSCI.4439-07.2008. http://dx.doi.org/10.1523/JNEUROSCI.4439-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rong LL, Trojaborg W, Qu W, Kostov K, Yan SD, Gooch C, Szabolcs M, Hays AP, Schmidt AM. Antagonism of RAGE suppresses peripheral nerve regeneration. FASEB J. 2004;18:1812–1817. doi: 10.1096/fj.04-1899com. http://dx.doi.org/10.1096/fj.04-1899com. [DOI] [PubMed] [Google Scholar]