Abstract
Introduction
Behçet’s disease is a chronic inflammatory disease of unknown aetiology that affects multiple organ systems. Since the diagnosis of this disease mainly relies on clinical criteria, a diagnostic laboratory test is required especially for neuro-Behçet’s patients without systemic involvement.
Method
In this study, we searched for the presence of autoantibodies against brain tissue, by means of indirect immunofluorescent staining technique in sera obtained from patients with neuro-Behçet’s disease, based on reports that humoral immune dysregulation may play a role in susceptibility to Behçet’s disease. After pre-absorbtion of sera with guinea pig liver powder to reduce nonspecific staining, serum samples were applied to mouse brain sections and immunoreactivity was detected with fluorescein (FITC)-conjugated goat antibody against human IgG.
Results
Ten sera from neuro-Behçet’s patients and 10 age-matched control sera were screened for immunoreactivity. We detected specific immunoreactivity to both parenchymal and vascular brain structures in the patients’ sera. Parenchymal vessel immunopositivity was detected in 8 of 10 patients, whereas only two of control sera showed no significant parenchymal vascular immunoreactivity (p=.025). In addition to vascular immunoreactivity, filamentous and reticular immunopositive structures were detected in brain sections of 5 out of 10 patients. No such immunoreactivity was detected in sections incubated with control sera (p=.016).
Conclusion
We detected a specific immunoreactivity against vascular and parenchymal filamentous structures in neuro-Behçet patients’ sera. Humoral autoimmunity may play a role in the pathogenesis of neuro-Behçet’s disease in addition to cellular immune response. Findings of this preliminary study will be evaluated with a large number of patients and controls, to determine whether it is the cause or the result and, further studies are underway to disclose the nature of epitope to which the immunoreactivity was directed against and to develop a diagnostic laboratory method for investigating central nervous system involvement in Behçet’s patients.
Keywords: Behçet’s Disease, Neuro-Behçet’s Disease, Autoimmunity, Immunohistochemistry
ÖZET
Amaç
Behçet Hastalığı çoklu sistem tutulumu ile giden, etiyolojisi ve fizyopatolojisi bilinmeyen, kronik, inflamatuar bir hastalıktır. Hastalığın tanısının klinik bulgulara dayanan kriterlere göre konulması ve özellikle diğer bulguların eşlik etmediği, nörolojik tutulumla başlayan hastalarda tanının belirlenmesindeki güçlükler, özgül bir laboratuvar testinin eksikliğine dikkati çekmektedir.
Yöntem
Bu çalışmada, immün sistem bozukluğuna bağlı geliştiği düşünülen durumlarda uygulanan, indirekt immünfloresan boyama tekniği kullanarak, SSS tutulumu olan Behçet hastalarının serumlarında, SSS’ye karşı otoantikor varlığı araştırılmıştır. Önce hasta ve kontrol serumları, özgül olmayan boyanmayı önleyebilmek için kobay karaciğeri ile preabsorbsiyona tabi tutulmuşlardır. Takiben fare beyin dokusu ile inkübe edilerek, floresan (FITC) ile konjuge, insan IgG’sine karşı keçide hazırlanmış IgG ile işaretlenmiştir. Beyin kesitleri floresan mikroskop ile değerlendirilmiştir.
Bulgular
On nöro-Behçet hastası ve 10 yaş uyumlu kontrol bireyin serumları immünreaktivite açısından incelenmiştir. Hasta serumları ile fare beyin dokusunun inkübasyonu sonucunda hem parankimal hem de vasküler yapılara karşı özgül immünreaktivite tespit edilmiştir. 10 hastanın 8’inde parankimal vasküler boyanma varken, kontrollerin sadece 2 tanesinde hastalarla karşılaştırıldığında çok belirgin olmayan immünreaktivite görüldü (p=0,025). Vasküler immünreaktiviteye ek olarak, 10 hastanın 5’inde filamentöz ve retiküler yapılarda immünreaktivite gözlenirken, kontrollerin serumlarıyla yapılan inkübasyonlarda, hiçbirinde benzer bir boyanma saptanmamıştır (p=0,016).
Sonuç
SSS tutulumu olan Behçet hastalarının serumlarında, hem parankimal hem de vasküler yapılara karşı özgül immünreaktivite tespit edilmiştir. Bu bulgular, Behçet hastalığı SSS patogenezinde hümoral otoimmünitenin rolü olabileceğini düşündürmektedir. Daha çok sayıda hasta ve kontrolün dahil edileceği bir çalışma ile, bu ön çalışmada tespit edilen bulguların, bir sebep mi yoksa sonuç mu olduğu araştırılacaktır. Bu tespit edilen özgün immünoreaktivitenin ne tür bir epitopa ait olduğu incelenerek, Behçet Hastalığının SSS tutulumu için klinikte kullanılabilecek bir laboratuvar yöntemi geliştirilmeye çalışılacaktır.
Introduction
Behçet’s disease is a systemic inflammatory disease charecterized with recurrent oral-genital afthous ulcers and eye involvement. Multi-system involvement may be observed; the skin, joints, eye, central nervous system (CNS) and gastrointestinal system (GIS) are involved most commonly. It may affect small, moderate and large arteries and veins (1). Although the etiology is not known exactly, it is thought to be an autoimmune disease triggered by environmental factors in individuals with genetic predisposition (2).
Although the disease may be observed at any age, it frequently occurs at the age of 20–35 years. In men and in individuals with an early age of onset, severe complications and mortality are observed more commonly (3,4,5). The frequency of neurological involvement in Behçet’s disease ranges between 2.2% and 47% (6,7,8). In one study, CNS involvement was observed with a rate of 34% in autopsy series in 170 patients who were diagnosed with neuro-Behçet’s with a rate of 10% while living. This variability is propably related not only with ethnic and geographical difference, but also with how neurological involvement is defined. Headache which is not questioned in detail may lead to confusion in terms of the frequency of neurological involvement (9). In a prospective study conducted in Turkey, neurological involvement was found with a rate of 5.3% similar to many studies (10,11,12).
In studies directed to investigate the etiology and pathogenesis of Behçet’s disease, the effect of different factors including genetic predisposition, infectious agents, heat schock proteins, humoral and cellular immunity changes, endothelial dysfunction and disorders in the coagulation and fibrinolytic system has been proposed, but the role of none of these factors in development of the disease could be demonstrated clearly (13,14,15,16,17,18,19, 20,21,22,23,24).
The diagnosis of Behçet’s disease is made according to the criteria based on the clinical findings specified by the international Behçet study group (25). However, there is no specific laboratory test for the diagnosis. Laboratory support is needed in the early periods of the disease when clinical findings do not meet diagnostic criteria yet or in cases which start with neurological involvement (26).
In Behçet’s disease, CNS involvement which is divided into parenchymal and non-parenchymal involvement is observed more commonly, whereas peripheral nervous system involvement is observed substantially rarely and generally occurs in relation with the drugs used in treatment. CNS involvement is observed in the whole of the nervous system together with diffuse and mild inflammation in the form of focal involvement more prominent in the parenchymal structures (27). The brainstem, basal ganglion, diencepahlic structures and internal capsula are the most commonly involved regions.
Information obtained from the clinical and imaging findings of patients with neuro-Behçet’s disease show that the disease tends to involve some regions in the CNS. This suggests that an antigenic stimulation belonging to those regions may be present.
In this study, it was aimed to demonstrate presence of autoantibody in sera of patients with Behçet’s disease to determine potential target structures and/or proteins by testing interaction (reactivity) of the serum samples which will be obtained from patients with animal tissues.
Methods
Ten ml blood samples were obtained from 10 patients with neuro-Behçet’s disease whose diagnoses were made according to the criteria specified by the international Behçet study group who had CNS involvement wnd who were being followed-up in Hacettepe University, Department of Neurology and Rheumatology outpatient clinics, 10 healthy individuals and 1 patient with seropositive neuromyelitis optica (NMO) after obtaining written informed consent. Obtaining sera from the patients and healthy controls with written informed consent and experimental procedures performed in animal brains were approved by the Hacettepe University ethics Committee (recording number FON 06/04). The blood samples obtained were kept at room temperature for 30 minutes, centrifuged at 4000 rpm, the sera were seperated and kept at −20°C until stainings were performed.
The brains of swiss albino mice weighing 22–34 g were used for the experiments. The mice were decapitated by using high dose chloralhydrate anesthesia. The brains were removed meticulously and were divided into two parts below the diencephalon separating the brainstem and cortex. Horizontal sections with a thickness of 10 μm were taken with cryostat from the brains frozen at −26°C. Two sections one from the brainstem and one from the hemisphere were placed on the same slide. The slides prepared were kept at −20°C until immunoflourescent staining was performed. Stainings were performed at room temperature (+23°C – + 25°C). Denaturation is loss of the three dimensional structure by proteins without disruption in the primary structure as a result of changes in environmental conditions including change in temperature and pH. Experiments were repeated at +37°C and +4°C to test presence of denaturation.
Preabsorption
Serum samples contain nonspecific antibodies. Before the immunoflourescence staining technique used in the experiments was realized, preabsorption with guinea pig liver (LV) was performed to prevent nonspecific labeling due to these antibodies. These types of applications are used widely in similar examination methods and enable non-specific staining to be abolished. With this objective, the patient sera were treated with the extract obtained from the guinea pig liver as described in the literature before immunoflourescent statining (28,29).
Immunoflourescent stating
The circumferences of the sections obtained before were marked with a pen called pappen (Zymed) preventing fluid flow and all stainings were performed at room temperature. The tissues were fixed with 10% formole solution prepared with PBS for 4 minutes. After the sections were washed with PBS, 1% CHAPS solution (AppliChem) prepared with PBS was applied. Following washing they were kept in 10% normal goat serum for one hour. Afterwards, the sections were incubated with patient and control serum and kept waiting. (before patient and control sera were placed, the preabsorption stage was completed). The sections which were retreated with PBS were incubated with flourescent-conjugated (FITC) Anti-Human IgG (Jackson immunoresearch) (1:500) prepared against human IgG in goat as secondary antibody for 70 minutes. Following pBS washings Hoechst solution (Hoeschst 33258, Molecular Probes, 10 mg/ml dissolved in distilled water) was dropped on the sections and closed with lamella. After the procedures were completed, the sections were examined by flourescent microscope (Nikon Eclipse E600). The sections were evaluated for two times at different times by a single reader who did not know the identities of the patients and controls. The stainings were not evaluated quantitatively, but as present or absent immunoreactivity. The cellular nuclei were evaluated at a wavelength of 330–380 nm by flourescent microscope and the other radiations were evaluated at a wavelength of 450–490 nm. Immunoflourescent imagings were recorded and examinations were performed by way of Nikon ACT program and figures were created with the help of the Adobe Photoshop 6.0 program.
Statistical analysis
The findings obtained from immunoflourescent imagings were marked as shown in Table 2 such as parenchymal vessel, parenchymal filament, non-parenchymal vessel and non-parenchymal ependim involvement for the patients and controls. SPSS 11.0 program was used for statistical calculations. Fisher exact test was used when the expected frequencies were smaller than 5 and Yates-corrected chi-square test was used when the expected frequencies were higher than 5. A p value of <.05 was considered statistically significant.
Table 2.
Immunoreactivities found in the sera of the patients with neuro-Behçet’s disease and healthy controls
| Parenchymal | Non-parenchymal | |||
|---|---|---|---|---|
|
| ||||
| Serum code | Vessel | Filament | Vessel | Epandim |
| Patient 1 | − | + | + | + |
| Patient 2 | − | − | + | − |
| Patient 3 | + | + | + | − |
| Patient 4 | + | − | − | + |
| Patient 5 | + | − | + | + |
| Patient 6 | + | + | + | − |
| Patient 7 | + | − | + | − |
| Patient 8 | + | − | + | − |
| Patient 9 | + | + | − | + |
| Patient 10 | + | + | − | − |
| Control 1 | − | − | + | − |
| Control 2 | − | − | − | − |
| Control 3 | − | − | − | − |
| Control 4 | − | − | − | − |
| Control 5 | − | − | − | − |
| Control 6 | + | − | − | − |
| Control 7 | + | − | − | + |
| Control 8 | − | − | − | − |
| Control 9 | − | − | + | − |
| Control 10 | − | − | + | − |
Results
Sera were obtained from a total of 10 patients diagnosed with neuro-Behçet’s disease, 10 healthy controls and 1 patient with NMO. The serum of the patient with NMO was used to show that the method described in the literature could be repeated appropriately and considered positive control (Figure 1).
Figure 1.
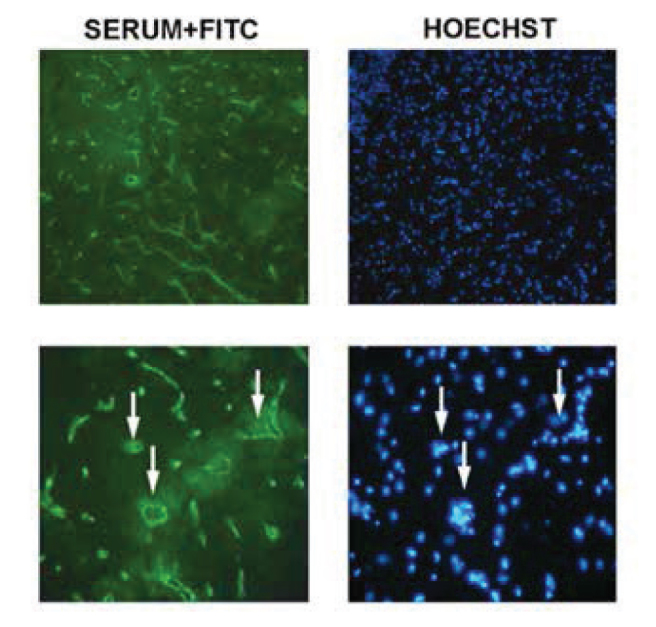
Intensive vascular immunoreactivity is observed with the serum of the NMO patient in sections obtained from the brainstem. Pictures with 100× magnification are shown in the upper column and pictures with 400× magnification are shown in the lower column. The arrows indicate some of the vessels observed on 400× magnification and the nuclei of these on Hoechst staining. Immunoflourescent labeling is present in all vascular structures as described in the literature and is compatible with the immunoreactivity pattern against AQP4.
Six of the patients with Behçet’s disease were male and 4 were female. The mean age was 37.7 (±7.3) years. 6 of the healthy controls were male and 4 were female. The mean age was 32.1 (±6.9) years. The mean duration of Behçet’s disease was observed to be 9.6 (±4.9) years and the mean duration of neurological involvement was observed to be 3.9 (±3.4) years. Half o the patients had findings related with brainstem involvement. Non-parenchymal involvement was observed in 2 patients, while parenchymal involvement was found in 8 patients (Table 1). When examined in terms of systemic involvement, it was found that all patients had a history of recurrent oral aphtous ulcers, genital aphtous ulcers, skin involvement and uveitis and all patients were in remission at the time when the sera were obtained.
Table 1.
Clinical and demographic properties of the patients diagnosed with Neuro-Behçet’s disease included in the study
| Patient | Age | Gender | Duration of Behçet’s disease | Duration of neurological involvement | Neurological involvement |
|---|---|---|---|---|---|
| Patient 1 | 31 | F | 14 years | 2 years | Subcortical white matter |
| Patient 2 | 36 | M | 5 years | 4 years | Brainstem |
| Patient 3 | 34 | M | 10 years | 2 years | Brainstem |
| Patient 4 | 47 | M | 17 years | 13 years | Subcortical white matter-basal ganglion |
| Patient 5 | 28 | F | 13 years | 3 years | Brainstem-thalamus |
| Patient 6 | 32 | M | 6 years | 5 years | Spinal cord |
| Patient 7 | 47 | F | 4 years | 4 years | Brainstem |
| Patient 8 | 48 | M | 15 years | 2 years | Brainstem |
| Patient 9 | 34 | F | 4 years | 1 years | Sinus vein thrombosis |
| Patient 10 | 40 | M | 8 years | 3 years | Sinus vein thrombosis |
The sera obtained from the patients and controls were incubated with mouse brain tissue to determine potential immunological target. Following incubation with sera, brain sections were examined in terms of parenchymal involvement (vessel involvement and filamentous involvement) and non-parenchymal involvement (large vessels and ependim involvement) and evaluated according to the severity of immunoreactivity (Table 2).
Intensive flourescent labeling which was determined visually around the large and small vessels localized in the brain tissue was considered parenchymal vessel involvement. All other filamentous immunoflourescent labelings outside the vessels in the brain tissue were considered parenchymal involvement. Positive immunoflourescent labeling in the superficial arteries and veins localized outside the brain tissue was considered non-parenchymal vessel involvement. Ependimal labelings including the brain tissue and ventricles were considered non-parenchymal involvement.
When examined in terms of parenchymal vessels, positive immunoflourescent labeling was found in 8 of 10 patients, whereas mild positive labeling was observed only in 2 individuals and the statistically significant difference between the groups was found with Yates-corrected chi-square test (p=.025). The vessels involved were mostly of venous character (Figure 2). When the other structures outside vessles were evaluated in the parenchyma, no cellular staining was found. However, filamentous immunoreactivity following dentrite-like structures was noted in the brain tissue in 5 patients (Figure 3 and 4). This filamentous immunoreactivity was not found in incubations performed with the sera of the control patients and the difference between the patients and controls was found to be significant with the Fisher exact chi-square test (p=.016) and the finding was interpreted to be specific for the disease.
Figure 2.

Intensive immunoflourescent labelings are noted around the vascular structures in addition to the filamentous and point pattern in the parenchyma in incubations performed with patients sera. Labelings with control serum are shown in the upper row. On the images taken with the same flourescence severity, occasional mild staining is found at the background and around the vessels in incubations performed with control sera, but negative immunoreactivity is present frequently as shown in the left column. In contrast, intensive immunoflourescent labeling is observed around the vessles following incubations with patients sera. Labelings with patient sera are shown in the left column and labelings with nuclear stain Hoechst are shown in the right column in the middle and lower rows. The arrows indicate vessels. Fusiform nuclei labeled with Hoechst are shown in the left column in the middle and lower rows. Pictures taken with 200× magnification.
Figure 3.
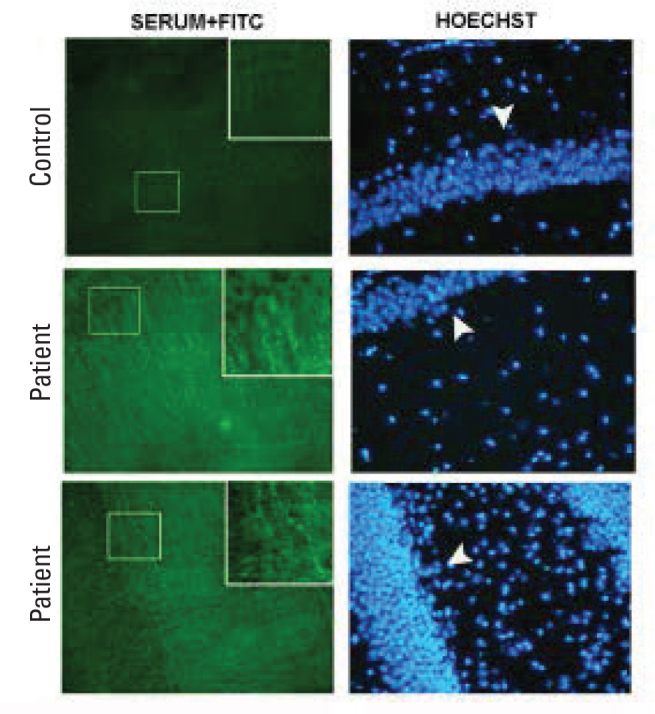
Immunoflourescence imagings performed with patient and control sera. The iamges are taken in the sections crossing the hyppocampus. Labelings with sera are shown in the left column and labelings with nuclear stain Hoechst are shown in the right column. It is noted that the patient sera show point and/or filamentous immune labelings in the pictures in the left column, but positive labeling is not present on examinations performed the with control serum. Cellular bodies and nuclei are not labeled in the patients and controls. Cellular nuclei in the sections are visualized with Hoechst staining in the right column. The arrow heads indicate hyppocampal cell layers. Pictures taken with 100× magnification.
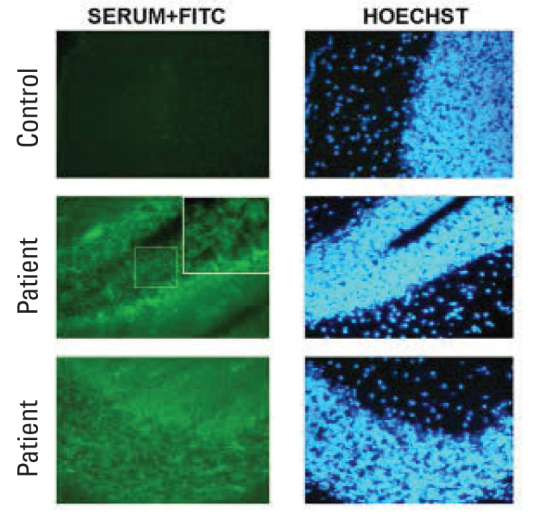
Figure 4.

Although filamentous and/or point immunoreactivity is observed in the sections taken from the striatum, staining is not found following incubations with control sera. The labelings performed with sera are shown in the left column and the labelings performed with nuclear stain Hoechst are shown in the right column. Pictures taken with 200× magnification.
When non-parenchymal vessel involvement was examined, labeling was observed in the structures showing arterial character. This kind of labeling was found in 7 of 10 patients following incubation with patient sera and similar findings were observed in 3 control sera. Yates-corrected chi-square test was applied for the results. Epandimal immunoreactivity was found in 4 of 10 patients and in 1 of the controls. The result was evaluated using Fisher exact chi-square test. Although non-parenchymal involvement (vessel and epandim involvement) was observed more commonly in the patients, no statistically significant difference was found in comparison with the controls (p=.18, p=.15).
Labelings performed without using patient serum and/or secondary antibody during each staining were controls for the specificity of the technique. No immunoreactivity was found for labelings performed in this way.
Parenchymal immunoreactivity showed similar staining pattern in all patients in both cortical (Figure 3) and deep white brain structures (Figure 4).
It was found that the patients with Behçet’s disease the patient with NMO carried different characteristics in terms of vascular involvement pattern. More intensive vascular involvement in comparison with the immunoreactivity observed in patients with Behçet’s disease similar to the literature was found and capillaries were also observed to be involved in the patient with NMO (Figure 1 and 2). This involvement is compatible with the immunoreactivty pattern described against AQP4 in the literature.
When the sections belonging to the brainstem and cerebellum were examined, similar findings were obtained (Figure 5).
Figure 5.
Data similar to the labeling pattern found in the other brain regions are found following incubations with patient sera in the sections takne from the cerebellum. Stainings following filametous or point, dendritic structures were present in the parenchyma. Pictures taken with 100× magnification.
Imagings with the assistance of confocal microscope were performed to examine the filamentous stuructures stained in more detail. This kind of imaging gives an idea abouıt the thre-dimensional structure of the structures stained. With this objective, a section with a thickness of 2.2 micron was screened with intervals of .2 micron. These screenings are shown as single sections one by one in Figure 6. Point and filamentous structures are noted. The 3-dimensional reconstruction of the serial sections in Figure 6 is shown in the X axis in Figure 7A and in the Z axis in Figure 7B. It is noted that filamentous structure is not observed in the fiber bundle of large axons, but is stained diffusely in the other areas and forms a netlike structure combining occasionally.
Figure 6.
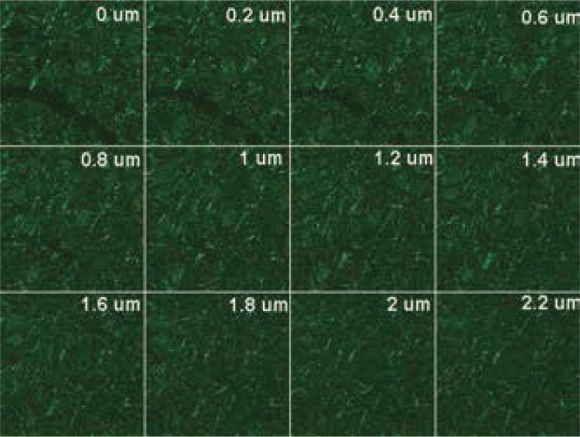
Serial .2 μm sections taken from the striatum with the help of confocal microscope following immune labeling with patient serum. Point and filamentous structure is noted.
Figure 7.
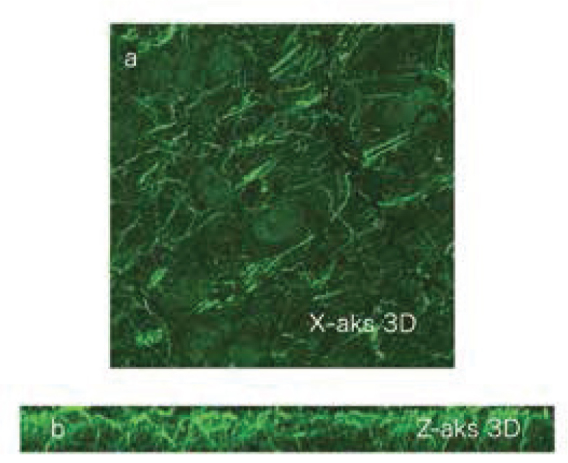
A) On imaging obtained by 3 dimensional overlapping of the sections in Figure 6 in the X axis, filamentous structures are noted clearly. B) Reconstruction of the sections in Figure 6 in the Z axis. The visualized brain tissue has a thickness of 2.2 μm and is an area passing through the striatum. It is noted that the filamentous structure has a netlike construction and extends deeply.
Discussion
In this study, specific immunoreactivity against both parenchymal and vascular stuructures was found in indirect immunoflouırescent stainings performed in mouse brains using the sera of patients with Behçet’s disease with CNS involvement. Although no tissue-specific antibody or biological marker has been identified yet for patients with Behçet’s disease, the results of this study suggest to presence of a common epitope and that an antibody responsible of the disease may be demonstrated in patients with neuro-Behçet’s disease.
Studies on Behçet’s disease have been conducted for a long time. However, no adequate information about its etiology and pathology has been obtained yet. The fact that the diagnosis of these disease is made based on clinical findings and difficulties in making the diagnosis especially in patients with an onset with neurological involvement without other findings indicate the lack of a specific laboratory test.
The fact that some patients have been reported to have benefited from plasma exchange in the literature, observation of maternal transplacental antibody trasfer and secondary neonatal Behçet’s disease, alternations in the functions of B cells, demonstration of circulating immune complexes in the sera of patients, deposition of IgM and C3 on the vascular wall on histopathological examinations of the lesions indicate that there is an immunological imbalance induced by activation of humoral immunity in addition to cellular immunity in Behçet’s disease (22,23,24,30,31,32,33,34,35,36). Many studies investigating presence of autoandibody have been conducted with different Behçet’s disease patient groups. Different methods have been used in these studies. Anti-endothelial cell antibodies, anti-Saccharomyces cerevisiae antibody, anti-alpha enolase antibody and putative kinase 1 antibodies induced by PTEN have been proposed by previous investigators ad candidate antibodies for Behçet’s disease, but none has been found to be specific and they have been demostrated in small groups (30,37,38,39). Studies on heat shock proteins (HSP) which are among the immune reactive proteins found in microorganisms or animal tissues have been conducted for a long time with patients with Behçet’s disease. HSP-60, HSP-65, HSP-70 and alpha B-cyrstallin are the HSPs reported in previous studies. In studies performed in recent years, stress-induced phosphoprotein-1 which is a member of the HSP family was also included in candidate antigenic targets. However, its specificity for Behçet’s disease is questionable, because it was also reported to be present in patients with rehumatoid arthritis (19,40,41,42,43,44).
NMO is a condition which is thought to arise from immune system disorder with selectivity for CNS. Lennon et al. showed the presence of autoantibody specific fort his disease using a method they developed (28,45). The basis of the method is based on flourescent labeling of the sera of NMO patients following incubation with Mouse brain tissue. As a result of their studies, this group found that the autoantibody in NMO developed against AQP4.
Information obtained from the clinical and imaging findings of patients with neuro-Behçet’s disease show that the disease tends to involve some regions in the CNS. This suggests that there could be an antigenic structure belonging to those regions. In this study, possible presence of brain tissue specific autoantibody was investigated in the sera of patients with Behçet’s disease with neuorlogical involvement using the technique used by Lennon et al.
In our study, the method which minimized nonspecific staining observed in control sera was seleceted as a result of the experiments performed with various preabsorption methods described in the literature and the procedures were continued. In the experiments repeated under conditions where the sera were applied at +37°C or +4°C in order to test the reliability of the method, it was obsevred that immunoreactivity observed with patient sear at room temperature did not occur. The fact that immunoreactivity we observed disappeared in experiments where the temperature was altered suggested that this was related with denaturation of specific IgGs in patient sera. Thus, this indicated that the staining we found was specific. Residual nonspecific staining following preabsorption also disappeared with increased dilution of secondary antibody. In addition, experiments were performed with the serum of a patient who was diagnosed clinically with NMO (optic neuritis, transverse myelitis and NMO IgG antibody positivity) as positive control to test the accuracy of the method used and intensive vascular immunoreactivity was obtained similar to what was described in the literature and the reproducibility of the method was demosntrated. Because of these factors, we think that the specificity of immunoflourescence labeling obtained as a result of long-term studies and repeated experiments is reliabile.
At the end of immunoflourescence labelings, parenchymal vascular staining was observed very rarely following incubation with control sera, while more intensive parenchymal vascular staining was observed in the patients. Staining in the filamentous structures was found only in the patients and not observed in the control group at all. This suggests that the staining pattern is specific for the disease. It can only be interpreted in further studies what kind of a structure this specific staining belongs to. In addition, it should be elucidated if filamentous and vascular staining is a monoclonal response to a single epitope or is related with a polyclonal reaction because of different epitopes.
Some aspects of the findings should be evaluated in further studies. For example, cortical and cerebellar involvement is a more rare finding in clinical practice in neuro-Behçet’s disease, but immunoreactivity was found also in these regions in our study. This may be related with the fact that the sera were applied to mouse tissue in the experiments and with the difference in the proteins expressed in the rodent and human brains and in the way of distribution of these proteins. Although limited clinical involvement including optic neuritis and transverse myelitis is observed in NMO patients, a diffuse AQP4 expression is found in immunohistochemical examinations (45). Another issue which should be evaluated is the fact that secondary antibody which would recognize human IgG structure was used in the experiments. However, mostly, IgM deposition in the tissues is mentioned in Behçet’s disease. The study should be repeated using secondary antibody against human IgM. Although it is currently thought that parenchymal and non-parenchymal involvement develop with different mechanisms, no marked difference was observed in our study. However, further interpretation on this issue is difficult, because there were only 2 patient sera with non-parenchymal involvement.
In future experiments, the number of patients and healthy individuals will be increased and other patient groups with autoimmune disease will also be used to elucidate if the findings are a cause or an outcome and to specifiy the sensitivity and specificity of this test.
Demonstration of brain tissue specific autoantibody and identification of the target structure in patients with Behçet’s disease will help in understaing the pathogenesis of the disease and will provide identification of a diagnostic laboratory test in addition to clinical criteria. Demonstration of the pathophysiology in detail will enable development of gene and protein targeted therapies with fewer side effects and with stronger efficiency.
Footnotes
Conflict of Interest: The authors reported no conflict of interest related to this article.
Çıkar Çatışması: Yazarlar bu makale ile ilgili olarak herhangi bir çıkar çatışması bildirmemişlerdir.
References
- 1.Ehrlich GE. Vasculitis in Behcet’s disease. Int Rev Immunol. 1997;14:81–88. doi: 10.3109/08830189709116846. [DOI] [PubMed] [Google Scholar]
- 2.Davatchi F, Shahram F, Akbarian M, Gharibdoost F, Nadji A, Chams H, Jamshidi AR. Behcet disease:analysis of 3443 cases. APLAR J Rheumatol. 1997;1:2–5. [Google Scholar]
- 3.Yazici H, Başaran G, Hamuryudan V, Hizli N, Yurdakul S, Mat C, Tüzün Y, Ozyazgan Y, Dimitriyadis I. The ten year mortality in Behçet’s Syndrome. Br J Rheumatol. 1996;35:139–141. doi: 10.1093/rheumatology/35.2.139. [DOI] [PubMed] [Google Scholar]
- 4.Kural-Seyahi E, Fresko I, Seyahi N, Ozyazgan Y, Mat C, Hamuryudan V, Yurdakul S, Yazici H. The long term mortality and morbidity of Behçet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine (Baltimore) 2003;82:60–76. doi: 10.1097/00005792-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Yazıcı H, Tüzün Y, Pazarlı H, Yurdakul S, Özyazgan Y, Özdoğan H, Serdaroğlu S, Ersanlı M, Ülkü BY, Müftüoğlu AÜ. Influence of age of onset and patient’s sex on the prevelance and severity of manifestations of Behçet’s Disease. Ann Rheum Dis. 1984;43:783–789. doi: 10.1136/ard.43.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gürler A, Boyvat A, Türsen Ü. Clinical manifestations of Behçet’s Disease: An analysis of 2147 patients. Yonsei Med J. 1997;38:423–427. doi: 10.3349/ymj.1997.38.6.423. [DOI] [PubMed] [Google Scholar]
- 7.Krause I, Uziel Y, Guedj D, Mukamel M, Molad Y, Amit M, Weinberger A. Mode of presentation and multisystem involvement in Behçet’s disease: The influence of sex and age of disease onset. J Rheumatol. 1998;25:1566–1569. [PubMed] [Google Scholar]
- 8.Krause I, Uziel Y, Guedj D, Mukamel M, Harel L, Molad Y, Weinberger A. Childhood Behçet’s disease: clinical features and comparison with adult-onset disease. Rheumatology (Oxford) 1999;38:457–462. doi: 10.1093/rheumatology/38.5.457. [DOI] [PubMed] [Google Scholar]
- 9.Aykutlu E, Baykan B, Akman-Demir G, Topcular B, Ertas M. Headache in Behçet’s disease. Cephalalgia. 2006;26:180–186. doi: 10.1111/j.1468-2982.2005.01017.x. [DOI] [PubMed] [Google Scholar]
- 10.Serdaroğlu P, Yazıcı H, Özdemir C, Yurdakul S, Bahar S, Aktin E. Neurologic involvement in Behçet’s syndrome: a prospective study. Arch Neurol. 1989;46:265–269. doi: 10.1001/archneur.1989.00520390031011. [DOI] [PubMed] [Google Scholar]
- 11.Siva A, Kantarci OH, Saip S, Altintas A, Hamuryudan V, Islak C, Koçer N, Yazici H. Behçet’s disease: diagnostic and prognostic aspects of neurological involvement. J Neurol. 2001;248:95–103. doi: 10.1007/s004150170242. [DOI] [PubMed] [Google Scholar]
- 12.Ashjazadeh N, Borhani Haghighi A, Samangooei Sh, Moosavi H. Neuro-Behcet’s disease: a masquerader of multiple sclerosis. A prospective study of neurologic manifestations of Behcet’s disease in 96 Iranian patients. Exp Mol Pathol. 2003;73:17–22. doi: 10.1016/s0014-4800(03)80004-7. [DOI] [PubMed] [Google Scholar]
- 13.Direskeneli H. Behcet’s disease: infectious aetiology, new autoantigens, and HLA-B51. Ann Rheum Dis. 2001;60:996–1002. doi: 10.1136/ard.60.11.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gül A. Behcet’s Disease: an update on the pathogenesis. Clin Exp Rheumatol. 2001;19(Suppl 24):6–12. [PubMed] [Google Scholar]
- 15.Sohn S, Lee ES, Bang D, Lee S. Behcet’s disease-like symptoms induced by the Herpes simplex virus in ICR mice. Eur J Dermatol. 1998;8:21–23. [PubMed] [Google Scholar]
- 16.The Behcet’s Disease Research Committee of Japan. Skin hypersensitivity to streptococcal antigens and the induction of systemic symptoms by the anti-gens in Behcet’s disease-a multicenter study. J Rheumatol. 1989;16:506–511. [PubMed] [Google Scholar]
- 17.Çalgüneri M, Kiraz S, Ertenli I, Benekli M, Karaarslan Y, Celik I. The effect of prophylactic penicillin treatment on the course of arthritis episodes in patients with Behcet’s disease. A randomized clinical trial. Arthritis Rheum. 1996;39:2062–2065. doi: 10.1002/art.1780391216. [DOI] [PubMed] [Google Scholar]
- 18.Isogai E, Isogai H, Yokota K, Hayashi S, Fujii N, Oguma K, Yoshikawa K, Sasamoto Y, Kotake S, Ohno S. Platelet aggregation induced by uncommon serotypes of Streptococcus sanguis isolated from patients with Behcet’s disease. Arch Oral Biol. 1991;36:425–9. doi: 10.1016/0003-9969(91)90132-e. [DOI] [PubMed] [Google Scholar]
- 19.Imamura Y, Kurokawa MS, Yoshikawa H, Nara K, Takada E, Masuda C, Tsukikawa S, Ozaki S, Matsuda T, Suzuki N. Involvement of Th1 cells and heat shock protein 60 in the pathogenesis of intestinal Behcet’s disease. Clin Exp Immunol. 2005;139:371–378. doi: 10.1111/j.1365-2249.2005.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mege JL, Dilsen N, Sanguedolce V, Gul A, Bongrand P, Roux H, Ocal L, Inanç M, Capo C. Overproduction of monocyte derived tumor necrosis factor alpha, interleukin (IL) 6, IL-8 and increased neutrophil superoxide generation in Behcet’s disease. A comparative study with familial Mediterranean fever and healthy subjects. J Rheumatol. 1993;20:1544–1549. [PubMed] [Google Scholar]
- 21.Li B, Yang P, Zhou H, Zhang Z, Xie C, Lin X, Huang X, Kijlstra A. T-bet expression is upregulated in active Behcet’s disease. Br J Ophthalmol. 2003;87:1264–1267. doi: 10.1136/bjo.87.10.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki N, Sakane T, Ueda Y, Tsunematsu T. Abnormal B cell function in patients with Behcet’s disease. Arthritis Rheum. 1986;29:212–219. doi: 10.1002/art.1780290209. [DOI] [PubMed] [Google Scholar]
- 23.Ekşioglu-Demiralp E, Kibaroglu A, Direskeneli H, Yavuz S, Karsli F, Yurdakul S, Yazici H, Akoglu T. Phenotypic characteristics of B cells in Behcet’s disease: increased activity in B cell subsets. J Rheumatol. 1999;26:826–832. [PubMed] [Google Scholar]
- 24.Lee KH, Chung HS, Kim HS, Oh SH, Ha MK, Baik JH, Lee S, Bang D. Human alphaenolase from endothelial cells as a target antigen of anti-endothelial cell antibody in Behcet’s disease. Arthritis Rheum. 2003;48:2025–2035. doi: 10.1002/art.11074. [DOI] [PubMed] [Google Scholar]
- 25.International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s Disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 26.Akman-Demir G, Serdaroglu P, Tasci B. Clinical patterns of neurological involvement in Behçet’s disease: evaluation of 200 patients. The Neuro-Behçet Study Group. Brain. 1999;122:2171–2181. doi: 10.1093/brain/122.11.2171. [DOI] [PubMed] [Google Scholar]
- 27.Sugihara H, Mutoh Y, Tsuchiama Y. Neuro-Behçet’s disease: report of two autopsy cases. Acta Pathol Jap. 1969;19:95–101. doi: 10.1111/j.1440-1827.1969.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 28.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 29.Jarius S, Franciotta D, Bergamaschi R, Wright H, Littleton E, Palace J, Hohlfeld R, Vincent A. NMO-IgG in the diagnosis of neuromyelitis optica. Neurology. 2007;68:1076–1077. doi: 10.1212/01.wnl.0000256822.01222.bd. [DOI] [PubMed] [Google Scholar]
- 30.Dinc A, Takafuta T, Jiang D, Melikoglu M, Saruhan-Direskeneli G, Shapiro SS. Antiendothelial cell antibodies in Behcet’s disease. Clin Exp Rheumatol. 2003;21(4 Suppl 30):27–30. [PubMed] [Google Scholar]
- 31.Briani C, Doria A, Marcolongo R, Tognon S, Ruggero S, Toffanin E, Ermani M, Ghirardello A, Zampieri S, Semenzato G. Increased titres of IgM anti-heparan sulfate antibody in Behcet’s disease. Clin Exp Rheumatol. 2006;24(Suppl 42):104–107. [PubMed] [Google Scholar]
- 32.David Weedon. Skin Pathology Textbook. 2nd Edition. Elsevier Health Sciences; 2002. [Google Scholar]
- 33.Lewis MA, Priestley BL. Transient neonatal Behcet’s disease. Arch Dis Child. 1986;61:805–806. doi: 10.1136/adc.61.8.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fam AG, Siminovitch K, Carette S, From L. Neonatal Behcet’s syndrome in an infant of a mother with the disease. Ann Rheu Disease. 1981;40:509–512. doi: 10.1136/ard.40.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta RC, O’Duffy JD, McDuffie FC, Meurer M, Jordon RE. Circulating immune complexes in active Behcet’s disease. Clin Exp Immunol. 1978;34:213–218. [PMC free article] [PubMed] [Google Scholar]
- 36.Abdallah MA, Ragab N, Khalil R, Kamel N. Circulating immune complexes in various forms of Behcet’s disease. Int J Dermatol. 1995;34:841–845. doi: 10.1111/j.1365-4362.1995.tb04418.x. [DOI] [PubMed] [Google Scholar]
- 37.Fresko I, Ugurlu S, Ozbakır F, Celik A, Yurdakul S, Hamuryudan V, Yazici H. Anti- Saccharomyces cerevisiae antibodies (ASCA) in Behçet’s syndrome. Clin Exp Rheumatol. 2005;23(Suppl 38):67–70. [PubMed] [Google Scholar]
- 38.Lee JH, Cho SB, Bang D, Oh SH, Ahn KJ, Kim J, Park YB, Lee SK, Lee KH. Human anti-alpha-enolase antibody in sera from patients with Behçet’s disease and rheumatologic disorders. Clin Exp Rheumatol. 2009;27(Suppl 53):63–66. [PubMed] [Google Scholar]
- 39.Vural B, Demirkan A, Ugurel E, Kalaylioglu-Wheeler Z, Esen BA, Gure AO, Gül A, Ozbek U. Seroreactivity against PTEN-induced putative kinase 1 (PINK1) in Turkish patients with Behçet’s disease. Clin Exp Rheumatol. 2009;27(Suppl 53):67–72. [PubMed] [Google Scholar]
- 40.Birtas-Atesoglu E, Inanc N, Yavuz S, Ergun T, Direskeneli H. Serum levels of free heat shock protein 70 and anti-HSP70 are elevated in Behçet’s disease. Clin Exp Rheumatol. 2008;26(Suppl 50):96–98. [PubMed] [Google Scholar]
- 41.Taşçi B, Direskeneli H, Serdaroglu P, Akman-Demir G, Eraksoy M, Saruhan- Direskeneli G. Humoral immune response to mycobacterial heat shock protein (hsp)65 in the cerebrospinal fluid of neuro-Behçet patients. Clin Exp Immunol. 1998;113:100–104. doi: 10.1046/j.1365-2249.1998.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Celet B, Akman-Demir G, Serdaroğlu P, Yentür SP, Taşci B, van Noort JM, Eraksoy M, Saruhan-Direskeneli G. Anti-alpha B-crystallin immunoreactivity in inflammatory nervous system diseases. J Neurol. 2000;247:935–939. doi: 10.1007/s004150070049. [DOI] [PubMed] [Google Scholar]
- 43.Vural B, Uğurel E, Tüzün E, Kürtüncü M, Zuliani L, Cavuş F, Içöz S, Erdağ E, Gül A, Güre AO, Vincent A, Ozbek U, Eraksoy M, Akman-Demir G. Anti-neuronal and stressinduced-phosphoprotein 1 antibodies in neuro-Behçet’s disease. J Neuroimmunol. 2011;239:91–97. doi: 10.1016/j.jneuroim.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Goëb V, Thomas-L’Otellier M, Daveau R, Charlionet R, Fardellone P, Le Loët X, Tron F, Gilbert D, Vittecoq O. Candidate autoantigens identified by mass spectrometry in early rheumatoid arthritis are chaperones and citrullinated glycolytic enzymes. Arthritis Res Ther. 2009;11:R38. doi: 10.1186/ar2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of opticspinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]


