Abstract
Introduction
Neurological involvements were shown in 20% of patients with Primary Sjogren’s Syndrome (pSS). Neurological symptoms may be the first signs of pSS in 57% of the cases. In addition, early diagnosis and treatment of neurological disorders may save or improve the quality of life of these cases. There have been reports about the neurologic manifestations of pSS but little is known about the details of neurologically presented cases.
Method
In this study, we described 11 pSS patients who presented with neurological manifestations.
Results
Central nervous system (CNS) involvement was recorded in 7 (63.7%) and peripheric nervous system (PNS) involvement in 4 cases (36.4%).
Conclusion
Our findings regarding the cases with neurological manifestations leading to the diagnosis of pSS suggest that: 1) The frequency of CNS involvement was higher than that of PNS, and the most frequent clinical pictures of CNS involvement are Multiple Sclerosis (MS)-like illnesses and optic neuritis, 2) Guillain Barre Syndrome (GBS) was the most frequent disease of PNS involvement; 3) Mononeuropathy multiplex (MM) might be the first sign of pSS; 4) Neurologists should consider pSS in the differential diagnosis of cases with MS, optic neuritis, GBS and neuropathies of unknown causes including MM; 5) There is an urgent need of therapeutical guidelines for the cases with neurological involvement associated with pSS.
Keywords: Primary Sjogren’s syndrome (pSS), neurological manifestations, central nervous system (CNS), peripheral nervous system (PNS)
ÖZET
Giriş
Primer Sjögren Sendromlu (PSS) hastaların %20’sinde nörolojik tutulum olduğu gösterilmiştir. Olguların %57’sinde ise ilk olarak nörolojik belirtiler ortaya çıkmaktadır. Nörologların bu belirtileri erkenden tespit etmesi ve PSS tedavisine başlanması ile hastaların yaşamı kurtarılabilmekte ve/veya yaşam kalitesi arttırılabilmektedir. PSS ile ilişkili nörolojik hastalıklar oldukça iyi tanımlanmıştır ancak nörolojik bulgularla prezante olan olgularda PSS tanısı için detaylı bilgi bulunmamaktadır.
Yöntem
Bu çalışmada nörolojik bulgular ile başvuran 11 hastada PSS tanımlanmıştır.
Bulgular
Yedi olguda (%63,7) merkezi sinir sistemi (CNS) tutulumu belirlenirken, 4 olguda (%36,4) periferik sinir sistemi (PNS) tutulumu saptanmıştır.
Sonuç
Nörolojik hastalıkları nedeniyle PSS tanısı alan bu olguların değerlendirmesi sonucu bulgularımız şunlardır: 1) PSS’in ilk bulgusu olarak CNS tutulumu, PNS tutulumundan daha sık ortaya çıkmaktadır, CNS tutulumu ile giden klinik tablolardan en sık Multiple Skleroz (MS) benzeri hastalık ve optik nevrit görülmektedir; 2) Guillain Barre Sendromu (GBS) PNS tutulumu ile ilişkili en sık gözlenen klinik tablodur; 3) Mononöropati multipleks (MM) PSS’nin ilk işareti olabilmektedir; 4) Nörologların MS, optik nevrit ve GBS yanı sıra MM dâhil tüm nedeni bilinmeyen nöropati olgularının ayırıcı tanısında PSS düşünmesi gerekmektedir; 5) PSS ile ilişkili nörolojik tutulumu olan olgular için acil olarak tedavi kılavuzuna ihtiyaç bulunmaktadır.
Introduction
Sjogren’s Syndrome (SS) is a chronic autoimmune disorder described as autoimmune epithelitis. It is characterized by dryness of the eyes (xerophtalmia) and mouth (xerostomia) due to lymphocytic infiltration of the exocrine glands (1). These two findings are called “sicca symptoms”. After rheumatoid arthritis, SS is the second most common systemic autoimmune disease affecting adults in the United States, with an annual incidence of 3.9–5.3/100 000 inhabitants (2). As with other autoimmune diseases, SS is more common in females than in males; female to male ratio is 9:1 (3). SS can occur alone (primary Sjogren’s syndrome: PSS) or secondary in association with other connective tissue disorders (mainly systemic lupus erythematosus, rheumatoid arthritis, or scleroderma) (4,5). Approximately 50% of the patients have PSS (3). The exact cause of SS is not known. It is known that genetic, infectious, endocrine and immunological mechanisms contribute to the development of the disease.
The American-European Consensus Criteria for SS are now widely accepted (6); this set of criteria includes two subjective measures of ocular and oral dryness and four objective measures of gland involvement and elevated autoantibody titers. Having PSS according to this classification requires four of six criteria, of which at least one to be a positive minor salivary gland biopsy or elevated titers of SS (A) and/or SS (B) antibodies. The diagnosis can also be made if three of the four objective criteria are present (6).
However, as a systemic rheumatic disease, there can be various “extra-glandular” complications of SS (similar mononuclear infiltrates invading visceral organs or vasculitic lesions), at least one third of the patients present with neurological, rheumatologic, pulmonary, or gastrointestinal manifestations (7). Systemic involvement can be divided as non-visceral (skin, arthralgia, and myalgia) and visceral involvement [liver, kidney, gastrointestinal, endocrine, central nervous system (CNS), peripheral nervous system (PNS)] (8). A wide variety of PNS and CNS complications are among some severe extraglandular complications of SS (9,10,11,12,13).
Prevalence of PNS involvement in PSS varies widely from 10 to 60%, with pure or predominantly sensory polyneuropathies as the most common neurological manifestations (sensory ataxic or small fiber sensory painful neuropathy) (12,14,15,16). Mononeuropathy multiplex (MM), polyradiculopathy, symptomatic dysautonomia, myopathy, cranial neuropathy and CNS involvement are less common.
CNS manifestations due to PSS are still a matter of discussion and the reported prevalence of CNS involvement ranges from 2% to 25% (10,11,12,13,15,17). According to Massara et al., CNS involvement is frequently the initial manifestation of PSS when mild complaints of sicca symptoms could be overlooked (18). CNS involvement in PSS can be seen in 4 clinical types:1) diffuse (encephalomyelitis, aseptic meningitis, neuropsychiatric dysfunctions) involvement-accounts for 40%-; 2) focal/multifocal (sensorial and motor deficits, brain stem, cerebellar lesions, seizure, migraine etc.) and spinal cord (myelopathy, transverse myelitis, motor neuron disease etc.) findings-about 36%-; 3) multiple sclerosis (MS) -like illnesses (seen in 20%-; and 4) isolated optic neuritis-observed in 4% of patients- (18).
Extra-epithelial involvement (glomerulonephritis, polyneuropathy, vasculitis, purpura) increases morbidity and mortality (19). Thus, it is important to diagnose and treat these complications. Neurologist should be aware of neurological involvements of PSS which can be seen either in the course of the disease or as a presentation sign at onset.
In this report, 11 cases diagnosed with PSS based on the neurological findings are presented. The patients’ clinical symptoms, neurological and laboratory findings, treatments and responses to the treatments are also discussed.
Methods
Eleven patients admitted to the neurology department with neurological findings were evaluated through detailed history and neurological examination. For the diagnosis, magnetic resonance imaging of the brain spine, electroencephalogram (EEG), electroneuromyography and cerebrospinal fluid (CSF) analyses were performed. Based on the clinical findings, further investigations were carried out for a definitive diagnosis.
All patients were evaluated for the presence of a connective tissue disorder, symptoms of xerostomia, xerophtalmia, and ulcers of external genitalia and/or the oral region, and they all underwent routine biochemical tests and urinalysis and fibrinogen, serum protein electrophoresis, antinuclear antibody (ANA), anti-double stranded nuclear antibody (anti-dsDNA), rheumatoid factor (RF), anti-SS (A), and anti-SS (B) antibodies tests. Later, they were evaluated by an ophthalmologist, and Schirmer tests were conducted. Salivary gland function tests were not carried out, but salivary gland biopsies from 6 patients were studied. Additionally, CSF analysis was made in 8 patients; 3 patients refused lumbar puncture.
Results
1. Clinical Results
Eight of the 11 cases (72.8%) admitted to the neurology department were female and 3 were (27.2%) male, with a mean age of 41.9 years (21–67 years). Neurological manifestations had started with either acute or subacute patterns. All the patients suffered from neurological symptoms first, and later, they were diagnosed as having PSS. Table 1 summarizes these patients’ clinical, laboratory and outcome findings. Table 2 illustrates the diagnostic criteria of PSS for each case.
Table 1.
Revised international classification criteria for Sjögren’s syndrome (6)
| I. Ocular symptoms: a positive response to at least one of the following questions: |
|
| II. Oral symptoms: a positive response to at least one of the following questions: |
|
| III. Ocular signs-that is, objective evidence of ocular involvement defined as a positive result for at least one of the following two tests: |
|
| IV. Histopathology: In minor salivary glands (obtained through normal-appearing mucosa) focal lymphocytic sialoadenitis, evaluated by an expert histopathologist, with a focus score ≥1, defined as a number of lymphocytic foci (which are adjacent to normal-appearing mucous acini and contain more than 50 lymphocytes) per 4 mm2 of glandular tissue. |
| V. Salivary gland involvement: objective evidence of salivary gland involvement defined by a positive result for at least one of the followinw diagnostic test: |
|
| VI. Autoantibodies: presence in the serum of the following autoantibodies: Antibodies to Ro (SSA) or La (SSB) antigens, or both. |
For primary SS in 7 patients without any potentially associated disease, primary SS may be defined as follows:
|
Table 2.
Clinical aspects, laboratory and outcome findings of 11 primary Sjogren’s Syndrome (pSS) patients with initial neurologic manifestations
| Patient | Age/sex | General signs/history | Neurological examination | Brain and Spine MRI | ENMG | CSF | Other Laboratory Tests | Treatment | Diagnosis/Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 34/F | Sense of oily taste, left hemiparesia, vertigo and ataxia, loss of weight | Dysarthria, left hemiparesia and cerebellar signs, bilateral Babinski, positive Romberg | Contrast enhanced lesions in pons, both cerebellar pedincles and left m. oblongata | ND | glucose: 76mg/dl (204) pro: 52 mg/dl 50 wbc (98%lymphocte, 2% PNML), 200 rbc |
ESR:34 mm CRP<3 |
Pulse methylprednisolone; pulse cyclophosphamide | Recurrent acute disseminated encephalomyelitis (ADEM) due to pSS Exitus due to pontine hemorhhage |
| 2 | 27/M | Ascendan numbness and paresia from legs to arms, skin eruptions on legs | Distally dominant paraparesia, loss of DTR and vibration, glove and socks type sensation disorder, steppage gait | Brain and whole spinal MRI: normal except multiple cysts on parotis glands | Mononeuropathy multiplex | NL | SEP; no response in lower extremities VEP-BAEP: NL |
Pulse methylprednisolone and azathioprine with klorokin | Mononeuropathy multiplex (MM) due to pSS Stabil neurologic state with mild distal paresia |
| 3 | 30/M | Acute onset right side hemiparesia | Right central facial paresia, moderate (3/5) right hemiparesia and dysarthria, hemihypoaesthesia | Brain MRI: acute infarction on posterior leg of the left internal capsule Carotid angiogram:NL | ND | ND | CRP:11 Homocystein:10.33 LDL:172 Triglyceride: 328 EKG and echo:NL Genetic predisposition to thrombosis tests: NL Carotid-vertebral Doppler USG: NL |
Acetylsalisilic aside | Cerebral ischemic stroke related to pSS Full recovery |
| 4 | 67/F | Acute onset loss of vision on right side | Right afferent pupil defect | Brain and Orbita MRI; expansion of subarachnoidal space around the right optic nerve with non specific ischemic lesions in cerebral hemispheres | NL | ND | VEP: Prechiasmatic visual pathway disorder Temporal artery Doppler: NL ESR:28 CRP:4 B12:93 |
Azathioprine after pulse cyclophosphamide | Optic Neuritis due to pSS Recovery with moderate neurologic deficits |
| 5 | 53/F | Loss of weight, difficulty in speaking and walking, right sided hemihypoaesthesia | Mild impairment of cerebellar tests, hyperactive DTR’s, positive Romberg, wide based-ataxic gait | Cerebral MRI; contrast enhanced lesions in mesencephalon, pons, two cerebellar pedincles | NL | NL | Serebral angiogram: NL, thorax and abdominal CT: NL, mammography: NL, biomarkers for neoplasia and antineuronal antibodies: NL, serum infectious tests: NL | Pulse cyclophosphamide and methylprednisolone | Central nervous system (CNS) involvement of pSS Recovery with mild neurologic deficits |
| 6 | 40/F | Ascending numbness of legs, ataxic gait difficulty | Moderate quadriparesia, hyperactive DTR’s, nonresponsive Babinski reflexes |
Brain MRI and angiogram: NL Spinal MRI: contrast enhancements of radixes on lumbar level |
Late responsive H reflexes and F responses due to polyradiculoneuritis | CSF/serum glucose: 65/122 mg/dl pro: 186 mg/dl acellular in microscopy, negative for oligoclonal bands, Ig G index: 0,54 |
SEP: delayed latency responses in upper extremities, no- response in lower extremities. Serum infectious test were negative for Lyme and brucella, HIV and CMV | Five courses of therapeutic plasma exchange (TPE), azathioprine after 8 cycles of pulse cyclophpsphamide | Guillain Barre syndrome due to pSS Full recovery |
| 7 | 39/F | Loss of vision in the right eye, she was previously diagnosed as Sjogren syndrome | Bilateral papilla edema with left hemifascial spasm | Brain and orbita MRI: bilateral enlargement of optic nerve sheats Brain MRA: NL |
ND | NL including pressure of CSF | VEP: Bilateral late latency and deformed responses Visual field: bilateral serious concentric restriction Thorax and abdomen CT: Fatty liver tissue with biliary stones |
Methylprednisolone with pulse cyclophos-phamide | Bilateral optic neuritis related to pSS Stable for disease course |
| 8 | 47/F | Right sided pins and neddles with spasms, rarely generalized spasms | Lhermitt’sign with DTR hyperactivity | Brain and spinal MRI: cord lesion extending from craniocervical junction to vertebrae C3 with left mesencephalic lesion, multiple spinal lesions (demyelinating) |
NL | pressure: 140 mm Ig G index: 0,49 CSF/serum glucose: 53/104 mg/dl pro: 24mg/dl 60 rbc and 10 wbc in microscopy oligoclonal bands, Antiaquaporine Antibody: (−) |
BAEP, VEP: NL SEP: small amplitude and deformed responses. Thorax and abdomen CT: NL Mammography: NL Tumoral markers: (−) Protein eletrophoresis: NL |
Pulse cyclophps-phamide with methylprednisolone | Multiple sclerosis-like illnesses due to pSS Full recover |
| 9 | 39/F | Progressive loss of strength in arms and legs causing gait difficulty | Gower’s sign due to proximal limb weakness, hypoactive DTR, positive Romberg sign with spastic and ataxic gait disturbance | Brain MRI: NL Spinal MRI: contrast enhancements of lumbar radixes with protrusion and indentation to cord in the level of C5–6 |
Late responsive H reflexes and F responses due to polyradiculoneuritis | CSF/serum glucose: 58/113 mg/dl pro: 67mg/dl 30 wbc in microscopy. Brusella and oligoclonal bands: negative Ig G index: 0,36 |
Abdominopelvic ultrasonography: fatty liver, renal calculi in the left, Hepatitis markers: anti HBs: (+) | 5 courses of TPE, azathioprine and prednisolone after 19 cycles of pulse cyclophosphamide | Guillain Barre syndrome due to pSS Full recovery |
| 10 | 64/F | Progressive loss of strength and, pin and needles with severe pain in arms and legs causing gait difficulty | Mostly distal, moderate quadriparesia, hyperactive DTR’s, nonresponsive Babinski, severe truncal ataxia | Brain, cervical and Lumbar spinal MRI; chronic parietooccipital ischemic lesions in cerebrum and, C6–7 narrow spinal cannel in cervical region and, grade I spondylolysthesis in the level of lumbar L4–5 | Left sided carpal tunnel syndrome and axonal type motor polyradiculoneuritis affecting lower extremities mostly | CSF/serum glucose: 80/82 mg/dl pro: 49mg/dl 20 wbc and 180 rbc in microscopy. Brusella and oligoclonal bands: negative |
Abdominopelvic ultrasonography: granular liver tissue, Hepatitis markers: anti HBs and anti HAV: (+); others were all negative including anti HIV. | IVIg 25 gr/day for 6 days for acute motor axonal neuropathy; after the diagnosis of pSS, methylprednisolone and plaquenyl were given by recommendations of Rheumatology clinic | Guillain Barre syndrome (GBS) due to pSS Full recovery |
| 11 | 21/M | Pins and needles sensation ascending from toes to chest; right hemifacial spasm | Non-specific and patchy sensation deficits throughout the body | Brain MRI: Contrast enhanced left parietal milimetric lesion with 3 spinal cord lesion (2 of them were contrast enhanced); from C2 to cervicomedullar junction, C3–4 and T3–4 | ND | ND (patient refused) | SEP; delayed median and tibial responses on right side. | IV pulse 1gr Methylprednisolone during 7 days, and tapered oral Methylprednisolone | Multiple sclerosis-like illnesses due to pSS Full recover |
Abbreviations: ND; not done, NL: in normal limits, MRI: magnetic resonance imagings, CSF; cerebrospinal fluid, ENMG; electoneuromyography, DTR; deep tendon reflexes, SEP;somatosensory evoked potentials, VEP; visual evoked potentials, BAEP; brainstem auditory evoked potentials, ESR; erythrocyte sedimentation rate, CRP; C reactive protein, IVIg; intravenous immunoglobulins
PNS manifestations were noted in 4 patients (36.4%): 3 were diagnosed with acute GBS due to PSS (80%), and 1 with MM.
Seven patients (63.7%) showed signs of CNS involvement, 2 were diagnosed with MS-like illnesses, 2 (18.2%) with cranial nerve involvements as isolated optic neuritis, 1 with recurrent form of ADEM, 1 with multiple contrast-enhanced lesions of the infratentorial cerebral region due to PSS, and 1 patient with right hemiparesia due to acute ischemic cerebral lesion in the posterior leg of the left internal capsule.
2. Laboratory Results
Electrophysiological examination was performed for 7 patients with signs and symptoms of peripheral neuropathies. While three of these patients showed normal results, 4 displayed findings compatible with peripheral neuropathy showing GBS in 3 cases, and MM in one patient. Of the patients with GBS, 2 had acute demyelinating type, and 1 was with motor axonal-type polyradiculoneuritis.
MRI of the brain (orbital MRI in 2 patients; spinal MRI in 6 patients) was performed for patients with signs and symptoms of CNS involvements in order to assess the brain, optic nerve, spinal column and type of such involvements. Brain MRIs were normal in 3 cases; enlargements of optic nerve sheaths were observed in 2, contrast-enhanced cerebral and/or spinal lesions in 4 patients, ischemic cerebral lesion was recorded in 1 patient with stroke, and contrast enhancements of lumbosacral radixes were established in 3 GBS patients. Figures 1,2,3,4 show the MRI results in PSS patients with CNS involvements.
Figure 1.
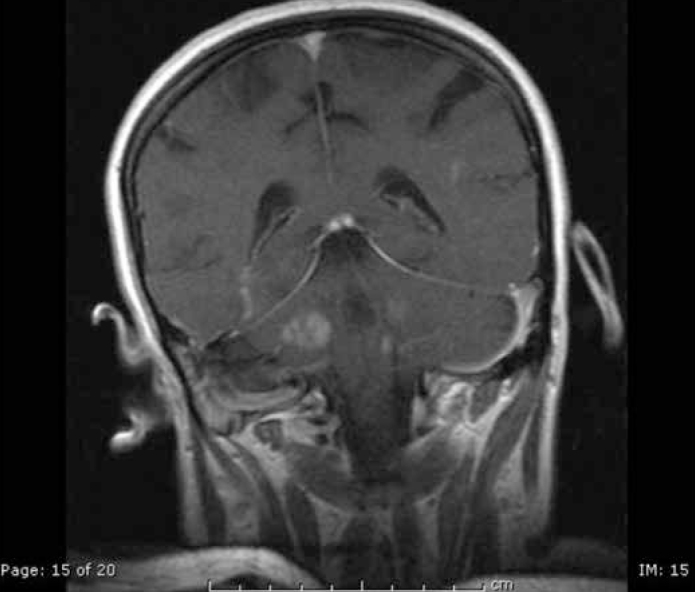
Contrast enhanced T1 weighted image revealed multiple, expansive hypointense lesions with contrast enhancements (Patient 1; Acute demyelinating encephalomyelitis (ADEM)-like picture due to primary Sjogren’s Syndrome)
Figure 2.
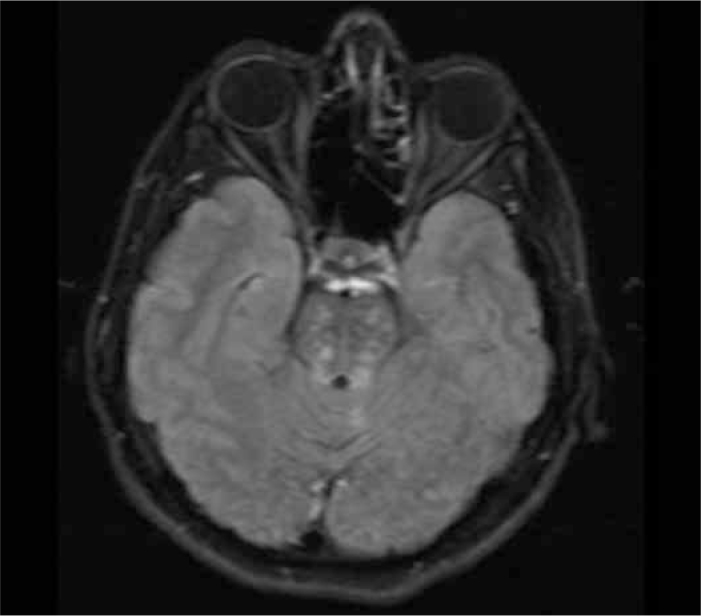
Axial fluid-attenuated inversion recovery (FLAIR) image demonstrates hyperintense lesions in pons (Patient 5; infratentorial cerebral lesions due to primary Sjogren’s Syndrome)
Figure 3.
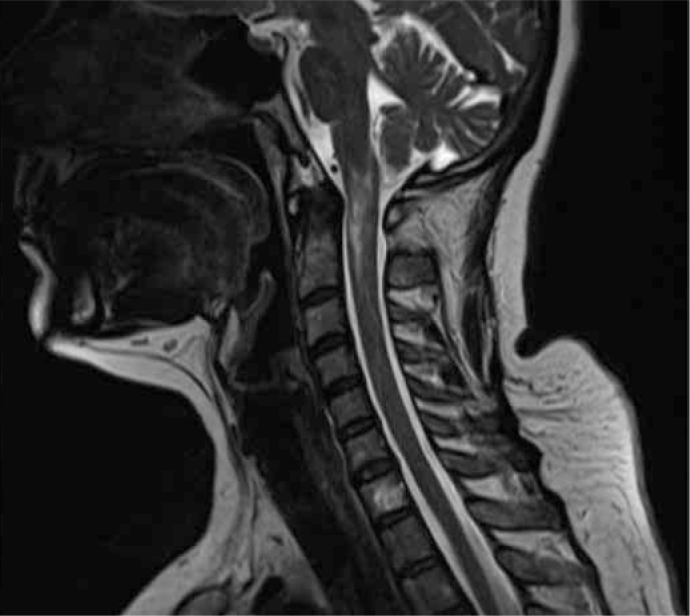
T2 weighted MRI images showed hyperintense lesion from medullar to C1 region (Patient 8; Multiple Sclerosis-like illness due to primary Sjogren’s Syndrome)
Figure 4.
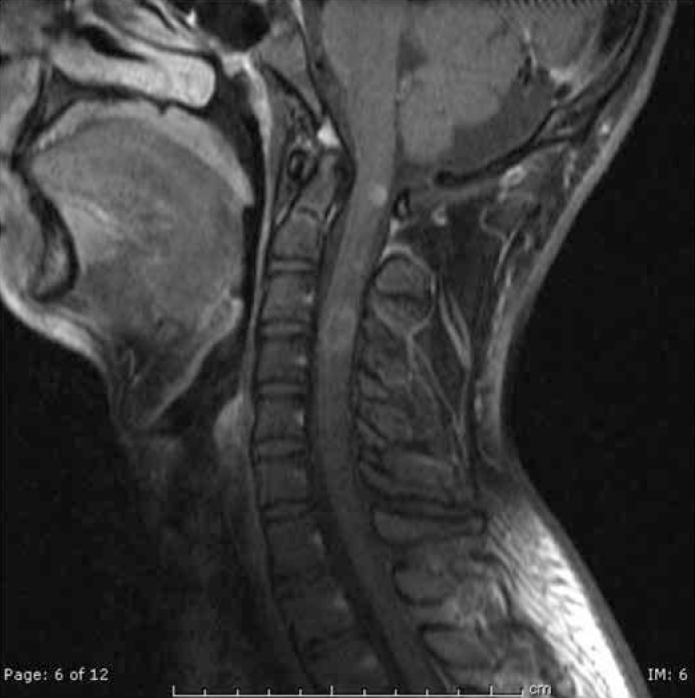
T1 weighted post-contrast image of the cervical spine revealed contrast enhancement at the C1 and C3-4 region (Patient 11; Multiple Sclerosis-like illness due to primary Sjogren’s Syndrome)
Lumbar puncture and CSF analysis were performed in eight patients (3 patients refused this procedure: 1 patient with cerebral infarction; 1 patient with optic neuritis who initially applied to the ophthalmology clinic and, 1 with a MS-like illness). CSF analysis revealed normal results in 4 patients; mild and moderate protein elevation and/or CSF pleocytosis were recorded in the remaining 4 patients. Somatosensory evoked potential (SEP) recordings of the patients with MM, GBS and MS-like illnesses showed delayed responses or unresponsiveness; visual evoked potential (VEP) responses were also pathological in 2 cases with optic neuritis.
3. Results of the treatments
Initially, all the patients with CNS involvements were prescribed pulse methylprednisolone 1 gram/kg for 5–7 days. The 2 of GBS patients were treated with 5 cycles of therapeutic plasma exchange (TPE), and 1 with intravenous immunoglobulin (IVIg), at a dose of .4 mg/kg/day for 5 days. Following the PSS diagnosis, treatment was conducted by a rheumatologist and all the patients were treated with pulse cyclophophosphamide at a dose of 600 mg/m2/month for 8–19 months. Then, the treatment was maintained with azathioprine at a dose of 2–3 mg/kg/day, alone or in combination with oral methylpredisolone. Oral methylprednisolone dosages were arranged as a dose of 1 mg/kg/day initially, and tapered to a maintenance dose of 10–20 mg/day.
Neurological deficits improved significantly in all patients; only the patient with ADEM-like picture died because of pontine hemorrhage during the course of treatment.
Discussion
In our study, eleven patients were diagnosed with PSS based on their neurological symptoms. Of these patients, 63.7% with initial neurological manifestations displayed CNS manifestations, while 36.4% had PNS manifestations. In the report of Massara et al., CNS involvement was detected in 25 (5.8%) of 424 PSS patients, of which 13 (52%) presented with neurological manifestations and were later diagnosed with PSS (18). Delalande et al. retrospectively studied 82 patients with neurological manifestations associated with PSS in which neurological findings were the preceding sign in 47% (11). It seems obvious that in half of PSS patients, the disease onset is in a neurological form. However, in Delalande’s study, all of the neurological involvements were discussed both before and after the diagnosis of PSS. Nevertheless, all of the 11 patients in our study population were diagnosed with PSS due to the development of neurological findings. For the previously diagnosed PSS cases, it was reported that neurological involvements had developed after a mean elapsing time of 7 (20) and 8.3 years (18).
Delalende and his colleagues reported similar rates of CNS (68%) and PNS (62%) involvements in PSS (11). As in our series, in their study, neurologic symptoms at the onset of PSS involved the CNS more frequently than PNS. Before the report of Delalende et al., PNS involvement was described more frequently than CNS involvement. They hypothesized that the highly heterogeneous CNS involvement in PSS in previous reports might be related to the different diagnostic criteria of PSS, the definition of neurological involvement, and the selection of patients from the neurology, internal medicine, or rheumatology departments. In our study group, all the patients were admitted to our neurology department, and they were later evaluated by a rheumatologist. It can help to reduce the potential bias in the proportion of CNS involvement at onset of PSS. Indeed, the high proportion of the CNS involvement may be related with selection of these 11 patients from the neurology outpatient clinic.
In our study, PNS complications were the first symptom of PSS in 4 patients (36.4%); 3 were diagnosed with acute GBS due to PSS (80%), and 1 with MM. Although there have been anecdotal reports about coexistence of GBS and PSS, Delalende et al. noted polyradiculoneuritis in only one patient with known PSS (11). MM is also a rare clinical entity and in the literature, to date, only 1 patient has been reported to be diagnosed with PSS after the diagnosis of MM (21). We suggest that the systematic screening for PSS is needed in cases of MM and polyradiculoneuritis as well as in cases of sensory, autonomic or trigeminal neuropathies of unknown causes.
Recent data concerning CNS involvement in PSS confirms the heterogeneity of clinical presentations that may mimic stroke or MS. In a report by Govoni et al., over a period of 5 years, CNS involvement was detected in 7 of 87 (8%) of unselected consecutive patients whose previous diagnosis was PSS (22). In our study, we identified 63.7% of patients (7/11) with CNS findings as initial clinical presentations of PSS; 5 of our patients had focal brain involvements yielding MS-like illnesses in 2 cases; one patient had recurrent ADEM-like disease, infratentorial cerebral involvement, and acute ischemic stroke. Optic neuritis was observed in 2 patients.
MS-like syndromes could represent a particular subset of neuro-Sjögren, and may thus affect the outcome. One tenth to one third of the neurological forms of PSS cases presents with pictures like MS (9,11). Some researchers have also reported an increased frequency of PSS in patients with MS (20). In our study, 18.2% of the cases were diagnosed with MS-like illnesses. Although the rate is fairly high, when the patient showing ADEM-like picture is included, the rate would be much higher (27.3%). Therefore, a screening test for PSS should be considered in suspected MS cases. Delalende and coworkers described 23 of 82 patients (28%) with a clinical feature suggesting MS; they also observed that the delay between onset and the diagnosis of PSS was significantly longer (9.8 years) for their 10 patients with recurrent deficits mimicking relapsing-remitting MS (11). Thus, this finding underlines the need for screening test in PSS for the exact diagnosis in such MS-like cases. The neuroradiological features with serologic and CSF analysis could help to distinguish between these two inflammatory CNS diseases. MRI lesions are generally smaller and less pronounced in PSS than in MS (23). There are some characteristic features of MS-like illnesses of PSS, however, affecting patients of older age, involvement of PNS and/or cranial nerve, spinal cord MRI lesions spanning multiple spinal segments, MRI lesions of cerebral cortex, and absence of lesions in the corpus callosum are all atypical in MS (20,23,24). As it was also observed in our patients, the CSF profile of PSS strongly resembles the profile described in MS patients, yet in MS, there are multiple bands, often more than 3, whereas 1 or 2 bands can mostly be detected in PSS patients (9). Oligoclonal band studies in our patients yielded negative results. Furthermore, Alexander et al. reported that the bands disappeared as a response to corticosteroid therapy in most neuro-Sjögren patients, contrary to that in those with MS (9).
Most of the cases with CNS manifestations associated with PSS had an acute, often recurrent course (11,18). It was concluded that severe disability was more frequent in cases of CNS involvement than in cases of PNS involvement, suggesting the need for intensive and early treatment in cases of CNS involvement (11). Contrary to this conclusion, spontaneous remission or only mild neurological impairment was deduced in patients with CNS involvement of PSS in another study (18). In line with the findings in the literature, the disease course of our patients with CNS involvements was in either acute or subacute fashion, and most of the patients recovered markedly (particular improvements were recorded in 4 patients) in spite of their serious clinical presentations. Only one patient with recurrent ADEM-like illness died due to a large pontine hemorrhage.
Bragoni reported a young woman with ischemic stroke as initial manifestations of SS (25). In our study, we defined PSS in a young man with right hemiparesia due to acute ischemic cerebral lesion in the posterior limb of the left internal capsule. Therefore, PSS should be considered among the causes of stroke, especially in young patients.
Although, optic neuritis was reported in 13 (15.9%) patients in Delalende’s series of 82 patients with PSS, abnormal VEPs were obtained in 61% of tested patients (11). They did not provide any information on whether these optic involvements were seen at the onset or during the course of the disease. We defined isolated either unilateral or bilateral optic neuritis in two cases (18.2%) as initial manifestations of PSS, and, we also recommend the systematic screening for PSS in cases with optic neuropathy.
Treatments usually comprise symptomatic approach in milder cases, nevertheless, pulse cyclophosphamide and steroids or other immunosuppressants (chlorambucil or azathioprine) are required in cases with progressive symptoms leading to neurological impairment. We achieved fairly reliable results with TPE and IVIg in cases with GBS and, we used corticosteroids in a certain number of our patients. The need for therapeutic guidelines is obvious, and further research should be conducted to gain deeper insight into this area. It was demonstrated that corticosteroid therapy is a likely candidate for MM and multiple cranial neuropathies (26,27), and favorable improvement may be seen in painful dysaesthesia of painful sensory neuropathy and radiculoneuropathy forms with IVIg therapy (27,28,29,30). In addition to corticosteroids and IVIg, plasmapheresis (31), D-penicillamine (32), anti-TNF agent, and infliximab (33) have been used with variable responses. Patients with myelopathy or MM have responded well to cyclic application of cyclophosphamide (11). Alexander recommends cyclophosphamide pulse therapy combined with a corticosteroid therapy for at least one year in the beginning (34). We applied 8–19 cycles of cyclophosphamide in our patients, and significant improvements were achieved. Randomized controlled studies are needed to assess the actual efficacy of these treatments for neuro-Sjögren syndrome.
Conclusions
In this study, we reported 11 patients with PSS, whose initial manifestations were neurological, and our conclusions from this series of patients are listed below;
CNS involvement was the major presenting form at a rate of 63.7% in this group of patients; PNS involvement was established in 36.4%. MS-like illnesses and optic neuritis were the most frequent forms of CNS involvements.
GBS was the top initial clinical diagnosis in patients with PNS involvements, unlike what is suggested by previous studies illustrating sensory ataxic neuropathy in cases with PSS. We can, thus, state that sensory ataxic neuropathy occurs in advanced PSS cases.
Differential diagnosis for PSS could be performed in cases with optic neuritis, MS, GBS and ischemic stroke. Complete laboratory testing, including SS (A&B) antibody studies, and ophthalmological and salivary gland evaluation may lead to more reliable diagnosis.
Suspicion of PSS should be accounted for when evaluating patients (particularly females) with unknown causes of neuropathy including MM. Additionally, patients with atypical neurological findings (either clinical or radiological) deserve this evaluation, and rheumatological consultation should be requested for such cases.
Multi-centered, randomized, prospective, carefully controlled trials with quantitative neurological and patient-centered outcome measures should be conducted in order to obtain therapeutic guidelines.
Footnotes
Conflict of interest: The authors reported no conflict of interest related to this article.
Çıkar çatışması: Yazarlar bu makale ile ilgili olarak herhangi bir çıkar çatışması bildirmemişlerdir.
References
- 1.Youinou Y. Sjögren’s syndrome: a quintessential B cell-induced autoimmune disease. Joint Bone Spine. 2008;75:1–2. doi: 10.1016/j.jbspin.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Binard A, Devauchelle-Pensec V, Fautrel B, Jousse S, Youinou P, Saraux A. Epidemiology of Sjogren’s syndrome: Where are we now? Clin Exp Rhumatol. 2007;25:1–4. [PubMed] [Google Scholar]
- 3.Pillemer SR, Matteson EL, Jacobsson LT, Martens PB, Melton LJ, 3rd, O’Fallon WM, Fox PC. Incidence of physician-diagnosed primary Sjögren syndrome in residents of Olmsted County, Minnesota. Mayo Clin Proc. 2001;76:593–599. doi: 10.4065/76.6.593. [DOI] [PubMed] [Google Scholar]
- 4.Carsons S. Sjögren’s Syndrome. In: Harris ED Jr, Budd RC, Firestein GS, Genovese MC, Sergent JS, Ruddy S, Sledge BC, editors. Kelley’s Textbook of Rheumatology. 6th ed. Philadelphia, USA: WB Saunders Company; 2005. pp. 1105–1124. [Google Scholar]
- 5.Ramos-Casals M, Font J. Primary Sjögren’s syndrome: current and emergent aethiopathogenic concepts. Rheumatology (Oxford) 2005;44:1354–1367. doi: 10.1093/rheumatology/keh714. [DOI] [PubMed] [Google Scholar]
- 6.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH European Study Group on Classification Criteria for Sjogren’s Syndrome. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moutsopoulos HM. Sjögren’s Syndrome. In: Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 16th ed. New York, USA: McGraw-Hill Medical Publishing Division; 2005. pp. 1990–1993. [Google Scholar]
- 8.Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 9.Alexander EL, Malinow K, Lejewski JE, Jerdan MS, Provost TT, Alexander GE. Primary Sjogren’s syndrome with central nervous system disease mimicking multiple sclerosis. Ann Intern Med. 1986;104:323–330. doi: 10.7326/0003-4819-104-3-323. [DOI] [PubMed] [Google Scholar]
- 10.Alexander EL. Central nervous system (CNS) manifestations of primary Sjogren’s syndrome: an overview. Scand J Rheumatol Suppl. 1986;61:161–165. [PubMed] [Google Scholar]
- 11.Delalande S, de Seze J, Fauchais AL, Hachulla E, Stojkovic T, Ferriby D, Dubucquoi S, Pruvo JP, Vermersch P, Hatron PY. Neurologic manifestations in primary Sjögren’s syndrome. A study of 82 patients. Medicine (Baltimore) 2004;83:280–291. doi: 10.1097/01.md.0000141099.53742.16. [DOI] [PubMed] [Google Scholar]
- 12.Chai J, Logigian EL. Neurological manifestations of primary Sjogren’s syndrome. Curr Opin Neurol. 2010;23:509–513. doi: 10.1097/WCO.0b013e32833de6ab. [DOI] [PubMed] [Google Scholar]
- 13.Créange A, Laplane D, Habib K, Attal N, Assuérus V. Dementia disclosing primary Gougerot-Sjogren syndrome. Rev Neurol (Paris) 1992;148:376–380. [PubMed] [Google Scholar]
- 14.Gemignani F, Marbini A, Pavesi G, Di Vittorio S, Manganelli P, Cenacchi G, Mancia D. Peripheral neuropathy associated with primary Sjogren’s syndrome. J Neurol Neurosurg Psychiatry. 1994;57:983–986. doi: 10.1136/jnnp.57.8.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafitte C, Amoura Z, Cacoub P, Pradat-Diehl P, Picq C, Salachas F, Léger JM, Piette JC, Delattre JY. Neurologic complications of primary Sjogren’s syndrome. J Neurol. 2001;248:577–584. doi: 10.1007/s004150170135. [DOI] [PubMed] [Google Scholar]
- 16.Mellgren SI, Conn DL, Stevens JC, Dyck PJ. Peripheral neuropathy in primary Sjögren’s syndrome. Neurology. 1989;39:390–394. doi: 10.1212/wnl.39.3.390. [DOI] [PubMed] [Google Scholar]
- 17.Alexander EL, Provost TT, Stevens MB, Alexander GE. Neurologic complications of primary Sjogren’s syndrome. Medicine (Baltimore) 1982;61:247–257. doi: 10.1097/00005792-198207000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Massara A, Bonazza S, Castellino G, Caniatti L, Trotta F, Borrelli M, Feggi L, Govoni M. Central nervous system involvement in Sjögren’s syndrome: unusual, but not unremarkable—clinical, serologic characteristic and outcomes in a large cohort of Italian patients. Rheumatology (Oxford) 2010;49:1540–1549. doi: 10.1093/rheumatology/keq111. [DOI] [PubMed] [Google Scholar]
- 19.Ramos-Casals M, Tzioufas AG, Font J. Primary Sjogren’s syndrome: new clinical and therapeutic concepts. Ann Rheum Dis. 2005;64:347–354. doi: 10.1136/ard.2004.025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soliotis FC, Mavragani CP, Moutsopoulos HM. Central nervous system involvement in Sjogren’s syndrome. Ann Rheum Dis. 2004;63:616–620. doi: 10.1136/ard.2003.019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viñas Gaya J. [3 cases of rare peripheral neuropathies associated with primary Gougerot-Sjögren syndrome]. Rev Neurol (Paris) 1993;149:481–484. [PubMed] [Google Scholar]
- 22.Govoni M, Padovan M, Rizzo N, Trotta F. CNS Involvement in Primary Sjogren’s syndrome: Prevalence, Clinical Aspects, Diagnostic Assessment and Therapeutic Approach. CNS Drugs. 2001;15:597–607. doi: 10.2165/00023210-200115080-00003. [DOI] [PubMed] [Google Scholar]
- 23.Morgen K, McFarland HF, Pillemer SR. Central nervous system disease in primary Sjogren syndrome: the role of magnetic resonance imaging. Semin Arthritis Rheum. 2004;34:623–630. doi: 10.1016/j.semarthrit.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum R, Barkhuizen A. Primary Sjogren’s syndrome. In: Noseworthy JH, editor. Neurological Therapeutics: Principles and Practice. 2nd ed. London, UK: Informa Healthcare; 2006. pp. 1587–1591. [Google Scholar]
- 25.Bragoni M, Di Piero V, Priori R, Valesini G, Lenzi GL. Sjogren’s syndrome presenting as ischemic stroke. Stroke. 1994;25:2276–2279. doi: 10.1161/01.str.25.11.2276. [DOI] [PubMed] [Google Scholar]
- 26.Griffin JW, Cornblath DR, Alexander E, Campbell J, Low PA, Bird S, Feldman EL. Ataxic sensory neuropathy and dorsal root ganglionitis associated with Sjögren’s syndrome. Ann Neurol. 1990;27:304–315. doi: 10.1002/ana.410270313. [DOI] [PubMed] [Google Scholar]
- 27.Mori K, Iijima M, Koike H, Hattori N, Tanaka F, Watanabe H, Katsuno M, Fujita A, Aiba I, Ogata A, Saito T, Asakura K, Yoshida M, Hirayama M, Sobue G. The wide spectrum of clinical manifestations in Sjogren’s Syndrome-associated neuropathy. Brain. 2005;128:2518–2534. doi: 10.1093/brain/awh605. [DOI] [PubMed] [Google Scholar]
- 28.Molina JA, Benito-León J, Bermejo F, Jiménez-Jiménez FJ, Oliván J. Intravenous immunoglobulin therapy in sensory neuropathy associated with Sjogren’s syndrome. J Neurol Neurosurg Psychiatry. 1996;60:699. doi: 10.1136/jnnp.60.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi Y, Takata T, Hoshino M, Sakurai M, Kanazawa I. Benefit of IVIG for long-standing ataxic sensory neuronopathy with Sjogren’s syndrome. Neurology. 2003;60:503–505. doi: 10.1212/01.wnl.0000046680.47883.7d. [DOI] [PubMed] [Google Scholar]
- 30.Taguchi Y, Takashima S, Takata M, Dougu N, Asaoka E, Inoue H. High-dose intravenous immunoglobulin in the treatment of sensory ataxic neuropathy with Sjogren’s syndrome: a case report. No To Shinkei. 2004;56:421–424. [PubMed] [Google Scholar]
- 31.Chen WH, Yeh JH, Chiu HC. Plasmapheresis in the treatment of ataxic sensory neuropathy associated with Sjogren’s syndrome. Eur Neurol. 2001;45:270–274. doi: 10.1159/000052142. [DOI] [PubMed] [Google Scholar]
- 32.Asahina M, Kuwabara S, Asahina M, Nakajima M, Hattori T. D-penicillamine treatment for chronic sensory ataxic neuropathy associated with Sjogren’s syndrome. Neurology. 1998;51:1451–1453. doi: 10.1212/wnl.51.5.1451. [DOI] [PubMed] [Google Scholar]
- 33.Caroyer JM, Manto MU, Steinfeld SD. Severe sensory neuronopathy responsive to infliximab in primary Sjogren’s syndrome. Neurology. 2002;59:1113–1114. doi: 10.1212/wnl.59.7.1113. [DOI] [PubMed] [Google Scholar]
- 34.Alexander EL. Central nervous system disease in Sjogren’s syndrome. New insights into immunopathogenesis. Rheum Dis Clin North Am. 1992;18:637–672. [PubMed] [Google Scholar]


