Abstract
Introduction
Vitamin D, the main function of which is thought to be the maintenance of calcium and phosphate homeostasis and bone structure, has been shown in recent studies to have important roles in brain development as well. A certain vitamin D receptor (VDR) gene haplotype was reported, for the first time by our group, to increase the risk of developing Alzheimer’s disease. Our studies also showed that vitamin D prevents beta amyloid-induced calcium elevation and toxicity that target nerve growth factor (NGF) release in cortical neurons; beta amyloid suppresses VDR expression and the disruption of vitamin D-VDR pathway mimics beta amyloid-induced neurodegeneration. In this study, our aim was to investigate the effects of vitamin D on the NGF release from hippocampal neurons.
Method
Primary hippocampal neuron cultures that were prepared from 18-day-old Sprague-Dawley rat embryos were treated with vitamin D for 48 hours. The alteration in the NGF release was determined with ELISA. Cytotoxicity tests were also performed for all groups.
Results
The NGF release in vitamin D-treated group was significantly higher than in untreated control group. The protective effect of vitamin D against cytotoxicity was also observed.
Conclusion
Our results indicated that vitamin D regulates the release of NGF, a very important molecule for neuronal survival of hippocampal neurons as well as cortical neurons.
Keywords: Alzheimer’s disease, vitamin D, nerve growth factor, primary hippocampal neuron culture
ÖZET
Amaç
Yapılan son çalışmalar, uzun yıllardır esas fonksiyonunun kalsiyum ve fosfat dengesini düzenlemek ve kemik yapısını korumak olduğu düşünülen vitamin D’nin, beyinde de önemli rollerinin olduğunu göstermiştir. Çalışmalarımızda kortikal nöronlarda vitamin D’nin, beta amiloid ile indüklenen ve kalsiyum artışı ile sinir büyüme faktörü (NGF) üretimini hedef alan toksisiteyi ortadan kaldırmada etkili olduğu; beta amiloidin vitamin D reseptörü anlatımını engellediği ve vitamin D-VDR yolağının bloke edilmesinin nörodejenerasyon benzeri değişikliklere sebep olduğu gösterilmiştir. Ayrıca vitamin D reseptörüne (VDR) ait belirli bir haplotipin Alzheimer Hastalığı riskini arttırdığı ilk kez grubumuz tarafından bildirilmiştir. Bu çalışmada kortikal nöronların NGF salınımını düzenleyen vitamin D’nin hippokampal nöronlarda da NGF salınımı üzerine etkisinin olup olmadığını saptamayı amaçladık.
Yöntem
Sprague Dawley cinsi sıçanların 18 günlük embriyolarından alınan hippokampus bölgelerinden hazırlanan primer hippokampal nöron kültürlerine, 48 saat süresince vitamin D uygulandı. NGF salınımındaki değişimler, ELISA yöntemi ile belirlendi. Tüm gruplara, sitotoksisite testi uygulandı.
Bulgular
Vitamin D uygulanan primer hippokampal nöronların NGF miktarlarının hiçbir uygulama yapılmayan kontrol grubuna göre anlamlı derecede arttığı saptandı. Ayrıca vitamin D’nin nöronları sitotoksisiteye karşı koruduğu belirlendi.
Sonuç
Sonuçlarımız kortikal nöronlarda olduğu gibi Alzheimer hastalığında büyük önem taşıyan hippokampal nöronlarda da vitamin D’nin nöron sağ kalımı için önemli bir molekül olan NGF salınımını etkilediğini göstermektedir.
Introduction
Vitamin D (1,25 dihydroxyvitamin D, 1,25 dihydroxycholecalciferol), the main function of which has been thought to be the maintenance of calcium and phosphate homeostasis and bone structure for long years, has been shown to have important roles in regulation of the immune system and renin-angiotensin system, in occurence of cardiovascular diseases and in the brain. Vitamin D which carries a cholesterol backbone and has steroid-like effects in the whole body regulates the expression of more than 100 genes by way of vitamin D receptor (VDR) which is a nuclear steroid receptor. It has been found that synthesis and destruction of vitamin D occur in the brain and VDR which is required for vitamin D to show its effect is found in different regions of the brain. In addition, it has been shown that vitamin D has protective effects on the nervous system in many studies (1,2,3,4,5,6).
The findings related with the effects of vitamin D on the nervous system can be collected under four titles (1,3,7,8,9): Prevention of oxydative stress: Vitamin D is an antioxydant which controls the process of detoxification in the brain by regulating the activity of γ glutamyl transpeptidase. Regulation of neurotrophic factors: Vitamin D regulates expression of neuron growth factor (NGF), neurotrphin-3 (NT-3), neurotrophin-4 (NT-4) and glial cell derived neurotrophic factors (GDNF). Ca2+ balance: Vitamin D regulates the expression of calcium binding proteins and L-type voltage sensitive calcium channels which have important roles in intracellular calcium balance. Regulation of the immune system: Vitamin D plays a role in autoimmune and cellular immune response.
Problems related with the amount of vitamin D in the body and intake of vitamin D can be associated with neuron damage in neurodegenerative diseases, because they will affect production of neurotrophic factors, intracellular calcium balance, oxydative stress mechanisms and the immune system. In addition, an association of vitamin D receptor gene polymorphisms and “TaubF” haplotype with late-onset Alzheimer’s disease was shown for the first time in the literature with our previous studies (3,10).
Recent studies have shown that vitamin D deficiency is substantially common in individuals living in developed countries including the young ones. In the elderly, the rate of vitamin D deficiency reaches up to 87%. Until recently, vitamin D has been regarded as only a simple vitamin and this may have hidden the fact that this bioactive substance is actually a multi-target secosteroid hormone and its deficiency may cause to serious problems in the elderly. Although the first findings in the brain extend back to 25 years ago, the information obtained is still substantially inadequate. Even this limited information presents data related with the fact that vitamin D plays a role in many pathways ranging from brain development to regulation of the immune system. Considering the intracellular pathways which participate in the effect of vitamin D on the nervous system, it can be thought that vitamin D deficiency may play a role in occurence of neurodegenerative diseases and a new perspective can be brought for explanation of the molecular mechanisms of neurodegeneration.
In our previous studies, it was shown that vitamin D was effective in abolishing the toxicity induced with beta amiloid in cortical neurons targeting increased calcium and NGF production, beta amiloid abolished the expression of vitamin D receptor and blocking the vitamin D-VDR pathway caused to neurodegeration- like changes (8,9,11). Other recent interesting studies showed that vitamin D strongly stimulated the macrophages of Alzheimer’s patients for phagosytosis and thus protected the neurons against apoptosis by enabling clearance of beta amiloid (12,13). Annweiler and Beauchet published a new treatment protocol (AD-IDEA) for Alzheimer’s disease by combining memantin which is a drug used in treatment of Alzheimer’s disease with vitamin D (14). However, there is still an insufficient number of studies related with the effect of vitamin D on neurons and neurodegeneration. The information about the relation between NGF and vitamin D is limited with the studies on glia cells, hippocampal neurons cultured in medium containing neuroblastoma cell lines and serum (6,15,16,17) and cortical neurons (8,9). It has been reported that media containin serum are not appropriate for neurotrophic factor and vitamin studies (18). Accordingly, there is no study related with how application of vitamin D and NGF release have an effect in cultures of hippocampal neurons prepared with medium containing no serum.
In the light of present biological data and genetic and cellular results we obtained in our previous studies, we planned to investigate the effect of vitamin D on hippocampal neurons especially in terms of NGF release in this study. With this objective, we aimed to determine the changes in the NGF protein which has significant roles in neuron survival by applying vitamin D to primary hippocampal neuron culture and to investigate the effect of vitamin D on NGF release in the hippocampus which is important for Alzheimer’s disease.
Methods
Preparation of Primary Hippocampal Neuron Culture
18-day-old Sprague-Dawley rat embryos were used to obtain hippocampal neurons. Using the method of Goslin and Banker (19) the hippocampi belonging to the right and left hemispheres of 18-day-old embryos were removed by clearing of membrane and inoculated by separating cells (8,9,11). Neuron culture was performed using 6 well discs with a diameter of 35 mm (Corning 3506. Corning Inc. New York, USA). The petris which would be used for inoculation were prepared by covering with poly-L-ornithine with a ratio of 1:50 (Sigma P-4957. Sigma-Alderich Chemie GmbH, Steinhemim, GE). The neurons were kept in leibovitz 15+ (L-15+; GibcoBRL 11415-064. Invitrogen Inc. New York, USA) culture medium containing 0.1 mg/ ml conalbumin (Sigma C-7786. Sigma-Alderich Chemie GmbH, Steinheim, GE), 7.5% Sodium bicarbonate (GibcoBRL 25080-094. Invitrogen Inc., New York, USA), 01 mM Putrescine (Sigma P-7505. Sigma-Alderich Chemie GmbH, Steinheim, GE), 10 ng/ ml Insulin (GibcoBRL 12585. Invitrogen Inc., New York, USA), 30 nM Sodium selenite (Sigma S-5261. Sigma-Alderich Chemie GmbH, Steinheim, GE), 20 nM Progesteron (Sigma P-6149. Sigma-Alderich Chemie GmbH, Steinheim, GE), 20 mM Glucose (Sigma G-7021. Sigma-Alderich Chemie GmbH, Steinheim, GE), and PenStrep (Sigma P-4333. Sigma-Alderich Chemie GmbH, Steinheim, GE) for one day. In the following day, the L-15+ medium was changed with Neurobasal medium-NBM which did not contain serum and which contained 1:50 B27, 9% sodium chloride and PenStrep. The cells in the primary hippocampal neuron culture become mature neurons by extending thier axons and dendrites in 7 days after inoculation (Figure 1).
Figure 1.
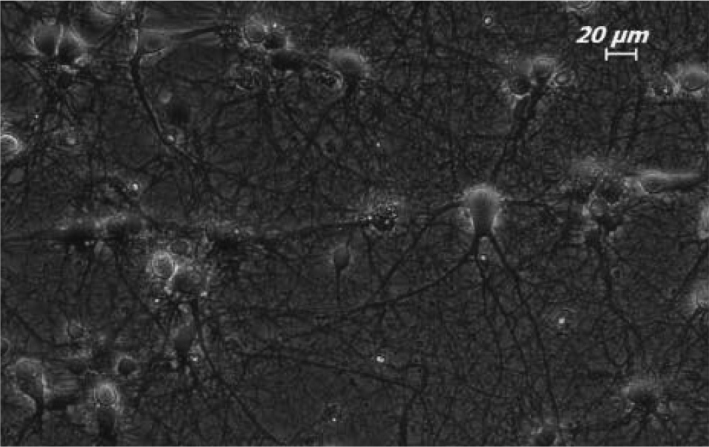
Appearance of 7-day old primary hippocampal neurons on phase-contrast microscope. 20× magnification.
In our strudy, we complied with the conditions stated in the “Guide for Use and Care of Laboratory Animals”. The study was approved by İstanbul University, Experimental Animals Unit Ethics Committee (approval number: 23797/20.09.2006).
Neuron/Glia Differentiation in Culture Cells
The cells in the culture were labeled with immunoflourescence method using neuron and glia cell specific antibodies. The differentiation of the neurons was made using pan Neuronal Marker primary antibody and FITC labeled secondary antibody. Glial cells were differentiated using glial fibrillary acidic protein (GFAP) primary antibody which is a glial cell marker and Texas Red (TR) labaled secondary antibodies. The culture cells which primary antibodies could not be applied to and which only secondary antibodies could be applied to were used as negative control. The primary hippocampal neuron culture which survived without any application for 14 days and thus in which glial cells were allowed to grow was used as positive control (Figure 2a). The cells in the culture were fixed in 3.7% paraformaldehyde (Sigma 15,812-7. Sigma-Alderich Chemie GmbH, Steinheim, GE). The cells were kept in 30% normal goat serum (Chemicon S26. Millipore Corp. California, USA) at rrom temperature for one hour and the primary abntibody diluted at a ratio of 1:50 Milli-MarkTM PAn Neuronal Marker (Millipore MAB2300. Millipore Corp. California, USA) was kept at +4°C for one night. Afterwards, it was incubated in 1.5% goat serum with goat anti-mouse IgG, FITC (fluorescein isothicyanate) (Millipore AP181F. Millipore Corp. California, USA) secondary antibody diluted at a ratio of 1:50 at room temperature for one hour. Then, it was kept in 10% normal goat serum at room temperature for 30 minutes. The cells were kept with 1:750 primary antibosy GFAP (Invitrogen AB5804. Invitrogen Inc., New York, USA) at room temperature for 2 hours. Finally, they were incubated with goat anti-rabbit IgG TR (Texas Red) (Santa Cruz sc-2780. SantaCruz Inc. California, USA) secondary antibosy diluted at a ratio of 1:100 at room temperature for one hour. They were kept in DAPI (4′,6′-Diamidino-2-Phenylindole) solution at a ratio of 1:48.000 for 5 minutes. The cells were examined wşth invert flourescence microscope (Leica DMIL, Leica Microsystems Ltd., Heerbrugg, GE) and their pictures were taken with flourescence camare system (Leica DFC 300 FX, Leica Microsystems Ltd., Heerburg, GE) using TR; I3 and A3 filters. The pictures belonging to the same region taken with different filters were overlapped using a special software (The Leica Application Suite Image Overlay Software, Leica Microsystems Ltd., Heerburg, GE). 10 pictures taken from random areas of each petri were overlapped and the neuron and glial cells were counted.
Figure 2. Specification of the neuron/glia ratio in culture cells.
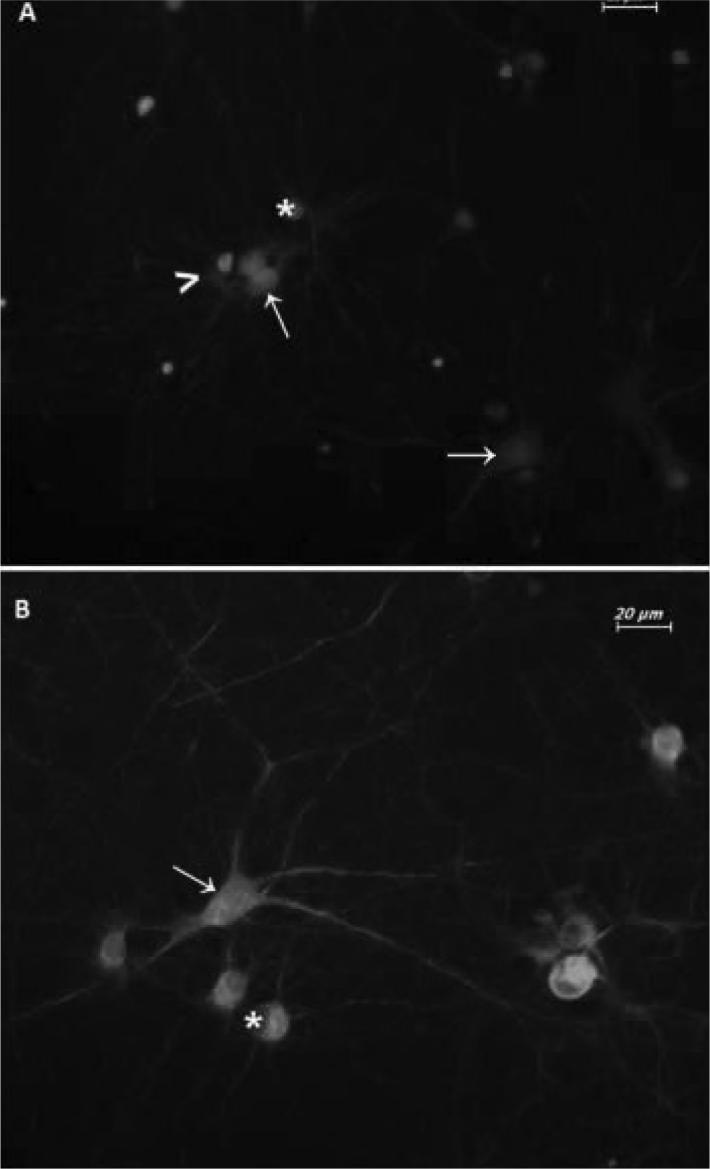
A)14-day old primary hippocampal neuron culture. 14-day old primary hippocampal neuron culure which was allowed to age and grow glia was used as positive control to determine the neuron/glia ratio. The glia ratio was found to be 50%. B)7-day old primary hippocampal neuron culture. The glia ratio was found to be 20%. FITC labeled Pan Neuronal Marker, TR labeled GFAP antibodies and triple staining with DAPI. Neuron: green (FITC), → (arrow); glia cells: red (TR), ι (arrow head); nucleus: blue (DAPI), *(astrix). 40× magnification.
Groups
The neurons matured on the 3rd day of culture were divided into 3 groups:
The first group: Vitamin D was applied. 10−8 M 1,25 dihydroxyvitamin D3 was applied for 48 hours on the 7th day.
The second group: Ethanol was applied. 10−8 M ethanol was applied for 48 hours on the 7th day.
The third group: Control group. Only the medium was changed at the same time as the groups in which vitamin D and ethonal in which vitamin D was dissolved were applied.
All groups were applied in 6 different petris and the experiments were repeated for three times.
Application of vitamin D
1,25 α-dihydroxyvitamin D3 (Sigma C-9756. Sigma-Alderich Chemie GmbH, Steinheim, GE) was diluted in absolute ethanol to obtain a 10−4 M solution. 1.25 α-dihydroxyvitamin D3 was applied to the petris containing 1.5 ml NBM+ for 48 hours such as a final concentration of 10−8 M was obtained. Following application, the medium was not changed until the media for which the NGF amount would be calculated were collected.
Cytotoxicity Test
Cytotoxicity test was performed by determining the amount of lactate dehydrogenase (LDH) released from the culture cells with damaged membrane or dead cells into the medium. The negative control, low-concentration control and high-concentration control groups which would be used in LDH release experiments were prepared with the other experiments. The negative control from NBM+ was prepared to determine LDH activity in the culture medium. The control groups which were used in each experiment and no substance was applied to were used as low-concentration control group to determine LDH release from the cells under normal conditions. 2% Triton X-100 was given to the culture medium to determine the highest amount of LDH which could be released from the high-concentration control group cells created with 24-hour incubation. At the end of application periods, samples of 500 μl were obtained from the culture media belonging to the control groups and experiment groups for ELISA studies and were kept at −80°C.
LDH release was determined using the Cytotoxicity Detection (LDH) Kit (Roche 11 644 793 001. Roche Diagnostics GmbH Roche Applied Science mannheim, GE) according to the protocol provided by the company.
Measurement of the Amount of NGF
The measurement of the amount of NGF released into the culture medium was performed using Chemikine Nerve Growth FActor (NGF) Sandwich ELISA kit (Chemicon CYT304. Millipore Corp. California, USA) according to the protocol provided by the company.
Statistical analysis
The mean value of 3 absorbance values read for each sample was calculated. The mean value for the negative control was extrapolated from these value and the mean value of the controls. The cytotoxicity percentage of the samples in comparison with the control was calculated using the following Formula and represented in graphic with Microsoft Office Excel software.
Seven different standards with known NGF amount provided by the company were used to determine the amount of NGF released from the high concentration control – low conxentration control cells. The NGF amounts between the groups were calculated with the Formula (R2:.935) obtained from the graphic of distribution drawn using the NGF amount contained by the standards and OD values and the graphic was drawn by converting to percentage NGF amount.
The raw data belonging to each group and to the controls were compared using one way ANOVA method with GraphPad InStat DTCG 3.06 sofware (GraphPad Software, Inc., San Diego USA). A pa value of <.05 was considered statistically significant difference.
Results
The Number of Glial Cells in The Culture
As a result of counting of the neurons and glial cells on 10 pictures taken from random areas of each petri, the number of glial cells were compared with the total cell count and the percentage of the glial cells was calculated to be 20% (Figure 2b.). thus, the culture was defined as neuron-rich culture.
Cytotoxicity results
It was found that the LDH release in the experimental group belonging to the primary hippocampal neuron culture which was applied vitamin D for 48 hours was decreased by 4.9% compared to the control group and this difference was statistically significant (95CI 2.330–6.074; p<.001). The LDH release in the experimental group belonging to the primary hippocampal neuron culture which was applied 10−8 M ethanol for 48 hours was increased by .9% compared to the control group and this difference was not statistically significant (95% CI −0.632–1.104; p>.05).
NGF measurement results
It was found that the NGF release in the experimental group belonging to the primary hippocampal neuron culture which was applied 10−8 M vitamin D for 48 hours was statistically significantly increased compared to the control group (95% CI 43–76; p<.0001) (Figure 3). the NGF release in the experimental group belonging to the primary hippocampal neuron culture which was applied 10-8 M ethanol was increased by 2.7% compared to the control group, but this increase was not statistically significant (95% CI −.921–3.526; p>.05).
Figure 3.
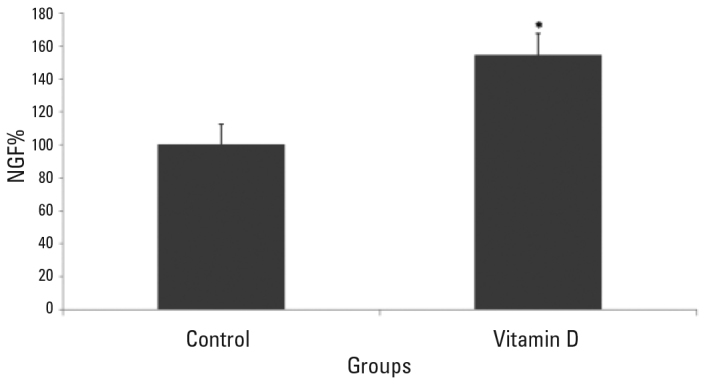
Release of NGF is significantly increased in the primary hippocampal neurons which were administered vitamin D compared to the control group* (p<.0001). The data belonging to the groups are given as mean±SD.
Discussion
According to the hypothesis of neurotrophic factor, the neurons extend their axons up to the target cell and target cells release a limited amount of neurotrophic factors. Neurotrophic factors bind to special cell receptors and the neurons which can nottake sufficient amount of neurotrophic factor die with apoptosis. For example, binding of NGF to its receptor trkA activates a molecule called Bcl-2. In this way, Apaf 1 which is the next molecule şn the apoptotic pathway is supressed and the caspases which would lead to apoptosis remain inactive. In cases where there is no sufficient NGF, this signal will not be received, Bcl-2 cam not become active and Apaf 1 can not be supressed. Thus, the caspases cut by Apaf 1 which remains active are activated and apoptosis occurs (20). Amiloid plaques and neurofibrillary tangles which occur in Alzheimer’s disease lead to damage to the neurons of the cholinergic system. The cholinergic system contain the nerve cells which process information and regulate brain functions. Degeneration of the neurons in this system leads to loss of acetylcholine and markedly increases cortical dementia which is observed in Alzheimer’s disease. Survival of these neurons substantially depends on intake of NGF (21).
Studies which were initiated with reporting that vitamin D regulated the synthesis of nerve growth factor (NGF) showed that vitamin D also regulated synthesis of other neurotrophins in the cells of the nervous system. Vitamin D stimulates the synthesis of NGF, neurotrophin 3 (NT3) and glial cell derived neurotrophic factor (GDNF), while it decreases the synthesis of neurotrophin 4 (NT4) (3,4,6,15). It was shown that production of neurotrophin regulated by vitamin D mediated neuron protection in a limited number of studies (8). The information about the relation between NGF and vitamin D is limited with the studies on glia cells, hippocampal neurons cultured in medium containing neuroblastoma cell lines and serum (6,15,16,17) and cortical neurons (8,9). Among these studies, an important charactersitic of the ones performed on hippocampal neuron culture was the fact that serum was not used in their media (16,17). Since it has been reported that serum containing media contain unknown amounts of growth factors, hormones, vitamins and proteins, use of serum is not recommended especially for neurotrophic factor and vitamin studies (18). It was found that the amount of NGF was increased in these studies performed in hippocampal neuron cultures prepared with serum containing media in accordance with our results. However, it should be kept in mind that the NGF change in these studies may not be related only with vitamin D applied to the culture because of the high possibility that 25-hydroxyvitamin D3 may be present in serum. Therefore, no medium containing serum was used in our study in order to detect the direct effect of vitamin D on NGF.
Our group showed that application of beta amiloid 1-42 which is one of the 2 main pathological structures particiapting in Alzheimer’s disease triggered neurodegeneration by way od a mechanism which toxically increases L-type voltage sensitive calcium channel-A1C (LVSCC-A1C), -A1D (LVSCC-A1D) and NGF levels and decreased VDR expression in culture neurons (8,9,11). Vitamin D applied on this Alzheimer’s like in vitro model protects the neurons by decreasing the expression of LVSCC A1C, LVSCC A1D, preventing decrease in VDR expression and increasng NGF levels (8). On the other hand, the vitamin D deficiency model which was created as a result of inhibition of the VDR gene by VDR siRNA application was reported by our group to function as a model similar to the neurodegeneration mechanisms related with aging (9). Substantially rapidly increasing LVSCC A1C and decreasing NGF in response to short-term vitamin D deficiency showed that the neurons would become unguarded against aging and degeneration with increased calcium, triggering of oxydative stress and decreased neurotrophic factors in cases where deficiency is persistent (9). The fact that the cells gave responses to vitamin D deficiency occuring as a result of inhibition of VDR gene in a similar way such as the cells which were applied beta amiloid suggested that beta amiloid caused to a kind of vitamin D deficiency by supressing VDR (9). In another study of us performed in 2012, it was shown for the first time in the literature that 25 hydroxyvitamin D3-24hydroxilase (24OHaz) enzyme which is considered the marker of use of vitamin D was expressed in the cortical and hippocampal neurons (11). In addition, it was found that 24OHaz enzyme was expressed in the hippocampal neurons with a higher rate compared to the cortical neurons and it was noted that the hippocampus which is a substantially important region for Alzheimer’s disease might need vitamin D to a greater extent (11).
The results obtained in the study presented show that NGF release is regulated by vitamin D in the hippocampal neurons as in the cortical neurons. On the other hand, decrease in LDH in the groups where 10−8 M vitamin D was applied for 48 hours compared to the control group where no substance was applied demonstrates that application of vitamin D prevents even the cellular damage occuring under normal culture conditions in the hippocampal neurons as in the cortical neurons. One of the most important changes observed in Alzheimer’s disease is the fact that the NGF level increases in the brain cortex and hippocampus and decreases in the basal anterior brain. It has been proposed that increased NGF is not transferred to the basal anterior brain because of a decrease in Trk A expression in the cortexand hippocampus (22). Accordingly, atrophy of the cholinergic neurons in AH arises from inability of some neurons to find trophic factor source as a result of disruption of NGF transfer and accumulation of NGF in certain areas (23). Hence, it was shown that vitamin D increased NGF expression and this increase porvided neuron protection in our study. Another affect of vitamin D is increase in the expression of p75 NTR receptors (24). Because of the variety of adaptor proteins which bind to special sites of p75 NTR which is mostly known for its stimulant effects vitamin D can activate the pathways which stimulate or inhibit apoptosis depending on the type and status of the cell (25).
When we interpreted these findings together with the data we obtained in our previous studies, we concluded that prevention of hippocampal involvement and NGF loss of the neurons which are a significant component of Alzheimer’s disease with administration of vitamin D would open new horizons in under- standing of the molecular mehanism of the disease and neurodegeneration and enable development of new approaches in treatment and prevention of the disease.
Acknowldgement
This study was supoorted by TUBITAK with the Project numbered 107S041 and by Istanbul University, BAP Unit with the Project numbered 548.
Footnotes
Conflict of Interest: The authors reported no conflict of interest related to this article.
Çıkar Çatışması: Yazarlar bu makale ile ilgili olarak herhangi bir çıkar çatışması bildirmemişlerdir.
References
- 1.Cekic M, Sayeed I, Stein DG. Combination treatment with progesterone and vitamin d hormone may be more effective than monotherapy for nervous system injury and disease. Front Neuroendocrin. 2009;30:158–172. doi: 10.1016/j.yfrne.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 4.Holick M. Noncalcemic actions of 1,25-dihydroxyvitamin D3 and clinical applications. Bone. 1995;17:107–111. doi: 10.1016/8756-3282(95)00195-j. [DOI] [PubMed] [Google Scholar]
- 5.Kato S, Sekine K, Matsumoto T, Yoshizawa T. Molecular genetics of vitamin D receptor acting in bone. J Bone Miner Metab. 1998;16:65–71. [Google Scholar]
- 6.Wion D, MacGrogan D, Neveu I, Jehan F, Houlgatte R, Brachet P. 1,25 dihydroxivitamin-D3 is a potent inducer of NGF synthesis. J Neurosci Res. 1991;28:110–114. doi: 10.1002/jnr.490280111. [DOI] [PubMed] [Google Scholar]
- 7.McCann J, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 8.Dursun E, Gezen-Ak D, Yilmazer S. A novel perspective for Alzheimer’s disease: vitamin D receptor suppression by Amyloid-β and preventing the Amyloid-β induced alterations by vitamin D in cortical neurons. J Alzheimers Dis. 2011;23:207–219. doi: 10.3233/JAD-2010-101377. [DOI] [PubMed] [Google Scholar]
- 9.Gezen-Ak D, Dursun E, Yilmazer S. The effects of vitamin D receptor silencing on the expression of LVSCC-A1C and LVSCC-A1D and the release of NGF in cortical neurons. PLOS ONE. 2011;6(3):e17553. doi: 10.1371/journal.pone.0017553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gezen-Ak D, Dursun E, Ertan T, Hanagasi H, Gurvit H, Emre M, Eker E, Ozturk M, Engin F, Yilmazer S. Association between vitamin D receptor gene polymorphism and Alzheimer’s disease. Tohoku J Exp Med. 2007;212:275–282. doi: 10.1620/tjem.212.275. [DOI] [PubMed] [Google Scholar]
- 11.Gezen-Ak D, Dursun E, Yilmazer S. Vitamin D inquiry in hippocampal neurons: Consequences of vitamin D-VDR pathway disruption on calcium channel and the vitamin D requirement. Neurol Sci. 2013;34:1453–1458. doi: 10.1007/s10072-012-1268-6. [DOI] [PubMed] [Google Scholar]
- 12.Masoumi A, Goldenson B, Ghirmai S, Avagyan H, Zaghi J, Abel K, Zheng X, Espinosa-Jeffrey A, Mahanian M, Liu PT, Hewison M, Mizwickie M, Cashman J, Fiala M. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloidbeta clearance by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2009;17:703–717. doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- 13.Mizwicki MT, Menegaz D, Zhang J, Barrientos-Durán A, Tse S, Cashman JR, Griffin PR, Fiala M. Genomic and Nongenomic Signaling Induced by 1α,25(OH)2-Vitamin D3 Promotes the Recovery of Amyloid-α Phagocytosis by Alzheimer’s Disease Macrophages. J Alzheimers Dis. 2011;29:51–62. doi: 10.3233/JAD-2012-110560. [DOI] [PubMed] [Google Scholar]
- 14.Annweiler C, Fantino B, Parot-Schinkel E, Thiery S, Gautier J, Beauchet O. Alzheimer’s disease--input of vitamin D with mEmantine assay (AD-IDEA trial): study protocol for a randomized controlled trial. Trials. 2011;12:230. doi: 10.1186/1745-6215-12-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neveu I, Naveilhan P, Jehan F, Baudet C, Wion D, De Luca HF, Brachet P. 1,25-dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res Mol Brain Res. 1994;24:70–76. doi: 10.1016/0169-328x(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 16.Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343:139–143. doi: 10.1016/s0304-3940(03)00303-3. [DOI] [PubMed] [Google Scholar]
- 17.Marini F, Bartoccini E, Cascianelli G, Voccoli V, Baviglia MG, Magni MV, Garcia-Gil M, Albi E. Effect of 1alpha,25-dihydroxyvitamin D3 in embryonic hippocampal cells. Hippocampus. 2010;20:696–705. doi: 10.1002/hipo.20670. [DOI] [PubMed] [Google Scholar]
- 18.Price P, Brewer GJ. Serum-Free Media for Neural Cell Cultures. In: Fedoroff S, Richardson A, editors. Protocols for Neural Cell Culture. 3 ed. New Jersey: Humana Press; 1998. pp. 255–264. [Google Scholar]
- 19.Goslin K, Asmussen H, Banker G. Rat Hippocampal Neurons in Low-Density Culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. 2 ed. Cambridge: MIT Press; 1998. pp. 339–371. [Google Scholar]
- 20.Jessel T, Sanes JR. The generation and survival of nerve cells editor. In: Kandel E, Schwarz JH, Jessel TM, editors. Principals of Neural Science. 4 ed. New York: McGraw-Hill; 2000. pp. 1149–1161. [Google Scholar]
- 21.Tuszynski MH, Hoi Sang U, Alksne J, Bakay RA, Pay MM, Merrill D, Thal LJ. Growth factor gene therapy for Alzheimer disease. Neurosurg Focus. 2002;13:1–5. doi: 10.3171/foc.2002.13.5.6. [DOI] [PubMed] [Google Scholar]
- 22.Capsoni S, Cattaneo A. On the Molecular Basis Linking Nerve Growth Factor (NGF) to Alzheimer’s Disease. Cell Mol Neurobiol. 2006;26:4–6. doi: 10.1007/s10571-006-9112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salehi A, Delcroix JD, Swaab DF. Alzheimer’s disease and NGF signaling. J Neural Transm. 2004;111:323–345. doi: 10.1007/s00702-003-0091-x. [DOI] [PubMed] [Google Scholar]
- 24.Naveilhan P, Neveu I, Baudet C, Funakoshi H, Wion D, Brachet P, Metsis M. 1,25-Dihydroxyvitamin D3 regulates the expression of the low-affinity neurotrophin receptor. Brain Res Mol Brain Res. 1996;41:259–268. doi: 10.1016/0169-328x(96)00103-9. [DOI] [PubMed] [Google Scholar]
- 25.Meldolesi J, Sciorati C, Clementi E. The p75 receptor: first insights into the transduction mechanisms leading to either cell death or survival. TiPS. 2000;21:242–243. doi: 10.1016/s0165-6147(00)01497-8. [DOI] [PubMed] [Google Scholar]


