Abstract
Introduction
Neurodegeneration is a process that is characterized by the loss of neuronal structure and function and eventually ends with neuronal death. An elevated level of inducible nitric oxide synthase (iNOS) is suggested to accompany this process by inducing oxidative and nitrosative damage. Vitamin D is reported to protect glial cells against neurotoxicity via suppressing iNOS synthesis. Though there was no data about whether iNOS is regulated by vitamin D in hippocampal neurons. In this study our aim was to determine any alteration in iNOS expression of hippocampal neurons in response to vitamin D treatment.
Method
Twenty four and 48 hours of vitamin D treatments were performed on primary hippocampal neuron cultures that were prepared from Sprague dawley rat embryos (E18). The alterations in the iNOS mRNA expression were determined with quantative real time polymerase chain reaction (qRT-PCR). The cytotoxicity levels of each group were investigated by the measurement of lactate dehydrogenase (LDH) that is released to culture medium.
Results
No difference was observed between groups in 24 hours of treatment regarding the iNOS expression. Though the iNOS mRNA level of vitamin D treated group was significantly lower than that of control group on the 48th hours of treatment (p<.001). Vitamin D treatment also attenuated the LDH release which is an indicator of cytotoxicity (p<.001).
Conclusion
Our results indicated that vitamin D has the potential to prevent oxidative damage by suppressing iNOS expression.
Keywords: Alzheimer’s disease, vitamin D, inducible nitric oxide synthase (iNOS), primary hippocampal neuron culture
ÖZET
Amaç
Nörodejenerasyon, sinir hücrelerinin yapı ve fonksiyonlarını kaybetmesine bağlı olarak nöron ölümü ile sonuçlanan bir süreçtir. Bu süreçte artan indüklenebilir nitrik oksit sentaz (iNOS) seviyelerinin, hücrelerin oksidatif ve nitrozatif hasarına sebep olarak nörodejenerasyona eşlik ettiği düşünülmektedir. Vitamin D’nin ise glia hücrelerinde iNOS sentezini baskılayarak hücreleri nörotoksisiteye karşı koruduğu ileri sürülmektedir. Ancak iNOS’un hippokampal nöronlarda vitamin D tarafından düzenlenip düzenlenmediğini gösteren herhangi bir çalışma yoktur. Bu çalışmadaki amacımız, hippokampal nöronların vitamin D uygulamasına cevap olarak iNOS anlatımlarında bir değişiklik olup olmadığını belirlemektir.
Yöntem
Sprague dawley cinsi sıçanların 18 günlük embriyolarının hippokampuslarından hazırlanan primer nöron kültürlerine 24 ve 48 saat süre ile vitamin D uygulandı. iNOS mRNA miktarlarındaki değişimler kantitatif gerçek zamanlı polimeraz zincir reaksiyonu (qRT-PCR) yöntemi ile belirlendi. Tüm grupların sitotoksisite seviyeleri kültür medyumuna salınan laktat dehidrogenazın (LDH) ölçülmesi ile belirlendi.
Bulgular
Yirmi dört saat boyunca vitamin D uygulanan grupla diğer gruplar arasında herhangi bir iNOS anlatımı açısından bir fark gözlenmezken, 48 saat süreyle vitamin D uygulanan hippokampal nöronların iNOS mRNA seviyelerinin kontrol gruplarına kıyasla anlamlı derecede düştüğü saptandı (p<0,001). Ayrıca, vitamin D uygulamasının sitotoksiste belirteci olan LDH salınımını da azalttığı saptandı (p<0,001).
Sonuç
Sonuçlarımız, vitamin D’nin iNOS anlatımındaki artışı engelleyerek, hippokampal nöronları oksidatif hasara karşı koruyabileceğini göstermektedir.
Introduction
The studies focusing on cognition indicated the hippocampus and temporal lobes as the sites for learning and memory (1). Hippocampus is very important for cognitive functions and is one of the sites that is effected earliest in Alzheimer’s disease (AD) (2). AD is the most frequent reason for dementia in the elderly (3,4) and this particular neurodegenerative disease is also accompanied by neuroinflammation (5). This inflammation in the brain is determined by astrogliosis, microgliosis and the alterations in the acute phase proteins (6). Elevation of the levels of inducible nitric oxide synthase (iNOS) due to the inflammation was reported in the brains of AD patients in various studies (5,7,8,9,10,11,12,13).
Vitamin D (1,25-dihydroxyvitamin D3) is a secosteroid hormone and recently named as a “neurosteroid”. The relation between vitamin D and neurodegeneration becomes a matter of interest in recent years. The insufficiency or deficiency of 25-hydroxyvitamin D3, the most common form of vitamin D in circulation was reported in AD, mood disorders, Parkinson’s disease (PD) and cognitive decline (14,15,16,17,18,19,20,21). Vitamin D supplementation was reported to induce axonogenesis (22), prevent amyloid beta 1–42 induced cytotoxicity (23), favor amyloid beta phagocytosis and clearance (24,25,26). Besides vitamin D regulated nerve growth factor (NGF) levels in cortical neurons and glial cells (27,28).
Vitamin D receptor (VDR) mediates cellular action of vitamin D and is a member of steroid hormone receptor superfamiliy. Our previous studies demonstrated the genetic relation between VDR and AD for the first time (29). We recently reported that “TaubF” haplotype of VDR gene increases the risk of devoloping AD (30). Our results was supported by other studies demonstrating the associaiton between VDR polymorphisms and cognitive decline (31,32), AD (33,34) and PD (35,36).
Present studies indicate that vitamin D may play a crucial role in mechanisms of neurodegeneration. Within this perspective, our aim was to determine whether the expression of iNOS, one of the major actors of oxidative damage, is regulated via vitamin D in primary hippocampal neurons.
Methods
Primary hippocampal neuron cultures
Neuronal cultures were prepared from the hippocampi of embryonic day 18 (E18) Sprague Dawley rat embryos as previously described in Goslin and Baker’s (37) and in our study (38). The cells were dissociated mechanically. The cells were plated onto poly-L-ornithine (Sigma P-4957. Sigma-Alderich Chemie GmbH, Steinheim, GE) covered 6-well plates (Corning 3506. Corning Inc. New York, USA)at a density of 6×105 per dish in Leibovitz 15 (GibcoBRL 11415-064) containing 0.1 mg/ml conalbumin (Sigma C-7786), 0.63 mg/ml sodium bicarbonate (GibcoBRL 25080-094), 0.1 mM putrescine (Sigma P-7505), 10 ng/ml insulin (GibcoBRL 12585), 30nM sodium selenite (Sigma S-5261), 20nM progesterone (Sigma P-6149), 20mM glucose (Sigma G-7021), 10 IU/ml Penicillin-Streptomycin (PenStrep, Sigma P-4333) and incubated for a day at 37°C and 5% CO2 in a humidified atmosphere. The next day L15+ medium replaced with neurobasal medium, NBM (GibcoBRL 21103-049), containing 1:50 B-27 (GibcoBRL 17504-044), 10 IU/ml PenStrep, 7 of 9% NaCl2 (Sigma S-3014). Cells were incubated at 37°C in 5% CO2 humidified atmosphere for 7 days until the neurons extend their neurites and became mature (Figure 1) and half of the medium was changed once per 3 days. The neuron/glia ratio of the cultures was determined by immunofluorescent labeling with neuronal (Millipore MAB2300. Millipore Corp. California, USA) and glial (Invitrogen AB5804. Invitrogen Inc., New York, USA) markers and nuclei staining DAPI (4’,6-Di-amidino- 2-Phenylindole) using The Leica Application Suite Image Overlay Software. The glia ratio to total cell number of the cultures were 20% (Figure 2). Thus the cultures were determined as neuron-rich cultures. The study was approved by the Animal Welfare and Ethics Committee of Istanbul University with the number of 11608/17.05.2006 and the procedures involving experimentation on animal subjects are done in accordance with both the guide of the Istanbul University, and with the National Research Council’s guide for the care and use of laboratory animals.
Figure 1.
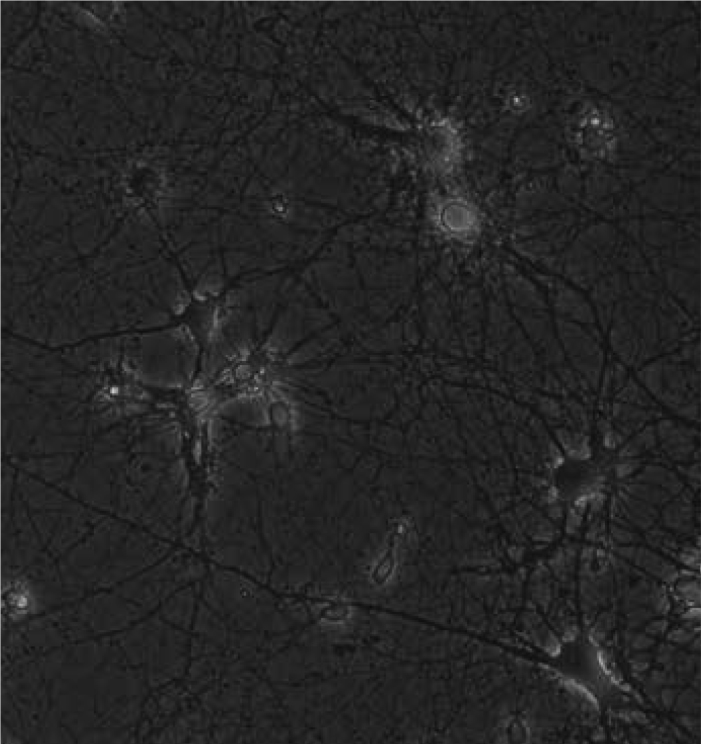
Phase-contrast image of seven-day-old primary hippocampal neurons ×20
Figure 2. Seven-day-old primary hippocampal neurons.
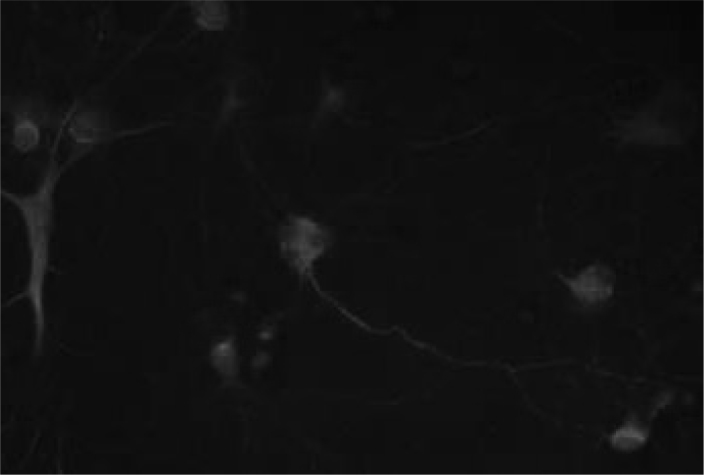
Neurons are green (FITC-labeled PAN neuronal marker antibody), glia are red (Texas Red-labeled GFAP antibody), the nuclei are blue (DAPI). Glia ratio in cultured cells was 20%. ×40.
Experimental design
On the 7th day of culture 3 groups were established: Vitamin D treated group; On the 7th day, 10−8 M 1,25 dihidroksivitamin D3 (calcitriol, 1,25-dihydroxycholecalciferol, Sigma C-9756. Sigma- Alderich Chemie GmbH, Steinheim, GE) which dissolved in absolute ethanol was administrated to the neurons for 24 and 48 hours. Ethanol treated grup; On the 7th day, 10−8 M absolute ethanol was administrated to the neurons for 24 and 48 hours. Untreated control group; On the 7th day, culture media was changed at the same time with other groups but no treatment was performed. Culture media was not changed until RNA isolations. Each group was established in 6 individual dishes. The experiments were repeated three times.
Total RNA isolation and cDNA syhthesis from cultured neurons
Total RNA isolation from cultured neurons were performed with RNA isolation kit (High Pure RNA isolation kit Roche 11 828 665 001. Roche Diagnostics GmbH Roche Applied Science Mannheim, GE) according to the manufacturer’s protocol. Concentration of RNAs were determined with NanoDrop- TM 1000 (Thermo Fisher ScientificTM Delaware, USA). Equal amount of total RNA (400 ng) for each group were used for cDNA sythesis (Transcriptor First Strand cDNA Synthesis Kit, Roche 04 379 012 001. Roche Diagnostics GmbH Roche Applied Science Mannheim, GE).
Quantitative real time polymerase chain reaction (qRT-PCR)
After the cDNA synthesis the changes in the mRNA levels of the target genes were investigated with qRT-PCR. Experiments were performed on Roche LIGHTCYCLER 480 (Roche Applied BiosystemsTM, California, USA), using UPL probes and Light-cycler 480 Probe Master Mix kit (Roche 04707494001, Roche Applied BiosystemsTM, California, USA). Relative expression levels of iNOS (NOS2- NM_012611.3 UPL Probe #128, Roche 04693647001) mRNA was determined after the normalization of the data with 3 reference (housekeeping) genes Actin Beta (ACTB) (Universal Probe Library (UPL) Rat ACTB Gene Assay, 05046203001 Roche Applied BiosystemsTM, California, USA), Glyceraldehyde 3 phosphate dehydrogenase (GAPDH)(UPL Rat GAPDH Gene Assay, 05046203001 Roche Applied BiosystemsTM, California, USA) and hypoxanthine guanine phosphoribosyl transferase (HPRT) (Rat HPRT NM_012583.2 UPL Probe #95, 04692128001 Roche Applied BiosystemsTM, California, USA). Reaction mixture excluding cDNA template was utilized as a negative control. Each PCR amplification was performed in triplicate.
Cytotoxicity
The amount of lactate dehydrogenase (LDH) released into the culture medium which represents the level of the cytotoxicity in all groups was determined with Cytotoxicity Detection kit (Roche 11 644 793 001. Roche Diagnostics GmbH Roche Applied Science Mannheim, GE) by ELISA according to the manufacturer’s protocol.
Statistical analysis
Every ELISA sample was read for three times and the mean values were used for percentage calculations. Mean value of negative control is subtracted from the mean values of samples and control groups. Cytotoxicity levels of the samples compared to control group were calculated according to the formula given below and Microsoft Office Excel was used for graphics.
Ct values obtained from qRT-PCR were used on the formula given below for determining the relative expression levels of the target genes and Microsoft Office Excel was utilized for calculations and graphics. ΔCt= 2 (Geometric mean of reference genes − Ct target gene) More sophisticated analyze for qRT-PCR results was done with “Relative Expression Software Tool, REST 2008” (Corbett Research Pty LtD and Michael Pfaffl, New South Wales, AU) whose algorithm has PCR efficiency, normalization factor and 95% confidence interval calculation.
Raw data of each group analyzed on GraphPad InStat DTCG 3.06 (GraphPad Software, Inc. San Diego USA) with oneway ANOVA method and p<0,05 mean statistically significant.
Results
Cytotoxicity
Lactate dehydrogenase (LDH) release which is also a marker for oxidative stress related cell death attenuated significantly in primary hippocampal neurons treated with vitamin D for 48 hours when compared with untreated control group (p<.001). On the other hand there was no difference for the LDH release between 10−8 M ethanol treated primary hippocampal neurons and untreated control group after 48 hours of treatment.
iNOS mRNA expression
No significant difference was observed for iNOS mRNA levels of any group in 24 hours of treatment (Figure 3). iNOS mRNA level of 10−8 M vitamin D treated group was significantly decreased in 48 hours of treatment compared to the untreated control group (p<.001) (Figure 3). There was no difference for the iNOS mRNA levels between 10−8 M ethanol treated primary hippocampal neurons and untreated control group after 48 hours of treatment.
Figure 3. iNOS mRNA expression of 24 and 48 hours vitamin D treated hippocampal neurons.
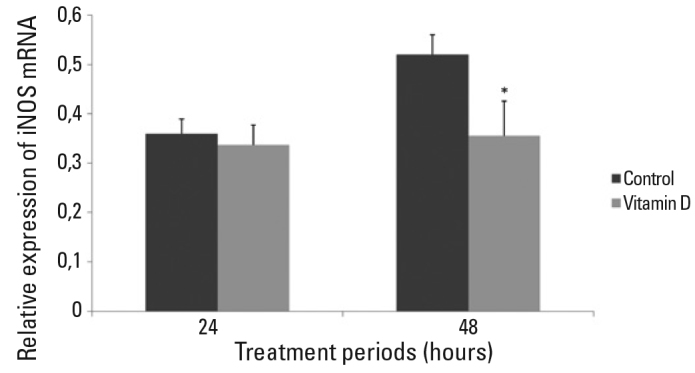
There was no statistically significant difference between vitamin D treated group and control group in 24 hours of treatment (p>0.05). iNOS mRNA level was significantly decreased in vitamin D treated group in 48 hours of treatment when compared with control group (*p<.001). Data were given as mean and SD.
Discussion
Neurodegeneration is accompanied by the immune response in neurodegenerative disorders like Alzheimer’s disease or multiple sclerosis. Due to this information these neurodegenerative disorders are also defined as “inflammatory brain disorders” besides being defined as progressive neuro-degenerative diseases (5,7). This particular immune response reveals itself by the elevation of cytokines and chemokines due to the induction of amyloid beta aggregations (7,8,9). While contributing to the immune response, the elevation of interleukin 1 β (IL1 β) and tumor necrosis factor alpha (TNF α) levels were demonstrated to induce the iNOS expression (39).
iNOS has been thought to cause neuronal damage and death by catalyzing NO production and NO-mediated neuronal damage and death is explained by the inhibition of mitochondrial cytochrome oxidases by NO over-production (40,41,42). The inhibition of mitochondrial cytochrome oxidases inhibit neuronal respiration and cause neuronal depolarization and glutamate release, followed by excitotoxicity via the N-methyl- D-aspartate (NMDA) receptors (41,42). Inhbition of iNOS was reported to attenuate the two major components of AD type pathology, amyloid beta and hyperphosphorylated tau aggregations (5).
Given its ability to regulate the mechanisms that control oxidative stress, intracellular calcium levels, immune response and neurotrophic factor synthesis, we suggest that the vitamin D might play a role in the mechanisms that cause neurodegenerative disorders and might also be a key molecule that suppresses neurodegenerative mechanisms and regulates the altered expressions of the related proteins (20,23,27,38,43,44).
In the peresent study, although it was statistically not significant, iNOS mRNA level of 24 hours vitamin D treated neurons was attenuated compared to the untreated control group. On the other hand, low levels of iNOS expression and LDH relase, which is also an indicator of oxidative stress related cell death, were both demonstrated in the conventional culture conditions in 48 hours of vitamin D treated hippocampal neurons. Besides vitamin D maintained iNOS expression in a precise level and did not let it to be elevated in those neurons. Our results are in accordance with our recent study which demonstrated dependence of iNOS gene expression to vitamin D-VDR pathway in cortical neurons. Vitamin D treatment attenuated the iNOS expression in cortical neurons also in 48 hours of treatment (45). In that study, we demonstrated with the siRNA mediated gene silencing experiments that this action of vitamin D is mediated trough VDR, not via 1,25-MARRS, vitamin D membrane receptor (45). Although the effect of vitamin D on iNOS was suggested to be mediated with cytokines, the fact that iNOS gene has VDR response element (VDRE) on it suggest that this action is more likely to be a direct effect (46,47). Attenuation of iNOS expression and the cytotoxicity after 48 hours of vitamin D treatment in the present study indicates the presence of similar mechanisms in hippocampal neurons. Regarding these results vitamin D might be considered as one of the ways to deal with the oxidative stress mechanisms that occur in the neurodegenerative disorders like Alzheimer’s disease.
Our previous study demonstrated that vitamin D suppressed beta amyloid induced cytotoxicity by suppressing the expression of L-type voltage sensitive calcium channel A1C (LVSCC A1C), inducing VDR epression and NGF release in cortical neurons (23). Another interesting result was beta amyloid 1–42 has a potential to attenuate the utilization of vitamin D by suppressing the expression of VDR (23). Elevation of LVSCC A1C protein in response to vitamin D-VDR pathway disruption by VDR siRNAs was observed in both hippocampal and cortical neurons (27,38). Higher expression of this channel was known to be associated with cell death related to disrupted calcium homeostasis in aging and neurodegeneration (48). Besides vitamin D-VDR pathway disruption by VDR siRNAs was demononstrated to mimic the beta amyloid induced toxicity in cortical neurons (23,27). We reported the presence of 25-hydroxyvitamin D3 24-hydroxylase (24OHase) enzyme which is an indicator of vitamin D utilization in cortical and hippocampal neurons in the literature for the first time (38). The study also pointed out higher requirement of vitamin D in hippocampus which is an important brain region especially for Alzheimer’s disease, given the higher expression of 24OHase in hippocampal neurons when compared with cortical ones (38). Supporting studies reported that vitamin D strongly induces phagocytosis and clearence of beta amyloid by macrophages of AD patients and protects neurons against apoptosis (24,25).
In 2011, Annweiler and Beauchet published a new AD treatment protocol (AD-IDEA) combining memantine, a drug used in AD treatment and vitamin D (49), and reported that 6 months of memantine+vitamin D supplementation incresed mini mental status examination (MMSE) scores by 4 points when compared with only memantine treated patients (50). The fact that vitamin D regulates iNOS, a major component of oxidative damage in hippocampal neurons which are crucial for cognitive functions, indicates its importance in Alzheimer’s disease. Although they are very limited in number, all of these recent studies underline the importance of vitamin D, a molecule synthesized from sun in prevention of neurodegeneration.
Acknowledgement
The present work was supported by the Scientific and Technological Research Council of Turkey- TUBITAK (Project No. 107S041) and by the Research Fund of Istanbul University (Project No. 548).
Footnotes
Conflict of Interest: The authors reported no conflict of interest related to this article.
Çıkar Çatışması: Yazarlar bu makale ile ilgili olarak herhangi bir çıkar çatışması bildirmemişlerdir.
References
- 1.Squire L. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 2.Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Molecular Neurodegeneration. 2011;6:85–94. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy J. Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 4.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 5.Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, Chen J, Vodovotz Y, Kipiani K, Beal MF. Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med. 2005;202:1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Moore AH, O’Banion MK. Neuroinflammation and anti-inflammatory therapy for Alzheimer’s disease. Adv Drug Deliv Rev. 2002;54:1627–1656. doi: 10.1016/s0169-409x(02)00162-x. [DOI] [PubMed] [Google Scholar]
- 8.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom PEM, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti GP-SC, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss- Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YB, Nagai A, Kim SU. Cytokines, chemokines and cytokine receptors in human microglia. J Neurosci Res. 2002;69:94–103. doi: 10.1002/jnr.10253. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Vizarra P, Fernandez AP, Castro-Blanco S, Encinas JM, Serrano J, Bentura ML, Munoz P, Martinez-Murillo R, Rodrigo J. Expression of nitric oxide system in clinically evaluated cases of Alzheimer’s disease. Neurobiol Dis. 2004;15:287–305. doi: 10.1016/j.nbd.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Heneka MT, Wiesinger H, Dumitrescu-Ozimek L, Riederer P, Feinstein DL, Klockgether T. Neuronal and glial coexpression of arginino-succinate synthetase and inducible nitric oxide synthase in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:906–916. doi: 10.1093/jnen/60.9.906. [DOI] [PubMed] [Google Scholar]
- 12.Luth HJ, Munch G, Arendt T. Aberrant expression of NOS isoforms in Alzheimer’s disease is structurally related to nitrotyrosine formation. Brain Res. 2002;953:135–143. doi: 10.1016/s0006-8993(02)03280-8. [DOI] [PubMed] [Google Scholar]
- 13.Wong A, Luth HJ, Deuther-Conrad W, Dukic-Stefanovic S, Gasic-Milenkovic J, Arendt T, Munch G. Advanced glycation endproducts co-localize with inducible nitric oxide synthase in Alzheimer’s disease. Brain Res. 2001;920:32–40. doi: 10.1016/s0006-8993(01)02872-4. [DOI] [PubMed] [Google Scholar]
- 14.Oudshoorn C, Mattace-Raso FUS, van der Velde N, Colin EM, van der Cammen TJM. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25:539–543. doi: 10.1159/000134382. [DOI] [PubMed] [Google Scholar]
- 15.Annweiler C, Schott AM, Allali G, Bridenbaugh SA, Kressig RW, Allain P, Herrmann FR, Beauchet O. Association of vitamin D deficiency with cognitive impairment in older women: cross-sectional study. Neurology. 2010;74:27–32. doi: 10.1212/WNL.0b013e3181beecd3. [DOI] [PubMed] [Google Scholar]
- 16.Cherniack EP, Florez H, Roos BA, Troen BR, Levis S. Hypovitaminosis D in the elderly: from bone to brain. J Nutr Health Aging. 2008;12:366– 373. doi: 10.1007/BF02982668. [DOI] [PubMed] [Google Scholar]
- 17.Evatt ML, DeLong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of vitamin D Insufficiency in patients with Parkinson Disease and Alzheimer Disease. Arch Neurol. 2008;65:1348–1352. doi: 10.1001/archneur.65.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llewellyn DJ, Langa KM, Lang IA. Serum 25-hydroxyvitamin D concentration and cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22:188–195. doi: 10.1177/0891988708327888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, Ferrucci L, Melzer D. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCann J, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 21.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriat Psychiat. 2006;14:1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 22.Chabas JF, Alluin O, Rao G, Garcia S, Lavaut MN, Risso JJ, Legre R, Magalon G, Khrestchatisky M, Marqueste T, Decherchi P, Feron F. Vitamin D2 potentiates axon regeneration. J Neurotrauma. 2008;25:1247–1256. doi: 10.1089/neu.2008.0593. [DOI] [PubMed] [Google Scholar]
- 23.Dursun E, Gezen-Ak D, Yilmazer S. A novel perspective for Alzheimer’s disease: vitamin D receptor suppression by Amyloid-β and preventing the Amyloid-β induced alterations by vitamin D in cortical neurons. J Alzheimers Dis. 2011;23:207–219. doi: 10.3233/JAD-2010-101377. [DOI] [PubMed] [Google Scholar]
- 24.Masoumi A, Goldenson B, Ghirmai S, Avagyan H, Zaghi J, Abel K, Zheng X, Espinosa-Jeffrey A, Mahanian M, Liu PT, Hewison M, Mizwickie M, Cashman J, Fiala M. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2009;17:703–717. doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- 25.Mizwicki MT, Menegaz D, Zhang J, Barrientos-Durán A, Tse S, Cashman JR, Griffin PR, Fiala M. Genomic and Nongenomic Signaling Induced by 1α,25(OH)2-Vitamin D3 Promotes the Recovery of Amyloid-α Phagocytosis by Alzheimer’s Disease Macrophages. J Alzheimers Dis. 2011;29:51–62. doi: 10.3233/JAD-2012-110560. [DOI] [PubMed] [Google Scholar]
- 26.Fiala M, Mizwicki MT. Neuroprotective and immune effects of active forms of vitamin D3 and docosahexaenoic acid in Alzheimer disease patients. Functional Foods in Health and Disease. 2011;12:545– 554. [Google Scholar]
- 27.Gezen-Ak D, Dursun E, Yilmazer S. The effects of vitamin D receptor silencing on the expression of LVSCC-A1C and LVSCC-A1D and the release of NGF in cortical neurons. PLOS ONE. 2011;6(3):e17553. doi: 10.1371/journal.pone.0017553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J, Gattoni-Celli M, Zhu H, Bhat NR, Sambamurti K, Gattoni-Celli S, Kindy MS. Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J Alzheimers Dis. 2011;25:295–307. doi: 10.3233/JAD-2011-101986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gezen-Ak D, Dursun E, Ertan T, Hanagasi H, Gurvit H, Emre M, Eker E, Ozturk M, Engin F, Yilmazer S. Association between vitamin D receptor gene polymorphism and Alzheimer’s disease. Tohoku J Exp Med. 2007;212:275–282. doi: 10.1620/tjem.212.275. [DOI] [PubMed] [Google Scholar]
- 30.Gezen-Ak D, Dursun E, Bilgiç B, Hanağasi H, Ertan T, Gürvit H, Emre M, Eker E, Ulutin T, Uysal Ö, Yilmazer S. Vitamin D Receptor Gene Haplotype Is Associated with Late-Onset Alzheimer’s Disease. Tohoku J Exp Med. 2012a;228:189–196. doi: 10.1620/tjem.228.189. [DOI] [PubMed] [Google Scholar]
- 31.Kuningas M, Mooijaart SP, Jolles J, Slagboom PE, Westendorp RGJ, Van Heemst D. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. 2009;30:466–473. doi: 10.1016/j.neurobiolaging.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Beydoun MA, Ding EL, Beydoun HA, Tanaka T, Ferrucci L, Zonderman AB. Vitamin D receptor and megalin gene polymorphisms and their associations with longitudinal cognitive change in US adults. Am J Clin Nutr. 2012;95:163–178. doi: 10.3945/ajcn.111.017137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmann DJ, Refsum H, Warden DR, Medway C, Wilcock GK, Smith DA. The vitamin D receptor gene is associated with Alzheimer’s disease. Neurosci Let. 2011;504:79– 82. doi: 10.1016/j.neulet.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 34.Beecham GW, Martin ER, Li YJ, Slifer MA, Gilbert JR, Haines JL, Pericak-Vance MA. Genome-wide Association Study Implicates a Chromosome 12 Risk Locus for Late-Onset Alzheimer Disease. Am J Hum Genet. 2009;84:35–43. doi: 10.1016/j.ajhg.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler MW, Burt A, Edwards TL, Zuchner S, Scott WK, Martin ER, Vance JM, Wang L. Vitamin D receptor gene as a candidate gene for Parkinson disease. Ann Hum Genet. 2011;75:201–210. doi: 10.1111/j.1469-1809.2010.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han X, Xue L, Li Y, Chen B, Xie A. Vitamin D receptor gene polymorphism and its association with Parkinson’s disease in Chinese Han population. Neurosci Lett. 2012;525:29–33. doi: 10.1016/j.neulet.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 37.Goslin K, Asmussen H, Banker G. Rat Hippocampal Neurons in Low-Density Culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. 2 ed. Cambridge: MIT Press; 1998. pp. 339–371. [Google Scholar]
- 38.Gezen-Ak D, Dursun E, Yilmazer S. Vitamin D inquiry in hippocampal neurons: Consequences of vitamin D-VDR pathway disruption on calcium channel and the vitamin D requirement. Neurol Sci. 2012b. [DOI] [PubMed]
- 39.Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric- oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TN-Falpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase- dependent signaling mechanism. J Biol Chem. 2000;275:7918–7924. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- 40.Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21:6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans. 2007;35:1119–1121. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- 42.Jekabsone A, Neher JJ, Borutaite V, Brown GC. Nitric oxide from neuronal nitric oxide synthase sensitises neurons to hypoxia-induced death via competitive inhibition of cytochrome oxidase. J Neurochem. 2007;103:346–356. doi: 10.1111/j.1471-4159.2007.04765.x. [DOI] [PubMed] [Google Scholar]
- 43.Cekic M, Sayeed I, Stein DG. Combination treatment with progesterone and vitamin d hormone may be more effective than monotherapy for nervous system injury and disease. Front Neuroendocrin. 2009;30:158–172. doi: 10.1016/j.yfrne.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 45.Dursun E, Gezen-Ak D, Yilmazer S. A New Mechanism for Amyloid- β Induction of iNOS: Vitamin D-VDR Pathway Disruption. J Alzheimers Dis. 2013;36:xx. doi: 10.3233/JAD-130416. [DOI] [PubMed] [Google Scholar]
- 46.Fernandes de Abreu DA, Eyles D, Féron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009;34:265–277. doi: 10.1016/j.psyneuen.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 47.Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med. 2007;13:117–124. doi: 10.1016/j.molmed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Annweiler C, Fantino B, Parot-Schinkel E, Thiery S, Gautier J, Beauchet O. Alzheimer’s disease--input of vitamin D with mEmantine assay (AD-IDEA trial): study protocol for a randomized controlled trial. Trials. 2011;12:230. doi: 10.1186/1745-6215-12-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Annweiler C, Herrmann FR, Fantino B, Brugg B, Beauchet O. Effectiveness of the combination of memantine plus vitamin D on cognition in patients with Alzheimer disease: a pre-post pilot study. Cogn Behav Neurol. 2012;25:121–127. doi: 10.1097/WNN.0b013e31826df647. [DOI] [PubMed] [Google Scholar]


