Abstract
Introduction
Persistent depressive disorder (PDD) introduced in the Diagnostic and Statistical Manual of Mental Disorders (DSM) 5 as a novel diagnostic category represents a consolidation of two separate DSM-IV categories, chronic major depressive disorder (MDD) and dysthymic disorder. The present study aims to investigate the frequency and clinical as well as socio-demographic correlates of PDD in comparison with those of episodic MDD among patients seeking treatment for depressive symptoms.
Methods
Participants were 140 depressive out-and in-patients under treatment at the psychiatry clinic of the Adnan Menderes University Research Hospital. Each patient was assessed by means of a structured clinical interview (SCID-I) and relevant psychometric instruments including the Hamilton Depression Inventory and Eskin Suicidal Behavior Inventory.
Results
Among the depressive patients, 61% fulfilled the criteria for PDD and 39% for episodic MDD. As compared with patients with episodic MDD, the PDD patients were older (d=.54), lower in educational attainment (d=.55), more likely to have comorbid generalized anxiety disorder (OR=3.7), and more prone to report symptoms of anxiety, hopelessness, pessimism, and somatic complaints. Nevertheless, the PDD patients displayed heterogeneous characteristics with respect to clinical severity and suicidal behavior.
Conclusion
Our findings suggest that majority of depressive patients, including those fulfilling the criteria for MDD, have been suffering from a persistent ailment rather than an episodic disorder. Clinicians with a cross-sectional perspective are more likely to diagnose MDD, whereas those with a longitudinal perspective are more likely to identify PDD in the majority of depressive patients. The incorporation of both of these perspectives into DSM-5 in a complementary manner will possibly enhance our insight into depressive disorders and improve our treatment results.
Keywords: Depression, dysthymic disorder, double depression, prevalence
INTRODUCTION
Until the Diagnostic and Statistical Manual of Mental Disorders (DSM) III was published in 1980, the most common diagnosis was neurotic depression. The development of DSM-III involved meticulous reduction of psychoanalytic terminology to constitute an atheoretical diagnostic guide. During this process, long discussions resulted in the final decision to exclude neurotic depression as a diagnostic category and to create two new categories to replace it: dysthymia and generalized anxiety disorder (GAD). Eventually, the diagnostic categories of major depressive episode (MDE) and major depressive disorder (MDD), introduced with DSM-III, have led to a universal perception that depression is an episodic disease in general (1).
In reality, depression is a disease not always with an episodic course but with a chronic course in some patients. A well-known example is the emergence of MDEs in dysthymic patients. DSM-III and DSM-IV, however, instructed clinicians involved with such cases to use codes of two separate diagnoses as if these patients had two separate diseases. Klein et al. (2,3,4,5,6) conducted rigorous longitudinal studies on such a group of patients and suggested that only one disease with a fluctuating course existed in these patients and that this disease should be referred to as chronic depression. The authors conceptualized “chronicity” and “severity” as two independent dimensions to describe the clinical features of depression. In other words, they suggested that the course of chronic depression is mild in some cases (as in pure dysthymia), severe (as in chronic MDD), or fluctuates (as in dysthymia with occasionally superimposed MDEs) in some others.
The proposal for the introduction of a novel diagnostic category of chronic depression consisting of dysthymia, double depression, and chronic MDD (6) was considered and accepted during the development of DSM-5. However, the new diagnostic category was named as persistent depressive disorder (PDD) instead of chronic depressive disorder. The DSM-5 diagnostic criteria for PDD starts with a brief elucidation that this disorder represents consolidation of chronic MDD and dysthymia defined in DSM-IV. The first three criteria for PDD are the same as the DSM-IV criteria for dysthymia. Requirement for diagnosis in criterion A is depressed mood that occurs for most of the day, for more days than not, and for at least 2 years (1 year for children/adolescents). Requirement for diagnosis in criterion B requires the presence of at least two of the following symptoms during periods of depressed mood: loss of appetite or over-eating, insomnia or hypersomnia, lack of energy or fatigue, low self-esteem, difficulties in gathering attention or decision-making, and hopelessness and that in criterion C is that during this 2-year period (1 year in children/adolescents), an individual has never been without the symptoms in criteria A and B for more than 2 months at a time. Criterion D affirms that criteria for MDD may be continuously present for two years, thus giving way to a diagnosis of PDD in patients who would have been diagnosed with chronic MDD previously.
In DSM-5, four different clinical types are described to be related to the course of PDD over the last two years: (1) with pure dysthymic syndrome, (2) with persistent MDE, (3) with intermittent MDEs with a current episode, and (4) with intermittent MDEs without a current episode. Given that PDD as a diagnostic category covers all these situations, the suggestions of Klein et al. seem to be entirely reflected in the DSM-5 classification system, except for the name of the category. Accordingly, the content of the MDD diagnostic category has been constricted in DSM-5 to include only those patients with episodic depression.
The present study aims to investigate in patients under treatment at our department for depressive complaints, (1) the frequency of those with unremitting complaints for the last 2 years (1 year for adolescents) and (2) the extent to which patients complying with the DSM-5 criteria for PDD comprise a homogenous group differing from an episodic depression group with respect to certain clinical and socio-demographic variables. Our data is thought to enhance information pertaining to the prevalence of PDD as a novel diagnostic category and to the clinical and socio-demographic characteristics associated with it.
METHODS
The present study was designed during the DSM-5 development process, and following approval by the Ethics Committee of Adnan Menderes University School of Medicine (Protocol No. 2011/042), it was conducted at the inpatient and outpatient psychiatry clinics of the university hospital.
Participants
The sample size was computed in line with our goal to examine clinical and demographic characteristics differentiating the patients with PDD from those with episodic depressive disorder. Because of source and time limitations, we decided to seek after medium effect size (Cohen’s d≥.50 or Cramer’s Φ≥.40) while estimating the required sample size. To detect the medium or larger differences between the persistent depression and episodic depression groups with sufficient power (80%) and type 1 error rate α less than .05, the minimum number of patients in each group was estimated at 49 (7). In accordance with this estimation, data collection phase was continued until at least this number was reached in each group.
Consequently, a total of 140 patients being treated for depressive complaints at adult psychiatry clinics and volunteering to participate by giving written consent were enrolled in this study. Patients with mental retardation, psychotic disorders, delirium, dementia, or amnestic syndrome and those rejecting to give written consent were excluded from the study. Because dysthymia is a disease that often begins during adolescence (3), patients between 16 and 71 (mean 37.9±13.2) years were included. Majority of the patients were females (n=113) and a minority were males (n=27). With respect to marital status, 36 patients were single, 80 were married, 13 were divorced, 6 were widowed, and 4 were separate.
Materials
DSM-IV Structured Interview for Axis-I Disorders (SCID-I)
This was developed by First et al. (8) as adjusted to DSM-IV diagnoses and was translated into Turkish by Çorapçıoğlu et al. (9) who also examined the reliability of the instrument and published a user’s guide in Turkish.
Hamilton Depression Rating Scale (HDRS)
This is a clinician-rated instrument used to evaluate the severity of depression (10). It consists of 17 items addressing depression symptoms experienced in the last week. Because HDRS was initially developed for patients who were hospitalized, it is a scale particularly focusing on the melancholic and physical symptoms of depression. The items related to difficulty in falling asleep, waking up at midnight or early in the morning, somatic symptoms, genital symptoms, weight loss, and insight are rated on a scale between 0 and 2, whereas the other items are rated on a scale between 0 and 4. The highest possible score to be yielded by the inventory being 53, the scores ranging from 0 to 7 are indicative of no depression; from 8 to 15, mild depression; from 16 to 28, moderate depression; and 29 or higher, severe depression. The validity and reliability of the Turkish translation of the inventory was tested by Akdemir et al. (11).
Eskin Suicidal Behavior Inventory
This index consists of five questions, two query suicidal attempt (lifetime and within the last year) and three querying suicidal thoughts (lifetime, in the last year, and present time). Hence, it reveals two composite scores, one indicating the overall prevalence rate of suicidal thoughts and the other indicating that of suicidal attempts (12). Only the composite rates were reported and discussed in this article because of space limitations.
Statistical Analysis
The patients who participated in the study were divided into four groups in accordance with the DSM-IV criteria (Figure 1): the episodic major depression (EMD) group consisting of patients with current MDD without dysthymia (n=55), the chronic major depression (CMD) group including patients with MDD without dysthymia over the last two years (n=20), the pure dysthymia (DYS) group consisting of patients with dysthymia without current MDD (n=20), and the double depression (DD) group including patients who meet the diagnostic criteria of dysthymia and also of acute or chronic MDD (n=45). We employed Kruskal–Wallis variance analysis to compare the four groups in terms of continuous variables (e.g., age, educational attainment, and HDRS score) given that two of these groups were too small in size required for parametric analysis. In analyses where a significant difference was detected among the groups, pairwise comparisons were made by combining groups as the data suggested. In comparisons between the EMD and PDD groups formed by combining the CMD, DYS, and DD groups in accordance with the DSM-5 classification, we employed the t-test as group sizes were adequate, and the data were normally distributed, calculated, and interpreted Cohen’s d referring to the effect size standards (small: d≥0.20, medium: d≥0.50, large: d≥0.80) defined by Cohen (7).
Figure 1.
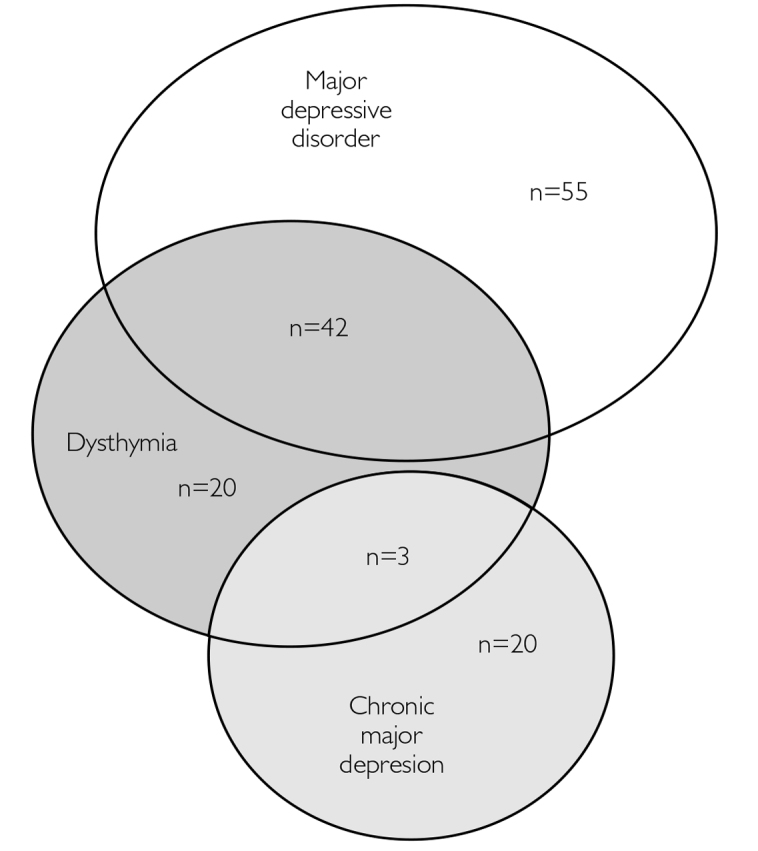
Distribution of depressive patients according to their DSM-IV diagnoses
We employed the chi-square test for comparisons among the four DSM-IV diagnostic groups in terms of categorical variables (gender, comorbidity, symptoms, and suicidal behavior). As an indicator of effect size, Cramer’s Φ (phi) was calculated and interpreted according to the conventional standards for cross-tables with two cells at the short edge (small: Φc≥.10, medium: Φc≥.30, large: Φc≥.50). When we observed at least a medium-sized effect, we combined the groups as data suggested to perform pairwise comparisons. In these pairwise comparisons, we employed odds ratio (OR) as the effect size indicator of the difference of the probability of observing a particular category in each group, and we interpreted the effect as significant when 95% confidence interval (CI) of the OR excluded 1.
We analyzed the data using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) 17 statistical program. Effect size indicators (Cohen’s d, Cramer’s Φ, and OR) were calculated and interpreted in accordance with the conventional standards to minimize type I error due to multiple comparisons and type 2 error due to a small sample size (7,13). Generally, inferential statistics used in quantitative research are means to deduce from the sample data whether the null hypothesis (H0) or research hypothesis (H1) is valid for the population from which the sample was taken. The statistical tests, however, were developed to test the validity of the H0 hypothesis. Thus, the p-value pertaining to the test value obtained from an analysis illustrates the probability of observing an effect (difference or association), same as or larger than the effect observed in the current sample, in studies to be performed with equally sized samples drawn from the same population, provided that the H0 hypothesis is valid. Conventionally, a p-values less than .05 is regarded a significant finding warranting rejection of the H0 hypothesis for the whole population. Nevertheless, whenever we follow this practice, we take the risk of making the so-called type I error as the p-value may never be exactly zero. The probability of making such an error (α) while interpreting the results of an analysis is equal to the p-value revealed by the analysis. Moreover, when the two groups were repeatedly compared with respect to several variables, the chance of making a type 1 error for multiple comparisons may considerably exceed .05; hence, the inflated α problem. In the present study, the overall chance of making a type 1 error through 39 comparisons is computed as α=1–.9539=.86. Given that the p-value is irrelevant for testing the H1 hypothesis, the recommended practice is to calculate and interpret the effect size to accept or reject the H1 hypothesis. By referring to the effect size standards coined by Cohen (7), we conclude whether the effect observed in the sample is trivial, small, moderate, or large. Investigators studying small samples in particular are prone to make a type 2 error, which is the failure to reject the null hypothesis when the research hypothesis is valid. The probability of making a type 2 error is denoted with β, and the power of an analysis is conceptualized as 1–β. Because effect size is not influenced by sample size, interpreting an effect size in addition to p-value in a study performed with a small sample permits a more accurate inference on which hypothesis (H0 or H1) is valid population-wise. Furthermore, the computation of the effect size renders it possible to figure the power of the analysis. In the current study, we used the G-power (3.1) program to estimate the required sample size while designing the project and to compute the post-hoc power of the conducted statistical tests while analyzing the data.
RESULTS
Frequency of Persistent and Episodic Courses among Depressive Patients
Figure 1 displays the classification of the patients according to their DSM-IV mood disorder diagnoses. Among the 140 patients enrolled, 120 (85.7%) met the diagnostic criteria of MDD. Of these 120 patients, however, 20 had chronic MDD, 42 had dysthymic disorder, and 3 had dysthymia+chronic MDD. Hence, among the patients diagnosed with DSM-IV MDD, 65 (54.2%) met the criteria for DSM-5 PDD. These rates suggest that more than half the patients diagnosed with MDD had a persistent rather than an episodic depressive disorder.
With regard to the DSM-IV dysthymia diagnosis, Figure 1 illustrates that 65 out of the 140 patients met the criteria for dysthymia, 57 of whom we were able to reliably identify the age of onset, and classifies 45.6% (n=26) as early-onset (before 21 years of age) dysthymics while 54.4% (n=31) as late-onset dysthymics. Of the dysthymic patients, 69.2% (n=45) had accompanying MDD (double depression). Given that the sum of the patients with DSM-IV dysthymia and/or chronic MDD was 85, the percentages of patients diagnosed with DSM-5 PDD and MDD among all patients enrolled in the study were calculated as 60.7% and 39.3%, respectively (Figure 2).
Figure 2.
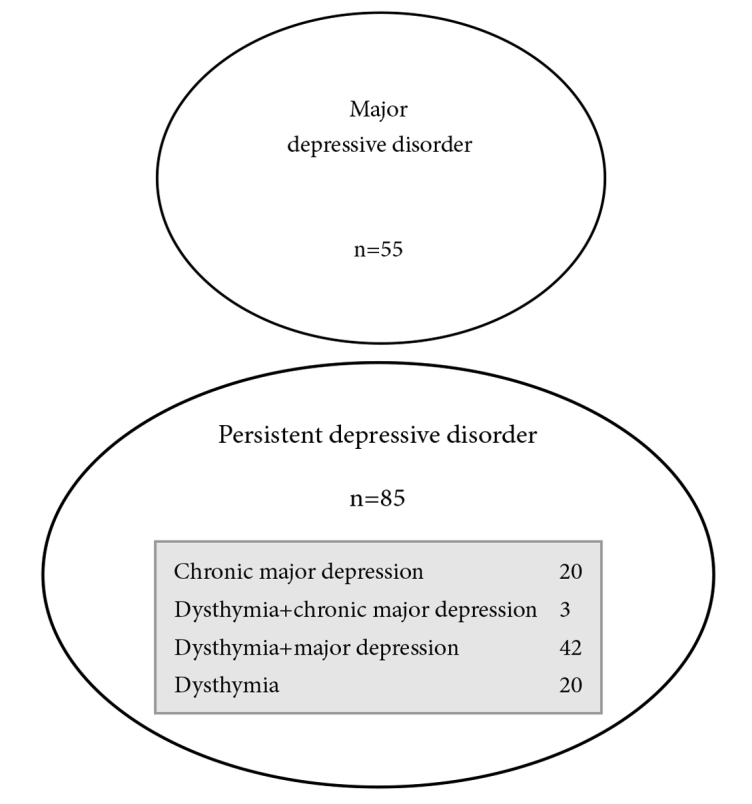
Distribution of depressive patients according to their DSM-5 diagnoses
Consequently, the four DSM-IV diagnostic groups to be compared in terms of socio-demographic and clinical variables were composed as follows: 55 patients with pure MDD comprising the EMD group; 20 patients having the sole diagnosis of chronic MD, the CMD group; 20 patients with dysthymia, the DYS group; and 45 patients diagnosed with both dysthymia and MD, comprising the DD group. Further analyses were performed to investigate the differences between combined diagnostic groups that were composed on the basis of the results of the preliminary comparisons.
Socio-Demographic Variables Associated with Persistent Depression
Table 1 summarizes the results of the preliminary comparisons among the four DSM-IV diagnostic groups. No significant difference was found among the groups in terms of gender. However, the groups significantly differed in age, mostly because of the lower mean age of the EMD group compared with that of the remaining three groups that could be combined to form the PDD group. The t-test conducted to determine the magnitude of difference in age revealed that the mean age of the EMD group (33.8±11.7) was significantly lower than that of the PDD group (40.6±13.5) and that the difference was medium sized (t138=3.08, p=.003, d=.54). Remarkably, the mean age of the early-onset dysthymic patients (33.0±13.4) was significantly lower than that of the late-onset patients (46.9±12.5) (t55=−4.07, p<.001). The 13 years difference in the mean ages of the respective groups, corresponding to nearly one standard deviation (d=1.07) revealed that the dysthymic patients and therefore, the patients in the PDD groups were not homogeneous in terms of age.
Table 1.
Distribution of depressive patients according to their DSM-5 diagnoses
| EMD n=55 |
CMD n=20 |
DYS n=20 |
DD n=45 |
Statistics | Significance | Effect size | |
|---|---|---|---|---|---|---|---|
| Gender (n and %) | |||||||
| Female | 48 (87.3) | 14 (70.0) | 15 (75.0) | 36 (80.0) | x2=3.43 | p=.33 | Φc=.16 |
| Male | 7 (12.7) | 6 (30.0) | 5 (25.0) | 9 (20.0) | |||
| Age (Mean±SD) | 33.76±11.73 | 41.80±11.26 | 42.20±17.48 | 39.36±12.54 | x2=9.43 | p=.02 | |
| Educational attainment (Mean±SD) | 10.95±4.05 | 8.45±4.01 | 9.53±3.78 | 8.45±4.37 | x2=11.56 | p=.009 | |
| HDRS (Mean±SD) | 16.69±4.83 | 18.41±6.04 | 14.67±4.32 | 20.46±5.19 | x2=16.78 | p=.001 | |
| Symptomatology (n and %) | |||||||
| Depressed mood | 53 (96.4) | 20 (100.0) | 20 (100.0) | 45 (100.0) | x2=3.14 | p=.37 | Φc=.15 |
| Loss of appetite | 34 (61.8) | 7 (35.0) | 5 (25.0) | 16 (35.6) | x2=11.9 | p=.01 | Φc=.29 |
| Insomnia | 41 (74.5) | 14 (70.0) | 7 (35.0) | 29 (64.4) | x2=10.34 | p=.02 | Φc=.27 |
| Hypersomnia | 7 (12.7) | 4 (20.0) | 1 (5.0) | 10 (22.2) | x2=3.82 | p=.28 | Φc=.16 |
| Low energy level-fatigue | 51 (92.7) | 20 (100.0) | 16 (80.0) | 44 (97.8) | x2=8.89 | p=.03 | Φc=.25 |
| Low self-esteem | 40 (72.7) | 18 (90.0) | 13 (65.0) | 34 (75.6) | x2=3.63 | p=.3 | Φc=.16 |
| Impaired concentration/decision-making | 46 (83.6) | 18 (90.0) | 15 (75.0) | 39 (86.7) | x2=2.00 | p=.6 | Φc=.12 |
| Hopelessness | 37 (67.3) | 17 (85.0) | 15 (75.0) | 42 (93.3) | x2=10.86 | p=.01 | Φc=.28 |
| Pessimism | 37 (67.3) | 18 (90.0) | 15 (75.0) | 43 (95.6) | x2=14.28 | p=.003 | Φc=.31 |
| Helplessness | 37 (67.3) | 15 (75.0) | 14 (70.0) | 41 (91.1) | x2=8.42 | p=.04 | Φc=.25 |
| Feelings of worthlessness/guilt | 34 (61.8) | 16 (80.0) | 7 (35.0) | 37 (82.2) | x2=16.21 | p=.001 | Φc=.34 |
| Loss of Interest | 50 (90.9) | 19 (95.0) | 12 (60.0) | 41 (91.1) | x2=15.58 | p=.001 | Φc=.33 |
| Anhedonia | 51 (92.7) | 19 (95.0) | 13 (65.0) | 42 (93.3) | x2=14.46 | p=.002 | Φc=.32 |
| Social withdrawal | 44 (80.0) | 16 (80.0) | 15 (75.0) | 35 (75.8) | x2=.26 | p=.97 | Φc=.04 |
| Subjective irritability/anger | 46 (83.6) | 14 (70.0) | 14 (70.0) | 40 (88.9) | x2=5.29 | p=.15 | Φc=.19 |
| Guilt-remorse | 32 (58.2) | 11 (55.0) | 9 (45.0) | 27 (60.0) | x2=1.39 | p=.71 | Φc=.10 |
| Somatic complaints | 27 (49.1) | 13 (65.0) | 13 (65.0) | 35 (77.8) | x2=8.8 | p=.03 | Φc=.25 |
| Anxiety | 23 (41.8) | 14 (70.0) | 11 (55.0) | 35 (77.8) | x2=14.43 | p=.002 | Φc=.32 |
| Contemplating death or suicide | 13 (23.6) | 8 (40.0) | 0 (0.0) | 12 (26.7) | x2=9.4 | p=.02 | Φc=.26 |
| Psychomotor agitation-retardation | 36 (65.5) | 17 (85.0) | 3 (15.0) | 31 (68.9) | x2=24.46 | p<0.001 | Φc=.42 |
| Comorbidity (n and %) | 17 (30.9) | 5 (25.0) | 15 (75.0) | 21 (46.7) | x2=14.53 | p=.002 | Φc= 0.32 |
| Generalized anxiety disorder | 5 (9.1) | 4 (20.0) | 7 (35.0) | 12 (26.7) | x2=8.15 | p=.04 | Φc=.24 |
| Suicidal behavior (n and %) | |||||||
| Suicidal thoughts | 38 (70.4) | 13 (65.0) | 7 (36.8) | 33 (75.0) | x2=9.30 | p=.03 | Φc=.26 |
| Suicidal attempts | 20 (37.0) | 5 (25.0) | 3 (15.8) | 23 (52.3) | x2=9.28 | p=.03 | Φc=.26 |
SD: standard deviation; EMD: episodic major depression; CMD: chronic major depression; DYS: dysthymia; DD: double depression
With regard to the education level, the EMD group with the highest educational attainment in years (mean=10.95, sd=4.05) differed from the remaining three groups in a significant manner. When the EMD group was compared with the combined PDD group (mean=8.7, sd=4.1), the difference was found to be significant and medium sized (t136=3.15, p=.002, d=.55). Together, these findings suggest that the depressive patients with a chronic course were relatively older and less educated when compared to patients with an episodic course.
Clinical Variables Associated with Persistent Depression
Table 1 displays the results of the statistical tests conducted to examine differences among the diagnostic groups with respect to a series of test scores and symptom ratings. The Kruskall–Wallis variance analysis revealed a significant difference among the four groups in terms of the HDRS scores indicating the severity of symptomatology. It is of interest that the lowest scores were observed in the EMD and DYS groups, whereas the highest scores were observed in the DD group. Owing to adequate sample sizes, the DD and EMD groups’ HDRS scores were compared with the t-test, and the difference was found to be significant and close to large size (t88=3.55, p=.001, d=.76).
The chi-square tests conducted to compare the diagnostic groups in terms of individual symptoms revealed medium size or close (Φc≥.30) differences for loss of appetite, psychomotor agitation/retardation, anhedonia, loss of interest, insomnia, suicidal thoughts, hopelessness, pessimism, feelings of worthlessness–guilt, somatic complaints, and anxiety. Together, these findings suggest that the depressive patients with a variety of chronic courses were more likely to present with somatic complaints, anxiety, hopelessness, and pessimism, but were less likely to present with loss of appetite when compared to the patients with episodic depression. Conversely, the patients with current major depression were more likely to present with psychomotor agitation/retardation, loss of interest, anhedonia, feelings of worthlessness–guilt, insomnia, and suicidal thoughts compared to the patients with pure dysthymia.
With regard to the psychiatric comorbidities among our depressed patients, GAD diagnosed in 28 patients (20.0%) emerged as the most common comorbidity, followed by obsessive-compulsive disorder, social phobia, and somatoform disorders each diagnosed in eight patients (5.7%), adjustment disorder in three patients (2.1%) and PTSD in two patients (1.4%). In general, the DYS group with a comorbidity rate of 75% differed from the EMD and CMD groups with 2–3 times lower rates. When the comorbidity rate of GAD was taken into consideration, a significant difference observed among the groups seemed to be related to the less frequent GAD diagnosis in the EMD group than in the remaining groups with varying courses of persistent depression. We computed the GAD comorbidity rate as 27.1% in the combined PDD group, which represented a 3-fold increase (OR=3.7, 95% CI=1.32–10.46) reaching statistical significance when compared with that of 9.1 in the EMD group.
Preliminary analyses pertaining to the prevalence of suicidal behavior in the diagnostic groups revealed that the pure dysthymia group with the lowest rates for suicidal thoughts and suicidal attempts differed from the remaining groups all with current MD (Table 1). Therefore, we compared the DYS group with the combined MD group, 71.2% of whom had suicidal thoughts and 40.7% of whom had attempted suicide. When compared with this combined MD group, the DYS group was less likely to report suicidal thoughts (OR=.24, 95% CI=.09–.65) and suicidal attempts (OR=.27, 95% CI=.08–.99), and the difference was significant in both comparisons.
DISCUSSION
Owing to the fact that MDD was diagnosed in 86% of the patients in this study, one may easily conclude that depressive patients with an episodic course comprise majority of our sample. However, the disease had a chronic rather than an episodic course in more than half (54%) of the patients diagnosed with MDD. With the addition of patients with pure dysthymia, the rate of persistent depression in our depressive sample climbed up to 61%. Clinical studies conducted in North America reported a chronic course in 54% and 60% of their samples consisting of (major) depressive patients (14,15). Likewise, a previous study performed in Turkey reported a chronicity rate of 56% (16). Thus, the converging findings of research from different countries suggest that at least half of the patients seeking treatment for depressive complaints in psychiatry clinics have a disease with a chronic course.
Our findings implying a lack of association between sex and course of depression are in line with previous reports from Turkey (17,18). As a recent review article concluded, the results of the studies investigating the effect of female sex on the development of chronic depression are inconsistent (19). Therefore, there is no substantial evidence suggesting an association of persistent depression with a patient’s sex.
The persistent depressive patients who participated in our study were on average nearly 7 years older than the episodic depressive patients with mean ages of 40.6 and 33.8 years, respectively. This finding converges with two previous reports from Turkey (16,20). Though a recent review of pertinent European and North American studies revealed discrepant results (19), evidence supporting an association between advanced age and chronic course of depression seem to prevail (21,22,23,24). An important finding of our study was the difference in age amounting to 13 years or almost one standard deviation between the early-onset and late-onset dysthymic patients, together accounting for majority of the persistent depressive patient group. While early-onset dysthymic patients and episodic major depressive patients, both with mean ages of approximately 33 years, represented the younger portion of our sample, the late-onset dysthymic patients with a mean age of 47 years represented the oldest portion. This finding implying that late-onset dysthymics were responsible for the relatively advanced age of the persistent depressive patient group is consistent with the notion that depression starts as an episodic disease and evidently turns into a chronic and intractable ailment. Tomba et al. (25) discussed the progression of depression in five stages and considered dysthymia, double depression, and chronic major depression as milestones of the third or later stages. It is not yet clear whether the gradual chronicity of depression is a result of the natural disease process or a result of serotonergic drug use. The latter explanation relies on recent observations suggesting that serotonergic drugs, contrary to what has been believed for years, exert an atrophic rather than a trophic effect on neurons, reversing neurons to their immature states (26). Some authors proposed to refer to this complication of serotonergic drugs as tardive dysphoria, a term synchronous with tardive dyskinesia (27).
The persistent depressive patients in the present study had an educational attainment of 2 years less than that of the episodic depressive patients on average, equaling to 0.55 pooled standard deviation or medium effect size. A previous Turkish study (16) reported that majority of patients in either group were primary school graduates. Various studies conducted in Europe and the United States reported findings supporting that low education level may be a risk factor for chronicity (22,24). Nevertheless, the existence of other reports with contrasting findings in literature renders research inconclusive on this matter (19,28).
The persistent depressive patients in this study were more likely to report anxiety, hopelessness, pessimism, and somatic complaints and were less likely to report loss of appetite when compared with episodic depressive patients. Though research addressing symptom profile revealed inconsistent results in general (2,29,30,31), a previous finding replicated in our study is the increased likelihood of somatic symptoms by chronic depressive patients (24). Our results implicating GAD as the leading comorbid diagnosis in the chronic depressive patients were also in line with repeated findings of previous reports from Europe and North America (19,22,23,24,32). Somatic complaints along with chronic anxiety and worry suggesting comorbid GAD diagnosis in this group of patients support neurotic depression as conceptualized by Ghaemi et al. (1,33) in context of four clinical subtypes of MDD. In their terminology, neurotic depression refers to a clinical type with the most chronic course and mildest symptoms, whereas melancholic depression refers to another type with the most episodic course and severe symptoms. According to the diagnostic criteria proposed by the authors, neurotic depression is a sub-threshold depression lasting for at least 6 months with accompanying anxiety and somatic complaints.
Severity of depression is most commonly measured with the HDRS score for clinical and research purposes. A remarkable finding of our study was the observation of the highest HDRS scores in the patients with double depression and the lowest scores in the patients with pure dysthymia. This finding suggests that the patients classified as persistent depressives comprise a heterogeneous group in terms of symptomatic severity. Given that our sample consisted of depressive patients receiving antidepressant drug therapy, however, it is not unlikely that partial responders to treatment may have taken place in the dysthymia group. Besides, the cross-sectional design of the study rendered it impossible to longitudinally monitor the severity of depressive symptoms of patients. Despite this limitation, our data concerning symptomatic severity notably revealed that the double depression patients constituted the group with the most severe symptoms. The HDRS scores of the episodic depression group were below those of the double depression group and above those of the dysthymia group, which may explain why several studies reported no difference between acute and chronic depression groups in terms of severity of symptoms (21,24,34). Our findings regarding symptom severity provided supportive evidence for Klein et al.’s view (6) that chronicity and severity are two independent clinical dimensions of depression. On the other hand, our data do not support Ghaemi et al.’s (1) view that chronicity is always accompanied by low severity. The clinically important aspect of our findings on severity was that the mean HDRS score of the double depression patients was approximately four points higher than that of the patients with episodic depression. On the basis of this difference representing a large effect (d=.76) and associated with statistical significance, we recommend that clinicians consider PDD as a diagnostic possibility, particularly when evaluating major depressive patients with relatively severe symptoms.
With regard to suicidal thoughts, two-thirds of our major depressive patients and only one-third of our purely dysthymic patients had positive life-time histories. The diagnostic groups differed with regard to suicidal attempts as well, with the lowest rate involving the pure dysthymia group (16%) and the highest rate involving the double depression group (54%). Pertinent literature includes reports of no difference between dysthymic and major depressive patients in terms of suicidal attempts (16,35,36) as well as reports of relatively frequent suicidal thoughts and attempts in cases of dysthymia and chronic depression (37,38) and of recurrent major depression (39). Collectively, the observed association of the lowest suicidal risk with pure dysthymia and of the highest risk with double depression in the present study provide support for Klein et al.’s (6) view that chronicity and severity are separate clinical dimensions of depression.
Study Limitations
This study has three limitations that should be taken into account while interpreting its findings. The first limitation involves the cross-sectional design of the investigation. A longitudinal design may be more instrumental in gaining insight into the clinical course and prognosis of depression. The second limitation involves our failure to collect information from the entire sample on the age of onset for depressive symptoms and the number of MDEs experienced. This omission rendered us unable to retrospectively examine the development of chronicity in our persistent depressive patients, despite the fact that we collected data regarding age of onset solely from the dysthymic patients to classify them as early-onset and late-onset dysthymics.
The third limitation involves the sample size of the study that was computed to detect at least medium size differences between patients with persistent depression and those with episodic depression (d≥.50 or Cramer’s Φ≥.40) with sufficient power (7). Data collection phase was continued in accordance with this estimation, until at least 50 patients were collected in each group. However, to investigate whether the patients with persistent depression constituted a homogenous group in terms of socio-demographic and clinical variables, this group was divided into three sub-groups (dysthymia, double depression, chronic MD), two of which consisted of 20 patients. Accordingly, the post-hoc power of this preliminary comparison to detect medium or large differences among the three persistent depression sub-groups and the episodic depression group was computed as 0.50 or slightly below (40); hence, there was a type 2 error probability (β) of approximately 50% for these comparisons. On the other hand, we were obliged to deal with an inflated type 1 error probability because of multiple comparisons (α=.86 for a total of 39 comparisons). We decided to not perform Bonferroni correction to push the type 1 error probability down to acceptable limits because it would have raised the type 2 error probability. Instead, we interpreted results of the analyses on the basis of associated effect sizes rather than associated p-values. Thus, while interpreting the results of particular comparisons among the four groups in terms of individual symptoms of depression, we accepted the result to be significant when it was associated with a Φc value implying a medium effect size. Therefore, the reader should be warned that the sample size and interpretative strategy of this study have been adjusted to detect medium or large effects but not small ones.
In conclusion, the findings of the present study support the idea that patients with persistent depression constitute a homogenous cluster differing from those with episodic depression in some aspects (educational attainment, GAD comorbidity, and symptomatology). Conversely, persistent depressive patients as a group seem to be heterogeneous in terms of age, symptomatic severity, and suicidal behavior. It is of interest that patients with double depression are likely to report more severe symptoms than those with episodic depression. Our data supports the validity of the concept of chronic depression and the diagnostic category of PDD, which may vary in severity from one patient to another or from one period to another. In contrast, the data at hand suggest that the concept of neurotic depression denoting a clinical subtype characterized by chronicity and invariably mild symptomatology is of questionable validity.
Understandably, clinicians performing cross-sectional clinical evaluations are inclined to attend to the signs and symptoms of current major depression because of the imminent treatment implications of this diagnosis. When in charge of treating and following depressive patients over a period of time, however, the same clinicians become more attentive to the signs of chronicity with long-term prognostic and treatment implications. Therefore, both perspectives are necessary and complementary for high-quality patient care. In this context, the introduction of PDD as a novel diagnostic category in DSM-5 represents a positive step forward encouraging clinicians to evaluate (unipolar) depressive patients’ current status as well as their long-term status in a complementary way, akin to already established diagnostic practices for bipolar disorder. Longitudinal studies are warranted to enhance our knowledge on clinical types, course, treatment, and prognosis of depression.
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Ghaemi SN, Vöhringer PA, Vergne DE. The varieties of depressive experience: diagnosing mood disorders. Psychiatr Clin North Am. 2012;35:73–86. doi: 10.1016/j.psc.2011.11.008. http://dx.doi.org/10.1016/j.psc.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Klein DN, Kocsis JH, McCullough JP, Holzer CE, Hirschfeld RM, Keller MB. Symptomatology in dysthymic and major depressive disorder. Psychiatr Clin North Am. 1996;19:41–53. doi: 10.1016/s0193-953x(05)70272-0. http://dx.doi.org/10.1016/S0193-953X(05)70272-0. [DOI] [PubMed] [Google Scholar]
- 3.Klein DN, Schatzberg AF, McCullough JP, Keller MB, Dowling F, Goodman D, Howland RH, Markowitz JC, Smith C, Miceli R, Harrison WM. Early-versuslate-onset dysthymic disorder: comparison in out-patients with superimposed major depressive episodes. J Affect Disord. 1999;52:187–196. doi: 10.1016/s0165-0327(98)00079-2. http://dx.doi.org/10.1016/S0165-0327(98)00079-2. [DOI] [PubMed] [Google Scholar]
- 4.Klein DN, Schwartz JE, Rose S, Leader JB. Five-year course and outcome of dysthymic disorder: a prospective, naturalistic follow-up study. Am J Psychiatry. 2000;157:931–939. doi: 10.1176/appi.ajp.157.6.931. http://dx.doi.org/10.1176/appi.ajp.157.6.931. [DOI] [PubMed] [Google Scholar]
- 5.Klein DN, Shankman SA, Rose S. Ten-year prospective follow-up study of the naturalistic course of dysthymic disorder and double depression. Am J Psychiatry. 2006;163:872–880. doi: 10.1176/ajp.2006.163.5.872. http://dx.doi.org/10.1176/ajp.2006.163.5.872. [DOI] [PubMed] [Google Scholar]
- 6.Klein DN. Classification of depressive disorders in the DSM-V: proposal for a two dimension system. J Abnorm Psychol. 2008;117:552–60. doi: 10.1037/0021-843X.117.3.552. http://dx.doi.org/10.1037/0021-843X.117.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen J. Statistical power analysis for the behavioral sciences. 2 Baskı. New Jersey: Lawrence Erlbaum; 1988. [Google Scholar]
- 8.First M, Spitzer R, GiVbbon M. Structured clinical interview for DSM-IV axis I disorders (SCID-I) Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- 9.Çorapçıoğlu A, Aydemir Ö, Yıldız M. DSM-IV Eksen I bozuklukları için yapılandırılmış klinik görüşme kullanım kılavuzu (SCID-I) Ankara: Hekimler Yayın Birliği; 1999. [Google Scholar]
- 10.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. http://dx.doi.org/10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akdemir A, Örsel S, Dağ İ, Türkçapar H, İşcan N, Özbay H. Hamilton depresyon derecelendirme ölçeğinin geçerliliği, güvenilirliği ve klinikte kullanımı. Psikiyatri Psikoloji Psikofarmakoloji Dergisi. 1996;4:251–259. [Google Scholar]
- 12.Eskin M, Voracek M, Stieger S, Altinyazar V. A cross-cultural investigation of suicidal behavior and attitudes in Austrian and Turkish medical students. Soc Psychiatry Psychiatr Epidemiol. 2011;46:813–823. doi: 10.1007/s00127-010-0254-7. http://dx.doi.org/10.1007/s00127-010-0254-7. [DOI] [PubMed] [Google Scholar]
- 13.Aron A, Aron EN, Coups EJ. Statistics for Psychology. 4 Baskı. New Jersey: Pearson Prentice Hall; 2006. [Google Scholar]
- 14.Yang T, Dunner DL. Differential subtyping of depression. Depress Anxiety. 2001;13:11–17. doi: 10.1002/1520-6394(2001)13:1<11::aid-da2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Riso LP, du Toit PL, Blandino JA, Penna S, Dacey S, Duin JS, Pacoe EM, Grant MM, Ulmer CS. Cognitive aspects of chronic depression. J Abnorm Psychol. 2003;112:72–80. http://dx.doi.org/10.1037/0021-843X.112.1.72. [PubMed] [Google Scholar]
- 16.Demirarslan P, Gökalp PG, Ögel K, Babaoğlu AN. Kronik depresyonda sosyodemografik ve klinik özellikler: İyileşen major depresyon olguları ile karşılaştırma. Düşünen Adam. 1999;12:4–11. [Google Scholar]
- 17.Özmen E, Ögel K, Sağduyu A, Boratav C. Birinci basamak sağlık hizmetlerinde distimik bozukluk. Turk Psikiyatri Derg. 2002;13:23–32. [PubMed] [Google Scholar]
- 18.Ülkeroğlu F, Kuloğlu M, Tezcan AE, Karabulut C, Ay M, Atmaca M, Doğan İ. Distimi tanılı hastalarda kliniğe eklenen major depresif epizodun yaşam boyu birlikteliği. Düşünen Adam. 1999;12:27–34. [Google Scholar]
- 19.Hölzel L, Härter M, Reese C, Kriston L. Risk factors for chronic depression--a systematic review. J Affect Disord. 2011;129:1–13. doi: 10.1016/j.jad.2010.03.025. http://dx.doi.org/10.1016/j.jad.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Aydemir A, Deveci OE, Taskin F, Taneli A, Esen-Danaci A. Serum brain-derived neurotrophic factor level in dysthymia: a comparative study with major depressive disorder Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1023–1026. doi: 10.1016/j.pnpbp.2007.02.013. http://dx.doi.org/10.1016/j.pnpbp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Rush AJ, Zimmerman M, Wisniewski SR, Fava M, Hollon SD, Warden D, Biggs MM, Shores-Wilson K, Shelton RC, Luther JF, Thomas B, Trivedi MH. Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. J Affect Disord. 2005;87:43–55. doi: 10.1016/j.jad.2005.03.005. http://dx.doi.org/10.1016/j.jad.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Rhebergen D, Beekman AT, Graaf Rd, Nolen WA, Spijker J, Hoogendijk WJ, Penninx BW. The three-year naturalistic course of major depressive disorder, dysthymic disorder and double depression. J Affect Disord. 2009;115:450–459. doi: 10.1016/j.jad.2008.10.018. http://dx.doi.org/10.1016/j.jad.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Murphy JA, Byrne GJ. Prevalence and correlates of the proposedDSM-5di-agnosis of Chronic Depressive Disorder. J Affect Disord. 2012;139:172–180. doi: 10.1016/j.jad.2012.01.033. http://dx.doi.org/10.1016/j.jad.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Gilmer WS, Trivedi MH, Rush AJ, Wisniewski SR, Luther J, Howland RH, Yohanna D, Khan A, Alpert J. Factors associated with chronic depressive episodes: a preliminary report from the STAR-D Project Acta Psychiatr Scand. 2005;112:425–433. doi: 10.1111/j.1600-0447.2005.00633.x. http://dx.doi.org/10.1111/j.1600-0447.2005.00633.x. [DOI] [PubMed] [Google Scholar]
- 25.Tomba E, Fava GA. Treatment selection in depression: the role of clinical judgment. Psychiatr Clin North Am. 2012;35:87–98. doi: 10.1016/j.psc.2011.11.003. http://dx.doi.org/10.1016/j.psc.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Andrews PW, Thomson JA, Jr, Amstadter A, Neale MC. Primum non nocere: an evolutionary analysis of whether antidepressants do more harm than good. Front Psychol. 2012;3:117. doi: 10.3389/fpsyg.2012.00117. http://dx.doi.org/10.3389/fpsyg.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Mallakh RS, Gao Y, Jeannie Roberts R. Tardive dysphoria: the role of long term antidepressant use in inducing chronic depression. Med Hypotheses. 2011;76:769–773. doi: 10.1016/j.mehy.2011.01.020. http://dx.doi.org/10.1016/j.mehy.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Subodh BN, Avasthi A, Chakrabarti S. Psychosocial impact of dysthymia: a study among married patients. J Affect Disord. 2008;109:199–204. doi: 10.1016/j.jad.2007.11.006. http://dx.doi.org/10.1016/j.jad.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Clark DA, Beck AT, Beck JS. Symptom differences in major depression, dysthymia, panic disorder, and generalized anxiety disorder. Am J Psychiatry. 1994;151:205–209. doi: 10.1176/ajp.151.2.205. http://dx.doi.org/10.1176/ajp.151.2.205. [DOI] [PubMed] [Google Scholar]
- 30.Joiner TE, Jr, Cook JM, Hersen M, Gordon KH. Double depression in older adult psychiatric outpatients: hopelessness as a defining feature. J Affect Disord. 2007;101:235–238. doi: 10.1016/j.jad.2005.03.019. http://dx.doi.org/10.1016/j.jad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Angst J, Gamma A, Rössler W, Ajdacic V, Klein DN. Long-term depression versus episodic major depression: results from the prospective Zurich study of a community sample. J Affect Disord. 2009;115:112–121. doi: 10.1016/j.jad.2008.09.023. http://dx.doi.org/10.1016/j.jad.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Klein DN. Chronic Depression: Diagnosis and Classification. Curr Dir Psychol Sci. 2010;19:96–100. http://dx.doi.org/10.1177/0963721410366007. [Google Scholar]
- 33.Ghaemi SN. Why antidepressants are not antidepressants: STEP-BD, STAR*D, and the return of neurotic depression. Bipolar Disord. 2008;10:957–968. doi: 10.1111/j.1399-5618.2008.00639.x. http://dx.doi.org/10.1111/j.1399-5618.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- 34.Ley P, Helbig-Lang S, Czilwik S, Lang T, Worlitz A, Brücher K, Petermann F. Phenomenological differences between acute and chronic forms of major depression in inpatients. Nord J Psychiatry. 2011;65:330–337. doi: 10.3109/08039488.2011.552121. http://dx.doi.org/10.3109/08039488.2011.552121. [DOI] [PubMed] [Google Scholar]
- 35.Beekman AT, Deeg DJ, Smit JH, Comijs HC, Braam AW, de Beurs E, van Tilburg W. Dysthymia in later life: a study in the community. J Affect Disord. 2004;81:191–199. doi: 10.1016/S0165-0327(03)00138-1. http://dx.doi.org/10.1016/S0165-0327(03)00138-1. [DOI] [PubMed] [Google Scholar]
- 36.Bernal M, Haro JM, Bernert S, Brugha T, de Graaf R, Bruffaerts R, Lépine JP, de Girolamo G, Vilagut G, Gasquet I, Torres JV, Kovess V, Heider D, Neeleman J, Kessler R, Alonso J ESEMED/MHEDEA Investigators. Risk factors for suicidality in Europe: results from the ESEMED study. J Affect Disord. 2007;101:27–34. doi: 10.1016/j.jad.2006.09.018. http://dx.doi.org/10.1016/j.jad.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Haykal RF, Akiskal HS. The long-term outcome of dysthymia in private practice: clinical features, temperament, and the art of management. J Clin Psychiatry. 1999;60:508–518. doi: 10.4088/jcp.v60n0802. http://dx.doi.org/10.4088/JCP.v60n0802. [DOI] [PubMed] [Google Scholar]
- 38.Satyanarayana S, Enns MW, Cox BJ, Sareen J. Prevalence and correlates of chronic depression in the Canadian community health survey: mental health and well-being. Can J Psychiatry. 2009;54:389–398. doi: 10.1177/070674370905400606. [DOI] [PubMed] [Google Scholar]
- 39.Witte TK, Timmons KA, Fink E, Smith AR, Joiner TE. Do major depressive disorder and dysthymic disorder confer differential risk for suicide? J Affect Disord. 2009;115:69–78. doi: 10.1016/j.jad.2008.09.003. http://dx.doi.org/10.1016/j.jad.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. http://dx.doi.org/10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]


