Abstract
Introduction
In this article, we report the data regarding treatment adherence of a group of patients with multiple sclerosis (MS) and relapsing–remitting or secondary progressive disease who were followed in the MS outpatient clinic of Bozyaka Education and Research Hospital, Izmir.
Methods
We collected the demographic data of 219 patients with MS who were treated with immunomodulatory drugs and the documentary data on the disease characteristics from the patient’ files. Each patient was provided a detailed questionnaire regarding treatment adherence in addition to the Beck depression scale (BDS) and Paced Auditory Serial Addition Test (PASAT). Nonadherence was defined as the discontinuation of the drug, i.e., more than one dose a month for intramuscular interferon, six doses a month for glatiramer acetate, and four doses a month for subcutaneous interferons. Statistical analyses were performed using Medcalc statistics package. For those parameters with an even distribution, the paired samples t-test was used to compare the results.
Results
Of the 219 [183 relapsing remitting multiple sclerosis (RRMS) and 36 secondary progressive multiple sclerosis (SPMS)] patients included in the study, 143 patients were women and 76 were men. The mean age of the patients was 40.77±10.36 years. The mean expanded disability status scale (EDSS) score was 2.90±1.88, and mean annualized attack rate (ARR) was .65±.55. Of the 219 patients, 75.1% continued the immunomodulatory treatment. Thirty-three patients in the RRMS group and 23 patients in the SPMS group abandoned the immunomodulatory treatment. Treatment adherences were similar between patients with RRMS and SPMS (53%). Adherence revealed no correlation with age, ARR, PASAT score, and disease duration. However, higher EDSS and depression scores had significant positive correlation with adherence. Moreover, treatment adherence was noted to be lower in the group with higher education levels. Treatment discontinuation did not correlate with age, ARR, BDS, or PASAT scores. The disease duration and EDSS scores were found to be significantly correlated with treatment discontinuation.
Conclusion
In this extensively followed up patients’ group with multiple sclerosis, the ones with extended disease duration, higher disability, and more educated had higher rates of treatment discontinuation and lower levels of treatment adherence. The patient-reported outcomes and well-documented treatment adherence data will contribute to the neurologists’ understanding of the patients’ inclinations regarding the injectable treatments and help in better management of the immunomodulatory treatments.
Keywords: Multiple sclerosis, immunmodulatory treatment, adherence
INTRODUCTION
The adherence to a particular long-term treatment in chronic diseases has always posed as a challenge in all medical branches. Multiple sclerosis (MS) is one of those diseases that requires frequent parenterally administered immunomodulatory drugs (IMD), such as daily or a few times a week for an undefined extended period. There are three kinds of interferons approved with their proven efficacy in MS treatment, subcutaneous (SC) IFN beta 1a, intramuscular (IM) IFN beta 1a, and SC IFN beta 1b. Glatiramer acetate (GA) is another type of immunomodulatory agent that is administered as daily SC injections. The adherence of patients with MS to the first-line immunomodulatory treatments (IMT) is estimated to be approximately 17%–46% in different series (1,2,3,4,5). The patients usually face various obstacles during the adaptation phase of their particular treatment. Among the reasons of treatment discontinuation, serious side effects, such as flu-like symptoms, depression, headache, laboratory abnormalities, and injection-site reactions; efficacy less than predicted; disease progression; and worsening of clinical picture may be listed. This study aims to examine the adherence of our patients to their IMTs and reveal the reasons for treatment discontinuation.
METHODS
We recruited 219 patients with MS who had been undergoing IMT and was being followed up in our demyelinating diseases outpatient clinic for the study. Their ages, gender, disease duration, annual relapse rate (ARR), EDSS scores, and the status of drug usage are recorded by means of a semi-structured questionnaire containing open-ended or yes/no-type items that we have developed in our clinic. Beck depression inventory (BDI) and Paced Auditory Serial Addition Test (PASAT) were applied to screen for any cognitive or depressive disorder. The study was accomplished according to Helsinki declaration, and patients were recruited after they gave their informed consent following a detailed description of the study. The patients were grouped according to their adherence level or continuation status of IMT, and the course of the disease. In the next step, they were compared for cognitive status, disability scores, and disease progression. In addition, comparisons between their level of education (8 years or less versus more than 8 years); disease duration; ARR; and adherence to the treatment were made. The criteria of nonadherence was to skip the injections four times a month for SC interferons, six times a month for GA, and once a month for IM interferon.
Statistical Analysis
Independent two samples t-test was used for comparing the continuous data between the groups. The continuous data was defined as arithmetic mean and standard deviation. Chi-square test was used for comparing the groups of categorical variables. The categorical variables were defined as absolute numbers and percentages. The analysis were made using MedCalc 12.3.0.0 biostatistical package programme. The p value for statistical significance was <.05 for all statistical tests.
RESULTS
We recruited 183 patients with relapsing–remitting MS (RRMS) and 36 patients with secondary progressive (SPMS) in this study. One hundred and forty three patients were females, whereas 76 were males. Their mean age was 40.77±10.36 years. The mean years of education were 9.38±4.08 years, mean ARR .65±.55, and mean EDSS score 2.90±1.88.
Approximately one fifth of our patients had switched among different IMDs during their treatment course. Therefore, most of the patients (80%) were already under treatment with the initial agent. Only 30% of the patients did not mention of any side effect with respect to IMDs. Of the reported side effects, the most common one was flu-like symptoms (31%) and the second was injection-site reactions (17.8%). Other side effects were fatigue (3.3%), psychological effects (3%), and abnormal blood test results (1.9%). Approximately three fourth of patients (77.5%) had injected themselves. For the rest, 12.2% got help from a family member, whereas 10.32% got help from a health professional.
Of the 219 patients, 75.1% were still under IMT. The rate of drug discontinuation was 24.9%. Thirty-three patients in the RRMS group and 23 in the SPMS had discontinued IMT. The most common reason of treatment discontinuation was refusal of the treatment in the RRMS group, whereas disease progression was the leading one in the SPMS group. Side effects were third in both groups. Higher EDSS and extended disease duration had a negative effect on maintaining the treatment (p<.0001, p=.03 respectively) (Figure 1,2). However no effect of age, gender, ARR, level of education, and existence of depression on treatment discontinuation was observed (Table 1).
Figure 1.
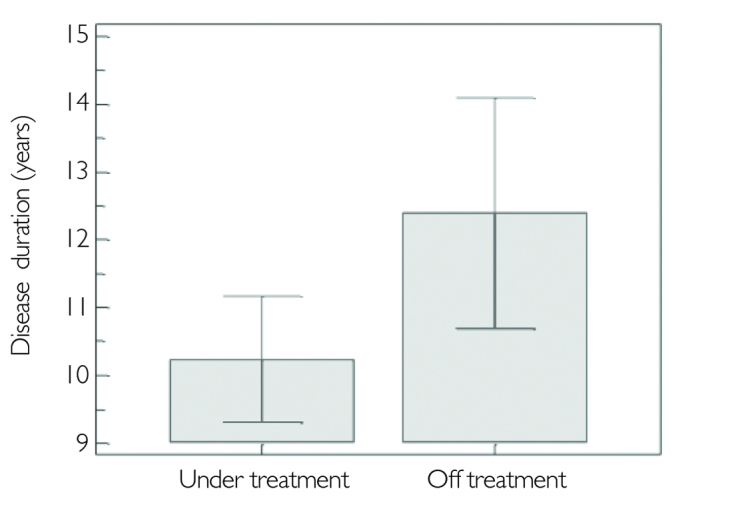
Disease duration is shown in MS patients who are under treatment or discontinued
MS: multiple sclerosis
Figure 2.
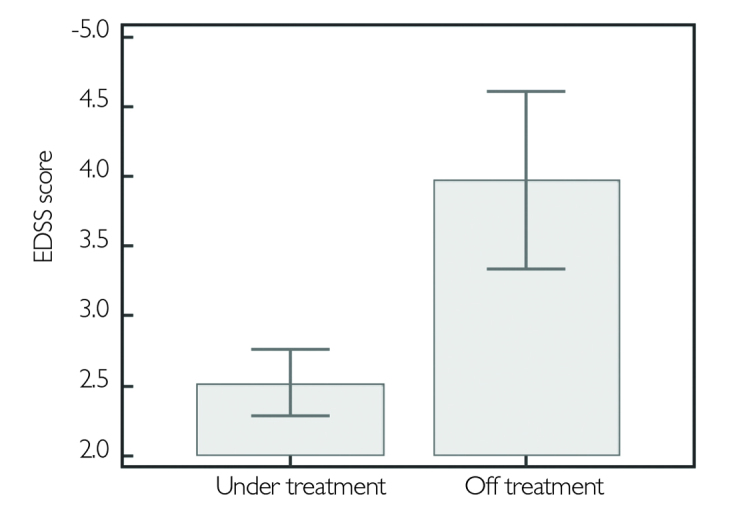
EDSS score comparison between patients under treatment and the ones who discontinued
EDSS: expanded disability status scale
Table 1.
Comparison of the various parameters between patients divided into two groups according to the continuation of the immunmodulatory treatment
| Group 1 | Group 2 | p | |
|---|---|---|---|
| Annualized relapse rate | .68±.82 | .61±.46 | .57 |
| BDI score | 12.84±9.55 | 13.24±9.85 | .88 |
| PASAT score | .62±.22 | .64±.27 | .2 |
| EDSS score | 2.52±1.57 | 3.98±2.30 | <.0001* |
| Mean age (years) | 39.99±10.2 | 42.90±10.69 | .08 |
| Education period (years) | 9.48±4.05 | 9.19±4.24 | .65 |
| Disease duration (years) | 10.24±6.04 | 12.40±6.20 | .03* |
Group 1: under treatment, Group 2: discontinued the treatment.
p<.05 statistically significant.
BDI: Beck depression inventory; PASAT: Paced Auditory Serial Addition Test; EDSS: expanded disability status scale
More than half of the patients adhered to their treatment in both MS groups, 53% in RRMS and 52% in SPMS. In all patients under IMT, gender, age, level of education, disease duration, or ARR were not found operative on treatment adherence. However, high EDSS and BDI scores were associated with nonadherence (p<.0001, p=.006, respectively) (Figure 3,4). No correlation was found between treatment adherence or treatment maintenance and PASAT scores (p=.42, p=.20, respectively). The parameters dealing with treatment adherence are given in Table 2 in detail.
Figure 3.
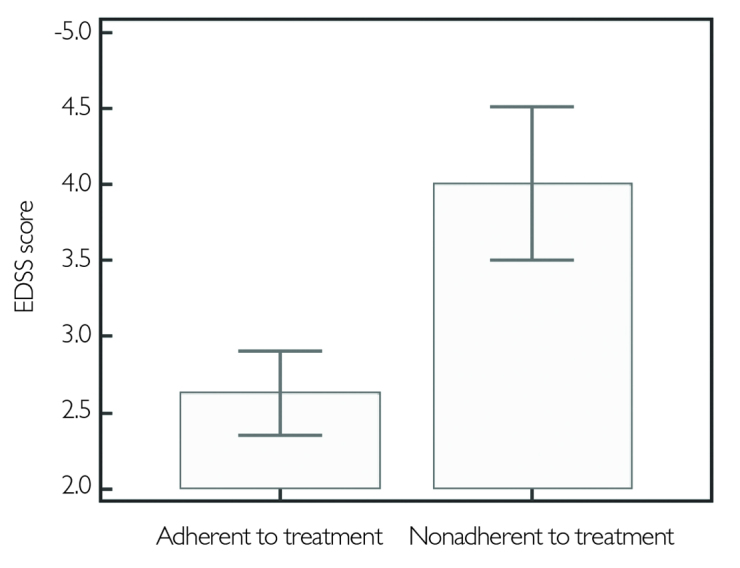
EDSS score comparison between treatment adherent and non adherent groups
EDSS: expanded disability status scale
Figure 4.
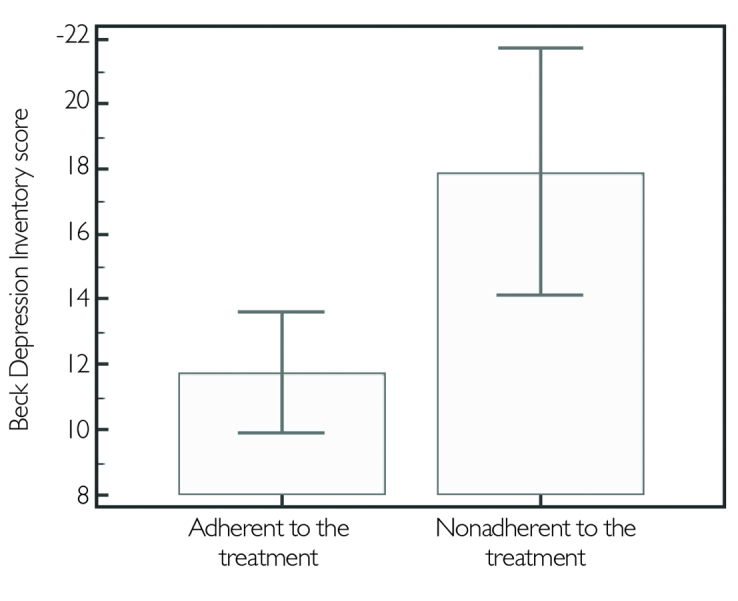
Comparison of the BDI scores between two groups; adherent and non adherent
BDI: Beck depression inventory
Table 2.
Various parameters compared between two groups
| Group 1 (Adherent) | Group 2 (Non adherent) | p | |
|---|---|---|---|
| Annualized relapse rate | .64±.82 | .73±.39 | .47 |
| BDI score | 11.75±9.47 | 17.91±8.67 | .006* |
| PASAT score | .63±.23 | .59±.22 | .42 |
| EDSS score | 2.63±1.83 | 4.01±1.58 | <.0001* |
| Mean age (years) | 40.98±10.51 | 39.26±9.91 | .34 |
| Education period (years) | 9.22±4.12 | 9.90±3.79 | .33 |
| Disease duration (years) | 10.52±6.40 | 11.93±5.13 | .19 |
Group 1: patients adherent to the treatment and Group 2: patients who are non adherent.
p<.005 statistically significant
BDI: Beck depression inventory; PASAT: Paced Auditory Serial Addition Test; EDSS: expanded disability status scale
When the patients were grouped according to their level of education, treatment nonadherence was 55% in higher educated group and 36% in the lower one. The difference was statistically significant (p=.007) (Figure 5). The reasons of nonadherence in the higher educated group were similar to the lower educated group, namely refusal of the treatment (26%), appearance of side effects (18%), and forgetfulness (17%). The RRMS and SPMS groups did not reveal any difference regarding the reasons of treatment nonadherence. In both the groups, the most common reason of nonadherence was refusal of treatment (27%) followed by side effects (27%), forgetfulness (18%), and various combinations in minor percentages.
Figure 5.
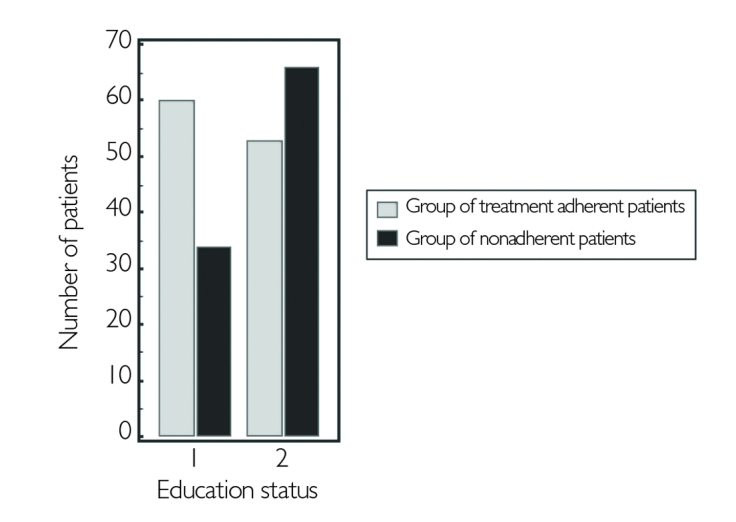
Comparison of educated patient group (1) and the uneducated group (2) for the treatment adherence
DISCUSSION
Multiple sclerosis is a chronic central nervous system disorder that mostly affects young adults as one of the leading causes of serious disability in this age group. Still, the first-line MS treatment mainly depends on IMDs (beta interferons and GA), which require a very long pursuance. In addition to parenteral application for extended periods, several side effects of IMDs and disease progression hampers treatment adherence.
In this study, we have investigated the rate and factors that affect adherence in our patient population with MS. During the study, it was observed that approximately one quarter of the patients had discontinued the treatment; this rate was higher in the SPMS group. The leading cause of discontinuation was refusal of treatment in the RRMS group, whereas in the SPMS group, it was extended disease duration and progression. When the patients who had terminated IMT were compared with patients continuing treatment, EDSS scores and disease duration of the former were statistically higher than the latter. As predicted, the high EDSS scores were related to progression of the disease, increasing disability, and lack of treatment response. MS Basis Study Group has estimated the treatment discontinuation rate as 40.3% (6). In this study, the increase of EDSS scores under IMT has appeared as an independent predicting factor for discontinuation. In the Global Adherence Project (GAP) study, treatment adherence was found to be related with duration of the treatment and disease, and the patients with a shorter disease duration had been more adherent (7). Similarly, in our study, patients with higher EDSS scores were also non adherent to their treatment, and patients with extended disease duration had discontinued their drugs more often. However, age, gender, level of education, ARR, BDI scores, and PASAT scores had no effect on maintenance of the treatment.
In the following analysis, patients who had discontinued IMDs had been excluded and the rest were grouped as adherent and non adherent. These two treatment groups were compared for age, level of education, disease duration, ARR scores, and PASAT scores. No significant difference was determined. However, EDSS and BDI scores were significantly higher in the non adherent group. The potential negative effect of depression on treatment adherence and frequent association with MS have been also reported in other studies (5,8). Two recent studies has demonstrated remarkable impact of treatment-related factors, such as disability, disease duration, depression, and quality of life on compliance (2,5). Furthermore, Mohr et al. (9) had found higher rates of nonadherence in depressed patients with MS. Treatment of comorbid depression in patients with MS for more than 6 months may have a positive impact on adherence (10). In contrast, Sidorenko et al. (11) reported no correlation between depression and rejection of treatment, disease duration, and type of MS.
When nonadherence to a parenterally administered IMD was encountered, factors such as the level of education of that particular patient or lack of treatment response must be considered besides difficulty of application (12). In our study, we observed a higher level of nonadherence in the higher educated group. Similar to our results, in the GAP study, patients with higher level of education were more often non adherent (7). In addition, we have found no gender difference in treatment continuation or treatment adherence. In MS Basis study, which included 44 centers and 2314 patients, female patients were found to be more non adherent than males (6). In contrast, GAP study had revealed a better degree of adherence in women (7).
In our study, the treatment adherence rates were noted to be quite low: 53% in the RRMS group and 52% in the SPMS group. Although all patients were monitored with regular follow-up visits and received individualized training and assistance service, the high rate of nonadherence was remarkable. In the literature relevant to the treatment compliance, different nonadherence rates may be reported from different countries. In a retrospective study from British Colombia, patients treated with interferon beta had mostly discontinued treatment in the first 6 months of the treatment, and the nonadherence rate was 39% as a whole (1). In an Italian MS study, 46% of patients abandoned their treatment during 4.2 years of mean follow-up (4). Rate of treatment adherence was determined to be 75% in the GAP study (7).
Treatment adherence in MS is related with many factors, such as depression, cognitive problems, treatment-related side effects, self-esteem of the patient related to the disease, faith in the treatment, difficulties in reaching the drug, and inefficient follow-up (13). Lugaresi et al. (14) had reported their observations on improving patient satisfaction and treatment adherence with the introduction of first autoinjector, RebiSmart™. However, in a long-running treatment, such as in MS, extended observational studies on compliance to such new devices must be conducted. In the Betaplus study, the rate of adherence to interferon beta-1b was 62% after two years, and the support of a nurse and application of auto injectors were found useful in improving drug compliance (15).
In the study of Treadaway et al. (5), forgetfulness was the most important reason to skip an injection. Cognitive deficiency may appear early during the MS course, and it was observed in 45%–60% of all patients (16). Compatibly, the rate of severe dementia late in the course of MS was 20%–30% (17). In the relevant literature, early cognitive dysfunction has been reported in the MS course and sometimes as the presenting symptom (18). In our study, forgetfulness was ranked third among treatment nonadherence disregarding the disease type. Distinct from the literature, we could find no significant effect of cognitive dysfunction on resuming or adhering to the treatment.
In this study, we aimed to review the level and factors affecting adherence to the treatment in patients with MS and to improve the efficient use of long-running IMT. The results reveal that besides an extended disease course, side effects, cognitive functions, and mood predict treatment adherence to a greater extent.
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Tremlett HL, Oger J. Interrupted therapy: stopping and switching for the B-interferons prescribed for MS. Neurology. 2003;61:551–554. doi: 10.1212/01.wnl.0000078885.05053.7d. http://dx.doi.org/10.1212/01.WNL.0000078885.05053.7D. [DOI] [PubMed] [Google Scholar]
- 2.Río J, Porcel J, Téllez N, Sánchez-Betancourt A, Tintoré M, Arévalo MJ, Nos C, Montalban X. Factors related with treatment adherence to interferon beta and glatiramer acetate therapy in multiple sclerosis. Mult Scler. 2005;11:306–309. doi: 10.1191/1352458505ms1173oa. http://dx.doi.org/10.1191/1352458505ms1173oa. [DOI] [PubMed] [Google Scholar]
- 3.O’Rourke KE, Hutchinson M. Stopping beta-interferon therapy in multiple sclerosis: an analysis of stopping patterns. Mult Scler. 2005;11:46–50. doi: 10.1191/1352458505ms1131oa. http://dx.doi.org/10.1191/1352458505ms1131oa. [DOI] [PubMed] [Google Scholar]
- 4.Portaccio E, Zipoli V, Siracusa G, Sorbi S, Amato MP. Long term adherence to interferon beta therapy in relapsing-remitting multiple sclerosis. Eur Neurol. 2008;59:131–135. doi: 10.1159/000111875. http://dx.doi.org/10.1159/000111875. [DOI] [PubMed] [Google Scholar]
- 5.Treadaway K, Cutter G, Salter A, Lynch S, Simsarian J, Corboy J, Jeffery D, Cohen B, Mankowski K, Guarnaccia J, Schaeffer L, Kanter R, Brandes D, Kaufman C, Duncan D, Marder E, Allen A, Harney J, Cooper J, Woo D, Stüve O, Racke M, Frohman EM. Factors that influence adherence with disease modifying therapy in MS. J Neurol. 2009;256:568–576. doi: 10.1007/s00415-009-0096-y. http://dx.doi.org/10.1007/s00415-009-0096-y. [DOI] [PubMed] [Google Scholar]
- 6.Meyniel C, Spelman T, Jokubaitis VG, Trojano M, Izquierdo G, Grand’Maison F, Oreja-Guevara C, Boz C, Lugaresi A, Girard M, Grammond P, Iuliano G, Fiol M, Cabrera-Gomez JA, Fernandez-Bolanos R, Giuliani G, Lechner-Scott J, Cristiano E, Herbert J, Petkovska-Boskova T, Bergamaschi R, van Pesch V, Moore F, Vella N, Slee M, Santiago V, Barnett M, Havrdova E, Young C, Sirbu CA, Tanner M, Rutherford M, Butzkueven H MS Basis Study Group. Country, sex, EDSS change and therapy choice independently predict treatment discontinuation in multiple sclerosis and clinically isolated syndrome. PLoS One. 2012;7:e38661. doi: 10.1371/journal.pone.0038661. http://dx.doi.org/10.1371/journal.pone.0038661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arroyo E, Grau C, Ramo-Tello C, Parra J, Sánchez-Soliño O GAP Study Group. Adherence to disease-modifying therapies in spanish patients with relapsing multiple sclerosis: two-year interim results of the global adherence project. Eur Neurol. 2011;65:59–67. doi: 10.1159/000323216. http://dx.doi.org/10.1159/000323216. [DOI] [PubMed] [Google Scholar]
- 8.Bruce JM, Hancock LM, Arnett P, Lynch S. Treatment adherence in multiple sclerosis: association with emotional status, personality and cognition. J Behav Med. 2010;33:219–227. doi: 10.1007/s10865-010-9247-y. http://dx.doi.org/10.1007/s10865-010-9247-y. [DOI] [PubMed] [Google Scholar]
- 9.Mohr DC, Goodkin DE, Likosky W, Gatto N, Baumann KA, Rudick RA. Treatment of depression improves adherence to interferon beta-1b therapy for multiple sclerosis. Arch Neurol. 1997;54:531–533. doi: 10.1001/archneur.1997.00550170015009. http://dx.doi.org/10.1001/archneur.1997.00550170015009. [DOI] [PubMed] [Google Scholar]
- 10.Tarrants M, Oleen-Burkey M, Castelli-Haley J, Lage MJ. The impact of comorbid depression on adherence to therapy for multiple sclerosis. Mult Scler Int. 2011;2011:271321. doi: 10.1155/2011/271321. http://dx.doi.org/10.1155/2011/271321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidorenko TV, Boiko AN, Davydovskaia MF, Zolotova SN, Lash Nıu, Popova NF, Khachanova NV, Shur SG, Gusev EI. Adherence to long-term therapy with disease modifying drugs. Zh Nevrol Psikhiatr Im S S Korsakova. 2009;109:107–113. [PubMed] [Google Scholar]
- 12.Bayas A. Improving adherence to injectable disease-modifying drugs in multiple sclerosis. Expert Opin Drug Deliv. 2013;10:285–287. doi: 10.1517/17425247.2013.763793. http://dx.doi.org/10.1517/17425247.2013.763793. [DOI] [PubMed] [Google Scholar]
- 13.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. http://dx.doi.org/10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 14.Lugaresi A. Rebismart TM (version 1,5) device for multiple sclerosis treatment delivery and adherence. Expert Opin Drug Deliv. 2013;10:273–283. doi: 10.1517/17425247.2013.746311. http://dx.doi.org/10.1517/17425247.2013.746311. [DOI] [PubMed] [Google Scholar]
- 15.Pozilli C, Schweikert B, Ecari U, Oentrich W Beta Plus Study group. Supportive strategies to improve adherence to IFN β-1b in multiple sclerosis-results of the βPlus observational cohort study. J Neurol Sci. 2011;307:120–126. doi: 10.1016/j.jns.2011.04.026. http://dx.doi.org/10.1016/j.jns.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Guimarães J, Sá MJ. Cognitive dysfunction in multiple sclerosis. Front Neurol. 2012;3:74. doi: 10.3389/fneur.2012.00074. http://dx.doi.org/10.3389/fneur.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao SM, Grafman J, DiGiulio D, Mittenberg W, Bernardin L, Leo GJ, Luchetta T, Unverzagt F. Memory dysfunction in multiple sclerosis: Its relation to working memory, semantic encoding, and implicit learning. Neuropsychology. 1993;7:364–374. http://dx.doi.org/10.1037/0894-4105.7.3.364. [Google Scholar]
- 18.Schulz D, Kopp B, Kunkel A, Faiss JH. Cognition in the early stage of multiple sclerosis. J Neurol. 2006;253:1002–1010. doi: 10.1007/s00415-006-0145-8. http://dx.doi.org/10.1007/s00415-006-0145-8. [DOI] [PubMed] [Google Scholar]


