Abstract
Introduction
Polycystic Ovary Syndrome (PCOS) is a syndrome of heterogeneous nature, affecting multiple systems, particularly the endocrine system. We propose to investigate the possible relationships among hormonal changes, levels of anxiety, depression, and anger in patients with PCOS.
Method
Forty-four female patients with PCOS and 44 body mass index (BMI )-matched healthy women participated in this study. We measured the sociodemographic features, some serum hormonal levels (insulin, gonadotropins, prolactin, dehydroepiandrosterone sulfate (DHEAS), thyroid-stimulating hormone (TSH), triiodothyronine (T3), thyroxine (T4), 17 OH-progesterone, and total and free testosterone), and some other biochemical parameters of the participants. Also, all participants completed the Trait Anger-Anger Expression Scale (STAS), Beck Depression, and Beck Anxiety Inventories. We evaluated the psychiatric scale scores obtained from PCOS patients and control subjects. We used the independent-samples t-test for parametric data to evaluate normal distribution, and Mann-Whitney U-test was used for both abnormally distributed and nonparametric data. We used Pearson correlation analysis to evaluate the potential connection between the two groups’ data.
Results
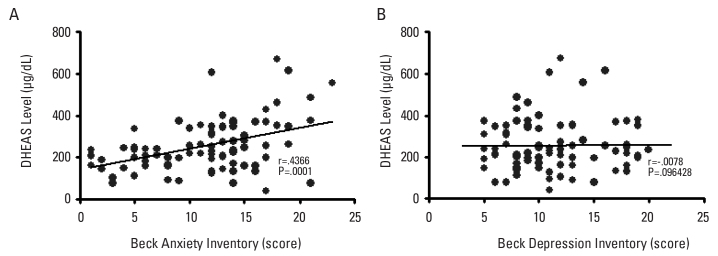
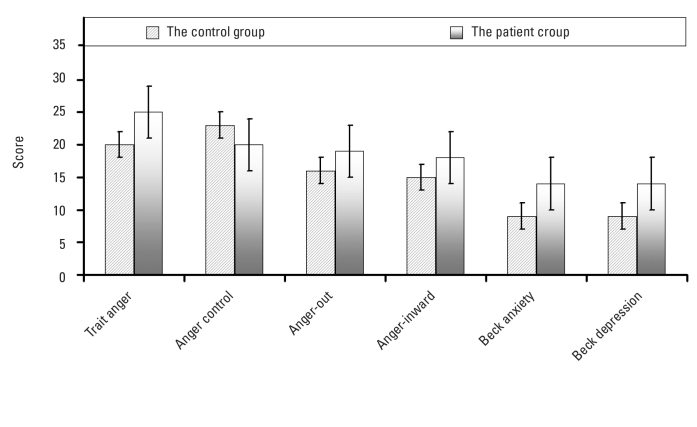
The mean ages of the patients with PCOS and control subjects who participated in this study were 27.3±5.6 and 27.4±6.1 years, respectively. The measures of BMI, insulin, luteinizing hormone (LH), DHEAS, and total testosterone serum levels in the patient group were significantly higher than in the control group (p<.05). There was a statistically significant positive correlation between Beck anxiety scores and serum DHEAS levels (Pearson r=.4366, P=.0001). We found significant differences between the two groups in terms of trait anger, anger control, outward and inward anger, anxiety level, and depression scores (P<.05).
Conclusion
Anxiety symptoms indicate a stronger relationship compared to depression with DHEAS serum levels via the autonomic nervous system, considering the gamma-aminobutyric acid (GABA)-antagonistic effect of DHEAS. Obesity, hirsutism, and infertility may reduce self-confidence and create depressive symptoms in patients with PCOS. In addition, changes in hormonal levels may lead to anxiety directly. Possibly, depressive symptoms are a secondary reflection of these changes.
Keywords: Polycystic ovary syndrome, anxiety, depression, anger, DHEAS, testosterone
Introduction
Polycystic ovary syndrome (PCOS) is common in reproductive age. Researchers characterize the syndrome with hirsutism, obesity, and amenorrhea. PCOS is a syndrome of heterogeneous nature, affecting multiple systems, particularly the endocrine system. Although, there is a lack of clarity regarding etiopathogenesis, increased dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) levels may have an essential role in the appearance of PCOS symptoms. PCOS and its relationships between psychiatric disorders have attracted much attention of researchers, especially in the last 20 years.
Investigators have hypothesized that hormones change the form or quantity of neurotransmitter receptors in the brain. They change the sensitivity to endogenous or exogenous circumstances, which may aggravate mood disorders ( 1 ). Fava et al. showed that hirsute women who were identified as having abnormally raised androgen levels reported negative feelings, such as depression, hostility, and irritability ( 2, 3 ). Also, women with PCOS reported an increase in assaultive behavior ( 4 ).
PCOS have a negative impact on psycho-social areas. For example, the psychological and social dimensions may influence females’ mental health in many ways ( 5 ). Assessing the relationship between different aspects of this network will contribute to the understanding of the complex nature of PCOS. The aim of this study was to understand the relationship of these heterogeneous biological parameters with anger, anxiety, and depression in females with PCOS during the reproductive period. The hypothesis of this study was that anxiety levels, depression, and anger would be greater in patients with PCOS than in healthy controls.
Method
Procedure
Forty-four female patients with PCOS and 44 body mass index (BMI)-matched healthy women participants were included in this study, which was planned cross-sectionally. Our PCOS diagnosis was on the basis of Rotterdam criteria ( 6 ). All of the patients had clinical and/or biochemical hyperandrogenism, chronic oligo-anovulation, and/or PCOS findings on ultrasonography. PCOS was defined as the presence of ≥12 follicles in each ovary, each measuring 2–9 mm in diameter, and/or increased ovarian volume (>10 ml; Rotterdam, 2004 ) ( 6 ). According to these criteria, at least two of the three criteria should be ensured for PCOS diagnosis. Exclusion criteria consisted of Cushing’s syndrome, hyperprolactinemia, nonclassical congenital adrenal hyperplasia, thyroid dysfunction, androgen-secreting tumors ( 6 ), and patients using drugs (steroids). BMI-matched healthy women who had regular menses and had no clinical or biochemical hyperandrogenism or PCOS were recruited as a control group from the general population. In order to avoid confounding factors, patients who were smoking or had type 2 diabetes mellitus uncontrolled hypertension and patients who were using an anti-depressant or anxiolytic or hormonal (e.g., oral contraceptive pill) or insulin-sensitizing medications for the last 3 months prior to the study were excluded from the study. All participants gave informed consent for the study. The research project was approved by the regional ethical committee of Gulhane Medical Military Academy. All participants were able to understand the questions and had the capability of filling out the self-report scales. All participants filled out the research questionnaire form that was prepared for the interview. The form consisted of this information: age, marital status, age at menarche, smoking status, and caffeine/alcohol use. Blood glucose (mg/dl), insulin (μIU/ml), follicle-stimulating hormone (FSH) (μIU/ml), luteinizing hormone (LH) (μIU/ml), Estradiol (E2) (pg/ml), prolactin (μIU/ml), DHEAS (mcg/dl), thyroid-stimulating hormone (TSH) (μIU/ml), triiodothyronine (T3) (nmol/L), thyroxine (T4) (nmol/L), 17 OH-progesterone (ng/ml), total testosterone (nmol/L), free testosterone (pg/ml), triglyceride (mg/dl), LDL cholesterol (mg/dl), and HDL cholesterol (mg/dl) levels of patients with PCOS and healthy women participants were measured from fasting blood samples on an Abbott I2000 SR immunology analyzer and Olympus AU 400 autoanalyzer. Participants completed the Trait Anger-Anger Expression Scale (STAXI) and Beck Depression and Anxiety Inventories under the supervision of an experienced specialist.
Beck Depression Inventory (BDI)
was created by Beck and his colleagues. The validity and reliability tests for the Turkish version were conducted by Hisli et al. This is a Likert type self-reported scale that consists of 21 items ( 7, 8 ). This scale measures and identifies emotional, cognitive, physical, and motivational symptoms of depression objectively. Each question has 4 options given, with scores between 0 and 3. The depression score is determined by the sum of all items. The total score ranges from 0–63. If BDI total scores are less than 9, it means that there is “no depression,” scores between 10 and 16 are considered “mild,” 17–23 is “medium,” and scores over 24 are “severe depression.” The cut off score for BDI was taken as 17 ( 8, 9 ).
Beck Anxiety Inventory (BAI)
The Beck Anxiety Inventory (BAI), created by Beck et al., is a 21-item multiple-choice self-reported inventory measuring the severity of an anxiety in adults and adolescents. The items in the BAI describe the emotional, physiological, and cognitive symptoms of anxiety but not depression; it can discriminate anxiety from depression. The BAI requires only a basic reading level, can be used with individuals who have intellectual disabilities, and can be completed in 5 to 10 minutes by using the preprinted paper form and a pencil. The validity and reliability study for the Turkish version of this scale was carried out by Ulusoy ( 10 ).
Trait Anger-Anger Expression Scale (STAXI)
STAXI was created by Spielberger and colleagues in order to determine students’ styles of anger expression and was applied to adolescents and adults; there is no time constraint for performing the test. The scale is a 34-item (0 to 4) Likert-type rating scale. The trait Anger-Anger Expression Scale consists of three subgroups: pulse anger, anger expression, and anger control ( 11 ). The validity and reliability study in Turkey was carried out by Ozer ( 12 ).
Statistical Analysis
The Statistical Package for Social Sciences 15.0 (SPSS Inc., Chicago, IL, USA) program was used for statistical analysis of all data. Independent samples t-test was used to compare parametric-impaired data for 2 groups, and Mann-Whitney U-test was used for 2 groups and nonparametric-impaired data. We used Pearson correlation analysis to determine the relation between parametric data and used Spearman relation analysis to determine the relation between nonparametric data.
Results
The mean age of patients with PCOS and control subjects in the study was 27.3±5.6 and 27.4±6.1 years, respectively. There was no difference between the patient and control groups in terms of the mean age of menarche (p=.2005). The rate of caffeine use (59%) in the patient group was higher than in the control group (39%); on the other hand, the rate of smoking was higher in the control group (43%) than in the patient group (25%). The value of BMI, insulin, LH, DHEAS, and total testosterone serum levels in the patient group were significantly higher than in the control group (p<.05). There was no significant difference in terms of glucose (p=.0904), although there was a significant difference in the level of serum insulin between the two groups (p=.0282). Similarly, the level of thyroid hormones and lipid profiles showed no difference in both groups (p>.05) (Table 1). There was a weak but statistically significant positive correlation between BAI score and serum DHEAS levels (Pearson r=.4366, p=.0001) (Figure 1). When comparing the two groups, there were significant differences between the two groups in terms of trait anger, anger control, outward and inward anger, anxiety, and BDI scores (p<.05) (Figure 2) (Table 2).
Table 1.
The data of both groups
| Control group mean and SD | Patient group mean and SD | P | |
|---|---|---|---|
| n | 44 | 44 | |
| Age (year) | 27.3±5.6 | 27.4±6.1 | .7030a |
| BMI (kg/m2) | 21.1±2.3 | 24.2±4.3 | <.0001a |
| Number of marriage | 26 (%59) | 30 (%68) | |
| Age of first menstruation | 12.9±1.0 | 13.0±.8 | .2005b |
| Smoking/caffeine/alcohol use | 19/17/3 | 11/26/4 | |
| Fasting blood glucose (mg/dL) | 85±12 | 91±20 | .0904a |
| Insulin (μIU/mL) | 5.9±2.2 | 7.97±5.66 | .0282a |
| FSH (μIU/mL) | 5.74±1.27 | 4.98±1.91 | .0299a |
| LH (μIU/mL) | 7.41±3.18 | 9.66±5.43 | .0200a |
| E2 (pg/mL) | 66±42 | 59±43 | .4978a |
| Prolactin (μIU/mL) | 10.42±4.99 | 10.73±5.17 | .8034a |
| DHEAS (mcg/dL) | 231±98 | 282±139 | .0492a |
| TSH (μIU/mL) | 1.69±.72 | 1.53±.73 | .2906a |
| T3 (nmol/L) | 2.89±.48 | 3.07±.59 | .1205a |
| T4 (nmol/L) | 95±25 | 93±20 | .8093a |
| 17 OH-progesterone (ng/mL) | 1.03±.71 | .80±.48 | .0828a |
| Total testosterone (nmol/L) | .34±.14 | .41±.17 | .0341a |
| Free testosterone (pg/mL) | 2.10±.99 | 2.08±.95 | .9468a |
| Triglycerides (mg/dL) | 107±52 | 110±52 | .7682a |
| LDL cholesterol (mg/dL) | 121±29 | 123±53 | .8318a |
| HDL cholesterol (mg/dL) | 46±10 | 45±11 | .6054a |
SD: Standard Deviation, BMI: body mass index, FSH: follicle-stimulating hormone, LH: luteinizing hormone, E2: Estradiol, DHEAS: dehydroepiandrosterone sulfate, TSH: thyroid-stimulating hormone, T3: triiodothyronine, T4: thyroxine.
Unpaired t test,
Mann-Whitney U test
Figure 1. a, b.
The figure shows the distribution of Beck Anxiety and Beck Depression scores in all study groups. The scatter diagram shows the significant correlation between DHEAS and anxiety scores. There was no significant correlation between DHEAS and Beck depression scores
Figure 2.
The figure shows the difference between the mean scores obtained from the various scales. The difference between the control and the patient groups can be observed in terms of scale scores
Table 2.
The scores of control and patient group calculated from the various scales
| Control Group Mean and SD | Patient Group Mean and SD | P | |
|---|---|---|---|
| Trait anger score | 20±2 | 25±4 | .0001a |
| Anger control score | 23±3 | 20±4 | .0007a |
| Anger-out score | 16±2 | 19±4 | .0001a |
| Anger-inward score | 15±3 | 18±4 | .0001b |
| Quality of life score | 102±17 | 95±22 | .1171a |
| Beck anxiety score | 9±6 | 14±4 | .0001a |
| Beck depression score | 9±2 | 14±4 | .0001a |
SD: Standard Deviation
Unpaired t test,
Mann-Whitney U test
Discussion
The relationship between psychological and biological variables for polycystic ovary syndrome is a poorly understood area. However, recent research on this complex issue is promising in understanding the relationship between biological and psychosocial parameters. DHEAS level and its psychological effects have a vital role in the etiopathogenesis of PCOS. Previous authors tried to explain this mechanism. According to these authors, the zona reticularis of the adrenal cortex synthesizes DHEAS from dehydroepiandrosterone. Sulfotransferase catalyzes this reaction ( 13, 14 ). DHEAS may cause some psychic symptoms, such as anxiety, overly concerned thoughts, fear, and some physical symptoms, like dry mouth, palpitation, headache, hyperventilation, sweating, and gastrointestinal symptoms. In addition, the authors described the relationships between those psychiatric symptoms, such as negative affect, feelings of worthlessness, sense of rejection, hypersensitivity to criticism, excessive self-examination, social unrest, and also disturbances of sleep or appetite, and DHEAS ( 15, 16 ). Progesterone levels in Day 21 of the menstrual cycle provide information about ovulation. If the follicle-stimulating hormone/luteinizing hormone (LH/FSH) ratio is above 3, this favors a diagnosis of PCOS. In PCOS cases, ovarian granulosa cells produce so much testosterone and androstenedione that estrogen cannot transform entirely. Increased LH and reduced FSH levels show the inhibition of aromatase activity; this inhibition causes an increase of androgen level and reduces the estrogen.
In this study, there was a significant correlation between anxiety scores and the serum levels of DHEAS. Otherwise, there was no correlation between depression score and the serum level of DHEAS (Figure 1). This is because symptoms are direct results of increased DHEAS in patients with PCOS. Anxiety and depression are indirect results of this condition. We could not find any correlation between DHEAS levels and depression scores. The depression scores of the patient group were significantly higher than in the control group. However, the finding that the depression score of the patient group was significantly higher than in the control group conflicted with result. This contradiction may be due to the low sample size of this study. Additionally, increased anxiety levels may be preliminary symptoms for depression, but this is a difficult issue to evaluate because of the cross-sectional study design. Meanwhile, increased physiological arousal and stress are the foreground, which is suitable with the explanations related to endocrine changes. On the other hand, negative mood and anhedonia are common signs in depression. Tignol et al. suggested that those depressive symptoms, such as sorrow, hopelessness, anhedonia, psychomotor retardation, and decreased appetite and sexual interest, show decreased arousal of the central nervous system (CNS). Otherwise, fear, stress, hyperactivity, agitation, premature ejaculation, and increased attention show an increased arousal of CNS and anxiety. Some researchers suggested that DHEAS has a GABA-antagonistic effect. Electrophysiological studies demonstrated the GABA-antagonistic effects of DHEA, a hormone secreted by the adrenal cortex, in response to adrenocorticotropic hormone (ACTH) ( 17 ). DHEA is metabolized to DHEAS, which also has a GABA-antagonist effect ( 17 ). Despite the importance of the GABA receptor complex (GABA-RC) for the normal function of the CNS, data from human studies describing the role of DHEA, DHEA-S, and other neurosteroid modulators of the GABA-RC are limited ( 18, 19 ).
GABA, the main inhibitor neurotransmitter in the CNS, plays a crucial role in the etiopathogenesis of anxiety disorders. In this study, we found a correlation between anxiety scores and serum DHEAS levels. On the other hand, we could not find any correlation between serum DHEAS and depression scores. However, the findings of this study arise from a cross-sectional study design and cannot eliminate the possible correlation with depressive symptoms over time. Additionally, the sample of this study was a young population, and patients with PCOS may develop more depressive symptoms afterward. On the other hand, the emergence of depressive symptoms may have a greater relationship with individual vulnerability and effects of life events. Possible correlations between DHEAS and depressive symptomatology should be investigated by longitudinal studies. Although there is no correlation between depressive symptom severity and serum DHEAS level, a significant difference attracted attention between groups in terms of depressive symptoms. This situation may be due to social, psychological, and biological causes. According to the World Health Organization, the incidence of infertility is 10%–15% in the community, and it causes medical, psychiatric, psychological, and social problems. Infertility may cause as a crisis in these fields ( 20, 21 ). When compared to the control group, trait anger, anger control, outward and inward anger, anxiety, and the BDI scores of patients with PCOS were significantly different. It may be suggested that in patients with PCOS, this fertile period, with the contribution of hormonal changes, may cause an unpleasant emotional state. Emotional changes, such as irritability, stress, and anxiety, may appear in these patients. Infertile women may also live in stress because of not having children in reproductive age. Stressors consist of different factors, as well as environmental factors, such as cold, infection, or trauma, as well as individual factors, such as obesity, age, gender, insulin, sex hormones, and adrenaline, as well as emotional factors, such as addiction, anxiety, and anger ( 22 ). The human body has strong adaptability to stressors, but it is not always entirely successful. This ability depends on a broad range, consisting of the autonomic nervous system, hypothalamic-pituitary-adrenal axis, cardiovascular, and metabolic processes ( 23, 24 ). The finding that the BAI score of women with PCOS is positively correlated with DHEAS also reveals that experienced anxiety may be due to hormonal imbalances. More specifically, there is no correlation between BDI scores, anger scale, and DHEAS levels. On the contrary, there is a correlation between BAI scores and DHEAS levels. One possible explanation of this condition is that BAI may be more successful in the prediction of somatic components of anxiety than BDI. Thus, hormone imbalances may affect the autonomic nervous system, and this may cause increased somatic symptoms of anxiety. According to recent research, while decreased DHEAS has GABA tonic effects ( 25 ), increased DHEAS levels show GABA-agonistic effects. In a study, animals showed aggressive behavior when exposed to DHEAS ( 26 ). As a result, anxiety symptoms show a stronger relationship, compared to depression symptoms, with the level of DHEAS via the autonomic nervous system, considering the GABA-antagonistic effect of DHEAS. The increase of androgen levels causes the suppression of FSH and leads to a vicious cycle. Some studies describe these mechanisms with the increase of androgen in patients with PCOS ( 27, 28 ). In this study, DHEAS and total testosterone levels in women with PCOS were significantly higher than in the controls. These findings show that women with PCOS are under hormonal oppression. After all, undoubtedly, these hormonal changes affect the emotional state of the patients. Dokras et al. suggested that the impaired psychosocial functions in patients with PCOS are associated with frustration feelings and anxiety ( 29 ).
In conclusion, while looking from a psychological aspect, obesity, hirsutism, and infertility may reduce self-confidence and cause depressive symptoms. In addition, hormonal changes may cause anxiety directly. Possibly, depressive symptoms are a secondary reflection of these changes.
Footnotes
Conflict of Interest: The authors reported no conflict of interest related to this article.
References
- 1.Steiner M. The Effects of Gonadal-Hormones on Brain and Behavior. Progress in neuro-psychopharmacology & biological psychiatry. 1987;11:115–119. doi: 10.1016/0278-5846(87)90048-0. http://dx.doi.org/10.1016/0278-5846%2887%2990048-0. [DOI] [PubMed] [Google Scholar]
- 2.FFava GA, Grandi S, Savron G, Bartolucci G, Santarsiero G, Trombini G, Orlandi C. Psychosomatic assessment of hirsute women. Psychother Psychosom. 1989;51:96–100. doi: 10.1159/000288142. http://dx.doi.org/10.1159/000288142. [DOI] [PubMed] [Google Scholar]
- 3.Shulman LH, DeRogatis L, Spielvogel R, Miller JL, Rose LI. Serum androgens and depression in women with facial hirsutism. J Am Acad Dermatol. 1992;27:178–181. doi: 10.1016/0190-9622(92)70166-d. http://dx.doi.org/10.1016/0190-9622%2892%2970166-D. [DOI] [PubMed] [Google Scholar]
- 4.Van de Poll NE, Van Goozen SH. Hypothalamic involvement in sexuality and hostility: comparative psychological aspects. Prog Brain Res. 1992;93:343–361. doi: 10.1016/s0079-6123(08)64584-7. http://dx.doi.org/10.1016/S0079-6123%2808%2964584-7. [DOI] [PubMed] [Google Scholar]
- 5.Weiss TR, Bulmer SM. Young women’s experiences living with polycystic ovary syndrome. J Obstet Gynecol Neonatal Nurs. 2011;40:709–718. doi: 10.1111/j.1552-6909.2011.01299.x. http://dx.doi.org/10.1111/j.1552-6909.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- 6.group TREAsPcw. Revised 2003 consensus on diagnostic criteria and long term health risks related to polycystic ovary syndrome (PCOS) Human Reproduction. 2004;19:41–47. doi: 10.1093/humrep/deh098. http://dx.doi.org/10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 7.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. http://dx.doi.org/10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 8.Hisli N. Beck depresyon envanterinin üniversite öğrencileri için geçerliği ve güvenirliği. Turk Psikoloji Dergisi. 1987;7:3–13. [Google Scholar]
- 9.Bostanci M, Ozdel O, Oguzhanoglu NK, Ozdel L, Ergin A, Ergin N, Atesci F, Karadag F. Depressive symptomatology among university students in Denizli, Turkey: Prevalence and sociodemographic correlates. Croat Med J. 2005;46:96–100. [PubMed] [Google Scholar]
- 10.Ulusoy M. Yayınlanmamış uzmanlık tezi. İstanbul: Bakırköy Ruh ve Sinir Hastalıkları Hastanesi; 1993. Beck Anksiyete Envanteri: Geçerlik ve güvenirlik çalışması. [Google Scholar]
- 11.Spielberger CDKS, Solomon EP. In: The experience, expression, and control of anger. MPJ, editor. New York: Springer-Verlag; 1988. [Google Scholar]
- 12.Özer AK. Sürekli öfke (SL-Öfke) ve öfke ifade tarzi (Öfke-Tarz) ölçeklerinin ön çalismasi. Türk Psikoloji Dergisi. 1994:26–35. [Google Scholar]
- 13.Bovenberg SA, van Uum SH, Hermus AR. Dehydroepiandrosterone administration in humans: evidence based? Neth J Med. 2005;63:300–304. [PubMed] [Google Scholar]
- 14.Rainey WE, Nakamura Y. Regulation of the adrenal androgen biosynthesis. J Steroid Biochem Mol Biol. 2008;108:281–286. doi: 10.1016/j.jsbmb.2007.09.015. http://dx.doi.org/10.1016/j.jsbmb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson S, Hahn S, Tan S, Mann K, Janssen OE, Schedlowski M, Elsenbruch S. Prevalence and implications of anxiety in polycystic ovary syndrome: results of an internet-based survey in Germany. Hum Reprod. 2009;24:1446–1451. doi: 10.1093/humrep/dep031. http://dx.doi.org/10.1093/humrep/dep031. [DOI] [PubMed] [Google Scholar]
- 16.Hahn S, Janssen OE, Tan S, Pleger K, Mann K, Schedlowski M, Kimmig R, Benson S, Balamitsa E, Elsenbruch S. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur J Endocrinol. 2005;153:853–860. doi: 10.1530/eje.1.02024. http://dx.doi.org/10.1530/eje.1.02024. [DOI] [PubMed] [Google Scholar]
- 17.Demirgoren S, Majewska MD, Spivak CE, London ED. Receptor binding and electrophysiological effects of dehydroepiandrosterone sulfate, an antagonist of the GABAA receptor. Neuroscience. 1991;45:127–135. doi: 10.1016/0306-4522(91)90109-2. http://dx.doi.org/10.1016/0306-4522%2891%2990109-2. [DOI] [PubMed] [Google Scholar]
- 18.McAuley JW, Reynolds IJ, Kroboth FJ, Smith RB, Kroboth PD. Orally administered progesterone enhances sensitivity to triazolam in postmenopausal women. J Clin Psychopharmacol. 1995;15:3–11. doi: 10.1097/00004714-199502000-00002. http://dx.doi.org/10.1097/00004714-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Friess E, Trachsel L, Guldner J, Schier T, Steiger A, Holsboer F. DHEA administration increases rapid eye movement sleep and EEG power in the sigma frequency range. Am J Physiol. 1995;268:E107–113. doi: 10.1152/ajpendo.1995.268.1.E107. [DOI] [PubMed] [Google Scholar]
- 20.Kraft AD, Palombo J, Mitchell D, Dean C, Meyers S, Schmidt AW. The psychological dimensions of infertility. Am J Orthopsychiatry. 1980;50:618–628. doi: 10.1111/j.1939-0025.1980.tb03324.x. http://dx.doi.org/10.1111/j.1939-0025.1980.tb03324.x. [DOI] [PubMed] [Google Scholar]
- 21.Green JA, Robins JC, Scheiber M, Awadalla S, Thomas MA. Racial and economic demographics of couples seeking infertility treatment. Am J Obstet Gynecol. 2001;184:1080–1082. doi: 10.1067/mob.2001.115222. http://dx.doi.org/10.1067/mob.2001.115222. [DOI] [PubMed] [Google Scholar]
- 22.McCance KL, SJ . In: Stress and Disease. McCance KL, HS, editors. 1994. [Google Scholar]
- 23.Selye H. The general adaptation syndrome and the diseases of adaptation. J Allergy. 1946;17:231, 89, 358. doi: 10.1016/0021-8707(46)90148-7. [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. http://dx.doi.org/10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 25.Ahboucha S, Talani G, Fanutza T, Sanna E, Biggio G, Gamrani H, Butterworth RF. Reduced brain levels of DHEAS in hepatic coma patients: Significance for increased GABAergic tone in hepatic encephalopathy. Neurochem Int. 2012;61:48–53. doi: 10.1016/j.neuint.2012.03.020. http://dx.doi.org/10.1016/j.neuint.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Nicolas LB, Pinoteau W, Papot S, Routier S, Guillaumet G, Mortaud S. Aggressive behavior induced by the steroid sulfatase inhibitor COUMATE and by DHEAS in CBA/H mice. Brain Res. 2001;922:216–222. doi: 10.1016/s0006-8993(01)03171-7. http://dx.doi.org/10.1016/S0006-8993%2801%2903171-7. [DOI] [PubMed] [Google Scholar]
- 27.Speroff LFM. Anovulation and the polycystic ovary. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 28.Azziz R. Diagnostic criteria for polycystic ovary syndrome: a reappraisal. Fertil Steril. 2005;83:1343–1346. doi: 10.1016/j.fertnstert.2005.01.085. http://dx.doi.org/10.1016/j.fertnstert.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 29.Dokras A, Clifton S, Futterweit W, Wild R. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstet Gynecol. 2011;117:145–152. doi: 10.1097/AOG.0b013e318202b0a4. http://dx.doi.org/10.1097/AOG.0b013e318202b0a4. [DOI] [PubMed] [Google Scholar]