Abstract
Objective
To assess the health risks associated with exposure to particulate matter (PM10), sulphur dioxide (SO2), nitrogen dioxide (NO2), carbon monoxide (CO) and ozone (O3).
Design
The study is an ecological study that used the year 2014 hourly ambient pollution data.
Setting
The study was conducted in an industrial area located in Pretoria West, South Africa. The area accommodates a coal-fired power station, metallurgical industries such as a coke plant and a manganese smelter.
Data and method
Estimate of possible health risks from exposure to airborne PM10, SO2, NO2, CO and O3 was performed using the US Environmental Protection Agency human health risk assessment framework. A scenario-assessment approach where normal (average exposure) and worst-case (continuous exposure) scenarios were developed for intermediate (24-hour) and chronic (annual) exposure periods for different exposure groups (infants, children, adults). The normal acute (1-hour) exposure to these pollutants was also determined.
Outcome measures
Presence or absence of adverse health effects from exposure to airborne pollutants.
Results
Average annual ambient concentration of PM10, NO2 and SO2 recorded was 48.3±43.4, 11.50±11.6 and 18.68±25.4 µg/m3, respectively, whereas the South African National Ambient Air Quality recommended 40, 40 and 50 µg/m3 for PM10, NO2 and SO2, respectively. Exposure to an hour's concentration of NO2, SO2, CO and O3, an 8-hour concentration of CO and O3, and a 24-hour concentration of PM10, NO2 and SO2 will not likely produce adverse effects to sensitive exposed groups. However, infants and children, rather than adults, are more likely to be affected. Moreover, for chronic annual exposure, PM10, NO2 and SO2 posed a health risk to sensitive individuals, with the severity of risk varying across exposed groups.
Conclusions
Long-term chronic exposure to airborne PM10, NO2 and SO2 pollutants may result in health risks among the study population.
Keywords: particulate matter, gaseous pollutants, health risk assessment, exposure groups, South Africa
Strengths and limitations of this study.
Large dataset spanning hourly ambient concentration of pollutants for a whole year.
This is the first study in Pretoria West, South Africa to estimate the health risks of human exposure to airborne pollutants using the US Environmental Protection Agency assessment model.
In our study, prediction of long-term and short-term health effects in infants, children and adults resulting from inhalation of pollutants was possible.
However, the health risk that could result from exposure to the combination of the pollutants could not be determined.
Introduction
Air pollution is a multifaceted mix consisting of suspended particulates and gaseous pollutants.1 Globally, air pollution continues to be a major environmental problem that has been recognised as an important public health risk.2 The increase in human population, industrialisation, urbanisation and modernisation, and its attendant increase in vehicular emissions and activities are the major contributors to the rising urban air quality problems.3
WHO in the year 2013 asserted that urban ambient air pollution resulted in 2 million deaths in the world.4 Epidemiological studies have linked exposure to ambient air pollution with adverse human health effects.5–7 Exposure to air pollution can result in acute (short-term) and chronic (long-term) health effects.8 9 The acute effects of air pollution on human health were sufficiently established in the twentieth century when severe air pollution scenarios in Europe and in the USA resulted in disease morbidities and mortalities in hundreds of thousands of people.10
Air pollution is a known trigger of chronic obstructive pulmonary disease (COPD)11 and has informed the establishment of air quality standards in many countries.12 13 The broad legislative framework for air quality assessment in populated areas was put in place by the European Union Directive on Air Quality 2008/50/EC.14 This framework recommended guideline limits for pollutants that have been identified to be injurious to the health of the public, including the environment and the built infrastructure.14 These injurious pollutants include particulate matter (PM) with a diameter of ≤10 µm (PM10), nitrogen dioxide (NO2), sulphur dioxide (SO2) and carbon monoxide (CO).15 The human health effects of exposure to SO2, NO2, O3 and PM10 have previously been reported.7 16–19 Ozone, NO2 and SO2 pollutants can all cause lethal effects on the airway20 such as an increase in bronchial reactivity,21 22 airway oxidative stress,23 pulmonary and systemic inflammation,24 amplification of viral infections25 and reduction in airway ciliary activity.26
South Africa is one of the largest industrialised economies in the Southern Hemisphere and is the only industrialised regional energy producer on the African continent with significant mining and metallurgical activities.27 It is an arid country with high naturally occurring dust levels, compounded by industrial and vehicular pollution emissions.28 Excessive high PM pollution levels have been observed in industrialised regions and urban areas which are said to contribute up to 30% of particulate pollution in the country.29 Significant associations between exposure to PM and respiratory, cardiovascular and cerebrovascular risks have been reported in South Africa.30
Increased emphasis on human health concerns resulting from air pollution necessitates the need for estimating the association between exposure and adverse health effects. The US Environmental Protection Agency (US EPA) human health risk assessment (HHRA) framework is a handy tool that has been used to estimate human health risk that can result from exposure to a given pollutant.31 In their studies,32 33 they reported that health risk assessment is useful for estimating the occurrence of adverse health effects in children and adults resulting from the direct inhalation of atmospheric particulates in urban areas. This framework was first introduced by the National Research Council in 199434 and has been previously used in few studies in South Africa.31 35–37 However, an HHRA framework on PM10, CO, NO2, SO2 and O3 has never been previously used in Pretoria West, South Africa. Hence, in view of the known health effects of exposure to sub-10 µm PM and other gaseous pollutants, this study aimed to quantify the health risk of people living in the urban area in Pretoria West using the HHRA framework.
Methods
Study area and population
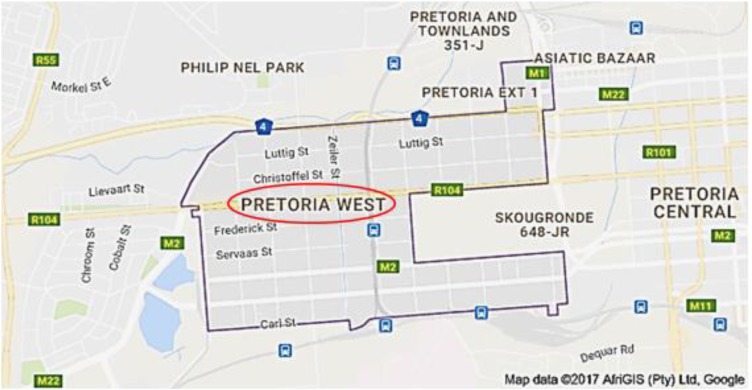
The study area was Pretoria West situated at 25° 44′ 46″ S 28° 11′ 17″ E (figure 1). Pretoria West is an industrial production area that accommodates a coal-fired power station, metallurgical industries such as a coke plant and a manganese smelter, fuel stations and a fuel tank farm. Pretoria is a city in the Northern part of Gauteng Province in Tshwane Metropolitan Municipality. It is situated ∼55 km (34 mi) north–northeast of Johannesburg in the Northeast of South Africa, in a transitional belt between the plateau of the Highveld to the South and the lower-lying Bushveld to the North. Pretoria has a population of 741 651 (49.75% men and 50.25% women) in 2011. This constitutes 23.2% young (0–14 years) persons, 71.9% of working age (15–64 years) and 4.9% of elderly (65+ years) persons.38
Figure 1.
Map of Pretoria West industrial area. The area is located in the Tshwane Metro and boasts a coal-fired power station, metallurgical industries and a fuel tank farm.
Data collection procedure
The study was an ecological study that focused on the comparison of groups, rather than individuals. Ecological study makes biological inferences about the effects of exposure on individual risks or groups. This study used secondary data obtained from the South African Weather Service (SAWS) through the South African Air Quality Information System (SAAQIS) website (http://www.saaqis.org.za) after the approval for its use was granted by the data originators, Environmental Management Services Department. The SAAQIS makes data available to stakeholders including the public and provides a mechanism to ensure uniformity in the way air quality data are managed, that is, captured, stored, validated, analysed and reported in South Africa.
The data originators obtained the data from a fixed ambient air quality monitoring station located at Pretoria West at longitude 28.146108, latitude −25.7555 and 1329 m above sea level. The monitoring station is routinely managed and maintained in order to achieve optimal results. The calibration of the monitoring unit is handled annually by the South African National Accreditation System. Moreover, at every quarter, the SAWS carries out a calibration certification of the monitoring unit using appropriate reference gases. Data requested by the researchers from the originators include hourly daily ambient level concentrations of PM10, CO, NO2, SO2 and O3 for the year 2014.
Data analysis
SPSS V.20 was used for the statistical analyses of the data. Descriptive statistics such as mean and SD were used to estimate the average concentration of pollutants that were monitored.
Human health risk assessment
Health risk assessment is an inclusive procedure by which possible adverse effects of human exposure to toxic agents are characterised.39 HHRA is predictive in nature and uses existing exposure data to measure the health effects of human exposure to a particular pollutant.40 The HHRA framework used in this study has four components: hazard identification, dose–response assessment, exposure assessment and risk characterisation.
Hazard identification
The identification of PM10, CO, NO2, SO2 and O3 as harmful and their attendant health risks was performed through a review of existing literature.
Dose–response assessment
Here, the amount of the pollutant taken into the body was estimated as a function of concentration and the length of exposure41 The dose–response assessment was not performed in this study. Rather, we compared the measured ambient concentration of pollutants in the study area with the South African National Ambient Air Quality Standard (NAAQS) which serves as the benchmark.
Exposure assessment
The exposure assessment identifies the population exposed to the hazard, the magnitude and duration of exposure to the hazard. Our study assumed the inhalation route as the major route of exposure to the monitored pollutants. As previously used in South Durban, South Africa,35 this study used a scenario assessment method. Normal (average exposure) and worst-case (continuous exposure) scenarios were computed for intermediate (24-hour) and chronic (annual) exposure periods. The normal acute (1-hour) exposure periods were also determined. These were determined among different age groups, namely infants (birth to a year), children (6–12 years) and adults (19–75 years).
For exposure to non-carcinogenic pollutants (PM10, CO, NO2, SO2, O3), the acute exposure rate equation is given as:
| 1 |
where AHD is the average hourly dose for inhalation (µg/kg/hour), C is the concentration of the chemical (µg/m3), IR is the inhalation rate (m3/hour) and BW is the body weight (kg).41
For exposure to non-carcinogenic pollutants (PM10, CO, NO2, SO2, O3), the chronic exposure equation used for the inhalation exposure route is:
| 2 |
where ADD is the average daily dose of the chemical of interest (µg/kg/day), C is the amount of the chemical in ambient air (µg/m3), IR is the inhalation rate (m3/day), ED is the exposure duration (days), BW is the body weight of the exposed group (kg) and AT is the averaging time (days).42
| 3 |
where ET is the exposure time (hour/day), EF is the exposure frequency (days/year) and DE is the duration of exposure (year). The standards for each age group are based on different assumptions, as shown in table 1.35 43
Table 1.
Exposure frequency, exposure duration and averaging time for different exposure groups
| Exposed group | EF (days/year) | DE (year) | AT (days) |
|---|---|---|---|
| Infant (birth to 1 year) | 350 | 1 | 365 (1×365) |
| Child (6–12 years) | 350 | 12 | 4380 (12×365) |
| Adult (19–75 years) | 350 | 30 | 10 950 (30×365) |
The EF value is calculated on the basis that a person will be absent from his place of abode (study area) for 14 days annually.43 The DE for an infant, child and adult was determined at 1, 12 and 30 years, respectively. The AT is estimated as the product of the duration of exposure and 365 days/year.
Table 2 shows the estimated ET values for each population group which was based on the average and continuous scenarios for acute, intermediate and chronic exposure periods.35 Default values were used for IR and BW43 and are given in table 3 for each exposure group.
Table 2.
Exposure time (hours) for normal and worst-case scenarios for acute, intermediate and chronic exposures
| Exposed group | Exposure time (hours) |
||||
|---|---|---|---|---|---|
| Intermediate |
Chronic |
||||
| Acute | Normal | Worst case | Normal | Worst case | |
| Infant (birth to 1 year) | 1 | 1 | 24 | 14.6 ((350/24)×1) | 350 (1×350) |
| Child (6–12 years) | 1 | 6 | 24 | 1050.0 ((4200/24)×6) | 4200 (12×350) |
| Adult (19–75 years) | 1 | 3 | 24 | 1312.5 ((10 500/24)×3) | 10 500 (30×350) |
Table 3.
Average inhalation rates and body weights of the exposed population
| Exposed group | Mean inhalation rate (m3/hour) |
Mean body weight (kg) | |
|---|---|---|---|
| Acute exposure | Chronic exposure | ||
| Infant (birth to 1 year) | 0.3 | 6.8 | 11.3 |
| Child (6–12 years) | 1.2 | 13.5 | 45.3 |
| Adult (19–75 years) | 1.2 | 13.3 | 71.8 |
Risk characterisation
Risk characterisation is the quantitative estimation of the health risk of exposure to a pollutant. Here, an estimate of possible non-carcinogenic effects from exposure to a known pollutant is determined using the hazard quotient (HQ).36 43 It reflects the probability of an adverse health outcome occurring among healthy and/or sensitive individuals. Non-cancer risks were calculated for acute and chronic exposure scenarios as:
| 4 |
| 5 |
where REL is the dose at which significant adverse health effects will occur in exposed groups compared with the unexposed group. In this study, we used the term ‘reference exposure level’ (REL), as adopted by the Office of the Environmental Health Hazard Assessment (OEHHA).44 The RELs that are used are presented in table 4.
Table 4.
Reference exposure levels for different pollutants
| Pollutant | 1 hour (µg/m3) | 8 hours (µg/m3) | 24 hours (µg/m3) | Annual mean (µg/m3) |
|---|---|---|---|---|
| PM10 | – | 75* | 40* | |
| NO2 | 200* | 188† | 40* | |
| SO2 | 350* | 125* | 50* | |
| CO | 29 770‡ | 10 305‡ | – | – |
| O3 | 226† | 120* | – | – |
Source: Department of Environmental Affairs.46
*NAAQS (National Ambient Air Quality Standard for South Africa).
†South Africa standards—Air Quality Act (Act 39 of 2004).
‡Default value was converted from ppm to µg/m3.
CO, carbon monoxide; NO2, nitrogen dioxide; PM10, particulate matter; SO2, sulphur dioxide; O3, ozone.
An HQ of 1.0 is considered to be the benchmark of safety. An HQ that is <1.0 indicates a negligible risk, that is, the pollutant under scrutiny is not likely to induce adverse health effects, even to a sensitive individual. An HQ>1.0 indicates that there may be some risks to sensitive individuals as a result of exposure.45
Results
PM10 concentration
The mean hourly, daily and annual concentration of PM10 in Pretoria West are 67.74, 52.01 and 48.26 µg/m3, respectively (table 5). Although, the daily (24 hours) guideline limit of 75 µg/m3 set by the NAAQS was not exceeded, the annual recommended mean limit of 45 µg/m3 that should not be exceeded was surpassed. The 1-hour (acute) scenario was not considered as a 1-hour REL value for PM10 was not found in the literature. The HQ from the health risk characterisation from exposure to PM10 is provided in table 6. The results showed that under the normal and worst-case scenario for average and continuous exposures, respectively, the risk of having health-related problems by the exposed population is low (HQ<1). This is because an HQ<1.0 indicates that PM10 is not likely to induce adverse health outcomes. However, infants (2.0×10−2 vs 4.2×10−1) followed by children (1.1×10−1 vs 4.2×10−1) are likely to be affected from exposure to PM10 compared with adults (3.0×10−2 vs 2.7×10−1) under the normal and worst-case scenario, respectively, for intermediate exposure. For the chronic (annual) exposure scenario for normal and worst-case exposures, HQ >1.0 for infants, children and adults. These results show that a sensitive exposed population may be at risk of developing health-related problems from chronic exposure to PM10. Infants are more likely to be affected than children and adults under the normal chronic exposure, while children will be more affected than infants and adults under the worst-case scenario.
Table 5.
Summary statistics of ambient concentrations of pollutants
| Averaging period | PM10 (µg/m3) Mean±SD |
NO2 (µg/m3) Mean±SD |
SO2 (µg/m3) Mean±SD |
CO (µg/m3) Mean±SD |
O3 (µg/m3) Mean±SD |
|---|---|---|---|---|---|
| 1 hour | 67.74±61.63 | 17.44±17.26 | 29.63±33.64 | 1442.6±1248.05 | 29.78±8.69 |
| 8 hours | – | – | – | 618.30±618.30 | 22.15±7.96 |
| 24 hours | 52.01±50.58 | 13.13±13.21 | 21.48±27.71 | – | – |
| Annual | 48.26±43.41 | 11.50±11.61 | 18.68±25.36 | – | – |
CO, carbon monoxide; NO2, nitrogen dioxide; PM10, particulate matter; SO2, sulphur dioxide; O3, ozone.
Table 6.
Hazard quotients for normal and worst-case exposure scenarios to particulate matter (PM10)
| Exposed group | Exposure |
|||
|---|---|---|---|---|
| Intermediate |
Chronic |
|||
| Normal | Worst case | Normal | Worst case | |
| Infant (birth to 1 year) | 2.0×10−2 | 4.2×10−1 | 1.0×101 | 2.44×102 |
| Child (6–12 years) | 1.1×10−1 | 4.2×10−1 | 3.62×102 | 1.45×103 |
| Adult (19–75 years) | 3.0×10−2 | 2.7×10−1 | 2.81×102 | 2.25×103 |
The 1-hour (acute) scenario was not considered since a 1-hour reference exposure level value for PM10 was not found in the literature.
SO2 concentration
The measured average concentration of SO2 for 1 hour, 24 hours and annual averages in the study area were 29.63, 21.48 and 18.68 µg/m3, respectively (table 5). These values are far less than the mean values of 350, 125 and 50 µg/m3 as provided by NAAQS for 1 hour, 24 hours and annual averages, respectively, that should not be exceeded (table 4). Estimation of risk for acute and intermediate (normal and worst-case) exposures to SO2 revealed that HQ<1.0 for infants, children and adults (table 7). This implies a negligible risk, even to a sensitive individual. For acute exposure, infants and children (2.0×10−3) are likely to be affected the same way from exposure to SO2 compared with adults (1.4×10−3). Under the normal and worst-case scenarios for chronic exposure, HQ>1.0 for the whole study population. This indicates that there may be some risks for sensitive individuals as a result of exposure to SO2. The severity of exposure differs for different age groups.
Table 7.
Hazard quotients for normal and worst-case exposure scenarios to sulphur dioxide (SO2) at different levels of exposures
| Exposed group | Exposure |
||||
|---|---|---|---|---|---|
| Acute | Intermediate |
Chronic |
|||
| Normal | Worst case | Normal | Worst case | ||
| Infant (birth to 1 year) | 2.0×10−3 | 4.0×10−3 | 1.1×10−1 | 31.5×10−1 | 7.55×101 |
| Child (6–12 years) | 2.0×10−3 | 3.0×10−2 | 1.0×10−1 | 1.12×102 | 4.49×102 |
| Adult (19–75 years) | 1.4×10−3 | 8.0×10−3 | 7×10−2 | 8.72×101 | 6.98×102 |
NO2 concentration
The monitored 1-hour, 24-hour and annual concentrations of NO2 shown in table 5 were 17.44, 13.13 and 11.50 µg/m3. The NAAQS 1-hour, 24-hour and annual guidelines of 200, 188 and 40 µg/m3, respectively, were not exceeded at Pretoria West (table 4). The HQ calculated for each of the acute and intermediate (normal and worst-case scenarios) exposures (shown in table 8) showed no likelihood of adverse health effects occurring at this level of exposure for an infant, child and adult (HQ<1.0). However, there is the likelihood that infants and children (2.3×10−3) might be affected by acute exposure to NO2 than adults (1.5×10−3). Moreover, having an adverse health outcome from normal and worst-case chronic exposure to NO2 was found to be higher (HQ>1.0) for all age groups. Children (3.05×102) appear more likely to be affected by normal chronic exposure than infants (8.6×101) and adults (2.37×102), whereas for worst-case chronic exposure, adults (1.893×103) are more likely to be affected.
Table 8.
Hazard quotients for normal and worst-case exposure scenarios to nitrogen dioxide (NO2) at different levels of exposures
| Exposed group | Exposure |
||||
|---|---|---|---|---|---|
| Acute | Intermediate |
Chronic |
|||
| Normal | Worst case | Normal | Worst case | ||
| Infant (birth to 1 year) | 2.3×10−3 | 6.0×10−3 | 1.5×10−1 | 8.6×101 | 2.05×102 |
| Child (6–12 years) | 2.3×10−3 | 4×10−2 | 1.5×10−1 | 3.05×102 | 1.218×103 |
| Adult (19–75 years) | 1.5×10−3 | 1.0×10−2 | 9.0×10−2 | 2.37×102 | 1.893×103 |
CO concentration
CO concentrations of 1442.6 µg/m3 (1-hour average) and 618.30 µg/m3 (8-hour average) (table 5) were not exceeded in comparison with the NAAQS guideline of 29 770 µg/m3 for 1-hour and 10 305 µg/m3 for 8-hour exposure limits. Estimation of risk for acute exposure to CO revealed that HQ <1.0 for infants, children and adults (table 9). This implies a negligible risk, even for sensitive infants, children and adults. However, infants and children (1.3×10−3) may suffer the effects compared with adults (8.0×10−4). Additionally, infants, children and adults living in the study area are not likely to experience adverse health effects associated with normal and worst-case exposure scenarios to 8-hour CO (HQ<1.0).
Table 9.
Hazard quotients for normal and worst-case exposure scenarios to carbon monoxide (CO) at different levels of exposures
| Exposed group | Exposure |
||
|---|---|---|---|
| Acute | Intermediate* |
||
| Normal | Worst | ||
| Infant (birth to 1 year) | 1.3×10−3 | 2.0×10−3 | 1.0×10−2 |
| Child (6–12 years) | 1.3×10−3 | 9.0×10−3 | 1.0×10−2 |
| Adult (19–75 years) | 8.0×10−4 | 3.0×10−3 | 8.0×10−4 |
*Intermediate—8-hour exposure period.
O3 concentration
The monitored concentration of O3 for 1-hour and 8-hour averages in the study area were 29.78 and 22.15 µg/m3, respectively (table 5). The NAAQS and annual guideline of 226 and 120 µg/m3, respectively, were not exceeded at Pretoria West (table 4). The HQ calculated for the acute and intermediate (normal and worst-case) exposure scenarios showed no likelihood of adverse health effects being experienced by any individuals (HQ<1.0) (table 10). During acute exposure, adults (2.2×10−2) are less likely to be affected than infants and children (3.0×10−3), while the reverse is the case for continuous exposure to O3 for 8 hours.
Table 10.
Hazard quotients for normal and worst-case exposure scenarios to ozone (O3) at different levels of exposures
| Exposed group | Exposure |
||
|---|---|---|---|
| Acute | Intermediate* |
||
| Normal | Worst | ||
| Infant (birth to 1 year) | 3.5×10−3 | 5.0×10−3 | 4.0×10−2 |
| Child (6–12 years) | 3.5×10−3 | 3.0×10−2 | 4.0×10−2 |
| Adult (19–75 years) | 2.2×10−2 | 9.0×10−3 | 2.0×10−2 |
*Intermediate—8-hour exposure period.
Discussion
Air pollution remains a global environmental threat and a public health risk. Researchers posited that health effects from exposure to ambient air pollution can occur at or below levels allowed by the national and international air quality standards. Findings from our study revealed that the 24-hour PM10 ambient quality standard of 75 µg/m3 was not exceeded on any of the days during the monitoring period. This is in contrast to other studies conducted elsewhere in South Africa. A 24-hour PM10 of 157.37 µg/m3 (highest peak) and 110 µg/m3 was reported by Thabethe et al31 and Matooaneand Diab35, respectively. The average annual concentration of PM10 recorded in our study was slightly above the guideline limit of 45 µg/m3 set by the NAAQS. This may account for the chronic (annual) HQ>1 recorded in our study, an indication of some level of risk to long-term exposure to PM10. The low concentration of pollutants recorded in our study may be due to the fact that industries in South Africa are required to submit their emission inventory to regulatory agencies monthly. This may compel these industries to ensure that their emission into the atmosphere is within stipulated guideline limits.
In South Africa, it was estimated that outdoor air pollution was responsible for 3.7% of the national mortality attributable to cancers of the trachea, bronchus and lung in adults aged 30 years and older, and 1.1% of mortality in children under 5 years of age.31 A review of 12 previous studies in the year 2001 affirmed that a 10 μg/m3 increase in PM10 causes an increase in hospital admissions for congestive heart failure and ischaemic heart disease.47 Among the vulnerable population (older people and those with a previous medical history of respiratory and cardiovascular diseases), long-term exposure to PM10 has been linked to an increase in morbidity and mortality from respiratory and cardiovascular diseases.48 Also for adults, large population studies have shown an association between respiratory (admissions for asthma, COPD and pneumonia) hospitalisation and ambient PM10.49 However, the effects seem to be stronger for older patients with even short-term exposures.50
This study further revealed that the 1-hour, 24-hour and annual mean concentration for NO2 are below the national standard. Evidence from the risk characterisation assessment shows a negligible risk for acute and intermediate exposure to ambient levels of NO2. However, 1-year exposure to ambient levels of NO2 could pose some risks to the sensitive individual. Recent epidemiological studies have revealed that exposure to low levels of NO2 could increase emergency room hospitalisation for acute and obstructive lung diseases in the general population.17 51 Studies conducted in Canada, Denmark and Italy found a significant association between exposures to levels of NO2 and acute ischaemic stroke.16 52 However, some studies did not find significant associations between exposure to ambient and personal levels of NO2 and health effects.53 54
Our study further shows low ambient value (compared with national standard) for SO2 in Pretoria West. Similarly, there is no likelihood of health risk (HQ<1) associated with 1-hour and 24-hour exposure to SO2. However, some levels of risk for sensitive individuals were found for chronic (annual) exposure to SO2 in the study area. The possibility of SO2 worsening childhood asthma at fairly modest concentration, that is, well below the US EPA standards and WHO guidelines, has been reported.55 Multicity studies conducted in Europe and Asia offer further proof supporting the short-term association of SO2 with adverse health outcomes, including mortality56 and morbidity.57
In this study, low ambient concentrations of CO and O3 were recorded. Researchers are of the opinion that exposure to ambient levels of CO is often not recognised; its toxicity is mostly under-reported and misdiagnosed due to its non-irritation and imperceptibility in the air we inhale.18 Exposure to CO has been linked to poison-correlated mortality in the USA.18 However, O3 is a strong oxidant that weakens biological tissues, thus resulting in increased use of medication, ailment and death.58 It has even been previously established that no level of exposure to O3 is safe since health risk has been found to be associated with O3 even at concentrations below the recommended standards.58
Furthermore, evidence from the risk characterisation assessment in this study shows that adults are less likely to be affected by acute and intermediate exposure to ambient concentrations of CO and O3 than infants and children. This was also true for acute and intermediate exposures to NO2 and SO2. It has been documented that children have a higher susceptibility to environmental pollutants than adults. They are considered a risk group for numerous reasons, including their relatively higher amount of air inhalation (the air intake per weight unit of a resting infant is twice that of an adult), and their immune system and lungs not being fully developed.31
Uncertainties and limitations
Although uncertainties occur in risk assessment, the risk assessment application has found usefulness in providing a quantitative and consistent framework for systematically evaluating environmental health risks and decisions for their control. Human health risk assessment as used in our study is conservative as it includes many safety factors that are built into the process. The final risk estimate is therefore likely to overstate the actual risk. To address these uncertainties in our study, we adopted equations from the US EPA, and applied benchmark values that were based on national and international standards and guidelines which were set based on the resulting human health effects from exposure to known pollutants.
The findings in our study should be interpreted in the light of the following limitations. The ecological nature of this study used populations or groups of people as the unit of analysis rather than individuals. The ecological technique assumes that individuals in the study area are all exposed to the same concentration of air pollutants without recourse to individual risk factors that may trigger the occurrence of disease outcomes. Such risk factors include sociodemographic factors, genetics, smoking habits and occupational exposure to respiratory hazards and pollutants in the workplace. Also, the health risk that could possibly result from exposure to the combination of the pollutants rather than individual pollutants as measured in our study could not be determined.
The strengths of this study are worthy of mention. First, the uniqueness of this study as it was the first conducted in the industrial area of Pretoria in South Africa that described the health risk associated with human exposure to PM and other gaseous pollutants. In addition, the study uses hourly ambient pollution data, with the method of data collection having undergone a validated process, and the study outcome is generalisable. Also, the use of the US EPA human health risk assessment framework which was first adopted by the National Research Council in 1994 allows our findings to be comparable to other studies.
Conclusions
Ambient air pollution is composed of suspended particulates and gaseous pollutants, with the gaseous components comprising O3, CO, NO2 and SO2. The acute, intermediate and chronic ambient concentrations of PM10 and the gaseous pollutants recorded in Pretoria West were within the South African NAAQS. No health risk was found to be associated with acute and intermediate exposure to the pollutants, though infants and children, rather than adults, are more likely to suffer the health effects. Long-term chronic (annual) exposure to normal and worst-case exposure scenarios to each of the pollutants posed some levels of risks for sensitive individuals, with the severity of risk differing across groups. Identification of the possibility of these pollutants to pose health hazards, as measured through the human health risk assessment framework, will make valuable contributions to government, environmental specialists and relevant stakeholders in taking more concrete steps to protect and prolong human lives. Additionally, these findings will assist policymakers in enforcing or strengthening existing legislation that limits the release of pollutants into the atmosphere or institutes risk management strategies.
Acknowledgments
The authors would like to thank the South African Weather Service, the South African Air Quality Information System and the Environmental Management Services Department of the City of Tshwane for granting the permission and releasing the data used for this study.
Footnotes
Contributors: OMM, MIM and MSM conceptualised the study. OMM designed the study, acquired the data and wrote the initial manuscript. ASA analysed the data and revised the manuscript. ASA, MIM and MSM critically reviewed the manuscript for important intellectual content. All authors proofread and approved the final version of the manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics approval: Human participants were not used in this study. However, the study was approved by the Tshwane University of Technology Senate Committee for Research Ethics, with reference number FCRE 2015/11/006 (SCI).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Mustafic H, Jabre P, Caussin C et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA 2012;307:713–21. 10.1001/jama.2012.126 [DOI] [PubMed] [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2224–60. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EPA, Environmental Protection Agency. Africa Air Quality (updated 2012; cited 15 June 2013). http://www.epa.gov/international/air/africa.htm
- 4.World Health Organization. Air Quality and Health Questions and Answers (cited 15 June2013). http://www.who.int/phe/air_quality_q&a.pdf
- 5.Rückerl R, Schneider A, Breitner S et al. Health effects of particulate air pollution: a review of epidemiological evidence. Inhal Toxicol 2011;23:555–92. 10.3109/08958378.2011.593587 [DOI] [PubMed] [Google Scholar]
- 6.Ostro B, Tobias A, Querol X et al. The effects of particulate matter sources on daily mortality: a case-crossover study of Barcelona, Spain. Environ Health Perspect 2011;119:1781–7. 10.1289/ehp.1103618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meister K, Johansson C, Forsberg B. Estimated short-term effects of coarse particles on daily mortality in Stockholm, Sweden. Environ Health Perspect 2012; 120:431–6. 10.1289/ehp.1103995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnell I, Potchter O, Yaakov Y et al. Human exposure to environmental health concern by types of urban environment: the case of Tel Aviv. Environ Pollut 2016;208:58–65. 10.1016/j.envpol.2015.08.040 [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Kindzierski W, Kaul P. Air pollution and acute myocardial infarction hospital admission in Alberta, Canada: a three-step procedure case-crossover study. PLoS ONE 2015;10:e0132769 10.1371/journal.pone.0132769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassanvand MS, Naddafi K, Faridi S et al. Characterization of PAHs and metals in indoor/outdoor PM10/PM2.5/PM1 in a retirement home and a school dormitory. Sci Total Environ 2015;527–528:100–10. 10.1016/j.scitotenv.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 11.Ko F, Hui D, Lai C. Worldwide burden of COPD in high and low-income countries. Part III. Asia-Pacific studies. Int J Tuberc Lung Dis 2008;12:713–17. [PubMed] [Google Scholar]
- 12.Adar SD, D'Souza J, Sheppard L et al. Adopting clean fuels and technologies on school buses. Pollution and health impacts in children. Am J Respir Crit Care Med 2015;191:1413–21. 10.1164/rccm.201410-1924OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schikowski T, Ranft U, Sugiri D et al. Decline in air pollution and change in prevalence in respiratory symptoms and chronic obstructive pulmonary disease in elderly women. Respir Res 2010;11:113 10.1186/1465-9921-11-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar P, Imam B. Footprints of air pollution and changing environment on the sustainability of built infrastructure. Sci Total Environ 2013;444:85–101. 10.1016/j.scitotenv.2012.11.056 [DOI] [PubMed] [Google Scholar]
- 15.Mouzourides P, Kumar P, Neophytou MKA. Assessment of long-term measurements of particulate matter and gaseous pollutants in South-East Mediterranean. Atmos Environ 2015;107:148–65. 10.1016/j.atmosenv.2015.02.031 [DOI] [Google Scholar]
- 16.Andersen ZJ, Kristiansen LC, Andersen KK et al. Stroke and long-term exposure to outdoor air pollution from nitrogen dioxide: a cohort study. Stroke 2012;43:320–5. 10.1161/STROKEAHA.111.629246 [DOI] [PubMed] [Google Scholar]
- 17.Chen R, Samoli E, Wong CM et al. Associations between short-term exposure to nitrogen dioxide and mortality in 17 Chinese cities: the China Air Pollution and Health Effects Study (CAPES). Environ Int 2012;45:32–8. 10.1016/j.envint.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 18.Iqbal S, Clower JH, Hernandez SA et al. A review of disaster-related carbon monoxide poisoning: surveillance, epidemiology, and opportunities for prevention. Am J Public Health 2012;102:1957–63. 10.2105/AJPH.2012.300674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalantzi EG, Makris D, Duquenne MN et al. Air pollutants and morbidity of cardiopulmonary diseases in a semi-urban Greek peninsula. Atmos Environ 2011;45:7121–6. 10.1016/j.atmosenv.2011.09.032 [DOI] [Google Scholar]
- 20.Ferrante M, Fiore M, Oliveri Conti G et al. Old and new air pollutants: an evaluation on thirty years experiences. Chapter 1 In: Haryanto B, ed. Air pollution—a comprehensive perspective. INTECH, 2012. 10.5772/47820 [DOI] [Google Scholar]
- 21.Happo MS, Salonen RO, Halinen AI et al. Inflammation and tissue damage in mouse lung by single and repeated dosing of urban air coarse and fine particles collected from six European cities. Inhal Toxicol 2010;22:402–16. 10.3109/08958370903527908 [DOI] [PubMed] [Google Scholar]
- 22.Ko FW, Hui DS. Air pollution and chronic obstructive pulmonary disease. Respirology 2012;17:395–401. 10.1111/j.1440-1843.2011.02112.x [DOI] [PubMed] [Google Scholar]
- 23.Antus B, Kardos Z. Oxidative stress in COPD: molecular background and clinical monitoring. Curr Med Chem 2015;22:627–50. 10.2174/092986732205150112104411 [DOI] [PubMed] [Google Scholar]
- 24.Ji X, Han M, Yun Y et al. Acute nitrogen dioxide (NO2) exposure enhances airway inflammation via modulating Th1/Th2 differentiation and activating JAK-STAT pathway. Chemosphere 2015;120: 722–8. 10.1016/j.chemosphere.2014.10.039 [DOI] [PubMed] [Google Scholar]
- 25.Kesic MJ, Meyer M, Bauer R et al. Exposure to ozone modulates human airway protease/antiprotease balance contributing to increased influenza A infection. PLoS ONE 2012;7:e35108 10.1371/journal.pone.0035108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ierodiakonou D, Zanobetti A, Coull BA et al. Ambient air pollution, lung function, and airway responsiveness in asthmatic children. J Allergy Clin Immunol 2016;137:390–9. 10.1016/j.jaci.2015.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rorich RP, Galpin JS. Air quality in the Mpumalanga Highveld region, South Africa. South Afri J Sci 1998;94:109–14. [Google Scholar]
- 28.DEAT, Department of Environmental Affairs and Tourism. PM2.5 as an emerging priority pollutant in South Africa—impacts on human health. Paper prepared for: Department of Environmental Affairs, Directorate: Information Management, 2010.
- 29.Engelbrecht JP, Swanepoel L, Chow JC et al. The comparison of source contributions from residential coal and low-smoke fuels, using CMB modeling, in South Africa. Environ Sci Policy 2002;5:157–67. 10.1016/S1462-9011(02)00029-1 [DOI] [Google Scholar]
- 30.Wichmann J, Voyi K. Ambient air pollution exposure and respiratory, cardiovascular and cerebrovascular mortality in Cape Town, South Africa: 2001–2006. Int J Environ Res Public Health 2012;9:3978–4016. 10.3390/ijerph9113978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thabethe NDL, Engelbrecht JC, Wright CY et al. Human health risks posed by exposure to PM10 for four life stages in a low socio-economic community in South Africa. Pan Afr Med J 2014;18:206 10.11604/pamj.2014.18.206.3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu X, Zhang Y, Ding ZH et al. Bio-accessibility and health risk of arsenic and heavy metals (Cd, Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2.5 in Nanjing, China. Atmos Environ 2012;57: 146–52. 10.1016/j.atmosenv.2012.04.056 [DOI] [Google Scholar]
- 33.Taner S, Pekey B, Pekey H. Fine particulate matter in the indoor air of barbeque restaurants: elemental compositions, sources and health risks. Sci Total Environ 2013;454–455:79–87. 10.1016/j.scitotenv.2013.03.018 [DOI] [PubMed] [Google Scholar]
- 34.NRC, National Research Council. Science and judgement in risk assessment. Washington, DC: National Academy Press, 1994. [Google Scholar]
- 35.Matooane M, Diab R. Health risk assessment for sulfur dioxide pollution in South Durban, South Africa. Arch Environ Health 2003;58:763–70. 10.3200/AEOH.58.12.763-770 [DOI] [PubMed] [Google Scholar]
- 36.Muller E, Diab RD, Binedell M et al. Health risk assessment of Kerosene usage in an informal settlement in Durban, South Africa. Atmos Environ 2003;37:2015–22. 10.1016/S1352-2310(03)00125-0 [DOI] [Google Scholar]
- 37.Oosthuizen MA, Wright CY, Matooane M et al. Human health risk assessment of airborne metals to a potentially exposed community: a screening exercise. Clean Air J 2015;25:51–7. 10.17159/2410-972X/2015/v25n1a5 [DOI] [Google Scholar]
- 38.Statistics South Africa. http://www.statssa.gov.za/ (accessed 2 Sep 2015).
- 39.US Environmental Protection Agency. Risk assessment guidelines of 1986. Washington, DC: Office of Health and Environmental Assessment, 1987, EPA 600-8.87/045. [Google Scholar]
- 40.Briggs D, Corvalan C and Nurminen M. Linkage methods for environmental health analysis: general guidelines. Geneva: World Health Organisation, 1996. [Google Scholar]
- 41.World Health Organization. Principles for the assessment of risks to human health from exposure to chemical s. Environmental Health Criteria 210. Geneva, Switzerland, 1999. [Google Scholar]
- 42.US Environmental Protection Agency. Human health risk assessment protocol for hazardous waste combustion facilities, 1988. http://www.epa.gov/epaoswer/hazwaste/combust/risk.htm [PubMed]
- 43.US Environmental Protection Agency. Exposure factors Handbook, 1997. http://www.epa.gov/ncea/expofac.htm
- 44.Office of Environmental Health Hazard Assessment (OEHHA). Acute toxicity summary: sulphur dioxide. In: Determination of acute reference exposure levels for airborne toxicants, 1999. California Environmental Protection Agency, OEHHA, 2001. http://www.oehha.org/air/acute_rels/pdf/7446095A.pdf [Google Scholar]
- 45.US Environmental Protection Agency. Risk assessment guidance for superfund, Vol 1: human health evaluation manual (Part A), 1989. http://www.epa.gov/superfund/programs/risk/ragsa/ [PubMed]
- 46.Department of Environmental Affairs. National Environmental Management: Air Quality Act, 2004 (ACT NO. 39 of 2004). National Ambient Air Quality Standards 2009.
- 47.Morris RD. Airborne particulates and hospital admissions for cardiovascular disease: a quantitative review of the evidence. Environ Health Perspect 2001;109(Suppl 4):495–500. 10.1289/ehp.01109s4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoek G, Krishnan RM, Beelen R et al. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health 2013;12:43 10.1186/1476-069X-12-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanobetti A, Franklin M, Koutrakis P et al. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health 2009;8:58 10.1186/1476-069X-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arena VC, Mazumdar S, Zborowski JV et al. A retrospective investigation of PM10 in ambient air and cardiopulmonary hospital admissions in Allegheny County, Pennsylvania: 1995–2000. J Occup Environ Med 2006;48:38–47. 10.1097/01.jom.0000183096.20678.f1 [DOI] [PubMed] [Google Scholar]
- 51.Santus P, Russo A, Madonini E et al. How air pollution influences clinical management of respiratory diseases. A case-crossover study in Milan. Respir Res 2012;13:95 10.1186/1465-9921-13-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vidale S, Bonanomi A, Guidotti M et al. Air pollution positively correlates with daily stroke admission and in hospital mortality: a study in the urban area of Como, Italy. Neurol Sci 2010;31:179–82. 10.1007/s10072-009-0206-8 [DOI] [PubMed] [Google Scholar]
- 53.Linaker CH, Chauhan AJ, Inskip HM et al. Personal exposures of children to nitrogen dioxide relative to concentrations in outdoor air. Occup Environ Med 2000;57:472–6. 10.1136/oem.57.7.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarnat JA, Schwartz J, Catalano PJ et al. Gaseous pollutants in particulate matter epidemiology: confounders or surrogates? Environ Health Perspect 2001;109:1053–61. 10.1289/ehp.011091053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.US Environmental Protection Agency (2002). Fourth external review for air quality criteria for particulate matter (Draft). EPA/600P-95/001aF-cF Research Triangle Park, NC: EPA Office of Research and Development. [Google Scholar]
- 56.Katsouyanni K, Touloumi G, Spix C et al. Short-term effects of ambient sulphur dioxide and particulate matter on mortality in 12 European cities: results from time series data from the APHEA project (Air Pollution and Health: a European Approach). BMJ 1997;314:1658–63. 10.1136/bmj.314.7095.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sunyer J, Ballester F, Tertre AL et al. The association of daily sulfur dioxide air pollution levels with hospital admissions for cardiovascular diseases in Europe (The Aphea-II study). Eur Heart J 2003;24:752–60. 10.1016/S0195-668X(02)00808-4 [DOI] [PubMed] [Google Scholar]
- 58.OECD, 2008. OECD Environmental outlook to 2030. Organization for Economic Co-Operation and Development; http://www.sourceoecd.org [Google Scholar]