Abstract
Objectives
The overuse of antimalarial drugs is widespread. Effective methods to improve prescribing practice remain unclear. We evaluated the impact of 10 interventions that introduced rapid diagnostic tests for malaria (mRDTs) on the use of tests and adherence to results in different contexts.
Design
A comparative case study approach, analysing variation in outcomes across different settings.
Setting
Studies from the ACT Consortium evaluating mRDTs with a range of supporting interventions in 6 malaria endemic countries. Providers were governmental or non-governmental healthcare workers, private retail sector workers or community volunteers. Each study arm in a distinct setting was considered a case.
Participants
28 cases from 10 studies were included, representing 148 461 patients seeking care for suspected malaria.
Interventions
The interventions included different mRDT training packages, supervision, supplies and community sensitisation.
Outcome measures
Analysis explored variation in: (1) uptake of mRDTs (% febrile patients tested); (2) provider adherence to positive mRDTs (% Plasmodium falciparum positive prescribed/given Artemisinin Combination Treatment); (3) provider adherence to negative mRDTs (% P. falciparum negative not prescribed/given antimalarial).
Results
Outcomes varied widely across cases: 12–100% mRDT uptake; 44–98% adherence to positive mRDTs; 27–100% adherence to negative mRDTs. Providers appeared more motivated to perform well when mRDTs and intervention characteristics fitted with their own priorities. Goodness of fit of mRDTs with existing consultation and diagnostic practices appeared crucial to maximising the impact of mRDTs on care, as did prior familiarity with malaria testing; adequate human resources and supplies; possible alternative treatments for mRDT-negative patients; a more directive intervention approach and local preferences for ACTs.
Conclusions
Basic training and resources are essential but insufficient to maximise the potential of mRDTs in many contexts. Programme design should respond to assessments of provider priorities, expectations and capacities. As mRDTs become established, the intensity of supporting interventions required seems likely to reduce.
Keywords: TROPICAL MEDICINE
Strengths and limitations of this study.
This analysis addresses the gap in knowledge around how to change prescribing practices, a key question in the era of resistance to antimicrobial medicines.
The analysis exploits indepth data from 10 intervention studies connected through the ACT Consortium in order to explore the reasons for variation in trial outcomes.
A comparative case study approach was used, allowing trends and patterns to be explored across contexts in a way not possible within single studies.
By analysing studies conducted within a consortium, access to unpublished documents, raw data and qualitative insights from the study teams allowed a deeper understanding of the studies and their contexts than is often found in systematic reviews of published reports.
The extent of variation across the study arms in terms of context, provider type, intervention content and study design allowed for exploration of a range of factors affecting outcomes, but also created challenges for comparability, necessitating a case study approach.
Background
The substantial overdiagnosis of malaria as a cause of acute febrile illness has been the focus of global attention in recent years,1–3 given concerns about the clinical effects of misdiagnoses, the cost of first-line artemisinin-based combination therapies (ACTs) and emerging malaria drug resistance.4 5 A policy of universal parasitological testing for malaria was introduced by the WHO in 2010,6 aiming to reduce overprescription of ACTs.2 Malaria rapid diagnostic tests (mRDTs) have been developed for use in low-resource settings, making parasite-based testing possible where microscopy may not be available or feasible.4
RDTs have been introduced with providers in a range of sectors.7 However, evidence from evaluations of mRDT introductions show mixed effects; mRDTs do not lead to improved targeting of ACTs if providers do not consistently use the tests or if they ignore test results.8–12 To maximise their potential for improving prescribing practices, evidence is required of the relative success and challenges of different types of mRDT intervention in different contexts.
This paper presents an analysis of the findings from 10 mRDT intervention studies conducted in Africa and Afghanistan, for which indepth information was available about interventions, outcomes and contexts. The studies, all from the ACT Consortium, represent a large proportion of the intervention studies on mRDTs recently conducted in areas of ongoing malaria transmission. This analysis aimed to identify how mRDTs can be used to improve prescribing in different contexts by exploring factors influencing providers' use of and adherence to test results and comparing results of interventions in different settings.
Methods
The ACT Consortium is an international research collaboration involving more than 20 institutions working on a systematic series of 25 studies in 10 countries in Africa and Asia, addressing practical questions in the delivery of malaria treatment.13 Intervention studies involving mRDTs were conducted in 10 sites in 6 countries. The analysis in this paper focuses on these studies because of the ability it gives to use raw outcome data (allowing comparable outcomes to be calculated), raw data from linked qualitative research, unpublished documentation about intervention content, implementation and contextual information as well as insights from the study teams. This allowed a more detailed and comparable analysis than could be achieved through reliance on publications or quantitative data alone.
This analysis used a comparative case study approach, where each study arm conducted in a distinct setting was considered a case and outcomes were interpreted in terms of the study design, intervention content, implementation and contextual factors.14 This approach suits investigation of ‘how’ and ‘why’ interventions have an effect and can highlight comparative general trends and distinct patterns that are not visible in single cases.15 17 The analysis explored three outcomes:
-
Provider uptake of mRDTs.
The proportion of patients presenting with fever, or history of fever in past 48 hours (unless specified otherwise), who were tested for malaria with an mRDT, as reported by the provider or patient.
-
Provider adherence to positive mRDT results.
The proportion of patients with a positive mRDT result (for Plasmodium falciparum malaria), who were prescribed or received an ACT, the first-line drug for non-severe malaria in all cases, as reported by provider or patient.
-
Provider adherence to negative mRDT results.
The proportion of patients with a negative mRDT result who were not prescribed, or did not receive, any antimalarial as reported by provider or patient (the effect of negative mRDT results on the use of other treatments, including antibiotics, in ACT Consortium studies has been presented in a separate paper).16
The analysis evaluated the impact of different interventions to introduce mRDTs in different contexts. Twenty-eight cases (ie, distinct settings or intervention arms) from the 10 studies were included, with a total of 148 461 patients (see table 1). Twenty cases from 7 studies analysed mRDT uptake, 24 cases from 9 studies evaluated provider adherence to positive mRDT results and all 28 cases analysed provider adherence to negative mRDT results.
Table 1.
Cases included in analysis
| Study | Study name | Country | Providers targeted | Cases* | Published results |
|---|---|---|---|---|---|
| Afgh1 | Strategies for expanding access to quality malaria diagnosis in south-central Asia where malaria incidence is low | Afghanistan | Government primary care providers | Afgh1/a: training; patients individually randomised to receive either mRDT or established microscopy, Eastern province | 18–20 |
| Afgh1/b: training; patients individually randomised to receive either mRDT or recently introduced microscopy, Northern province | |||||
| Afgh1/c: training; patients individually randomised to receive either mRDT or clinical diagnosis (no microscopy available), Northern province | |||||
| Cam1 | Cost-effectiveness of interventions to support the introduction of malaria rapid diagnostic tests in Cameroon | Cameroon | Government and mission providers (in hospitals and primary care) | Cam1/a1: basic training, Bamenda | 21–27 |
| Cam1/b1: basic training, Yaoundé | |||||
| Cam1/a2: enhanced training, Bamenda | |||||
| Cam1/b2: enhanced training, Yaoundé | |||||
| Ghan1 | How the use of rapid diagnostic tests influences clinicians' decision to prescribe ACTs | Ghana | Government primary care providers | Ghan1/a: training; patients individually randomised to receive either mRDT or microscopy | 28–30 |
| Government and private primary care providers | Ghan1/b: training; patients individually randomised to receive either mRDT or clinical diagnosis | ||||
| Nig1 | Costs and effects of strategies to improve malaria diagnosis and treatment in Nigeria | Nigeria | Government primary care providers, private pharmacies and private medicine dealers | Nig1/a1: basic training, Enugu | 27 31–34 |
| Nig1/b1: basic training, Udi | |||||
| Nig1/a2: enhanced training, Enugu | |||||
| Nig1/b2: enhanced training, Udi | |||||
| Nig1/a3: enhanced training + school activities, Enugu | |||||
| Nig1/b3: enhanced training + school activities, Udi | |||||
| Tanz1 | IMPACT 2: Evaluating policies in Tanzania to improve malaria diagnosis and treatment | Tanzania | Government healthcare providers (in hospitals and primary care) | Tanz1/a: standard MoH† training, Mwanza, moderate transmission | 35 |
| Tanz1/b: standard MoH training, Mbeya, low transmission | |||||
| Tanz1/c: standard MoH training, Mtwara, moderate transmission | |||||
| Tanz2 | Targeting ACT drugs: the TACT trial | Tanzania | Government primary care providers | Tanz2/a1: pilot study, low transmission | 36–38 |
| Tanz2/b1: pilot study, moderate transmission | |||||
| Tanz2/2: basic training | |||||
| Tanz2/3: enhanced training | |||||
| Tanz2/4: enhanced training + patient sensitisation | |||||
| Tanz3 | Effectiveness of malaria rapid diagnostic tests in fever patients attending primary healthcare facilities in Zanzibar | Tanzania | Government primary care providers | Tanz3: enhanced training, Zanzibar | 39 40 |
| Uga1 | The PRIME trial: improving health centres to reduce childhood malaria in Uganda | Uganda | Government primary healthcare providers | Uga1: training, Tororo | 41–44 72 73 |
| Uga2 | Use of rapid diagnostic tests to improve malaria treatment in the community in Uganda | Uganda | Community health volunteers | Uga2/a: training, low transmission | 45 74 |
| Uga2/b: training, moderate transmission | |||||
| Uga3 | Introducing rapid diagnostic tests in drug shops to improve the targeting of malaria treatment | Uganda | Private drug shop vendors | Uga3: training, Mukono | 46–51 |
*The initial letters refer to the study country, the first number refers to the (country-specific) study number, the subsequent letter refers to the specific context if a study took place in multiple geographical or epidemiological settings and the final number refers to the intervention arm.
†MoH, Ministry of Health.
The studies took place between 2007 and 2012. Studies were either individual (n=2) or cluster-randomised controlled trials (n=6); observational (n=2) or preintervention/postintervention studies (n=1) (Tanz2 used different designs in their pilot and main study, so n=11). Providers targeted were governmental or non-governmental healthcare workers, private retail sector workers or community health volunteers. Six studies took place in East Africa, three in West AfricaCam1,Nig1,Ghan1 and one in south-central AsiaAfgh1. One focused only on children under 5 yearsUga2; the rest included children and adults. See online supplementary file 1 for more detailed information about each study.
bmjopen-2016-012973supp1.pdf (574.4KB, pdf)
All the interventions included basic training on malaria testing with RDTs for healthcare providers, however the content, duration and approach varied. Some interventions included additional activities and materials such as extra training, supervision and feedback, patient information leaflets or school-based activities (see table 2 and online supplementary file 1).
Table 2.
Intervention content
| Scenario | mRDT/malaria training | Supervision | mRDT/ACT supplies | Other intervention activities |
|---|---|---|---|---|
| Afgh1/a | One and a half day training, following the national training package. This covered performing mRDTs (most, but not all, practiced testing) and prescribing antimalarials | None | mRDTs supplied by study | None |
| Afgh1/b | ||||
| Afgh1/c | ||||
| Cam1/a1 | One day, didactic session covered three modules: malaria diagnosis, mRDTs, and malaria treatment | Monthly | mRDTs and ACTs supplied by study | None |
| Cam1/b1 | ||||
| Cam1/a2 | Same as Cam1/1, plus: Interactive two day training on adapting to change (focused on WHO malaria treatment guidelines), professionalism and effective communication |
Monthly | mRDTs and ACTs supplied by study | None |
| Cam1/b2 | ||||
| Ghan1/a | Two day training about the sensitivity and specificity of mRDTs, alternative causes of febrile illness and the Ghana national guidelines (which indicated presumptive treatment for children who are <5 years old) | None, but study team were present | mRDTs supplied by study | None |
| Ghan1/b | ||||
| Nig1/a1 | Half day demonstration on how to use mRDTs, which included practising conducting one test. They also received a copy of the WHO job aid, which shows the steps in using an mRDT | None | mRDTs supplied by study | None |
| Nig1/b1 | ||||
| Nig1/a2 | Same as Nig1/1, plus: Two day interactive, seminar-style training, covering how to test, appropriate treatment for positive and negative results and effective communication. Those attending were given job aids (eg, treatment algorithm) |
Monthly | mRDTs supplied by study | None |
| Nig1/b2 | ||||
| Nig1/a3 | Same as Nig1/2 | Monthly | mRDTs supplied by study | School-based activities |
| Nig1/b3 | ||||
| Tanz1/a | Two day training (standard MoH), covering performing mRDTs (including practical) and prescribing antimalarials | Routine MoH supervision only | mRDTs supplied by MoH | None |
| Tanz1/b | ||||
| Tanz1/c | ||||
| Tanz2/a1 | One day training on how to use the mRDT and read the result. Antimalarial drug use guidelines were reviewed and job aids provided | None | mRDTs supplied by study | None |
| Tanz2/b1 | ||||
| Tanz2/2 | Two day, didactic, MoH training on how to use mRDTs, including practical | Six-weekly, focused on supplies and reporting | mRDTs supplied by study | None |
| Tanz2/3 | Same as Tanz2/2, plus: Three additional 90 min interactive training workshops, with one session repeated 6–7 months later. These covered: adapting to the change in the diagnosis and management of malaria; practice with confidence when using mRDTs: tools to enable change in managing febrile illness; sustaining the change in practice. Training on communication skills was included |
Six-weekly, focused on supplies and reporting | mRDTs supplied by study | SMS feedback on own mRDT uptake and adherence at 5 months Two times per day motivational SMS for 15 days |
| Tanz2/4 | Same as Tanz2/3 | Six-weekly, focused on supplies and reporting | mRDTs supplied by study | SMS feedback on own mRDT uptake and adherence at 5 months Two times per day motivational SMS for 15 days. Patient leaflets and posters |
| Tanz3 | Six to 11 days IMCI training (depending on whether refresher training or for new health workers) which included malaria diagnosis and treatment, plus 1-week study-specific training (including good clinical practice, provision of informed consent, performance and interpretation of mRDT according to the manufacturer's instructions). One day of the IMCI training focused specifically on malaria. Training covered communication skills | None | mRDTs and ACTs supplied by MoH, with study back up in the case of stockouts | IMCI training, additional study salary for providers |
| Uga1 | Two day training session followed a week later by on-site training in facilities. Training was interactive and included performing and reading an mRDT, management of a patient with fever and either a positive or negative mRDT as well as patient communication. All health workers were invited to attend the training | Supervision at 6 weeks and 6 months | mRDTs supplied by MoH, with study back up in the case of stockouts | Training on patient-centred services; training in-charges in health centre management |
| Uga2/a | Four day interactive training, covering performing and reading an mRDT, how to prescribe antimalarials, how to deal with negative cases and communication skills. Providers were also given pictorial job aids | Close supervision for first 6 months (prior to evaluation) | mRDTs and ACTs supplied by study | Community sensitisation |
| Uga2/b | ||||
| Uga3 | Four day interactive training to all drug shop vendors, which covered performing and reading mRDTs, prescribing antimalarials, how to deal with mRDT negatives and communicating and negotiating with patients | Close supervision for first 2 months (prior to evaluation) | mRDTs and ACTs supplied by study | Community sensitisation |
Three studies compared different training packagesNig1,Cam1,Tanz2. Six studies compared intervention effects in different epidemiological contextsUga2,Tanz1,Nig1,Cam1,Afgh1,Ghan1. Seven studies evaluated an intervention against a control arm where mRDTs were not made availableUga1,Uga2,Uga3,Nig1,Cam1,Afgh1, Ghan1.
Comparability of findings
Although the studies were co-designed and largely similar, because of differences in primary study questions and differences in epidemiology, data collection methods and evaluation timing, mean pooled analyses would be inappropriate. For example, mRDT uptake was reported through provider-completed registers in some projects and patient exit interviews in others. Some studies reported adherence in terms of the percentage of patients prescribed ACTs or antimalarials, while others reported the percentage of patients who received them. Stockouts may have affected receipt of medication; whether prescriptions were affected is unknown, as alternative medication may or may not have been offered when there was a known stockout. The analysis presented therefore focuses on understanding the reasons for variation in the results, rather than seeking pooled point estimates.
Quantitative outcome data were extracted from each study's raw data set and reanalysed to maximise comparability across studies, using the most comparable denominators and numerators possible. Study, intervention and context characteristics were extracted from published and unpublished documents. Where available, thematic content analysis was undertaken on qualitative data from providers involved in the studies (ie, focus group discussionsUga2,Uga3 or interviewsAfgh1,Ghan1,Tanz1/a,Tanz1/b,Tanz2,Uga1 with health workers, drug shop vendors or volunteers). In Tanz3, interviews from a later, related study were analysed, which included six study providers and six similar providers who had not been involved in the study but had comparable mRDT experiences.
The analysis drew on the approaches informing intervention component analysis (ICA)52 and qualitative comparative analysis (QCA),53 which seek to identify critical features of interventions. As with ICA, we sought to identify how interventions differed from one another and then, as with QCA, identify which factors appeared to be important. Our initial stage involved gathering as much information about the interventions as possible, going broader than the ICA approach by also capturing information about their delivery and context. However, our analysis differed from ICA and QCA, which attempt to characterise and apply scores to interventions and their characteristics and cross-tabulate these with outcomes. We found our data were not amenable to scoring in a quantitative sense, due to wide variation in the extent and types of information available. Therefore, our analysis was qualitative, using a meaning-based approach. Tables were created for each outcome of interest, with explanatory factors relating to the intervention, context and study design (see online supplementary file 2 for an example). These were shared with study teams and the ACT Consortium core scientific team, with ongoing discussions about the findings and other potential explanatory factors.
bmjopen-2016-012973supp2.pdf (98KB, pdf)
Results
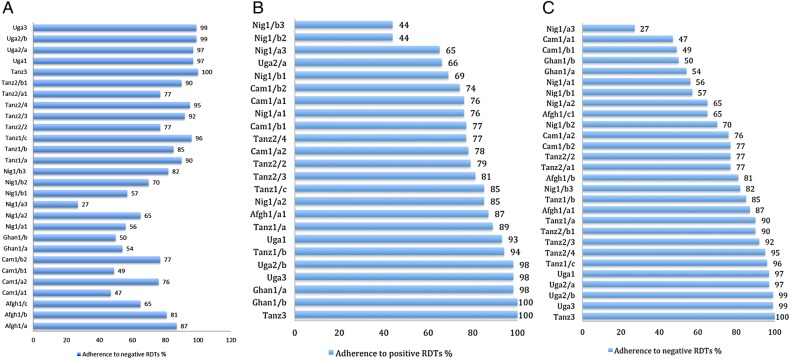
There was wide variation across cases in all three outcomes: 12–100% mRDT uptake (figure 1A); 44–98% adherence to positive mRDTs (figure 1B); 27–100% adherence to negative mRDTs (figure 1C). All outcomes were universally high in some casesUga1,Uga2/b,Uga3 and universally low in othersNig1/a1,Nig1/a3, but in many cases, the three outcomes did not correspond—for example, testing was infrequent but adherence to results highTanz1/a,Tanz1/b,Tanz2/3 or adherence to positives high, but negatives lowGhan1/a,Ghan1/b,Cam1/a1,Cam1/b1, or vice versaUga2/a,Nig1/b3.
Figure 1.
(A) Uptake of malaria rapid diagnostic tests (mRDTs) (% patients with fever or history of fever who were tested for malaria with an mRDT).(B) Adherence to positive mRDT results (% of patients with a positive mRDT who did receive ACTs). (C) Adherence to negative mRDTs (% of patients with a negative mRDT results who did NOT receive antimalarials).
There were no single factors which alone accounted for any of the outcomes; successful mRDT uptake and adherence appeared to result from a combination of context and intervention characteristics. The analysis identified several factors which, taken together, may account for the heterogeneity observed. The appeal of the intervention to providers was crucial for all three outcomes, but each was additionally shaped by other factors.
Factors affecting mRDT uptake
There was wide variation between cases in the use of mRDTs for febrile patients (see figure 1A). Providers' motivation to perform well in the intervention was associated with uptake, as were familiarity with testing, adequate human resources and supplies, and the cost of mRDTs.
Motivation to perform well in the intervention
The range of sectors and contexts in which providers worked meant that their own priorities varied between cases. For example, government health workers' priorities may have included some or all of the following: treating ill patients, managing their workload in the light of staff shortages, managing (or ‘rationing’) their medicine supplies in the face of future shortages, maintaining their position of authority as a clinician. In contrast, while private providers may also have prioritised treating ill patients, some viewed their role as more of a business than a healthcare service. As such, their priorities may have been more business-oriented, such as making a profit and ensuring sufficient customers.
Data on provider priorities were not available for all cases; for some, qualitative data were available but for others, anecdotal evidence and study team perceptions were used. Nevertheless, where the intended use of mRDTs and associated intervention activities aligned well with providers' own priorities, they appeared more motivated to participate and ‘perform’ well in the intervention, and we observed higher uptake and adherence. There were a number of explanations for, and/or factors associated with, higher motivation but political and financial support were often critical. For example, in Tanz2, carefully developed messages addressing existing provider principles and practices, as well as Ministry of Health branding of the intervention (an institution known to influence the government health workers in this setting), appeared to motivate providers. In Uga3, the drug shop vendors were previously not permitted to offer testing and this new service, along with the associated training, supervision and visible involvement of the Ministry of Health, gave them a legitimacy they had previously lacked.48 These vendors also reported increased customer numbers and associated profits, enhanced by the study's free provision of mRDTs and ACTs for them to sell at a subsidised rate. In Tanz3, government providers were paid a supplement to participate in the study. Additional unintentional aspects of studies, such as regular visits or perceived support from evaluators, may have also helped to improve outcomesUga3,Tanz2.38
In contrast, where mRDT interventions were not aligned with provider priorities, we saw lower uptake and adherence. For example, in Nig1 in the private sector, providers saw themselves more as vendors than healthcare practitioners. Here, there were anecdotal reports that they were particularly concerned about losing money from sales if mRDT results were negative and wondered whether the public would consider them legitimate to test. This was the case in spite of the free provision of mRDTs to providers by the study team. When providers viewed the intervention as extra unpaid work (eg, conducting tests or recording test results), this affected their motivation. In Uga3, some drug shops declined to participate in the trial for this reason and in Uga1, some health facilities hesitated to continue participating when they felt the work was too much without remuneration. Here, a misalignment between the providers' priorities and the intentions of the intervention led to a lack of motivation for providers to perform in line with guidelines.
Familiarity with testing
In most cases, there was little prior experience of malaria testing, either using mRDT or microscopy. Although patients were generally keen to be tested for malaria, it was not typically part of providers' routine habits to test. In cases where testing had become part of the established process of care, mRDT uptake tended to be higher. For example, in Tanz1/c, mRDTs had already been scaled up in other districts in recent years, and at baseline there was substantial microscopy testing, unlike the other two cases in this study where uptake was lowerTanz1/a,Tanz1/b. Wide-scale public awareness of testing may have facilitated uptake, for example, in Cameroon, where mass communication campaigns coincided with the studyCam1, which saw an increase in malaria testing in all study arms from baseline.23 Some interventions incorporated local community sensitisation activities to increase familiarityUga2,Uga3,Tanz2/4,Nig1/3, although this appeared insufficient on its own to ensure high uptake.
Adequate human resources and supplies
Where staff workload was high, or patient numbers exceeded capacity, particularly in small facilities with only one staff member, mRDTs were not always usedUga1,Tanz2/1.
There were adequate stocks of mRDTs in facilities in most studies, in several cases due to study provision of additional supplies to avert stockouts. However, stockouts did occur in some studiesCam1,Tanz1,Tanz2, which was associated with lower uptake to some extent. Nevertheless, even when mRDTs were available, they were not always used, suggesting other factors were also influential.
Cost of mRDTs to patients
In most studies, mRDTs were provided free to patients. In those cases where providers were permitted to charge patients for mRDTs, higher prices may have affected their uptake. For example in Nig1, where mRDT uptake was among the lowest observed, patients were charged more than the recommended price on average, particularly in the private sector.
Factors affecting adherence to positive mRDT results
ACTs were not consistently prescribed to patients with positive mRDT results (see figure 1B). Given the expectation for antimalarial overuse based on previous data, this finding was not anticipated and reasons for low adherence to positive results were therefore not explicitly explored during the studies. However, some explanatory factors driving this outcome did emerge, in addition to the motivation to perform well in the intervention (discussed above). These were the stability of ACT supplies and local preferences for different types of antimalarial.
Stability of ACT supplies
Stockouts of ACTs were associated with variation in adherence to positive mRDT results; however, this could not explain all the variation. In some cases, ACT use was relatively low despite no or few stockouts, whereas in others, use was high despite stockouts occurring. It may be that provider confidence in the stability of ACT supplies also influenced the use and rationing of ACTs, even when ACTs were available. For example, in Tanz2, lower rates of adherence to positive mRDTs were observed in the case where stockouts were most frequentTanz2/4, even after periods of stockouts were excluded from the analysis.
Pre-existing antimalarial preferences
Information on pre-existing antimalarial preferences was gathered from baseline and preintervention surveys,32 49 interview transcriptsTanz1 and unpublished reports,54 although no data were available for five studiesAfgh1,Ghan1,Tanz3,Uga1,Uga2. The data suggest an association between the use of ACTs for positive mRDTs and baseline preferences for, or use of, ACTs rather than other antimalarials. For example, in Nig1, where ACT use was generally low, prior to the intervention, other antimalarials were asked for by patients, prescribed and purchased more commonly than ACTs.34 In contrast, in Tanz1, where adherence to RDT positive results was higher, according to stakeholder interviews, ACTs were patients' preferred antimalarial. This may have been due to greater exposure to community sensitisation around ACTs55 or cultural norms around provider authority such that patients felt more inclined to change their preferences in the light of providers' guidance than was the case in Nigeria. An alternative explanation relates to the different roles of the public sector in these countries and therefore, the different influence that the choice of official first-line medicines has on preferences. For example, in Tanzania, public facilities are much more widely used that they are in Nigeria, so people will have become used to the idea of ACTs. In Nigeria, the public sector is a more limited provider, so making a drug officially first line may have much less effect on preferences.
Factors affecting adherence to negative mRDT results
There was also wide variation in the proportion of patients prescribed or given antimalarials in spite of negative mRDT results (see figure 1C). In addition to being motivated to perform well in the intervention (discussed above), the analysis suggests adherence to negative mRDTs was also driven in part by the extent to which mRDTs fitted—or were helped by intervention activities to fit—into the existing landscape of care (existing diagnostic and consultation practices). This included providers' perceptions of the role of mRDTs in the diagnostic process and possibilities for alternative diagnoses and treatment. In addition, the analysis suggests that adherence was affected by the extent to which the interventions attempted to control clinical practice.
Malaria tests were usually the only diagnostics available in study facilities. In most cases, test-based malaria diagnosis required a substantial shift from reliance on clinical judgement. In a minority of cases, this shift had already begun before the evaluation started, for example, in Tanzania and Zanzibar where mRDT introductions had begun nationallyTanz1,Tanz3, or where malaria testing using microscopy was establishedAfgh1/a,Afgh1/b,Tanz1/c. Here, mRDTs appeared to fit into the landscape of care more easily and adherence to negative mRDT results was higher. Where testing was new and did not fit into the landscape of care so well, even if mRDT use was attractive, adhering to negative results appeared more difficultAfgh1/c,Cam1,Ghan1,Nig1.
Two factors appeared to facilitate integration of mRDTs into the landscape of care: providers' perceptions of the role of mRDTs in the diagnostic process and whether alternative management of illnesses, not involving antimalarials, was possible for those with negative mRDT diagnoses.
Perceived role of mRDTs in diagnostic process
Two main factors influenced providers' perceptions of the role of mRDTs within the process of malaria diagnosis: how well mRDTs fitted with the dynamic of consultations and whether the mRDT results matched their expectations.
In some cases, providers saw mRDTs as central to the diagnostic process. For example, community health volunteers in Uga2, whose adherence was very high, described the mRDTs as working as ‘a judge’, and drug shop vendors in Uga3 saw taking blood as crucial to their enhanced role. Conversely, some providers felt clinical judgement should play a more important role in making a diagnosis than mRDTs. Qualitative data suggested that where mRDTs challenged clinicians' expertise and disrupted traditional consultation practices, this led to lower adherence to negative results Afgh1,Ghan1,Tanz2/1. By questioning the test's accuracy, providers were able to reassert their authority and manage the consultation as usual.18 36
Some interventions aimed to help mRDTs ‘fit’ with the dynamics of consultations. For example, training included role-play activities or reflections about how mRDTs would work in practiceCam1/2,Uga1,Uga3, experimentationTanz2/3. Tanz2/4 and reflection facilitated by multiple training and feedback sessions with peersCam1/2,Tanz2/3,Tanz2/4,Uga1,Uga2,Uga3; and training on communicating with patientsCam1/2,Nig1/2, Tanz2/3,Tanz2/4,Uga1,Uga 2,Uga3. Providers reported positive impressions of the training's impact on their interactions with patients including the importance of talking to patients and explaining the need for mRDTs or the meaning of their resultsGhan1,Tanz2/1,Tanz2/3,Tanz1/a,Uga2.
In some cases, mRDT results did not match expectations; typically, fewer mRDTs were positive than had been expected, particularly when the tests were first introducedUga3,Tanz2/4,Ghan,1/2. When this happened, providers placed less emphasis on mRDTs in the diagnostic process, preferring to rely more heavily on clinical judgement. For example, in Cam1/a1, mRDT positivity rates were just 9%, despite the local perception that malaria prevalence was high in that area. Several interviewees from different cases explained that it was hard to trust mRDTs when so many results were negativeGhan1/b,Nig1,Tanz1/b,Tanz2/4,Uga3, or that they only trusted them once they had seen some positive mRDT resultsUga2,Tanz2/4. Providers described a fear of missing malaria diagnoses, particularly when the frequency of positive results was lower than expected, and this was associated with lower adherenceGhan1/1,Ghan1/2,Tanz1/b. In contrast, providers in Tanz3, where adherence to negative mRDTs was high, appeared less concerned about malaria, recognising that prevalence had declined. Some interventions explicitly aimed to raise awareness of current malaria epidemiology during trainingTanz2/3,Tanz2/4,Uga1 in order to (re)set expectations of mRDT positivity rates; this was also associated with higher adherence to negative results.
In several cases, providers reported that their trust in mRDTs grew over timeTanz3, Tanz2/2, Tanz2/3, Uga3. Some described deliberate ‘experimentation’ to build trust in results, either by testing with microscopy as well as mRDTsAfgh1 or by seeing whether mRDT-negative patients recovered without antimalarialsGhan1,Uga2. Indeed in one study, this was explicitly encouragedTanz2/3, Tanz2/4. Conversely, some providers' accounts showed mistrust of mRDTs was reinforced by experiences of seeing patients, or indeed themselves, recover when taking antimalarials in spite of a negative mRDT resultUga2/b,Ghan1/a. Patient follow-up was considered another useful means of building trustUga2, Ghan1/b. Two interventions aimed to increase the perceived role of mRDTs by providing information about mRDTs' sensitivity and specificityTanz1,Tanz2/3,Tanz2/4.36
Alternative treatments for non-malarial fever patients
Interventions offered different options for dealing with mRDT-negative patients (as mentioned above, data on the use of alternative treatments are presented in a separate paper). It appeared that expectations and options for alternative management of negative cases—in terms of providers' role, knowledge of case management and availability of other medicines—were important in antimalarial prescribing to mRDT-negative patients. In the public facility interventions where detailed guidance was given to aid alternative diagnosesUga1,Tanz2,Tanz3, adherence was higher than in public facilities where no substantial guidance was providedGhan1,Afgh1 or where it was recommended that providers only offer antipyretics to mRDT-negative patientsNig1/2,Nig1/3. At the community level, where volunteer providers were not expected (or permitted) to provide medicines beyond antimalarialsUga2, adherence to negative results was high. In private shops in Uganda, where no training on non-malarial febrile illness management was provided, adherence to mRDT-negative results was still high in terms of ACT prescription, although here mRDT-negative patients ended up being sold other medicinesUga3.
Directive intervention approach
Some interventions were more directive about provider practices, particularly regarding the use of unambiguous guidance and supervision or surveillance.
Adherence was typically higher if interventions instructed that no antimalarial should be given to those with negative mRDT resultsUga1,Uga2,Uga3,Tanz3. In contrast, adherence was lower when an intervention allowed exceptions for when antimalarials could be given in spite of a negative result, for example, if a febrile patient was under 5 years and had travelled a long distance to seek careAfgh1,Tanz2/2,Cam1.
The highest adherence was observed among providers who had been closely supervised—either for an intense period after trainingUga2,Uga3 or throughout the evaluation periodTanz3. Providers receiving feedback by text message experienced these as a form of surveillance, and reported responding by feeling they should follow guidelines even if their clinical judgement was at odds with thisTanz2/3,Tanz2/4.
Discussion
This analysis addresses the persisting gap in knowledge around how to change prescribing practices. This is a key question in this time of international concern over resistance to antimicrobial medicines, with the imperative to optimise medicine use agreed on by United Nations signatories.56 57 By analysing indepth data from 10 co-designed intervention studies from the ACT Consortium, we identify factors affecting the uptake of mRDTs and adherence to test results in different contexts. The varied findings suggest that to improve prescribing through mRDTs, interventions must go beyond basic training in mRDT use and must be tailored to the needs of providers in particular contexts. Uptake and adherence were highest where providers were motivated by the intervention and the tests fitted with the landscape of care. Intervention characteristics that aligned mRDTs with provider priorities included interactive training that addressed how to manage test-negative patients in practice, including clinical and interpersonal aspects of care. Where malaria endemicity is overestimated locally, experimentation and feedback on frequent test-negative cases was important. A directive approach supported by feedback or supervisory instruction can yield high adherence to guidelines but may affect patient-centred care. The results suggest that as mRDTs become established, the intensity of supporting interventions required is likely to reduce.
A strength of this analysis was its use of rich data sources which enabled a more indepth and comprehensive analysis. Although additional insights may have emerged from inclusion of a wider set of studies, synthesising findings from published healthcare interventions is often challenging, with diverse and poorly described interventions, contexts and methods.58 59 Nevertheless, our analysis was limited by the fact that not all included studies were able to provide information on all characteristics of interest, while for other characteristics (eg, year and duration), there was too much variation to identify any patterns. While study samples were generally sizeable, in some cases where testing rates and/or malaria prevalence were low, the denominator for adherence outcomes was small. With one exception, where a government mRDT policy was evaluatedTanz1, all of the evaluated interventions in this analysis were instigated by the study teams. As such, there may be aspects of the interventions, such as RDT supply sources and costs to providers, which may not apply at scale.
Previous studies have identified capacity issues as important in mRDT implementation, such as staffing levels or overworked staff,9 12 60–64 mRDT or ACT supplies,9 12 61–65 and providers' confidence in mRDT results.12 61–66 Our synthesis shows that beyond these issues, the introduction of the tests had to make sense in context. Some interventions in our analysis additionally included a more directive approach. While these interventions did achieve the highest rates of adherence to negative results, the consequences of restricting the autonomy of clinicians in favour of standardised guidelines need to be weighed up against the need for clinicians to consider individual patients on a case-by-case basis.67 Our finding, that settings where testing was more familiar used mRDTs more appropriately, echoes observations from country-level roll-out of mRDTs,68 69 and suggests that the interventions required will change over time. Our finding, that basic training alone is insufficient to ensure use of the tests as intended, aligns with findings from studies of interventions aiming to change clinical practice in general.4 70
Prior to introducing mRDTs, initial assessments should be carried out to understand providers' priorities and capacities, as well as how easily tests might integrate into landscapes of care. Although our analysis suggests that a process of tailoring is required to formulate the intervention to best fit each context, certain broad intervention features are likely to be applicable across settings (see box 1). As these recommendations arise directly from the data available in our studies, they are not exhaustive.
Box 1. Examples of recommended intervention features.
Planning
Recognise and address providers’ priorities
Staffing
Ensure sufficient staff numbers for increased workload
Training
Offer longer, more detailed training, incorporating interactive activities
Include training on communicating with patients
-
Address process of change to test-based care:
plan a series of interactive training and/or supervision sessions
incorporate role-play activities which address local challenges
use reflective activities
-
Build trust in mRDTs by including:
discussion of data on changes in malaria prevalence in the area
discussion of sensitivity and specificity of mRDTs
encouragement to cross-check these data with experience of tests in practice
Guidance
Provide detailed guidance and resources for acceptable case management for mRDT-negative patients
Consider how directive mRDT guidance should be, balancing clarity with the need for clinician judgement to make exceptions (eg, if patients have travelled far, with limited means of transportation to return if their condition worsens)
Medical supplies
Ensure providers can be confident in supplies of mRDTs and ACTs
Keep costs to patients low
Community/patient sensitisation
-
Conduct patient-oriented sensitisation activities
where familiarity with testing is low, where frequent false-positive microscopy has overestimated prevalence, or if ACTs are not the most common antimalarial used or demanded by patients
These findings can inform broader antimicrobial stewardship efforts. Malaria is the first disease for which interventions have been systematically evaluated in order to understand how to change routine prescribing through rapid diagnostics. The lessons learnt in attempting to shift from presumptive to test-directed treatment are relevant for interventions beyond malaria. The intervention and contextual characteristics identified here highlight that apparently simple technological solutions can require complex supporting apparatus when implemented in real life.71 However, these findings suggest that as mRDTs become established, the intensity of supporting interventions required is likely to reduce. Further research could explore whether an initial investment in mRDTs could establish patterns of care that allow for other diagnostic tests to be introduced more easily in the future.
Conclusion
This analysis shows that uptake and adherence to mRDTs can be high, but this requires either existing contexts where integrating the tests into practice already makes sense, or tailored interventions to encourage this. Basic training and supplies are essential but insufficient to maximise the potential of mRDTs in contexts where they do not fit well with the landscape of care. Apparently simple technological solutions such as mRDTs can require complex supporting interventions that take account of how they will be interpreted and used.
Acknowledgments
This research was funded by the ACT Consortium through a grant from the Bill and Melinda Gates Foundation to the London School of Hygiene and Tropical Medicine. The authors gratefully acknowledge the contribution of colleagues involved in each of the studies included in the analysis, in particular those who collected data, conducted analysis or contributed to the concept of this analysis: Bonnie Cundill, Catherine Maiteki, Clarence Mkoba, Evelyn Ansah, Ismail Mayan, Lindsay Mangham Jefferies, Mark Rowland, Mwinyi Msellem, Patrick Kachur, Rebecca Thomson, Renata Mandike, Richard Ndyomugyenyi, Seth Owusu-Agyei, Shunmay Yeung, Toby Leslie, Jo Reynolds, Hugh Reyburn, David Lalloo and David Schellenberg. The authors would also like to thank all other participants in the included studies: the patients and their guardians, providers, data collectors and other study team members. LSV is an employee of the WHO and DRA is an employee of the Centres for Disease Control and Prevention.
Footnotes
Disclaimer: The views expressed in this article are the views of the authors and may not necessarily reflect the views of the WHO or CDC.
Contributors: HEDB and CIRC designed the study. HEDB conducted the analysis and drafted the paper; CIRC contributed to analysis and drafting. BL, FB, KB, AB, KBr, SC, DDL, KE, CG, HH, SL, PM, AM, WM, AMb, OO, DRA, DS, SS and LSV contributed to data collection. All authors contributed to study design, analysis and the final write-up and approved the manuscript.
Funding: This analysis, as well as the projects it included, was funded by the Bill and Melinda Gates Foundation, grant number 39640.
Competing interests: None declared.
Patient consent: No.
Ethics approval: ZAMREC, Zanzibar; Ghana Health Service Ethical Review Committee; UNCST; MU SOMREC; Ministry of Health and National Institute of for Medical Research, Tanzania; Ministry of Health Institutional Review Board, Afghanistan; University Committee on Medical and Scientific Research Ethics, Nigeria; National Ethics Committee, Cameroon; Makerere University IRB; Uganda National Council for Science and Technology; LSHTM; University of California San Francisco Committee on Human Research; CDC; IHI, NIMR.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data from the studies included in this analysis can be found at the ACTc repository: https://actc.lshtm.ac.uk. This includes outcome data, description of intervention and data collection tools.
References
- 1.World Health Organization (WHO). New Perspectives: Malaria Diagnosis. Report of a joint WHO/USAID Informal consultation, 25–27 October 1999 Geneva, Switzerland: World Health Organization (WHO), 2000. [Google Scholar]
- 2.World Health Organization (WHO). T3. Test. Treat. Track. Scaling up diagnostic testing, treatment and surveillance for malaria. Geneva: Global Malaria Programme, 2012. http://www.who.int/malaria/publications/atoz/t3_brochure/en/ [Google Scholar]
- 3.World Health Organization (WHO). The use of malaria rapid diagnostic tests. 2nd edn Geneva, Switzerland: WHO, 2006. [Google Scholar]
- 4.Bell D, Perkins MD. Making malaria testing relevant: beyond test purchase. Trans R Soc Trop Med Hyg 2008;102:1064–6. 10.1016/j.trstmh.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 5.Drakeley C, Reyburn H. Out with the old, in with the new: the utility of rapid diagnostic tests for malaria diagnosis in Africa. Trans R Soc Trop Med Hyg 2009;103:333–7. 10.1016/j.trstmh.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO). Guidelines for the treatment of malaria. 2nd edn Geneva: WHO, 2010. [Google Scholar]
- 7.Mendelson M, Rottingen JA, Gopinathan U et al. . Maximising access to achieve appropriate human antimicrobial use in low-income and middle-income countries. Lancet 2016;387:188–98. 10.1016/S0140-6736(15)00547-4 [DOI] [PubMed] [Google Scholar]
- 8.Odaga J, Lokong JA, Donegan S et al. . Rapid diagnostic tests versus clinical diagnosis for managing people with fear in malaria endemic settings. Cochrane Database Syst Rev 2014;(4):CD008998 10.1002/14651858.CD008998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao VB, Schellenberg D, Ghani AC. Overcoming health systems barriers to successful malaria treatment. Trends Parasitol 2013;29:164–80. 10.1016/j.pt.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 10.Johansson EW, Gething PW, Hildenwall H et al. . Diagnostic testing of pediatric fevers: meta-analysis of 13 national surveys assessing influences of malaria endemicity and source of care on test uptake for febrile children under five years. PLoS ONE 2014;9:e95483 10.1371/journal.pone.0095483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson EW, Gething PW, Hildenwall H et al. . Effect of diagnostic testing on medicines used by febrile children less than five years in 12 malaria-endemic African countries: a mixed-methods study. Malar J 2015;14:194 10.1186/s12936-015-0709-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochodo E, Garner P, Sinclair D. Achieving universal testing for malaria. BMJ 2016;352:i107 10.1136/bmj.i107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. http://www.actconsortium.org/
- 14.Pope C, Mays J, Popay J. Synthesizing qualitative and quantitative health evidence: a guide to methods. Berkshire, England: McGraw Hill/Open University Press, 2007. [Google Scholar]
- 15.Yin RK. Case study research: design and methods. 4th edn London: SAGE Publications, 2009. [Google Scholar]
- 16.Hopkins H, Bruxvoort KJ, Cairnes ME et al. . The impact of introducing malaria rapid diagnostic tests on antibiotic prescribing: a nine-site analysis in public and private health care settings. BMJ 2017, in press. [Google Scholar]
- 17.Crowe S, Cresswell K, Robertson A et al. . The case study approach. BMC Med Res Methodol 2011;11:100 10.1186/1471-2288-11-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds J, Wood M, Mikhail A et al. . Malaria “diagnosis” and diagnostics in Afghanistan. Qual Health Res 2013;23:579–91. 10.1177/1049732312470761 [DOI] [PubMed] [Google Scholar]
- 19.Leslie T, Mikhail A, Mayan I et al. . Overdiagnosis and mistreatment of malaria among febrile patients at primary healthcare level in Afghanistan: observational study. BMJ 2012;345:e4389 10.1136/bmj.e4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leslie T, Mikhail A, Mayan I et al. . Rapid diagnostic tests to improve treatment of malaria and other febrile illnesses: patient randomised effectiveness trial in primary care clinics in Afghanistan. BMJ 2014;348:g3730 10.1136/bmj.g3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandler CI, Mangham L, Njei AN et al. . ‘As a clinician, you are not managing lab results, you are managing the patient’: how the enactment of malaria at health facilities in Cameroon compares with new WHO guidelines for the use of malaria tests. Soc Sci Med 2012;74:1528–35. 10.1016/j.socscimed.2012.01.025 [DOI] [PubMed] [Google Scholar]
- 22.Mangham LJ, Cundill B, Achonduh OA et al. . Malaria prevalence and treatment of febrile patients at health facilities and medicine retailers in Cameroon. Trop Med Int Health 2012;17:330–42. 10.1111/j.1365-3156.2011.02918.x [DOI] [PubMed] [Google Scholar]
- 23.Mbacham WF, Mangham-Jefferies L, Cundill B et al. . Basic or enhanced clinician training to improve adherence to malaria treatment guidelines: a cluster-randomised trial in two areas of Cameroon. Lancet Glob Health 2014;2:e346–58. 10.1016/S2214-109X(14)70201-3 [DOI] [PubMed] [Google Scholar]
- 24.Wiseman V, Mangham LJ, Cundill B et al. . A cost-effectiveness analysis of provider interventions to improve health worker practice in providing treatment for uncomplicated malaria in Cameroon: a study protocol for a randomized controlled trial. Trials 2012;13:4 10.1186/1745-6215-13-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Achonduh OA, Mbacham WF, Mangham-Jefferies L et al. . Designing and implementing interventions to change clinicians’ practice in the management of uncomplicated malaria: lessons from Cameroon. Malar J 2014;13:204 10.1186/1475-2875-13-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangham-Jefferies L, Wiseman V, Achonduh OA et al. . Economic evaluation of a cluster randomized trial of interventions to improve health workers’ practice in diagnosing and treating uncomplicated malaria in Cameroon. Value Health 2014;17:783–91. 10.1016/j.jval.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 27.Mangham-Jefferies L, Hanson K, Mbacham W et al. . What determines providers’ stated preference for the treatment of uncomplicated malaria? Soc Sci Med 2014;104:98–106. 10.1016/j.socscimed.2013.12.024 [DOI] [PubMed] [Google Scholar]
- 28.Ansah EK, Narh-Bana S, Epokor M et al. . Rapid testing for malaria in settings where microscopy is available and peripheral clinics where only presumptive treatment is available: a randomised controlled trial in Ghana. BMJ 2010;340:c930 10.1136/bmj.c930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ansah EK, Reynolds J, Akanpigbiam S et al. ‘Even if the test result is negative, they should be able to tell us what is wrong with us’: a qualitative study of patient expectations of rapid diagnostic tests for malaria. Malar J 2013;12:258 10.1186/1475-2875-12-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandler CI, Whitty CJ, Ansah EK. How can malaria rapid diagnostic tests achieve their potential? A qualitative study of a trial at health facilities in Ghana. Malaria J 2010;9:95 10.1186/1475-2875-9-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ezeoke OP, Ezumah NN, Chandler CC et al. . Exploring health providers’ and community perceptions and experiences with malaria tests in South-East Nigeria: a critical step towards appropriate treatment. Malar J 2012;11:368 10.1186/1475-2875-11-368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangham-Jefferies L, Hanson K, Mbacham W et al. . Mind the gap: knowledge and practice of providers treating uncomplicated malaria at public and mission health facilities, pharmacies and drug stores in Cameroon and Nigeria. Health Policy Plan 2015;30:1129–41. 10.1093/heapol/czu118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiseman V, Ogochukwu E, Emmanuel N et al. . A cost-effectiveness analysis of provider and community interventions to improve the treatment of uncomplicated malaria in Nigeria: study protocol for a randomized controlled trial. Trials 2012;13:81 10.1186/1745-6215-13-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mangham LJ, Cundill B, Ezeoke O et al. . Treatment of uncomplicated malaria at public health facilities and medicine retailers in south-eastern Nigeria. Malar J 2011;10:155 10.1186/1475-2875-10-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruxvoort K, Kalolella A, Nchimbi H et al. . Getting antimalarials on target: impact of national roll-out of malaria rapid diagnostic tests on health facility treatment in three regions of Tanzania. Trop Med Int Health 2013;18:1269–82. 10.1111/tmi.12168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandler CI, Meta J, Ponzo C et al. . The development of effective behaviour change interventions to support the use of malaria rapid diagnostic tests by Tanzanian clinicians. Implement Sci 2014;9:83 10.1186/1748-5908-9-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cundill B, Mbakilwa H, Chandler CI et al. . Prescriber and patient-oriented behavioural interventions to improve use of malaria rapid diagnostic tests in Tanzania: facility-based cluster randomised trial. BMC Med 2015;13:118 10.1186/s12916-015-0346-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leurent B, Reyburn H, Muro F et al. . Monitoring patient care through health facility exit interviews: an assessment of the Hawthorne effect in a trial of adherence to malaria treatment guidelines in Tanzania. BMC Infect Dis 2016;16:59 10.1186/s12879-016-1362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baltzell K, Elfving K, Shakely D et al. . Febrile illness management in children under five years of age: a qualitative pilot study on primary health care workers’ practices in Zanzibar. Malar J 2013;12:37 10.1186/1475-2875-12-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shakely D, Elfving K, Aydin-Schmidt B et al. . The usefulness of rapid diagnostic tests in the new context of low malaria transmission in Zanzibar. PLoS ONE 2013;8:e72912 10.1371/journal.pone.0072912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandler CI, DiLiberto D, Nayiga S et al. . The PROCESS study: a protocol to evaluate the implementation, mechanisms of effect and context of an intervention to enhance public health centres in Tororo, Uganda. Implement Sci 2013;8:113 10.1186/1748-5908-8-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staedke SG. Evaluating the impact of a public health centre intervention on management of malaria and health outcomes of children in Uganda—results from the PRIME & PROCESS studies. Policy Brief 2014. http://www.actconsortium.org/data/files/resources/109/PRIME-and-PROCESS-policy-brief-English.pdf (accessed 7 Jun 2016) [Google Scholar]
- 43.Staedke SG, Chandler CI, DiLiberto D et al. . The PRIME trial protocol: evaluating the impact of an intervention implemented in public health centres on management of malaria and health outcomes of children using a cluster-randomised design in Tororo, Uganda. Implement Sci 2013;8:114 10.1186/1748-5908-8-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiLiberto DD, Staedke SG, Nankya F et al. . Behind the scenes of the PRIME intervention: designing a complex intervention to improve malaria care at public health centres in Uganda. Glob Health Action 2015;8:29067 10.3402/gha.v8.29067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lal S, Ndyomugyenyi R, Alexander ND et al. . Health facility utilisation changes during the introduction of community case management of malaria in South Western Uganda: an interrupted time series approach. PLoS ONE 2015;10:e0137448 10.1371/journal.pone.0137448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mbonye AK, Ndyomugyenyi R, Turinde A et al. . The feasibility of introducing rapid diagnostic tests for malaria in drug shops in Uganda. Malar J 2010;9:367 10.1186/1475-2875-9-367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chandler CI, Hall-Clifford R, Asaph T et al. . Introducing malaria rapid diagnostic tests at registered drug shops in Uganda: limitations of diagnostic testing in the reality of diagnosis. Soc Sci Med 2011;72:937–44. 10.1016/j.socscimed.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutchinson E, Chandler C, Clarke S et al. . ‘It puts life in us and we feel big’: shifts in the local health care system during the introduction of rapid diagnostic tests for malaria into drug shops in Uganda. Crit Public Health 2015;25:48–62. 10.1080/09581596.2014.886762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mbonye AK, Lal S, Cundill B et al. . Treatment of fevers prior to introducing rapid diagnostic tests for malaria in registered drug shops in Uganda. Malar J 2013;12:131 10.1186/1475-2875-12-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mbonye AK, Magnussen P, Chandler CI et al. . Introducing rapid diagnostic tests for malaria into drug shops in Uganda: design and implementation of a cluster randomized trial. Trials 2014;15:303 10.1186/1745-6215-15-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mbonye AK, Magnussen P, Lal S et al. . A cluster randomised trial introducing rapid diagnostic tests into registered drug shops in Uganda: impact on appropriate treatment of malaria. PLoS ONE 2015;10:e0129545 10.1371/journal.pone.0129545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutcliffe K, Thomas J, Stokes G et al. . Intervention component analysis (ICA): a pragmatic approach for identifying the critical features of complex interventions. BMC Syst Rev 2015;4:f3755 10.1186/s13643-015-0126-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas J, O'Mara-Eves A, Brunton G. Using qualitative comparative analysis (QCA) in systematic reviews of complex interventions: a worked example. Syst Rev 2014;3:67 10.1186/2046-4053-3-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meta J, Nasuwa F, Kessy J et al. . TACT Formative Research Analysis Report, 2010.
- 55.Willey BA, Tougher S, Ye Y et al. . Communicating the AMFm message: exploring the effect of communication and training interventions on private for-profit provider awareness and knowledge related to a multi-country anti-malarial subsidy intervention. Malar J 2014;13:46 10.1186/1475-2875-13-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organisation. Global Action Plan on Antimicrobial Resistance. Geneva, 2015, http://www.who.int/drugresistance/global_action_plan/en/ [Google Scholar]
- 57.United Nations. Draft political declaration of the high-level meeting of the General Assembly on antimicrobial resistance 2016. http://www.un.org/pga/71/wp-content/uploads/sites/40/2016/09/DGACM_GAEAD_ESCAB-AMR-Draft-Political-Declaration-1616108E.pdf
- 58.Michie S, Fixsen D, Grimshaw JM et al. . Specifying and reporting complex behaviour change interventions: the need for a scientific method. Implement Sci 2009;4:40 10.1186/1748-5908-4-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffmann TC, Erueti C, Glasziou PP. Poor description of non-pharmacological interventions: analysis of consecutive sample of randomised trials. BMJ 2013;347:f3755 10.1136/bmj.f3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kyabayinze DJ, Asiimwe C, Nakanjako D et al. . Programme level implementation of malaria rapid diagnostic tests (RDTs) use: outcomes and cost of training health workers at lower level health care facilities in Uganda. BMC Public Health 2012;12:291 10.1186/1471-2458-12-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Febir LG, Baiden FE, Agula J et al. . Implementation of the integrated management of childhood illness with parasitological diagnosis of malaria in rural Ghana: health worker perceptions. Malar J 2015;14:174 10.1186/s12936-015-0699-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mubi M, Kakoko D, Ngasala B et al. . Malaria diagnosis and treatment practices following introduction of rapid diagnostic tests in Kibaha District, Coast Region, Tanzania. Malar J 2013;12:293 10.1186/1475-2875-12-293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bastiaens GJ, Bousema T, Leslie T. Scale-up of malaria rapid diagnostic tests and artemisinin-based combination therapy: challenges and perspectives in sub-Saharan Africa. PLoS Med 2014;11:e1001590 10.1371/journal.pmed.1001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boadu NY, Amuasi J, Ansong D et al. . Challenges with implementing malaria rapid diagnostic tests at primary care facilities in a Ghanaian district: a qualitative study. Malar J 2016;15:126 10.1186/s12936-016-1174-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diggle E, Asgary R, Gore-Langton G et al. . Perceptions of malaria and acceptance of rapid diagnostic tests and related treatment practises among community members and health care providers in Greater Garissa, North Eastern Province, Kenya. Malar J 2014;13:502 10.1186/1475-2875-13-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asiimwe C, Kyabayinze DJ, Kyalisiima Z et al. . Early experiences on the feasibility, acceptability, and use of malaria rapid diagnostic tests at peripheral health centres in Uganda-insights into some barriers and facilitators. Implement Sci 2012;7:5 10.1186/1748-5908-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Armstrong D. Clinical autonomy, individual and collective: the problem of changing doctors’ behaviour. Soc Sci Med 2002;55:1771–7. 10.1016/S0277-9536(01)00309-4 [DOI] [PubMed] [Google Scholar]
- 68.Faust C, Zelner J, Brasseur P et al. . Assessing drivers of full adoption of test and treat policy for malaria in Senegal. Am J Trop Med Hyg 2015;93:159–67. 10.4269/ajtmh.14-0595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zurovac D, Githinji S, Memusi D et al. . Major improvements in the quality of malaria case-management under the “test and treat” policy in Kenya. PLoS ONE 2014;9:e92782 10.1371/journal.pone.0092782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greenhalgh T, Swinglehurst D. Studying technology use as social practice: the untapped potential of ethnography. BMC Med 2011;9:45 10.1186/1741-7015-9-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beisel U, Umlauf R, Hutchinson E et al. . The complexities of simple technologies: re-imagining the role of rapid diagnostic tests in malaria control efforts. Malar J 2016;15:64 10.1186/s12936-016-1083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandler CIR, Webb EL, Maiteki-Sebuguzi C et al. . The impact of an intervention to introduce malaria rapid diagnostic tests on fever case management in a high transmission setting in Uganda: A mixed-methods cluster-randomised trial (PRIME). Plos One. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Staedke SG, Maiteki-Sebuguzi C, DiLiberto DD. The impact of an intervention to improve malaria care in public health centers on health indicators of children in Tororo, Uganda (PRIME): A cluster-randomised trial. Am J Trop Med Hyg 2016;95:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ndyomugyenyi R, Magnussen P, Lal S et al. . Appropriate targeting of artemisini-based combination therapy by community health workers using malaria rapid diagnostic tests: findings from randomized trials in two contrasting areas of high and low malaria transmission in south western Uganda. Tropical Medicine and International Health 2016;21:1157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-012973supp1.pdf (574.4KB, pdf)
bmjopen-2016-012973supp2.pdf (98KB, pdf)