Abstract
Introduction
Therapeutic antibodies to immune checkpoints show promising results. Programmed death-ligand 1 (PD-L1), an immune checkpoint ligand, blocks the cancer immunity cycle by binding the PD-L1 receptor (programmed death 1). We investigated PD-L1 protein expression and messenger RNA (mRNA) levels in SCLC.
Methods
PD-L1 protein expression and mRNA levels were determined by immunohistochemistry (IHC) with SP142 and Dako 28-8 PD-L1 antibodies and in situ hybridization in primary tumor tissue microarrays in both tumor cells and tumor-infiltrating immune cells (TIICs) obtained from a limited-disease SCLC cohort of 98 patients. An additional cohort of 96 tumor specimens from patients with extensive-disease SCLC was assessed for PD-L1 protein expression in tumor cells with Dako 28-8 antibody only.
Results
The overall prevalence of PD-L1 protein expression in tumor cells was 16.5%. In the limited-disease cohort, the prevalences of PD-L1 protein expression in tumor cells with SP142 and Dako 28-8 were 14.7% and 19.4% (tumor proportion score cutoff ≥1%) and PD-L1 mRNA ISH expression was positive in 15.5% of tumor samples. Increased PD-L1 protein/mRNA expression was associated with the presence of more TIICs (p < 0.05). The extensive-disease cohort demonstrated a 14.9% positivity of PD-L1 protein expression in tumor cells with Dako 28-8 antibody.
Conclusions
A subset of SCLCs is characterized by positive PD-L1 and/or mRNA expression in tumor cells. Higher PD-L1 and mRNA expression correlate with more infiltration of TIICs. The prevalence of PD-L1 in SCLC is lower than that published for NSCLC. The predictive role of PD-L1 expression in SCLC treatment remains to be established.
Keywords: PD-L1, SCLC, IHC, mRNA in situ hybridization
Introduction
SCLC is a highly malignant disease that represents approximately 15% of lung cancer cases. Although the majority of SCLC tumors are initially sensitive to chemotherapy and radiation therapy, SCLC is associated with an aggressive clinical course characterized by rapid growth and a tendency to early metastasis. Unlike the genomic landscape of lung adenocarcinoma, that of SCLC is not broadly characterized by a set of mutually exclusive, targetable driver oncogenes involved in activation of kinase signaling. The most frequently recurrent mutations seen in this disease are inactivating mutations in the tumor suppressor genes tumor protein p53 gene (TP53) and retinoblastoma 1 gene (RB1), which cannot be targeted directly.1–3 Over the past few decades, developments in therapeutic regimens have not substantially improved patient survival.4 Novel therapies for SCLC have been understudied and are urgently needed.
The recent successes obtained with immunotherapy in several types of tumors have renewed hopes for treatment of SCLC. In theory, tumors with a high somatic mutation load are more likely to respond to immunotherapy, as they should have a higher diversity of neoantigens that can trigger an immune response. SCLC is most strongly linked to long-term high exposure to tobacco carcinogens, leading to an exceptionally high degree of genomic alterations, including mutations, insertions, deletions, large-scale copy number alterations, and gross interchromosomal and intrachromosomal rearrangements.5–7 Immunotherapy has also shown encouraging efficacy in SCLC.8,9 In the Checkmate 032 study, in which patients were treated irrespective of programmed death ligand 1 (PD-L1) status, nivolumab alone demonstrated 10% partial response and 22% stable disease rates, whereas the combination of nivolumab and ipilimumab demonstrated 2% complete response, 21% partial response, and 21% stable disease rates. In that study, tumor responses occurred in patients irrespective of PD-L1 status. Durable responses were seen in patients with relapsed SCLC, and survival results are thus far encouraging. Although data from SCLC clinical trials to date have not suggested that PD-L1 expression by immunohistochemistry (IHC) is a promising predictive biomarker for anti–programmed cell death 1 (PD-1)/PD-L1 treatment in SCLC, PD-L1 expression may still have meaningful biological impact on SCLC given the observed effect of anti–PD-1 therapy. For NSCLC, pembrolizumab (Merck, Kenilworth, NJ) was recently approved by the U.S. Food and Drug Administration (FDA) with PD-L1 expression as a companion diagnostic (Dako 22C3 [Dako, Carpinteria, CA]). Nivolumab (Bristol-Myers Squibb, New York, NY) was approved in the same patient setting without a required predictive assay, but instead using a complementary diagnostic assay (Dako 28-8 [Dako]). This assay has a significant predictive value for nivolumab therapy in patients with NSCLC previously treated with chemotherapy.10 As for atezolizumab (Genentech, South San Francisco, CA), the Ventana PD-L1 assay (SP142 [Spring Bioscience, Pleasanton, CA]) has also recently been approved by the FDA as a complementary diagnostic to provide PD-L1 status of patients with metastatic urothelial cancer.11–16 Because predictive biomarkers may help select patients most likely to benefit from immunotherapy, further studies are needed to determine how PD-L1 IHC assays predict response and clinical outcome compared with mRNA- or genome-based assays.
The aim of this study was to describe the protein and mRNA expression patterns of PD-L1 in SCLC and to better understand the relationship between PD-L1 tumor expression and immune cell infiltration. We assessed PD-L1 protein expression by two complementary diagnostic assays (SP142 and Dako 28-8) and mRNA expression using formalin-fixed, paraffin-embedded (FFPE) tumor tissue samples from two cohorts of patients with SCLC and analyzed their association with clinical characteristics and clinical outcomes. We also studied PD-L1 expression in SCLC cell lines (tissue microarray) for comparison.
Materials and Methods
Patient Populations and Tumor Specimens
The first cohort, referred to as the limited-disease (LD)-SCLC cohort, consisted of archival FFPE tumor samples obtained from a unique series of 98 patients who underwent pulmonary resection between 1982 and 2002 at the Medical University of Gdańsk, Poland.17,18 All primary diagnoses of LD-SCLC were retrospectively reviewed by three experienced pathologists (H.Y., T.B., and M.K.) according to the 2015 World Health Organization criteria.19 In all patients, surgery was followed by standard chemotherapy. For 78 patients, medical records were reviewed to obtain clinical characteristics, including age, sex, tumor diameter, and TNM stage.
The independent second cohort, referred to as the extensive-disease (ED)-SCLC cohort, consisted of 96 archival tumor biopsy and resection specimens collected from patients with documented ED-SCLC. These patients represent a subset from an ongoing prospective lung cancer cohort study with enrollment from 39 community oncology sites in the United States in which documentation of ED-SCLC, including mixed histologic types, was based on medical record pathology reports. Among the samples analyzed, 54 of 96 were obtained at the time of ED-SCLC diagnosis, 30 of 96 within 1 month before diagnosis, three of 96 more than 1 month before diagnosis, seven of 96 within 1 month after diagnosis, and one of 96 more than 1 month after diagnosis. The time of biopsy for one sample was not available. Before biopsy, four of 96 patients were documented to have received chemotherapy and one of 96 to have received radiation therapy; none had received prior surgery.
TMA Construction from LD-SCLC Cohort
A tissue microarray (TMA) was constructed with 98 surgically resected SCLC specimens from the LD-SCLC cohort using a Beecher MTA-1 manual tissue arrayer (Beecher Instruments, Sun Prairie, WI). Morphologically representative areas of SCLC were identified and annotated on hematoxylin and eosin (HE)-stained slides under the microscope by a pathologist. The annotated slides were used to guide dissection of three 1.5-mm-diameter cores from different tumor areas of the paraffin-embedded blocks. Duplicate cores were set into TMA blocks. Two additional custom-designed lung cancer cell line TMAs were constructed for reagent titration, assay validation, and reproducibility assessment. These FFPE TMA blocks contained core samples from 54 NSCLC cell lines and 18 SCLC cell lines, with samples from term human placenta as positive controls for endogenous PD-L1 expression.
HE, IHC, Antibodies, and Scoring
IHC on 4-μm FFPE sections from the LD-SCLC cohort, control placenta, and cell line TMAs was performed using a commercially available antibody directed against the intracellular domain of PD-L1 (monoclonal rabbit clone SP142 1:100) and the UltraVIEW detection kit (Ventana, Tucson, AZ). Slides from the LD-SCLC cohort were labeled with a bar-coded, standardized antibody-specific protocol and loaded into a Benchmark XT automated staining machine (Ventana). Another primary rabbit monoclonal antibody directed against the extracellular domain of human PD-L1 (Dako clone 28-8) was used for staining the LD-SCLC cohorts. The Dako 28-8 pharmDx kit was previously validated for detection of PD-L1 in NSCLC. Slides were stained on the Dako Link 48 autostainer, with deparaffinization, rehydration, and target retrieval performed on the Dako PT Link. For the LD-SCLC cohort, two pathologists (H.Y. and T.B.) independently scored the specimens. Specimens were deemed adequate if at least one core had adequate tissue. For discrepant results, a final score was determined by a consensus conference of the pathologists. Scores for specimens with multiple cores were averaged. Tumor and tumor-infiltrating immune cells (TIICs) were scored for PD-L1 expression separately. Scoring was determined according to tumor proportion score (TPS) criteria on the basis of percentage of cells with partial or complete cell membrane staining at any intensity. The degree of TIICs infiltration was assessed with a semiquantitative score from 0 to 3 as follows: 0 = none (no immune infiltrates), 1 = focal (mostly perivascular in tumor with some intratumoral extension), 2 = moderate (prominent extension of immune infiltrates away from perivascular areas and among tumor cells), and 3 = severe (immune infiltrates obscuring tumor).20
For the ED-SCLC cohort, samples were analyzed under a protocol validated for detection of PD-L1 in NSCLC.18 The IHC assay utilized Dako 28-8 to detect cell-membrane expression of PD-L1 in viable tumor cells. Slides were stained as already described. Dako automated staining runs included slides containing a PD-L1–positive cell line (NCI-H226), a PD-L1–negative cell line (MCF-7), and tonsil tissue with PD-L1–positive crypt epithelium to serve as run controls.21 All staining for the ED-SCLC cohort was performed at Mosaic Laboratories (Lake Forest, CA). The scoring of tumor cells was also calculated from the raw data as already described for the LD-SCLC cohort. In contrast to the LD-SCLC cohort, this cohort consisted of both primary tumor (52 of 96) and metastatic (44 of 96) tissue specimens. The metastatic samples tested included samples from the liver (16 of 44), lymph nodes (18 of 44), or other sites (10 of 44). HE staining, analysis, and quantification of the percentage of intratumoral inflammatory cells were performed by pathologists from Q2 Solutions (Morrisville, NC). Of 87 samples, 83 were deemed acceptable for evaluation of the intratumoral immune infiltrate percentage by HE analysis, as tumor tissue content represented at least 10% of the tissue on the slide. The intratumoral immune infiltrate inferred from HE analysis was composed of mononuclear cells, including lymphocytes, macrophages, and plasma cells. Intraalveolar macrophages were not considered part of the immune infiltrate. The 10% cutoff used to define a high degree of intratumoral immune infiltration was selected to represent the median observed across the cohort in which approximately 45% of the samples had at least 10% intratumoral immune infiltrate.
mRNA ISH
mRNA in situ hybridization (ISH) was performed on the tumor tissue from the LD-SCLC cohort and cell line TMAs by using the RNAscope 2.0 assay system (Advanced Cell Diagnostics, Hayward, CA) with recommended probes (Probe-Hs-CD274, RNAscope Reagent Kit for pretreatments 1, 2, and 3 and RNAscope FFPE Reagent Kit, 2.0 HD Detection Kit [Brown]). ISH scores were generated at ×200 magnification and recorded using the RNAscope system scoring guidelines: 0 = no staining; 1 = 1 to 3 dots per tumor cell; 2 = 4 to 10 dots per tumor cell; 3 = more than 10 dots per cell with less than 10% of tumor cells with dot clusters; and 4 = more than 10 dots per cell with more than 10% of tumor cells with dot clusters.22
Statistical Analysis
Statistical analyses were performed with SAS version 9.4 (SAS Institute, Inc., Cary, NC). The analyses were conducted in the LD-SCLC and ED-SCLC cohorts, with available variables in each. Samples in each cohort were grouped into two groups based on PD-L1 expression and the two groups were compared by using Student’s t test for continuous variables, including age, and Fisher’s exact test for categorical variables, including sex, smoking status, and categorized percentage of TIIC (<10% versus ≥10%). Spearman’s correlation coefficient was calculated to evaluate the correlation between PD-L1 protein expression, mRNA expression, and degree of TIIC infiltration. For ED-SCLC, survival curves comparing a TPS less than 1 versus at least 1 were created after 3:1 patient matching for sex, smoking, and age (dichotomized at 70 years of age). These criteria were selected on the basis of their known role in patient prognosis and treatment decisions in SCLC.23–25 Survival curves were generated on the basis of overall survival, which was defined as time from initial diagnosis with ED-SCLC to last follow-up date or date of death. Survival functions between groups were compared using the log-rank test. All tests were considered statistically significant at p less than 0.05.
Results
PD-L1 Protein Expression in SCLC
Samples were collected from 194 patients with SCLC (98 LD-SCLC cases and 96 ED-SCLC cases). The distribution of PD-L1 expression in the SCLC cohorts by two antibodies (SP142 and Dako 28-8) is shown in Table 1. Those specimens that could not be evaluated had either inadequate tissue or insufficient viable tumor cells. Because there is no standardized definition for positivity for PD-L1 protein expression, we evaluated a cutoff of at least 1% cell staining (on the basis of the published association of this cutoff with clinical response to anti–PD-1 therapy).8,16,26–30 The overall prevalence of PD-L1 expression in tumor cells in these SCLC cohorts was 16.5% (41 of 249 cases [95 slides stained with SP142 and 67 slides stained with Dako 28-8 for the LD-SCLC cohort and 87 slides stained with Dako 28-8 for the ED-SCLC cohort]) with a TPS cutoff of at least 1%.
Table 1.
Prevalence of PD-L1 Protein and mRNA Expression in SCLC Tumor Cells
| PD-L1 IHC (Protein, TPS)
|
|||||||
|---|---|---|---|---|---|---|---|
| SCLC Cohort | Antibody | <1% (n) | ≥1%–<5% (n) | ≥5%–<10% (n) | ≥10%–<50% (n) | ≥50% (n) | PD-L1 mRNA ISH (mRNA) RNA Score >2 (n) |
| LD-SCLC (n = 98) | SP142 (n = 95) | 85.3% (81) | 11.6% (11) | 0% (0) | 2.1% (2) | 1.1% (1) | 15.5% (15 of 97) |
| Dako 28-8 (n = 67) | 80.6% (54) | 10.4% (7) | 3.0% (2) | 3.0% (2) | 3.0% (2) | ||
|
| |||||||
| ED-SCLC (n = 96) | Dako 28-8 (n = 87) | 83.9% (73) | 12.6% (11) | 0% (0) | 1.1% (1) | 1.1% (1) | ND |
PD-L1, programmed death-ligand; mRNA, messenger RNA; IHC, immunohistochemistry; TPS, tumor proportion score; ISH, in situ hybridization; LD, limited disease; ED, extensive disease; ND, not determined.
In the LD-SCLC cohort, specimens with a predominantly membranous staining pattern were scored as positive; however, many of them also exhibited cytoplasmic staining and the staining intensities were usually faint. Of the specimens with LD-SCLC evaluated using SP142, 14 of 95 cases (14.7%) had PD-L1 expression in tumor cells with a TPS cutoff of at least 1%, three of 95 cases (3.2%) had PD-L1 expression with a TPS of at least 10%, and only one of 95 cases (1.1%) had PD-L1 expression in tumor cells with a TPS of at least 50%. As for the specimens evaluated by Dako 28-8 antibody, 19.4% (13 of 67) had at least 1% of tumor cells that expressed PD-L1 protein, six of 67 cases (9.0%) had PD-L1 expression with a TPS of at least 5%, four of 67 cases (6.0%) expressed PD-L1 in at least 10% tumor cells, and two of 67 cases (3.0%) had PD-L1 expression with a TPS of at least 50% (Table 1).
A similar prevalence was observed in the ED-SCLC cohort: PD-L1 was evaluable in 87 of 96 cases. Scoring for the ED-SCLC cohort was based on PD-L1 membrane staining in viable tumor cells (Fig 1A). Samples with nonevaluable PD-L1 levels were mainly primary tumor samples (eight of nine cases), most of which (six of eight cases) had fewer than 100 viable tumor cells for counting. Paralleling the results with LD-SCLC, 13 of 87 cases (14.9%) exhibited tumor cell expression of PD-L1 at a TPS cutoff of at least 1%, and two of 87 cases (2.9%) exhibited tumor cell expression with a TPS of at least 10% (TPS equal to 20% and 100%, respectively). The likelihood of observing a TPS of at least 1% did not correlate with the average time difference between the date of slide staining and the date of slide cut (average of 110 versus 137 days; t test p < 0.33). Although there was a slightly higher proportion of PD-L1–positive samples at the 1% cutoff for slides cut and stained within 90 days, these results were not significant (Fisher’s exact test p < 0.19). Specifically, six of 25 samples aged 90 days or less were positive for PD-L1 versus seven positive samples of 65 aged 90 days or more. Frequencies of PD-L1–positive and PD-L1–negative tumor samples were equally similar when aged 90 to 180 days (six of 51 [11%)] or 180 days or more (one of 10 [10%]). The interval between the date of staining and the date of tumor biopsy was equally not significant (378 versus 340 days; t test p < 0.65). PD-L1 expression in tumor cells did not correlate with samples being obtained from primary versus metastatic biopsy specimen (Fisher’s exact test p < 0.77). Seven of 43 metastatic tumor samples and six of 44 primary tumor samples were associated with a TPS of at least 1%.
Figure 1.
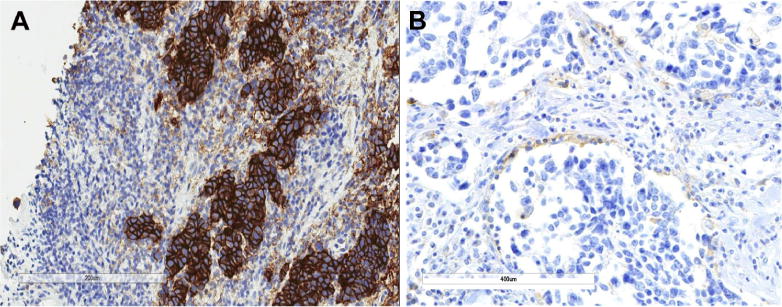
Programmed death ligand 1 (PD-L1) protein expression in SCLC (immunohistochemistry). (A) Positive PD-L1 protein expression in SCLC tumor cells from the extensive-disease SCLC cohort (Dako 28-8). (B) Positive PD-L1 protein expression in tumor-infiltrating immune cells from the limited-disease SCLC cohort (Spring Bioscience SP142).
We also scored PD-L1 protein expression in 18 SCLC cell line TMAs with the SP142 antibody. Three of 18 lines (16.7%) had PD-L1 expression with a TPS cutoff of at least 1%, two of 18 (11.1%) had PD-L1 expression with a TPS cutoff of at least 5%, and one of 18 (5.6%) had PD-L1 expression with a TPS cutoff of at least 50%.
Association of PD-L1 Expression with TIICs
Scoring of PD-L1 protein expression in TIICs was performed in the LD-SCLC cohort. PD-L1 protein expression in TIICs was evaluable in 96 of 98 of specimens with SP142 (98%) and in 67 of 98 of specimens with Dako28-8 (68%) (Table 2). Scoring criteria were based on clinical trials of atezolizumab in NSCLC.30 With a cutoff of at least 1%, 54 of 96 specimens (56.3%) demonstrated PD-L1 expression in TIICs with SP142 and 44.8% (30 of 67) with Dako 28-8 (Fig. 1B). A distinct membranous staining pattern was observed in TIICs that had clearly discernible cytoplasm, which is suggestive of macrophages and dendritic cells. TIICs with PD-L1 staining were typically seen as variably sized aggregates toward the periphery of the tumor mass, as stromal bands dissecting the tumor mass, and as single cells scattered in the stroma. The PD-L1–stained TIICs were mostly located at the periphery of the tumors (see Fig. 1B). Overall, by combining tumor cells and/or TIICs as a criterion to define PD-L1 protein expression based on a cutoff at least 1%, 56 of 98 cases (57.1%) were positive according to SP142 and 34 of 67 cases (50.7%) were positive with Dako 28-8. There was a significantly higher occurrence of PD-L1 protein expression in TIICs than in SCLC tumor cells (p < 0.0001). PD-L1 protein expression in TIICs correlated with the degree of TIIC infiltration based on a semiquantitative assessment of the presence and geography of TIICs (p < 0.0001, r = 0.625 and p < 0.0001, r = 0.538, with SP142 and Dako 28-8 antibody, respectively [Fig. 2]).
Table 2.
Prevalence of PD-L1 Protein Expression on Tumor-Infiltrating Immune Cells in LD-SCLC
| Antibody | PD-L1 IHC | |||
|---|---|---|---|---|
| <1% (n) | ≥1%–<5% (n) | ≥5%–<10% (n) | ≥10% (n) | |
| SP142 (n = 96) | 43.8% (42) | 26.0% (25) | 4.2% (4) | 26.0% (25) |
| Dako 28-8 (n = 67) | 55.2% (37) | 0% (0) | 13.4% (9) | 31.4% (21) |
PD-L1, programmed death-ligand 1; LD, limited disease; IHC, immunohistochemistry; TPS, tumor proportion score.
Figure 2.
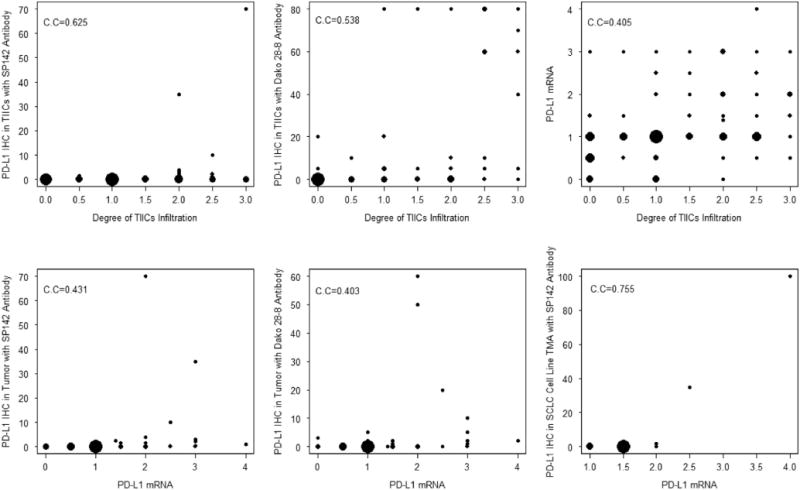
The bubble plots of Spearman’s correlation of programmed death ligand 1 (PD-L1) expression in the limited-disease SCLC Cohort. The size of the bubble is proportional to the number of the repeats at a value. IHC, immunohistochemistry; TIICs, tumor-infiltrating immune cells; C.C, correlation coefficient.
In ED-SCLC, IHC and HE analysis revealed an association between tumor cell expression of PD-L1, immune infiltration, and predominant PD-L1 staining of macrophages. Upon integration of PD-L1 tumor cell staining by IHC with the degree of intratumoral immune cell infiltration inferred through HE, we observed that samples positive for PD-L1 expression were associated with a higher degree of intratumoral immune infiltrate. Ten of 13 samples (77%) positive for PD-L1 tumor cell expression (TPS ≥1%) had at least 10% intratumoral inflammatory cells according to HE analysis (see the “Methods” section). In contrast, only 28 of 70 PD-L1–negative samples (40%) had this degree of intratumoral immune cell infiltration. We next examined the association between PD-L1 expression in tumor cells and the immune infiltrate by IHC. Distinction of the immune infiltrate and predominant PD-L1 staining of macrophage or lymphocyte populations was ascertained by a trained pathologist using morphologic evidence upon slide review. In samples associated with positive PD-L1 tumor cell expression (TPS ≥1%), all samples (13 of 13) were associated with membrane staining of PD-L1 in the intratumoral immune cell compartment. Specifically, in all 13 samples the predominant intratumoral immune cell types associated with membrane staining of PD-L1 were macrophages over lymphocyte populations. In samples not expressing PD-L1 in tumor cells, PD-L1 staining of the immune cell compartment was absent in 33 of 74 samples, as either no immune infiltrate was detected (five of 74 samples) or PD-L1 staining was absent in both tumor-infiltrating lymphocytes and macrophages (28 of 74 samples). The remaining samples had either predominant PD-L1 staining of macrophage (38 of 74 samples) or lymphocyte populations (three of 74 samples). One may therefore conclude that PD-L1 expression in tumor cells is associated with the presence of an abundant inflammatory infiltrate in which PD-L1 expression was predominantly observed in macrophage cells (13 of 13 versus 38 of 74, Fisher’s exact test p < 0.0005).
Prevalence of PD-L1 mRNA Levels by ISH in LD-SCLC
In the LD-SCLC cohort, analysis of PD-L1 mRNA expression was studied using RNAscope ISH. Because there is no standard definition for positivity of mRNA ISH, we defined an average staining score higher than 2 in the TMA cores as the cutoff for mRNA ISH positivity on the basis of the presence of mRNA signal dot clusters with RNA scores higher than 2 (Fig. 3). Evaluation of 97 SCLC specimens by PD-L1 mRNA ISH in tumor cells revealed a prevalence of 15.5% (15 of 97 cases) with RNA scores higher than 2. Qualitatively, we did not observe a difference between PD-L1 mRNA expression in tumor cells and TIICs. Both tumor cells and TIICs had a homogeneous expression pattern throughout the slides. In SCLC cell line TMA, the prevalence of PD-L1 mRNA ISH was 11.1% (two of 18 cases) when RNA scores higher than 2 were chosen as positive cutoff values. The two cell lines with PD-L1 mRNA scores higher than 2 also showed PD-L1 protein expression at a TPS of at least 5% with the SP142 antibody. Analysis of an SCLC cell line TMA revealed a positive correlation between PD-L1 protein expression on tumor cells with SP142 antibody and mRNA expression (p < 0.00001, r = 0.755). PD-L1 protein expression in tumor cells in the LD-SCLC cohort also showed a positive correlation with mRNA expression (SP142: p < 0.0001, r = 0.431; Dako 28-8: p = 0.0007, r = 0.403). The degree of TIICs infiltration correlated with PD-L1 mRNA expression in tumor cells (p < 0.0001, r = 0.405) as well.
Figure 3.
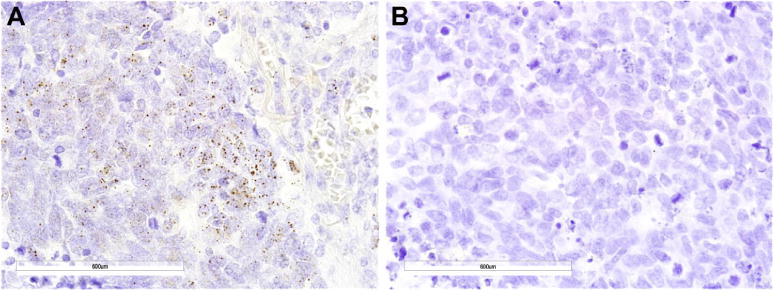
Programmed death ligand 1 (PD-L1) messenger RNA (mRNA) in SCLC (SCLC) in situ hybridization (ISH) of the limited disease SCLC cohort. (A) PD-L1 mRNA ISH-positive expression in SCLC tumor cells (original magnification, 600). (B) PD-L1 mRNA ISH-negative expression in SCLC (original magnification, 600). Brown dots in cells represent mRNA expression.
Association Between PD-L1 Protein Expression or mRNA Levels and Clinical Characteristics
Among the 78 patients with LD-SCLC with clinical data, no significant association was observed between PD-L1 protein expression in tumor cells and clinical characteristics or prognosis (≥1% cutoff [Table 3]). However, at the mRNA level, a significant association was observed in LD-SCLC. The mean ages of patients for PD-L1 mRNA expression higher than 2 (n = 11) and 2 or lower (n = 67) on tumor cells were 64 and 57 years, respectively (p = 0.0006).
Table 3.
Clinicopathological Characteristics of LD-SCLC and ED-SCLC Cohorts according to PD-L1 Protein Status in Tumor Cells
| Parameter | LD-SCLC Cohort (n = 98)a TPS≥1%
|
ED-SCLC Cohort (n = 87) TPS≥1%
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SP142 Antibody (n = 95)
|
Dako 28-8 Antibody (n = 67)
|
Dako 28-8
|
|||||||
| PD-L1-Pos Pts | PD-L1-Neg Pts | p Value | PD-L1-Pos Pts | PD-L1-Neg Pts | p Value | PD-L1-Pos Pts | PD-L1-Neg Pts | p Value | |
| All patients | 13 (14%) | 82 (96%) | 13 (19%) | 54 (81%) | 13 (15%) | 74 (85%) | |||
|
| |||||||||
| Age of biopsy specimen (mean ± SD), d | ND | ND | ND | ND | 378 ± 351 | 340 ± 258 | 0.64b | ||
|
| |||||||||
| Age of cut slide (mean ± SD), d | ND | ND | ND | ND | 110 ± 60 | 137 ± 96 | 0.33b | ||
|
| |||||||||
| Patient age (mean ± SD), y | 59.7 ± 7.8 | 58.3 ± 8.9 | 0.59b | 61.7 ± 5.2 | 58.0 ± 9.0 | 0.21b | 71.3 ± 8.0 | 65.9 ± 7.6 | 0.02b |
|
| |||||||||
| Sex | |||||||||
| Male | 9 (16%) | 48 (84%) | 1.0c | 10 (24%) | 32 (76%) | 0.18c | 6 (13%) | 40 (87%) | 0.76c |
|
| |||||||||
| Smoking | |||||||||
| Ever | ND | ND | ND | ND | 13 (15%) | 70 (80%) | 1.0c | ||
| Never | ND | ND | ND | ND | 0 (0%) | 4 (5%) | |||
|
| |||||||||
| % TIICs | |||||||||
| <10% | ND | ND | ND | ND | 3 (3%) | 42 (48%) | 0.02c | ||
| ≥10% | ND | ND | ND | ND | 10 (12%) | 28 (32%) | |||
| ND | ND | ND | ND | ND | 0 (0%) | 4 (5%) | |||
PD-L1 protein expression was evaluated in 95 of 98 specimens from the LD-SCLC cohort using SP142 antibody, with clinical records from 78 patients reviewed; 67 of 98 specimens were evaluable for staining with Dako 28-8, with clinical records from 51 patients reviewed.
p Values from pooled two-sample t test (equal variance).
p Values from Fisher’s exact test.
LD, limited disease; ED, extensive disease; PD-L1, programmed death-ligand 1; TPS, tumor proportion score; Pos, positive, Neg, negative; Pts, patients; ND, not determined; TIIC, tumor-infiltrating immune cell.
Among the 87 patients in the ED-SCLC cohort, the mean age at biopsy was 71 years for those with PD-L1 protein expression (TPS ≥1% [n = 13]) and 65 years for those without PD-L1 protein expression (n = 74) (Table 3). There was a significant association of PD-L1 expression with age at TPS cutoffs of at least 1% (t test p = 0.02), which is consistent with the mRNA findings in LD-SCLC.
For ED-SCLC, for which the cohort survival data were available and patients could be matched by age, sex, and smoking status, 3:1 (TPS <1% versus ≥1%) patient-matched analyses demonstrated that patients with a PD-L1 TPS of at least 1% trended toward longer overall survival (log-rank p < 0.0511 [Fig. 4]); however, caution in interpretation of these results is required on account of the small numbers. Median overall survival was 16.1 months for these patients versus 9.9 months in patients with a TPS lower than 1%. Alteration to a 2:1 matching ratio (16.1 versus 11.4 months, p < 0.0965) or a 4:1 matching ratio (16.1 versus 10 months, p < 0.055) had little impact on the significance of this finding.
Figure 4.
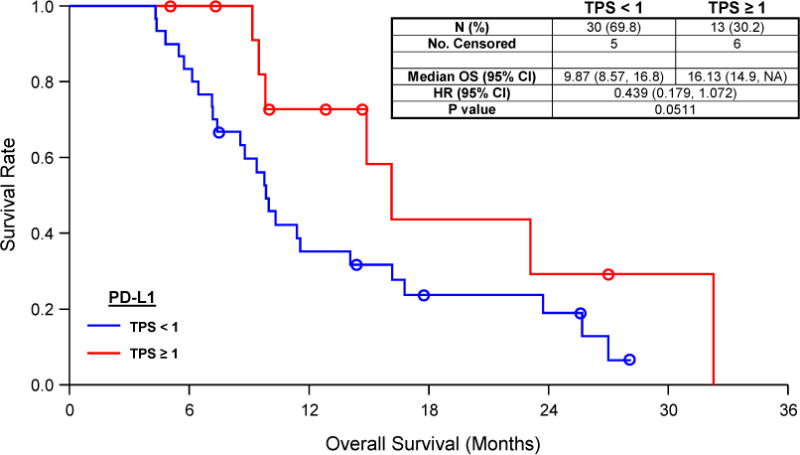
Kaplan–Meier survival analysis of overall survival (OS) according to tumor cell membrane staining (immunohistochemistry) of programmed death ligand 1 (PD-L1) in the extensive-disease SCLC cohort. The patients described in this analysis were matched 3:1 (PD-L1 <1 to PD-L1 ≥1) by sex, smoking status (current versus prior/never), and age (dichotomized at 70 years) before analysis for OS by PD-L1 expression. TPS, tumor proportion score; HR, hazard ratio; CI, confidence interval.
Discussion
In this study we examined two independent SCLC cohorts, one with LD-SCLC and the other with ED-SCLC. This gave us the opportunity to compare independent cohorts and compare potential differences in PD-L1 expression. The assessment was done independently by two different institutions, with different antibodies used for assessment.
According to our research, the overall prevalence of PD-L1 protein expression in tumor cells was 16.5% (41 of 249 slides at a TPS cutoff ≥1%), which is similar to that of a recent clinical trial of nivolumab in SCLC (16.9% at a TPS cutoff ≥ 1%).8 However, PD-L1 expression in SCLC is lower than that reported in NSCLC.10,26 It is well known that SCLC is strongly associated with tobacco use and has high mutation rates without known oncogenic drivers.31 These high mutation rates might produce more diversity in tumor antigens and increase the possibility of eliciting a mutation-specific immune response. There is evidence that the onset of neurologic paraneoplastic syndromes in a subset of patients with SCLC is related to the T-cell response to onconeural antigens expressed by the tumor.32 Effector T cells have been found to be more numerous in patients with limited-stage disease and long-term survival, and lower in patients with recurrent disease.33 Because of the disease-specific molecular profiling and complicated immunologic mechanisms in SCLC, the association between PD-L1 expression and the response to immunotherapy in SCLC requires further investigation.
The choice of antibody for IHC likely plays an important role in the detection of PD-L1 expression by IHC. Two separate studies using anti–PD-L1 antibodies from the same company (Abcam, Cambridge, UK) reported a high prevalence of PD-L1 expression in SCLC (71.6% and 82.8% with a 5% cutoff for positivity).34,35 By contrast, Schultheis et al.,27 who used two different PD-L1 antibodies (5H1 and E1L3N [Cell Signaling Technology, Danvers, MA]) to stain specimens from another SCLC cohort, reported a complete lack of PD-L1 protein positivity in tumor cells but 18.5% PD-L1 positivity in TIICs. In the current study, both antibodies (SP142 and clone 28-8) have been approved by the FDA as complementary diagnostics for PD-1/PD-L1 immunotherapy. With these two alternative PD-L1 antibodies, we showed that the prevalence of PD-L1 expression in tumor cells evaluated by Dako 28-8 (19.4%) was marginally higher than that by SP142 (14.7%) at a TPS cutoff at least 1% in the LD-SCLC cohort. This result may be partially due to the difference in the binding domains of the antibodies and the staining conditions. SP142 antibody binds to the cytoplasmic domain of PD-L1, whereas Dako 28-8 binds to the extracellular domain. This is not yet well characterized, but it is possible that this difference in binding domains alters the sensitivity and specificity of the detection assay.36 In our current study, the comparison of antibodies was limited to the LD-SCLC cohort. However in ongoing projects, different PD-L1 assays are being compared on the same set of tumors to better understand the similarities and differences between antibodies and staining platforms.
As a complementary diagnostic assay in metastatic urothelial cancer, the SP142 antibody has been used to evaluate PD-L1 expression not only in tumor cells but also in TIICs in a series of clinical trials of atezolizu-mab.8,12–14 These studies showed that response to atezolizumab correlated with PD-L1 IHC expression on tumor cells and TIICs. On the basis of these data, we evaluated PD-L1 expression in TIICs in our study as well. We observed a relatively low prevalence of PD-L1 protein expression in SCLC tumor cells versus in TIICs (14%–19% versus 51%–57%). However, further studies are needed to better understand the relationship between the PD-L1 expression on tumor cells and the TIICs in the tumor microenvironment.
The role of storage on the results of retrospective analysis of archival material is another unknown factor in the study of PD-L1 protein expression by IHC. The stability of the PD-L1 protein over time has not been clearly elucidated in SCLC. In the current study, PD-L1 expression in ED-SCLC tumor cells was assessed up to 306 days after slide preparation and up to 1370 days after biopsy specimen collection. Neither variable had a statistically significant impact on the likelihood of PD-L1 staining, but this effect may be masked, owing to the low prevalence of PD-L1 expression in SCLC. Caution may be warranted in the use of old slides given that it was noted that slides stained within 90 days had a slightly higher prevalence for positive PD-L1 staining (six of 25 samples or 24%) over samples aged 90 days or more (seven of 61 samples or 11%). Research studies using renal cell carcinoma specimens showed that the prevalence of PD-L1 protein expression in fresh-frozen renal cell carcinoma tumor tissue was higher than the prevalence of PD-L1 in FFPE tissue (37% versus 24%) when the 5H1 antibody was used with a cutoff of at least 5% of tumor cells with membranous tumor staining.37–39 This discrepancy may reflect the denaturant effect of formalin fixation on protein, which frequently compromises antigen staining during IHC. As with all biomarkers, it will be important to develop standardized methods for evaluation of PD-L1 by IHC.
PD-L1 mRNA in situ may potentially be a sensitive and stable biomarker for prediction of response to anti–PD-1/PD-L1 immunotherapy. We performed mRNA ISH in LD-SCLC to better understand the expression of PD-L1 mRNA in SCLC tumor specimens. The 15.5% positivity rate for PD-L1 mRNA expression by ISH (RNA score cutoff >2) was close to the 14.7% to 19.4% positivity rate of PD-L1 protein expression in tumor cells with a TPS cutoff of at least 1%. However, the prevalence of PD-L1 mRNA positivity in SCLC (15.5%) that we observed was lower than the mRNA positivity prevalence of 35.7% in SCLC reported by Schultheis et al.27 This difference may reflect the use of different techniques with different definitions of positivity (RNAscope assessment of RNA on a glass slide versus RNA-seq).
mRNA evaluation using the RNAscope method is a relatively new technology. We used the RNAscope approach with archival (FFPE) tissue samples on glass slides and visualized the stained slides under a standard bright-field microscope. The RNAscope mapped the observed signals to individual cells and allowed the integration of molecular information with histopathological findings for optimal clinical interpretation.22
We observed a positive correlation between PD-L1 protein and mRNA expression in tumor cells, both in SCLC tissue specimens from LD-SCLC (SP142: r = 0.431; Dako 28-8: r = 0.403) and an even stronger correlation in lung cancer cell lines (SP142: r = 0.755). A comparison of PD-L1 protein and mRNA expression in two NSCLC cohorts also showed a positive association between protein and mRNA levels.40 One explanation for the higher correlation between PD-L1 protein and mRNA expression in SCLC cell lines than in SCLC tissue may be the differences in specimen processing. However, another intriguing possibility is that the tumor microenvironment in the SCLC tissue may affect the translation of PD-L1 mRNA to protein, causing a lower direct correlation between mRNA and protein levels.
Our results showed that both PD-L1 mRNA and protein expression in TIICs correlate with the degree of infiltration by TIICs in both LD-SCLC and ED-SCLC. This is consistent with a study of metastatic melanoma41 that found an association between tumor infiltration by CD8-positive T lymphocytes and increased PD-L1 protein/ mRNA expression in melanoma. There may be a functional distinction between tumors with intrinsic PD-L1 expression and those in which PD-L1 is induced through adaptive immune cells.41 We propose that expression of PD-L1 by TIICs in SCLC might reflect the presence of adaptive antitumor immune pressure. As reported for melanoma,41 immunotherapy targeting checkpoints may also be preferentially beneficial in lung cancers that have a preexisting T-cell–activated tumor microenvironment.
In summary, we characterized PD-L1 protein and/or mRNA expression in tumor cells and TIICs for two independent cohorts of patients with SCLC using two distinct methods with different antibodies for detection and found similar results in the two cohorts, with significantly lower PD-L1 prevalence than that reported in NSCLC. IHC is a commonly used and convenient method for detecting protein expression, but there is an urgent need for standardized methods of assessment and definitions of positivity for PD-L1. Although PD-L1 protein expression detected by IHC is currently being pursued as a predictive assay for PD1/PD-L1 immunotherapy, in this study we have also demonstrated the clinical feasibility of using an mRNA in situ assay based on FFPE tissue, which could be further studied in clinical trials. Although our study is retrospective in nature and has a relatively small sample size, our results are relatively consistent between independent cohorts and provide a rationale for future clinical investigations of the PD-1/PD-L1 axis as a therapeutic target for patients with SCLC with detectable PD-L1 mRNA and/or protein expression. However, owing to the lower expression of PD-L1 in SCLC than in NSCLC, despite encouraging preliminary clinical results with immune checkpoint inhibitors, it is possible that different immune mechanisms might be operative in SCLC treatment compared with in NSCLC treatment.
Acknowledgments
Data presented on the ED-SCLC cohort are from a study funded by Bristol-Myers Squibb. We would like to acknowledge Dr. Jason Hipp (Bristol Myers-Squibb) along with the pathologists at Mosaic Laboratories (Dr. Eric P. Olsen) and Q2 solutions (Drs. John Cochran, Michael Koch, Lena Kubbafor, Mary Schneck, Alan Stevenson, and Dino Vallera) for their contributions toward the pathological analyses. We would also like to thank the members of Biostats Solutions (Ena Bromley, Brian Burdi, Grace Li, Lin Li, and Qian Wu) for their contributions to the statistical analyses of ED-SCLC samples.
Footnotes
Disclosure: Drs. Batenchuk and Kulig reports employment with Bristol-Myers Squibb. Dr. Batenchuk also reports owning stock in Bristol-Myers Squibb. Ms. Lu and Dr. Gao are partially supported by Specialized Programs of Research Excellence in Lung Cancer -Biostatistics and Informatics Core grant 51P50 CA8187. Dr. Zhou reports receiving payment for lectures from Lilly, Roche, Henri. Dr. Hussein has served on the Speaker Bureau for Bristol-Myers Squibb. Drs. Goldschmidt and Jassem have received consulting fees from Bristol-Myers Squibb. Dr. Close reports a contract with Bristol-Myers Squibb. Dr. Hirsch has received research support (through the University of Colorado) from Ventana-Roche, Imclone-Lilly, Genentech, Celgene, and Amgen, and he has also participated in advisory boards for Novartis, Genentech/Roche, Lilly-Imclone, Pfizer, Celgene, Boehringer-Ingelheim, Syntha Pharmaceuticals, Amgen, and Bristol-Myers Squibb. The remaining authors declare no conflict of interest.
References
- 1.Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol. 2001;28:3–13. [PubMed] [Google Scholar]
- 2.Mori N, Yokota J, Akiyama T, et al. Variable mutations of the RB gene in small-cell lung carcinoma. Oncogene. 1990;5:1713–1717. [PubMed] [Google Scholar]
- 3.Arriola E, Canadas I, Arumi M, Rojo F, Rovira A, Albanell J. Genetic changes in small cell lung carcinoma. Clin Transl Oncol. 2008;10:189–197. doi: 10.1007/s12094-008-0181-1. [DOI] [PubMed] [Google Scholar]
- 4.Spigel DR, Socinski MA. Rationale for chemotherapy, immunotherapy, and checkpoint blockade in SCLC: beyond traditional treatment approaches. J Thorac Oncol. 2013;8:587–598. doi: 10.1097/JTO.0b013e318286cf88. [DOI] [PubMed] [Google Scholar]
- 5.Pleasance ED, Stephens PJ, O’Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463:184–190. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. 2012;44:1111–1116. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peifer M, Fernandez-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44:1104–1110. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 9.Ott PA, Fernandez MEE, Hiret S, et al. Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): preliminary safety and efficacy results from KEYNOTE-028 [abstract] J Clin Oncol. 2015;33(suppl):7502. [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spira AI, Park K, Mazieres J, et al. Efficacy, safety and predictive biomarker results from a randomized phase II study comparing MPDL3280A vs docetaxel in 2L/3L NSCLC (POPLAR) [abstract] J Clin Oncol. 2015;33(suppl):8010. [Google Scholar]
- 13.Spigel DR, Chaft JE, Gettinger SN, et al. Clinical activity and safety from a phase II study (FIR) of MPDL3280A (anti-PDL1) in PD-L1-selected patients with non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol. 2015;33(suppl):8028. [Google Scholar]
- 14.Horn L, Spigel DR, Gettinger SN, et al. Clinical activity, safety and predictive biomarkers of the engineered antibody MPDL3280A (anti-PDL1) in non-small cell lung cancer (NSCLC): update from a phase Ia study [abstract] J Clin Oncol. 2015;33(suppl):8029. [Google Scholar]
- 15.Liu SV, Powderly JD, Camidge DR, et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with platinum-based doublet chemotherapy in patients with advanced non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol. 2015;33(suppl):8030. [Google Scholar]
- 16.McDermott DF, Sosman JA, Sznol M, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;34:833–842. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 17.Badzio A, Wynes MW, Dziadziuszko R, et al. Increased insulin-like growth factor 1 receptor protein expression and gene copy number in small cell lung cancer. J Thorac Oncol. 2010;5:1905–1911. doi: 10.1097/JTO.0b013e3181f38f57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Yu H, Badzio A, et al. Fibroblast growth factor receptor 1 and related ligands in small-cell lung cancer. J Thorac Oncol. 2015;10:1083–1090. doi: 10.1097/JTO.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travis WD. 2015 WHO Classification of the pathology and genetics of tumors of the lung. J Thorac Oncol. 2015;10:S68. [Google Scholar]
- 20.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips T, Simmons P, Inzunza HD, et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol. 2015;23:541–549. doi: 10.1097/PAI.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf M, Holle R, Hans K, Drings P, Havemann K. Analysis of prognostic factors in 766 patients with small cell lung cancer (SCLC): the role of sex as a predictor for survival. Br J Cancer. 1991;63:986–992. doi: 10.1038/bjc.1991.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Smoking and lung cancer survival—the role of comorbidity and treatment. Chest. 2004;125:27–37. doi: 10.1378/chest.125.1.27. [DOI] [PubMed] [Google Scholar]
- 25.Takhar HS, Sukumaran S, Ly M, et al. Impact of age on treatment and survival in patients with small cell lung cancer. J Thorac Oncol. 2013;8:S933. [Google Scholar]
- 26.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultheis AM, Scheel AH, Ozretic L, et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer. 2015;51:421–426. doi: 10.1016/j.ejca.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 30.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 31.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121:664–672. doi: 10.1002/cncr.29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 33.Koyama K, Kagamu H, Miura S, et al. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14:6770–6779. doi: 10.1158/1078-0432.CCR-08-1156. [DOI] [PubMed] [Google Scholar]
- 34.Ishii H, Azuma K, Kawahara A, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol. 2015;10:426–430. doi: 10.1097/JTO.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 35.Komiya T, Madan R. PD-L1 expression in small cell lung cancer. Eur J Cancer. 2015;51:1853–1855. doi: 10.1016/j.ejca.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 expression in lung cancer. J Thorac Oncol. 2016;11:964–975. doi: 10.1016/j.jtho.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104:2084–2091. doi: 10.1002/cncr.21470. [DOI] [PubMed] [Google Scholar]
- 38.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 40.Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor micro-environment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]


