Abstract
In 2012, a zoonotic coronavirus was identified as the causative agent of Middle East Respiratory Syndrome (MERS), called MERS Coronavirus (MERS-CoV). Since then, the virus has infected 1728 patients as of 12 May 2016, with a mortality rate of 36%. While MERS-CoV generally causes subclinical or mild disease, infection can result in serious outcomes, including acute respiratory distress syndrome (ARDS) and multi-organ failure in patients with co-morbidities. The virus is endemic in camels in the Arabian peninsula and Africa and thus poses a consistent threat of frequent reintroduction into human populations. Disease prevalence will increase substantially if the virus mutates to increase human-to-human transmissibility. No therapeutics or vaccines are approved for MERS; thus, development of novel therapies is needed. Further, since many MERS cases are acquired in healthcare settings, public health measures and scrupulous attention to infection control practices are required to prevent additional MERS outbreaks.
Keywords: host-virus interactions, camels, immune response, outbreak, animal models, antiviral therapies
The Outbreaks
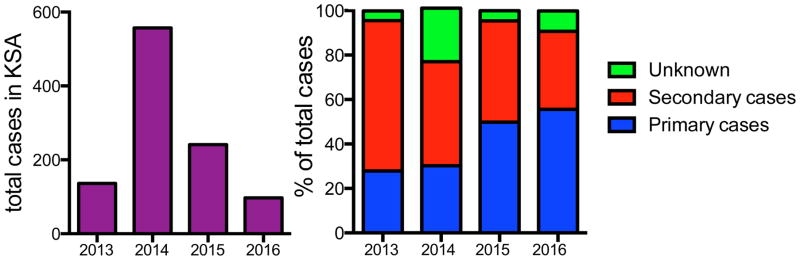
In June of 2012 a man in Jeddah, Saudi Arabia was admitted to a hospital with severe pneumonia. A novel coronavirus (CoV) related to the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) was isolated from this patient and shown to be the etiological agent (1). Further studies showed that it was related to several bat CoV, including HKU4 and HKU5 and it was named the Middle East Respiratory Syndrome-CoV (MERS-CoV) (2). Retrospective studies showed that the same virus was also responsible for an outbreak of respiratory disease in Zarqa, Jordan in April 2012. Since these initial outbreaks, MERS-CoV infection has been identified in 1728 patients (12 May 2016), with 624 associated deaths (3). All of the cases were geographically linked to the Middle East, and cases that occurred outside of the Middle East involved travelers from this region. Many MERS cases occurred in hospitals on the Arabian peninsula, usually in the setting of inadequate infectious control practices. In the summer of 2015, an outbreak of 186 MERS cases occurred in South Korea. This outbreak stemmed from a single patient who had returned from the Middle East. Diagnosis of MERS was delayed, as he sought treatment at multiple medical facilities, which facilitated virus spread to susceptible individuals at these healthcare facilities (4). As was the case for the Middle East patients, a majority of severely ill patients had underlying diseases, as described below in more detail. Fortunately, increased surveillance for MERS-CoV and improved infection control measures in hospitals has slowed the spread of this highly pathogenic virus (Fig. 1).
Figure 1.
Case distribution of MERS-CoV in Saudi Arabia, 2013–2016. The total (A) and percentage (B) of primary, secondary, or unknown MERS cases are plotted per year in Saudi Arabia. While secondary cases of MERS predominated during the early stages of the outbreak, improved infection and healthcare controls have significantly reduced the number and percentage of secondary cases in the last two years (13; 81).
The Virus
MERS-CoV is a member of the family Coronaviridae, which are enveloped, positive-sense, single stranded RNA viruses. CoVs are the largest known RNA viruses to date, with genomes of ~30 kb. The CoV family is divided into four genera based on phylogenetic clustering: alpha, beta, gamma, and delta coronaviruses. These genera are further subdivided into distinct lineages. MERS-CoV is a lineage C β-Coronavirus while SARS-CoV is a lineage B β-Coronavirus (2). Coronaviruses cause a variety of diseases in mammalian species, including respiratory, hepatic, enteric, and neurologic pathologies of differing severity in mammals ranging from humans to domesticated and companion animals (5).
All CoVs have a highly conserved genome organization with a 5′ replicase polyprotein followed by structural and accessory genes interspersed through the 3′ end of the genome (5). The polyprotein is cleaved by viral proteases into 16 non-structural proteins largely mediating virus replication. Genes at the 3′ end of the genome encode for several virion structural proteins, including the nucleocapsid (N), matrix (M) and envelope (E) proteins as well as the spike (S) glycoprotein. The S protein mediates binding of the virus to the host cell receptor and is a major determinant of virulence. Finally, the accessory proteins encode for proteins largely involved in modulating the innate immune response to CoVs. MERS-CoV encodes for 5 unique accessory proteins, with at least two, 4A and 4B, that modulate interferon production when expressed in isolation (6).
Soon after the discovery of MERS-CoV, the receptor that mediates cell entry was identified as dipeptidyl peptidase four (DPP4), a large ectopeptidase present at the surface of many different cell types (7). The use of DPP4 distinguishes MERS-CoV from SARS-CoV, which uses angiotensin-converting enzyme 2 (ACE2), another large ectopeptidase, as its receptor (8). While there has been extensive recombination of MERS-CoV in camels (9) and genetic changes in the virus have been noted since 2012, there is no evidence that the virus has mutated to enhance binding to DPP4. Rather, in one instance, a virus with diminished affinity for the receptor was described (10). This suggests that adaptation of MERS-CoV spike protein to human DPP4 is not occurring, unlike SARS-CoV, which underwent extensive mutation to adapt to the human ACE2 protein (11).
Epidemiology
Since 2012, MERS cases have been identified in 27 countries, although most occur in the Kingdom of Saudi Arabia (KSA) and other countries on the Arabian peninsula. Furthermore, all cases outside of the Middle East can be traced back to patients who had recently traveled from this region. The largest series of outbreaks occurred in the spring of 2014 in KSA, when ~500 hospital-acquired cases appeared throughout the country within a few months due to breaches in infection control. These outbreaks were terminated by increased awareness and reporting and scrupulous attention to infection control measures. The 2015 MERS outbreak in South Korea was halted by placing approximately 17,000 individuals under quarantine. 36 patients succumbed to the infection, with a case fatality rate of 19%, substantially less than that reported in the Middle East (12). This lower case fatality rate likely reflects careful screening to identify all infected patients, including those with subclinical disease. This outbreak was alarming as it was the first time MERS-CoV spread to a large number of patients outside of the Middle East.
As described in more detail below, camels are the probable source for zoonotic transmission of the virus, but the source for infection in many patients with primary (i.e., not associated with MERS patient contact) disease remains undetermined. Several epidemiologic studies demonstrated that secondary cases occur in healthcare settings and to a lesser extent, in households (13). Human-to-human spread requires close contact and is likely through large droplets, although aerosol or fomite transmission has not been ruled out. MERS-CoV may persist in the environment for up to 24 h, which may also be source for human infection (14). Sequences from MERS-CoV isolated from single outbreaks are very similar, if not identical, consistent with interhuman transmission (15). Sequence analyses of virus isolated from patients that were infected at different times and places revealed little evidence for directed mutation, suggesting that the virus is not adapting to human populations, as occurred with SARS-CoV (15) MERS-CoV is transmitted inefficiently among humans. For example, one study found that the transmission rate of infected patients to household contacts was only 4% (13; 16). Many of these household contacts were asymptomatic or had mild disease. The R0 factor for MERS-CoV has been estimated to be less than 0.7 and likely closer to 0.5, significantly lower than an R0 of 1, a mark of epidemic potential (17). Other serologic and RT-PCR studies showed that as many as 45,000 people in Saudi Arabia were infected by MERS-CoV (18). Although not clinically ill, these individuals may play a role in spreading MERS-CoV.
To date, almost two-thirds of all MERS cases have been males; however, males and females have similar case fatality rates (13). This sex-based difference in case numbers is believed to reflect difference in MERS-CoV exposure. Age is also a risk factor for developing severe MERS, as the average MERS patient is ~50 years old. Primary cases and secondary hospital-acquired cases tend to be older, while infected secondary household members and healthcare personnel are generally younger. Elderly patients have a much greater likelihood of dying from the disease, with a fatality rate of nearly 90% for patients over 80 compared to ~10% for those under the age of 20 (13).
Perhaps the most consistently described risk factor for MERS disease is the presence of underlying co-morbidities such as diabetes (68%), chronic renal disease (49%), obesity, hypertension (34%) chronic cardiac diseases (28%) and lung disease such as asthma and chronic obstructive pulmonary disease (13; 19). In total, approximately 75% of all documented cases occurred in patients with co-morbidities.
Pathogenesis
MERS pathogenesis begins with entry of virus via the respiratory tract where the spike (S) protein interacts with its cellular receptor DPP4. DPP4 is expressed in the respiratory tract on type I and II pneumocytes, non ciliated bronchial epithelial cells, endothelial cells, and some hematopoietic cells (7; 20). DPP4 is less abundant on the surface of upper airway (nasal cavity to conducting airways) epithelia, but is highly expressed on the epithelial cells of distal airways and alveoli (21). Relatively low abundance of DPP4 in the upper respiratory tract may limit human-to-human transmission. DPP4 is also widely expressed on the epithelial cells of several other organs and tissues such as kidneys, intestine, liver, thymus and bone marrow (22). Immunohistochemical examination of lungs from patients with chronic lung disease, such as chronic obstructive pulmonary disease revealed increased DPP4 expression on alveolar epithelia (type I and II cells) and alveolar macrophages compared to controls (20). These findings suggest that pre-existing pulmonary disease results in augmented DPP4 expression, which may predispose individuals to more severe MERS.
Lack of patient autopsy or surgical pathology samples from the Middle East or the Korean outbreak has limited studies of MERS-CoV pathogenesis. So far, understanding of MERS-CoV pathogenesis is based on in vitro studies, infection of human lung explants and laboratory animals, and description of a single autopsy(23). In vitro studies revealed robust MERS-CoV replication in non-differentiated and differentiated human primary epithelial cells and several tissue culture cell lines (24–26). MERS-CoV antigen was detected in type I and type II pneumocytes and non ciliated and possibly ciliated bronchial and bronchiolar epithelial cells, endothelial cells and alveolar macrophages in infected human lung explants (27; 28). Ex vivo infection of lung tissues corroborated findings observed in the single MERS autopsy case, where immunohistochemical examination of lungs revealed MERS-CoV antigen in pneumocytes (type I and II), airway epithelial cells, endothelial cells and rarely in macrophages (23; 27).
Host Immune Response to MERS-CoV
A rapid and well-coordinated innate immune response is the first line of defense against viral infections; dysregulated and exuberant innate immune responses may cause immunopathology. MERS-CoV infection of human epithelial cells induced significant but delayed type I, II and II interferon (IFN) responses (29). Similarly, pro-inflammatory cytokines and chemokines such as IL-1β, IL-6 and IL-8 showed delayed but marked induction upon MERS-CoV infection (30). In addition to infecting epithelial cells, in vitro studies show that MERS-CoV infects hematopoietic cells such as monocyte-macrophages, dendritic cells and activated T cells (31–33). This is in contrast to SARS-CoV, which abortively infects monocyte-macrophages and DCs (34; 35). Similar to the situation in epithelial cells, MERS-CoV infection of human peripheral blood monocyte-derived macrophages and dendritic cells induced delayed but elevated levels of proinflammatory cytokines and chemokines such as CCL-2, CCL-3, RANTES, IL-2 and IL-8 (30; 32). However, induction of type I IFN by monocyte-macrophages and DCs was not substantial except for plasmacytoid dendritic cells, which produced copious amounts of type I and III interferons upon infection (31; 32; 36). MERS-CoV infection of activated T cells induced apoptosis via both intrinsic and extrinsic pathways (33), which could contribute to the lymphopenia observed in MERS patients with poor outcomes. Limited data available from MERS patients with poor outcome shows elevated CXCL-10, IL-17 and IL-23 expression, with a paucity of IFN-α and IFN-γ expression (37). At this point, it is difficult to determine factors contributing to protective versus pathologic immune responses because of insufficient clinical data and limited autopsy samples.
Clinical Features of MERS in Humans
MERS-CoV infection causes a wide range of clinical manifestations in humans. The clinical symptoms of MERS disease range from asymptomatic to mild respiratory illness to severe acute pneumonia, which rapidly progresses to acute lung Injury and ARDS, multi-organ failure and death (19; 38–42).
Incubation period
Studies of human-to-human MERS-CoV transmission from clusters of MERS patients revealed a median incubation period of 5–7 days, with a range of 2–14 days (19; 43). In MERS patients, the median time from the onset of illness to hospitalization is approximately 4 days and the median time from onset of symptoms to intensive care unit (ICU) admission is approximately 5 days (19; 39).
Clinical manifestations
In hospitalized patients, the most common manifestations include flu-like symptoms such as fever, sore throat, non-productive cough, chills/rigors, chest pain, headache, myalgia, shortness of breath, and dyspnea, which rapidly progress to pneumonia in those that develop severe disease. MERS in humans often causes gastrointestinal symptoms such as abdominal pain, vomiting and diarrhea(19; 39–42). Patients with milder disease often present with disease manifestations confined to the upper respiratory tract infection.
Laboratory findings
Samples obtained from the upper respiratory tract of MERS patients showed lower virus titers and RNA copy numbers compared to those collected from the lower respiratory tract. In some patients, low levels of MERS-CoV genomic RNA were detected in blood, urine and stool (44; 45). Hematological analysis of MERS patients revealed lymphocytopenia and thrombocytopenia. Other laboratory findings include elevated creatinine, lactate dehydrogenase, and alanine and aspartate aminotransferase suggestive of renal and liver disease (19). Chest radiographic and computerized tomography (CT) findings demonstrated minor to extensive unilateral and bilateral abnormalities including enhanced bronchovascular markings, airspace opacities, patchy infiltrates and airspace consolidations. Airspace opacities were either nodular or reticular with reticulonodular shadowing and pleural effusions (46).
Gross and histopathological examination
Despite numerous deaths related to MERS-CoV infection in humans in Saudi Arabia, South Korea and other parts of the world, only one autopsy report of MERS in humans is available. Gross examination revealed extensive lung consolidation with generalized congestion and edema (23). The most prominent histological findings were exudative type diffuse alveolar damage (DAD), necrosis of alveolar epithelial cells, hyperplasia of type II pneumocytes, sloughing of bronchiolar epithelium, alveolar edema, alveolar fibrin deposition, hyaline membrane formation, thickening of alveolar septa and inflammatory cell infiltration (monocyte/macrophages, neutrophils and lymphocytes)(23). While pathological changes were also observed in the kidney, heart, liver bone marrow and lymphoid organs, it is not known whether these findings reflected direct virus infection or, alternatively, occurred non-specifically in the context of terminal disease.
The Camel Connection
MERS-CoV is most closely related to the Tylonycteris and Pipistrellus bat CoVs HKU4 and HKU5, respectively, suggesting that bats could have been the ultimate source for the virus. In support of this, a single CoV isolate from an adult female Neormocia cf. zuluensis bat from South Africa was found to harbor a β-Coronavirus that was highly related to MERS-CoV. This virus was shown to root the phylogenetic tree of MERS-CoV (47), suggesting that MERS-CoV distantly originated in African bats.
Bats were considered an unlikely source of repeated direct transmission to humans due to the lack of contact between bats and humans. Thus, many other animals found in the Arabian peninsula such as goats, horses, chickens, sheep, poultry, and camels were tested for MERS-CoV seropositivity. Only dromedary camels were found to be positive for anti-MERS-CoV antibody. Dromedary camels are primarily present throughout Africa and the Arabian peninsula and camels at all sites were shown to be seropositive with rates as high as 80% in some populations (reviewed in (48). Surprisingly, serum samples from as far back as 1982 in Africa and 1992 in Saudi Arabia were positive for MERS-CoV antibodies. This suggests that MERS-CoV has infected camels for an extended amount of time and raises the question as to why MERS was not detected in patients in Saudi Arabia before 2012. Of note, camels from Australia, Canada, USA, Germany, the Netherlands and Japan are seronegative for MERS-CoV (48). These data all point to a likely transmission of MERS-CoV to camels in Africa many years ago with subsequent spread to the Middle East. Although the majority of primary human cases are not associated with camel exposure, transmission from camels has been documented in several cases. Sequence analysis of virus collected from two humans and at least 3 camels at a barn in Qatar found them to be infected with the same strain of virus. Other studies found that a prominent risk factor for MERS disease was contact with camels, and in Saudi Arabia and Qatar those who worked with camels were much more likely to be seropositive for MERS-CoV (~3.6–6.4%) than the general population (0.15%) ((18), reviewed in (49)). Another study reported that camels in 2014/2015 in Saudi Arabia harbored at least 5 different lineages of MERS-CoVs as well as β-Coronavirus 1-HKU23 and camelid CoVs related to the human CoV 229E (9). Some of the MERS-CoV strains were recombinants between different camel isolates. These camel isolates were very similar to those circulating in human populations at that time, providing strong support for the link between camel and human infection.
While camels are clearly the primary zoonotic intermediate for MERS-CoV infection, the route of transmission into the human population is not entirely clear and is likely multi-factorial. In contrast to humans, DPP4 is highly expressed in the upper respiratory tract of camels facilitating MERS-CoV transmission from camel to camel (21). The highest levels of virus are found in nasal discharge from camels (50), suggesting that direct contact with a sick animal may lead to human transmission (49). While there is an increased rate of seropositivity for MERS-CoV amongst slaughterhouse workers (18), smaller prospective studies found no evidence for MERS-CoV infection for those with occupational exposure (51), indicating that transmission from camels to humans is inefficient. In addition to respiratory spread, the virus may also be transmitted through contaminated camel milk and even meat. Camel milk is not pasteurized in many parts of the Middle East, and MERS-CoV RNA has been detected in raw milk collected in the marketplace of Qatar (52). Virus may be naturally present in milk or meat, or could be introduced by poor hygienic conditions. Prevention of camel to human transmission will require extensive implementation of precautionary measures such as the use of personal protective equipment as well as increased awareness of the likelihood of infection. As camels are important in many aspects of the Arabian culture, eliminating the threat of MERS-CoV will be difficult and will require changing local customs.
What is Being Done to Prevent MERS-CoV
Diagnosis of MERS-CoV
Patients with MERS-CoV and other respiratory virus infections present with similar clinical manifestations, making it difficult to diagnose MERS on clinical grounds. Formal guidelines have been established to diagnose MERS. During the acute phase of the illness, MERS-CoV may be detected in respiratory tract specimens by detection of either infectious virus or viral RNA. For the latter, the diagnosis of MERS-CoV infection requires two positive qRT-PCR tests on two different specific genomic segments or a single positive qRT-PCR with a sequence of another positive genomic segment. qRT-PCR detection of MERS-CoV genomic mRNA in upper and lower airway samples by targeting a gene segment upstream of E gene (UpE) and in ORF1ab is recommended (53). Efforts should be made to collect samples from the lower respiratory tract since virus loads are higher at this site (21). MERS can also be diagnosed by detecting virus-specific antibodies in serum samples collected at least 2–3 weeks after disease onset. A positive ELISA assay followed by a positive immunofluorescence and/or microneutralization test is required for diagnosis (54). Of note, MERS-CoV antibody titers appear to wane with time, especially in patients with mild disease (55).
Vaccine development
No MERS-CoV-specific vaccines are currently approved for use in humans. Inactivated virus vaccines, live-attenuated virus vaccines, viral vector vaccines, subunit vaccines and DNA vaccines have all been developed but none have reached clinical trials (reviewed in (56; 57). Live-attenuated vaccines induce both antibody and T cell responses, which tend to persist for long times, but are not likely to be clinically useful because of the concern that they could recombine with circulating CoV, generating new pathogenic CoV strains. Vaccinating camels is also a potential strategy to prevent MERS-CoV transmission to humans (58).
Anti-MERS-CoV therapies
Currently there is no specific approved therapeutic agent or vaccine available to treat or prevent MERS. As a result, supportive care is the mainstay of treatment. Since type I interferons effectively inhibit MERS-CoV replication in vitro (24; 59), the timely administration of IFNa2 with another agent such as ribavirin may reduce lung virus load and diminish lung injury. Early (8 h.p.i.) administration of IFNa2 and ribavirin reduced MERS-CoV titers and lung immunopathology in experimentally infected rhesus macaques (60). In one clinical study, administration of IFNa2 and ribavirin showed enhanced survival in critically ill patients at 14 days but not at 28 days (61). However, in another study, late administration of IFNa2 and ribavirin in critically ill patients had no effect on mortality (62). Early administration, which is critical for therapeutic efficacy is difficult to achieve in a clinical setting, probably explaining the disparity in results described above.
Passive immunotherapy with convalescent patient plasma or MERS-CoV-specific antibodies represents a potentially promising approach to treat MERS patients (63). Plasma from MERS survivors with high titers of neutralizing antibodies could be used for passive immunotherapy, although this approach is hindered by the lack of MERS survivors with high titer antibodies. Substantial progress has been made in producing neutralizing antibodies specific to MERS-CoV. In one study, Corti et al. isolated a potent MERS-CoV neutralizing antibody from memory B cells of a MERS patient and showed that therapeutic treatment with this antibody inhibited MERS-CoV replication in a mouse model (64). Similarly, MERS-CoV neutralizing antibodies developed using naïve human antibody-phage display libraries were shown to effectively inhibit MERS-CoV replication and reduced lung pathology in MERS-CoV challenged rhesus macaques (65; 66). In another approach, polyclonal antibodies produced from transchromosomic bovines effectively inhibited MERS-CoV replication both in vitro and in vivo in a mouse model (67). All of these antibodies target the receptor-binding domain (RBD) of the MERS-CoV S protein. Use of neutralizing antibodies for therapeutic purposes relies on rapid identification of patients and administration at early times after disease onset. These antibodies may be most useful for prophylaxis in high-risk hospital and healthcare settings.
In addition to interferon and neutralizing antibodies, several antiviral drugs are under investigation for clinical use (68; 69). MERS-CoV-specific peptide fusion inhibitors function by efficiently inhibiting virus entry and replication in vitro and were shown to reduce lung virus loads when used as prophylactic or therapeutic agents in mouse and macaque models (70; 71). Drugs targeting viral enzymes (papain-like protease (PLpro) and 3C-like protease (3CLpro)) inhibit CoV replication and are potential targets to reduce virus load. Lopinavir, a drug with anti-HIV protease activity, has been used in SARS and MERS patients although efficacy has not been proven. Additionally, several antiviral drugs such as chloroquine, chlorpromazine, mycophenolic acid, nitazoxanide have been identified in broad drug screens, but these drugs need further investigation before they can be used in MERS patients.
Animals models of MERS-CoV infection
Suitable animal models are necessary to screen antiviral therapeutics and vaccines and for studies of pathogenesis. Small laboratory animals, particularly rodents, do not support MERS-CoV replication due to inability of MERS-CoV-S to bind to human DPP4 orthologs in these animals. The first MERS-CoV mouse model was generated by sensitizing mice to infection by intranasal transduction of human DPP4 (72). These transduced mice developed mild to moderate pneumonia, after MERS-CoV challenge. Subsequently, several human DPP4 transgenic mouse models were developed. These mice generally developed a fatal encephalitis (73; 74), providing a platform for stringent evaluation of vaccines and anti-MERS-CoV drugs. MERS-CoV challenged rabbits displayed subclinical disease (75; 76), while rhesus macaques displayed mild-moderate respiratory disease. Marmosets are also susceptible to infection and have been reported to develop severe disease (77), although these results are controversial (78) The basis for these differences is not clear, but may reflect differences in virus stocks, route of administration and source and age of marmosets. Experimentally infected camels developed mild rhinitis without overt pneumonia and are useful for studies of camel-to-camel and camel-to-human transmission (50).
Control of MERS-CoV spread
MERS-CoV transmission in healthcare settings accounts for many MERS cases although transmission in this setting has diminished as infection control measures have been rigorously applied (79; 80). Since the early signs of MERS-CoV resemble those of other respiratory infections, health-care workers in the Middle East and elsewhere should follow standard procedure consistently in all patients. Droplet and contact precautions should be practiced when treating probable or confirmed cases of MERS-CoV infection. Since people with diabetes, renal failure, chronic lung disease, and immunocompromised individuals are at high risk of developing severe disease after MERS-CoV infection, these people should avoid close contact with camels, when visiting farms or markets, where the virus is known to be potentially circulating. Following general hygiene measures, such as regular hand washing before and after touching animals and avoiding contact with sick animals will reduce the risk of contracting MERS-CoV. As stated above, food hygiene practices including avoiding raw camel milk or camel urine (used medicinally), and eating only well-cooked meat should be practiced. In combination, these and other WHO recommendations appear to be having a positive effect, as the number of secondary cases of MERS in Saudi Arabia is dropping (Fig. 1).
Conclusions and Future Directions
In the nearly 4 years since MERS-CoV appeared as a novel virus with potential epidemic threat, several advances have been made and many questions answered. For instance, we now are reasonably certain that the zoonotic source of the virus is the dromedary camel. However, there are many questions and issues that remain to be addressed.
In order to prevent the spread and spread of future outbreaks of MERS, it will be critical to continue to increase our surveillance for MERS. It will be important to closely monitor circulating MERS-CoV for mutations that enhance its transmission or virulence: coronaviruses are known to have high rates of recombination (5) and thus MERS could adapt to become more transmissible in humans. Furthermore, physicians must be aware of MERS as a possible diagnosis if a patient with a febrile respiratory disease has recently returned from the Middle East. Finally, stringent infection control measures, as discussed above, are critical to control the spread of this virus in healthcare settings.
As with all other CoVs, there are currently no therapeutics or vaccines available to control the infection. In order to do develop therapeutics and vaccines, new small animal models that more closely resemble lethal human disease will be required. Using these models we will be able to better evaluate repurposed and new drugs, vaccines and antibodies for their efficacy. Determining novel host and viral determinants of pathogenesis will help to identify additional drug targets as well as aid in vaccine development.
Acknowledgments
Supported in part by grants to S.P. from the NIH (AI060699, AI091322). A.R.F. was supported by an individual NRSA F32-AI113973.
References
- 1.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. The New England journal of medicine. 2012;367:1814–20. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. Journal of virology. 2013;87:7790–2. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. 2016 http://www.who.int/emergencies/mers-cov/en/
- 4.Lee SS, Wong NS. Probable transmission chains of Middle East respiratory syndrome coronavirus and the multiple generations of secondary infection in South Korea. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2015;38:65–7. doi: 10.1016/j.ijid.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masters PS, Perlman S. Coronaviridae. In: Howley PM, Knipe DM, editors. Fields Virology. Vol. 6. Philadelphia, PA: Lippincott Williams and Wilkins, a Wolters Kluwer business; 2013. pp. 825–58. [Google Scholar]
- 6.Yang Y, Zhang L, Geng H, Deng Y, Huang B, et al. The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists. Protein & cell. 2013;4:951–61. doi: 10.1007/s13238-013-3096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–4. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–4. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabir JS, Lam TT, Ahmed MM, Li L, Shen Y, et al. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science. 2016;351:81–4. doi: 10.1126/science.aac8608. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Cheon S, Min CK, Sohn KM, Kang YJ, et al. Spread of Mutant Middle East Respiratory Syndrome Coronavirus with Reduced Affinity to Human CD26 during the South Korean Outbreak. mBio. 2016;7:e00019–16. doi: 10.1128/mBio.00019-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinese SMEC. Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303:1666–9. doi: 10.1126/science.1092002. [DOI] [PubMed] [Google Scholar]
- 12.Korean Society of Infectious D, Korean Society for Healthcare-associated Infection C, Prevention. An Unexpected Outbreak of Middle East Respiratory Syndrome Coronavirus Infection in the Republic of Korea, 2015. Infection & chemotherapy. 2015;47:120–2. doi: 10.3947/ic.2015.47.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsahafi AJ, Cheng AC. The epidemiology of Middle East respiratory syndrome coronavirus in the Kingdom of Saudi Arabia, 2012–2015. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2016;45:1–4. doi: 10.1016/j.ijid.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Doremalen N, Bushmaker T, Munster VJ. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2013:18. doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- 15.Cotten M, Watson SJ, Zumla AI, Makhdoom HQ, Palser AL, et al. Spread, circulation, and evolution of the Middle East respiratory syndrome coronavirus. mBio. 2014:5. doi: 10.1128/mBio.01062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drosten C, Meyer B, Muller MA, Corman VM, Al-Masri M, et al. Transmission of MERS-coronavirus in household contacts. The New England journal of medicine. 2014;371:828–35. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- 17.Poletto C, Pelat C, Levy-Bruhl D, Yazdanpanah Y, Boelle PY, Colizza V. Assessment of the Middle East respiratory syndrome coronavirus (MERS-CoV) epidemic in the Middle East and risk of international spread using a novel maximum likelihood analysis approach. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014:19. doi: 10.2807/1560-7917.es2014.19.23.20824. [DOI] [PubMed] [Google Scholar]
- 18.Muller MA, Meyer B, Corman VM, Al-Masri M, Turkestani A, et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. The Lancet Infectious diseases. 2015;15:629. doi: 10.1016/S1473-3099(15)00029-8. [DOI] [PubMed] [Google Scholar]
- 19.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. The Lancet Infectious diseases. 2013;13:752–61. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyerholz DK, Lambertz AM, McCray PB., Jr Dipeptidyl Peptidase 4 Distribution in the Human Respiratory Tract: Implications for the Middle East Respiratory Syndrome. The American journal of pathology. 2016;186:78–86. doi: 10.1016/j.ajpath.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Widagdo W, Raj VS, Schipper D, Kolijn K, van Leenders GJ, et al. Differential Expression of the Middle East Respiratory Syndrome Coronavirus Receptor in the Upper Respiratory Tracts of Humans and Dromedary Camels. Journal of virology. 2016;90:4838–42. doi: 10.1128/JVI.02994-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boonacker E, Van Noorden CJ. The multifunctional or moonlighting protein CD26/DPPIV. European journal of cell biology. 2003;82:53–73. doi: 10.1078/0171-9335-00302. [DOI] [PubMed] [Google Scholar]
- 23.Ng DL, Al Hosani F, Keating MK, Gerber SI, Jones TL, et al. Clinicopathologic, Immunohistochemical, and Ultrastructural Findings of a Fatal Case of Middle East Respiratory Syndrome Coronavirus Infection in the United Arab Emirates, April 2014. The American journal of pathology. 2016;186:652–8. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zielecki F, Weber M, Eickmann M, Spiegelberg L, Zaki AM, et al. Human cell tropism and innate immune system interactions of human respiratory coronavirus EMC compared to those of severe acute respiratory syndrome coronavirus. Journal of virology. 2013;87:5300–4. doi: 10.1128/JVI.03496-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan JF, Chan KH, Choi GK, To KK, Tse H, et al. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation. The Journal of infectious diseases. 2013;207:1743–52. doi: 10.1093/infdis/jit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dijkman R, Jebbink MF, Koekkoek SM, Deijs M, Jonsdottir HR, et al. Isolation and characterization of current human coronavirus strains in primary human epithelial cell cultures reveal differences in target cell tropism. Journal of virology. 2013;87:6081–90. doi: 10.1128/JVI.03368-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hocke AC, Becher A, Knepper J, Peter A, Holland G, et al. Emerging human middle East respiratory syndrome coronavirus causes widespread infection and alveolar damage in human lungs. American journal of respiratory and critical care medicine. 2013;188:882–6. doi: 10.1164/rccm.201305-0954LE. [DOI] [PubMed] [Google Scholar]
- 28.Chan RW, Chan MC, Agnihothram S, Chan LL, Kuok DI, et al. Tropism of and innate immune responses to the novel human betacoronavirus lineage C virus in human ex vivo respiratory organ cultures. Journal of virology. 2013;87:6604–14. doi: 10.1128/JVI.00009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menachery VD, Eisfeld AJ, Schafer A, Josset L, Sims AC, et al. Pathogenic influenza viruses and coronaviruses utilize similar and contrasting approaches to control interferon-stimulated gene responses. mBio. 2014;5:e01174–14. doi: 10.1128/mBio.01174-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau SK, Lau CC, Chan KH, Li CP, Chen H, et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. The Journal of general virology. 2013;94:2679–90. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 31.Tynell J, Westenius V, Ronkko E, Munster VJ, Melen K, et al. Middle East respiratory syndrome coronavirus shows poor replication but significant induction of antiviral responses in human monocyte-derived macrophages and dendritic cells. The Journal of general virology. 2016;97:344–55. doi: 10.1099/jgv.0.000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J, Chu H, Li C, Wong BH, Cheng ZS, et al. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. The Journal of infectious diseases. 2014;209:1331–42. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu H, Zhou J, Wong BH, Li C, Chan JF, et al. Middle East Respiratory Syndrome Coronavirus Efficiently Infects Human Primary T Lymphocytes and Activates the Extrinsic and Intrinsic Apoptosis Pathways. The Journal of infectious diseases. 2015 doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, et al. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366–74. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung CY, Poon LL, Ng IH, Luk W, Sia SF, et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. Journal of virology. 2005;79:7819–26. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheuplein VA, Seifried J, Malczyk AH, Miller L, Hocker L, et al. High secretion of interferons by human plasmacytoid dendritic cells upon recognition of Middle East respiratory syndrome coronavirus. Journal of virology. 2015;89:3859–69. doi: 10.1128/JVI.03607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faure E, Poissy J, Goffard A, Fournier C, Kipnis E, et al. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PloS one. 2014;9:e88716. doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. The Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Tawfiq JA, Hinedi K, Ghandour J, Khairalla H, Musleh S, et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;59:160–5. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Annals of internal medicine. 2014;160:389–97. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 41.Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. The New England journal of medicine. 2013;369:407–16. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2014;29:301–6. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virlogeux V, Park M, Wu JT, Cowling BJ. Association between Severity of MERS-CoV Infection and Incubation Period. Emerging infectious diseases. 2016:22. doi: 10.3201/eid2203.151437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poissy J, Goffard A, Parmentier-Decrucq E, Favory R, Kauv M, et al. Kinetics and pattern of viral excretion in biological specimens of two MERS-CoV cases. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2014;61:275–8. doi: 10.1016/j.jcv.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Memish ZA, Al-Tawfiq JA, Makhdoom HQ, Assiri A, Alhakeem RF, et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. The Journal of infectious diseases. 2014;210:1590–4. doi: 10.1093/infdis/jiu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A, Madani TA. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR American journal of roentgenology. 2014;203:782–7. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- 47.Corman VM, Ithete NL, Richards LR, Schoeman MC, Preiser W, et al. Rooting the phylogenetic tree of middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. Journal of virology. 2014;88:11297–303. doi: 10.1128/JVI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omrani AS, Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus (MERS-CoV): animal to human interaction. Pathogens and global health. 2015;109:354–62. doi: 10.1080/20477724.2015.1122852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reusken CB, Raj VS, Koopmans MP, Haagmans BL. Cross host transmission in the emergence of MERS coronavirus. Current opinion in virology. 2016;16:55–62. doi: 10.1016/j.coviro.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adney DR, van Doremalen N, Brown VR, Bushmaker T, Scott D, et al. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerging infectious diseases. 2014;20:1999–2005. doi: 10.3201/eid2012.141280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hemida MG, Al-Naeem A, Perera RA, Chin AW, Poon LL, Peiris M. Lack of middle East respiratory syndrome coronavirus transmission from infected camels. Emerging infectious diseases. 2015;21:699–701. doi: 10.3201/eid2104.141949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reusken CB, Farag EA, Jonges M, Godeke GJ, El-Sayed AM, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014:19. doi: 10.2807/1560-7917.es2014.19.23.20829. [DOI] [PubMed] [Google Scholar]
- 53.Corman VM, Muller MA, Costabel U, Timm J, Binger T, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2012:17. doi: 10.2807/ese.17.49.20334-en. [DOI] [PubMed] [Google Scholar]
- 54.Coronavirus WLtfMERS. http://www.who.int/csr/disease/coronavirus_infections/mers-laboratory-testing/en/IgJ.
- 55.Alshukairi ANKI, Ahmed WA, Dada AM, Bayumi DT, Malic LS, et al. Antibody response and disease severity in healthcare worker MERS survivors. [May 3, 2016];Emerg Infect Dis. 2016 Jun; doi: 10.3201/eid2206.160010. http://dx.doi.org/10.3201/eid2206.160010. [DOI] [PMC free article] [PubMed]
- 56.Graham RL, Donaldson EF, Baric RS. A decade after SARS: strategies for controlling emerging coronaviruses. Nature reviews Microbiology. 2013;11:836–48. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perlman S, Vijay R. Middle East respiratory syndrome vaccines. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2016;(16):31021–9. doi: 10.1016/j.ijid.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haagmans BL, van den Brand JM, Raj VS, Volz A, Wohlsein P, et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2016;351:77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- 59.Kindler E, Jonsdottir HR, Muth D, Hamming OJ, Hartmann R, et al. Efficient replication of the novel human betacoronavirus EMC on primary human epithelium highlights its zoonotic potential. mBio. 2013;4:e00611–12. doi: 10.1128/mBio.00611-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Falzarano D, de Wit E, Rasmussen AL, Feldmann F, Okumura A, et al. Treatment with interferon-alpha2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nature medicine. 2013;19:1313–7. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. The Lancet Infectious diseases. 2014;14:1090–5. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2014;20:42–6. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. The Journal of infectious diseases. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corti D, Zhao J, Pedotti M, Simonelli L, Agnihothram S, et al. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:10473–8. doi: 10.1073/pnas.1510199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang XC, Agnihothram SS, Jiao Y, Stanhope J, Graham RL, et al. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E2018–26. doi: 10.1073/pnas.1402074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ying T, Du L, Ju TW, Prabakaran P, Lau CC, et al. Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies. Journal of virology. 2014;88:7796–805. doi: 10.1128/JVI.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luke T, Wu H, Zhao J, Channappanavar R, Coleman CM, et al. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Science translational medicine. 2016;8:326ra21. doi: 10.1126/scitranslmed.aaf1061. [DOI] [PubMed] [Google Scholar]
- 68.Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses - drug discovery and therapeutic options. Nature reviews. Drug discovery. 2016;15(5):327–47. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Public Health England and ISARIC. Treatment of MERS-CoV: Information for clinicians. Clinical decision-making support for treatment of MERS-CoV. https://http://www.gov.uk/government/uploads/system/uploads/attachment_data/file/360424/MERS_COV_information_for_clinicians_17_July.pdf.
- 70.Channappanavar R, Lu L, Xia S, Du L, Meyerholz DK, et al. Protective Effect of Intranasal Regimens Containing Peptidic Middle East Respiratory Syndrome Coronavirus Fusion Inhibitor Against MERS-CoV Infection. The Journal of infectious diseases. 2015;212:1894–903. doi: 10.1093/infdis/jiv325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lan J, Yao Y, Deng Y, Chen H, Lu G, et al. Recombinant Receptor Binding Domain Protein Induces Partial Protective Immunity in Rhesus Macaques Against Middle East Respiratory Syndrome Coronavirus Challenge. EBioMedicine. 2015;2:1438–46. doi: 10.1016/j.ebiom.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:4970–5. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agrawal AS, Garron T, Tao X, Peng BH, Wakamiya M, et al. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. Journal of virology. 2015;89:3659–70. doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, et al. Middle East Respiratory Syndrome Coronavirus Causes Multiple Organ Damage and Lethal Disease in Mice Transgenic for Human Dipeptidyl Peptidase 4. The Journal of infectious diseases. 2016;213:712–22. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haagmans BL, van den Brand JM, Provacia LB, Raj VS, Stittelaar KJ, et al. Asymptomatic Middle East respiratory syndrome coronavirus infection in rabbits. Journal of virology. 2015;89:6131–5. doi: 10.1128/JVI.00661-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Houser KV, Gretebeck L, Ying T, Wang Y, Vogel L, et al. Prophylaxis With a Middle East Respiratory Syndrome Coronavirus (MERS-CoV)-Specific Human Monoclonal Antibody Protects Rabbits From MERS-CoV Infection. The Journal of infectious diseases. 2016;213:1557–61. doi: 10.1093/infdis/jiw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baseler LJ, Falzarano D, Scott DP, Rosenke R, Thomas T, et al. An Acute Immune Response to Middle East Respiratory Syndrome Coronavirus Replication Contributes to Viral Pathogenicity. The American journal of pathology. 2016;186:630–8. doi: 10.1016/j.ajpath.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson RF, Bagci U, Keith L, Tang X, Mollura DJ, et al. 3B11-N, a monoclonal antibody against MERS-CoV, reduces lung pathology in rhesus monkeys following intratracheal inoculation of MERS-CoV Jordan-n3/2012. Virology. 2016;490:49–58. doi: 10.1016/j.virol.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scientific Advisory Council MoH, Saudi Arabia. Infection prevention/control and managament guidelines for patients with Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection. (2) http://www.moh.gov.sa/en/CCC/StaffRegulations/Corona/Documents/GuidelinesforCoronaPatients.pdf.
- 80.http://www.who.int/csr/disease/coronavirus_infections/MERS_CoV_RA_20140613.pdf WUoM-CTfAtHaIRfA-RGLaoMAf.
- 81.http://www.moh.gov.sa/en/CCC/Pages/Weekly-Monitor.aspx.