Abstract
Recent studies reveal 2-aminoadipic acid (2-AAA) is both elevated in subjects at risk for diabetes and mechanistically linked to glucose homeostasis. Prior studies also suggest enrichment of protein-bound 2-AAA as an oxidative post-translational modification of lysyl residues in tissues associated with degenerative diseases of aging. While in vitro studies suggest redox active transition metals or myeloperoxidase (MPO) generated hypochlorous acid (HOCl) may produce protein-bound 2-AAA, the mechanism(s) responsible for generation of 2-AAA during inflammatory diseases are unknown. In initial studies we observed that traditional acid- or base-catalyzed protein hydrolysis methods previously employed to measure tissue 2-AAA can artificially generate protein-bound 2-AAA from an alternative potential lysine oxidative product, lysine nitrile (LysCN). Using a validated protease-based digestion method coupled with stable isotope dilution LC/MS/MS, we now report protein bound 2-AAA and LysCN are both formed by hypochlorous acid (HOCl) and the MPO/H2O2/Cl− system of leukocytes. At low molar ratio of oxidant to target protein Nε-lysine moiety, 2-AAA is formed via an initial Nε-monochloramine intermediate, which ultimately produces the more stable 2-AAA end-product via sequential generation of transient imine and semialdehyde intermediates. At higher oxidant to target protein Nε-lysine amine ratios, protein-bound LysCN is formed via initial generation of a lysine Nε-dichloramine intermediate. In studies employing MPO knockout mice and an acute inflammation model, we show that both free and protein-bound 2-AAA, and in lower yield, protein-bound LysCN, are formed by MPO in vivo during inflammation. Finally, both 2-AAA and to lesser extent LysCN are shown to be enriched in human aortic atherosclerotic plaque, a tissue known to harbor multiple MPO-catalyzed protein oxidation products. Collectively, these results show that MPO-mediated oxidation of protein lysyl residues serves as a mechanism for producing 2-AAA and LysCN in vivo. These studies further support involvement of MPO-catalyzed oxidative processes in both the development of atherosclerosis and diabetes risk.
Keywords: Lysine, 2-aminoadipic acid, lysine nitrile, neutrophil, myeloperoxidase, hypochlorous acid, atherosclerosis, diabetes, inflammation, chloramine
Graphical Abstract
1. Introduction
2-Aminoadipic acid (2-AAA) is a low abundance amino acid previously suggested as an intermediate in some minor pathways of lysine catabolism in humans [1, 2]. Interest in this relatively uncommon amino acid has increased recently, however, due to its suggested enrichment both in disease-associated tissues and plasma in subjects at risk for development of diabetes. For example, using an untargeted metabolomics approach, Gerszten and colleagues recently reported that plasma levels of 2-AAA herald increased future risk of developing diabetes [3]. Moreover, physiological levels of 2-AAA were shown to promote pancreatic beta cell insulin secretion and lower plasma glucose levels in murine models, further suggesting potential involvement of 2-AAA as a modulator of glucose homeostasis [3]. In earlier studies it was suggested that protein lysyl residue oxidation by myeloperoxidase (MPO) – generated halogenating oxidants [4–10] may serve as a possible mechanism for generation of 2-AAA as a post translational modification of protein and lipoprotein lysyl residues [11]. Subsequent studies by both our group and Monnier and colleagues suggested not only MPO, but also metal catalyzed oxidative processes may participate in 2-AAA formation [11–14]. Indeed, protein bound 2-AAA has been reported to be enriched at sites known to harbor enhanced levels of protein oxidation products, including high density lipoprotein recovered from human atherosclerotic lesions, and insoluble collagen from aged skin, particularly from subjects with diabetes or renal disease [11–14]. Interestingly, while biosynthetic pathways for generating 2-AAA are not observed in mammals, 2-AAA is a reported intermediate in the synthesis of lysine in certain fungi, as well as in the biosynthesis of penicillin in β-lactam-producing fungi [15, 16]. It has also been reported as a metabolite produced from bacteria of the genus Thermus [17]. Despite the association between 2-AAA levels and the risk for developing diabetes [3], and its potential links to both vascular disease and degenerative diseases of aging [11–14], neither direct demonstration of oxidative pathway(s) that may participate in 2-AAA formation in vivo in mammals, nor detailed investigation of the biochemical pathway and structural intermediates involved in 2-AAA formation from protein lysyl residues, have been reported.
Wilson and colleagues recently reported that the major product of protein lysyl residue oxidation by reagent hypochlorous acid (HOCl) is not 2-AAA, but instead the unusual adduct lysine nitrile (LysCN, 2-amino-5-cyanopentanoic acid) [18]. While these purely in vitro studies used a high HOCl relative to target (protein lysyl residue) ratio, they reported up to 80% yield of LysCN when a maximal HOCl to target ratio is used (~100 to 600-fold excess) [18]. Nitriles in general can be acid labile [19], and acid hydrolysis was used in all (including our own) prior reported studies detecting protein-bound 2-AAA enrichment in tissues [11–14]. These provocative results suggesting LysCN as a potential HOCl generated oxidation product of protein lysyl residues therefore raised concerns to us of whether protein-bound 2-AAA is even a post translational oxidation product that exists in vivo, and whether 2-AAA is formed by the MPO/H2O2/Cl− system in vivo.
In this paper we report on our pursuit to elucidate the products and reaction mechanisms of protein lysyl residue oxidation by HOCl [6, 20–25], MPO-generated chlorinating oxidants [4, 5, 7–10], and activated leukocytes both in vitro and in animal models employing wild type (WT) and MPO knockout (MPO-KO) mice. The studies presented herein establish that 2-AAA is a major, and LysCN a minor, post translational oxidation product of lysyl residues formed at sites of inflammation. Our studies further demonstrate substantial enrichment of both 2-AAA and LysCN within human aortic atherosclerotic plaque, a site previously shown to harbor enriched content of both MPO and alternative MPO-generated oxidation products[5, 10, 26–29], and elucidate the reaction pathway responsible for MPO-generated chlorinating oxidants in forming these lysine oxidation products.
2. Materials and Methods
2.1. Materials
Nα-Boc-L-Lysine and Nα-acetyl-L-Lysine used as surrogates for protein lysyl residues were purchased from Chem-Impex International, Inc. (Wood Dale, Illinois). D3-2-aminoadipic acid was purchased from C/D/N isotopes Inc. (Pointe-Claire, Quebec). 13C6,15N2-Lysine was purchased from Cambridge isotope laboratories, Inc. (Andover, Massachusetts). Sodium hypochlorite (NaOCl), H2O2, ammonium hydroxide (NH4OH), trifluoroacetic acid and organic solvents were obtained from Fisher Scientific Co. Pronase was purchased from Roche life science (Indianapolis, Indiana). SAX SPE cartridges were obtained from Jordi lab (Mansfield, Massachusetts). Axis-Shield Polymorphprep was purchased from Cosmo Bio USA (Carlsbad, California). All other materials were obtained from Sigma-Aldrich, unless otherwise indicated.
2.2. Ethical considerations
All animal model studies were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic. All study protocols and informed consent for human subjects were approved by the Cleveland Clinic Institutional Review Board. Informed consents were obtained for isolation of human MPO, neutrophils and all other subject samples.
2.3. General procedures
In vitro oxidation reactions were performed in 50 mM phosphate buffer (PB), pH=7.0 supplemented with 100 μM diethylene triamine pentaacetic acid (DTPA). The concentrations of NaOCl and H2O2 were determined spectrophotometrically (ε292 (NaOCl) = 350 M−1 cm−1 [30] and (ε240 (H2O2) = 39.4 M−1 cm−1 [31]). Human MPO was isolated, characterized and quantified as described [7]. Human neutrophils were isolated by ficoll-hypaque buoyant density centrifugation as described previously [32]. Phorbol myristate acetate was prepared in dimethyl sulfoxide (DMSO). Cell experiments were performed in Hanks’ balanced salt solution, pH 7.2 (no calcium, magnesium, or phenol red) (Thermo Fisher) with 100 μM DTPA.
2.4. Clinical specimens
Human aortic tissues were recovered at the time of vascular or heart transplantation surgery. All tissues were rinsed in ice-cold normal saline immediately, submerged in buffer (65 mM sodium phosphate, pH 7.4, 100 μM DTPA, 100 μM butylated hydroxytoluene), snap frozen with liquid N2 and stored at −80°C under argon until used for analysis.
2.5. Synthesis of lysine nitrile, Nα-acetyl-2-aminoadipic acid, Nα-acetyl-lysine nitrile
Lysine nitrile (2-amino-5-cyanopentanoic acid) synthesis was carried out according to the method of Yamazaki [33] with modifications. Generally, NaOCl (4.5 mmol) was added to the stirring solution of Nα-Boc-L-Lysine (500 mg, 2 mmol) in ethanol (10 mL). The mixture was stirred at room temperature for 24 h. Afterwards, the reaction mixture was poured into H2O (50 mL) and then was extracted with dichloromethane (CH2Cl2, 3×30 mL). The solution was concentrated by rotary evaporation and the residue purified on a silica gel column and eluted with CH2Cl2. The Boc blocking group was removed with a solution of 50% trifluoroacetic acid in CH2Cl2. The resultant lysine nitrile was analyzed by high resolution mass spectrometry (MS) and showed the anticipated elemental composition and fragmentation pattern. The theoretical exact mass to charge ratio (m/z) for LysCN [MH]+ (C6H22N2O2) is 143.0821 au, the detected m/z was [MH]+= 143.0820 au, Δppm= 0.4 au.
Nα-acetyl-2-aminoadipic acid (Ac-2AAA) was synthesized following the method of Ravindranath [34]. Due to the low solubility of 2-AAA in water, triethylamine (TEA) (6 mmol) was added to H2O (2 mL) to solubilize 2-AAA (320 mg, 2 mmol). Acetic anhydride (4 mmol) was then added and the mixture was sonicated for 30 min in an FS60 sonic water-bath (Fisher Scientific). This process was repeated after adding another aliquot of acetic anhydride (4 mmol). After sonication, the Ac-2AAA was purified by reverse phase HPLC. The product was confirmed by high resolution MS analysis and showed the anticipated elemental composition and fragmentation pattern (m/z [M-H] − = 202.0715 au and the theoretical exact mass ([M-H]−) of Ac-2AAA = 202.0715 au, Δppm= 0.0 au).
Nα-acetyl-lysine nitrile (Ac-LysCN) was synthesized by adding NaOCl (5 mM) to Nα-Ac-Lys (2 mM) in 50 mM sodium phosphate, pH=7.0. The solution was heated at 40°C for 72 h. Ac-LysCN was isolated from the solution by reverse phase HPLC and dried under reduced pressure in a vacuum centrifuge. The product was analyzed by high resolution MS and showed the anticipated elemental composition and fragmentation pattern (m/z = 183.0769 au; theoretical exact mass [M-H] − = 183.0770 au, Δppm=0.07 au).
2.6. High performance liquid chromatography for isolating Ac-2AAA and Ac-LysCN
HPLC separation of Ac-2AAA and Ac-LysCN were performed using preparative C18 columns (10×250 mm, 5 μm particle diameter, Beckman, Ultrasphere ODS) pre-equilibrated with solvent A (0.1% propionic acid in water, pH=3.3). Products were monitored by absorbance (A205) and eluted at a flow rate of 4 mL/min with a nonlinear gradient generated with solvent B (0.1% acetic acid in methanol) as follows: 0–5% solvent B for 5 min; 5–100% solvent B for 10 min; isocratic 100% solvent B for 4 min. The pH of the mobile phase was ~2.0. For solid phase extraction (SPE) we loaded the column with sample in 1% NH4OH (pH=11) and eluted with 1% formic acid (pH=2.7).
2.7. Lysine nitrile stability test
1 mM LysCN was incubated with either 6N HCl at 110 °C or 4N NaOH at 120 °C in a sand bath. At the indicated times the reactions were cooled in an ice bath and then processed with strong anion exchange (SAX) solid phase extraction (SPE) and further analyzed by HPLC with online electrospray ionization tandem mass spectrometry (LC/MS/MS).
2.8. Examining the mechanism of Nα-Acetyl-L-Lysine oxidation by HOCl
Ac-Lys was used as the target for HOCl oxidation. Three different ratios of oxidant to target were used: 1 mM HOCl to 1 mM Ac-Lys (1:1), 2 mM HOCl to 1 mM Ac-Lys (2:1), and 1 mM HOCl to 1M (pH 7.0) Ac-Lys (1:1000). All of the oxidation reactions were performed in 50 mM PB, pH 7.0. The formation of monochloramine (RNHCl) and dichloramine (RNCl2) intermediates were monitored by UV at 254 nm or 304 nm, respectively [7]. The decay of RNHCl and RNCl2 was measured by HPLC with a photodiode array detector, by monitoring the absorbance (A254 for RNCl and A304 for RNCl2). HPLC with on line triple-TOF mass spectrometry (AB Sciex tripleTOF 5600 system, Framingham, MA) was used to analyze and quantify Ac-Lys, Ac-2AAA and Ac-LysCN in the reaction. We used N-acetyl-glutamic acid (Ac-Glu) as an internal standard and calibration curves were prepared using varying Ac-2AAA and Ac-LysCN levels and a fixed amount of internal standard Ac-Glu. At the indicated times, internal standard (20 μL Ac-Glu, 100 μM) was mixed with 20 μL reaction solution. A portion (5 μL) of the mixture was injected onto a silica column (2.0×150 mm, 5 μm particle, Phenomenex Luna silica) at a flow rate of 0.2 mL/min with the following HPLC gradient: 100% solvent A (0.1% propionic acid in H2O) for 5 min; 0–100% solvent B (0.1% propionic acid in methanol) for 5 min; isocratic a 100% solvent B for 3 min; 100–0% solvent B for 2 min; the column was then equilibrated with 100% A for 10 min. The quantity of each analyte was measured by peak area corresponding to their exact mass relative to peak area of internal standard. TripleTOF MS was auto calibrated after every three injections.
In order to identify the proposed reaction intermediates, the TripleTOF mass spectrometer was set to simultaneously scan analytes with an exact mass to charge ratio from 100 to 1000 in the reaction, as well as the product ion of the identified compounds. The identities of the intermediates were confirmed by matching the exact mass of the precursor and the potential product ions with the proposed compounds, as well as their fragmentation patterns.
2.9. Protein enzymatic digestion
Protein samples were first dialyzed against 10 mM Tris buffer, pH=7.5 prior to enzymatic digestion. The digestion (1 mg protein/mL) was carried out in 0.1 M Tris, pH=7.5 and 10 mM CaCl2. To the reaction solutions we added stable isotope labeled internal standards (d3-2-AAA, and [13C6,15N2]Lys) prior to addition of protease. Samples were pre-warmed at 45°C in a water bath and the digestion started with pronase addition (specific activity = 7 unit/mg). The same amount of pronase (50 μg/mL) was added to the reaction every four hours to a final concentration of 300 μg/mL at the 20th hour. The digestion was stopped 24 hours after the first addition of pronase by cooling on ice, and then by adding 2 mL of 1% ammonium hydroxide to the solution, followed by solid phase extraction (SPE) column chromatography. Digested samples were analyzed by LC/MS/MS following addition of internal standards.
2.10. Quantification of protein lysine oxidation products after exposure to chlorinating oxidants
Three different chlorinating oxidant systems were used: (i) reagent HOCl; (ii) the MPO/H2O2/Cl− system; (iii) and activated human neutrophils. Human serum albumin (HSA) (1 mg/mL or 15 μM) was used as a source of protein for oxidation studies. HOCl or H2O2 (for the MPO/H2O2/Cl− system) was used at molar ratios from 1/8 to equal amount of protein target as indicated (1.9–15 μM). When using the MPO/H2O2/Cl− oxidation system, final reaction conditions included 32 nM of isolated human MPO and 100 mM of Cl−. For oxidation reactions using neutrophils, 1×106/mL isolated human neutrophils were incubated with HSA and, where indicated, activated by addition of 200 nM (final) phorbol-12-myristate-13-acetate (PMA). Neutrophils were maintained in suspension at 37°C for 2 hour by intermittent inversion The media used was Hank’s balance salt solution (pH was 7.4, and the chloride concentration was approx. 140 mM). All reactions were terminated by adding 2 mM methionine. After reaction, recovered HSA was placed in hydrolysis tubes, internal standards for mass spectrometry quantification were added, and then subjected to protease digestion for protein bound lysine, 2-AAA, and LysCN quantification by LC/MS/MS.
2.11. Mouse inflammation model
Age and sex-matched C57BL/6J (wild type, WT) and MPO knockout (MPO-KO) mice (back-crossed > 50 generations onto C57BL/6J) were used for peritonitis model studies [35]. Mice were intraperitoneally injected with 1 mM 4% thioglycollate (TG) broth to recruit neutrophils. After twenty hours, the mice received a second intraperitoneal injection with zymosan (250 mg/kg). Four hours later peritoneal lavage was collected with PBS containing 100 μM DTPA and 100 μM butylated hydroxytoluene. In control groups, normal saline (1 mL) was injected intraperitoneally, and peritoneal lavage was collected after 24 hours. Lavage fluid was separated from cells by centrifugation at 800 × g for 10 min at 4 °C, overlaid with argon, and (both cells and supernatants) stored at −80 °C until analysis.
2.12. Sample preparation for quantification of protein bound 2-AAA and LysCN
After protease digestion, the sample mixture was diluted with 2 mL 1% ammonium hydroxide solution. This solution was passed over a mini SAX SPE cartridge (3.0 mL, 25–35 μm, 60 mg; Jordi lab), which was pre-equilibrated with 2 × 2 mL methanol followed by 2×2 mL 1% NH4OH solution. Columns were washed with 2 × 2 mL H2O and 2 × 2 mL 70% methanol, and amino acids were eluted with 2 × 2 mL 70% methanol supplemented with 1% formic acid. The eluted solution was dried under speed vacuum, and then reconstituted in 100 μL H2O for LC/MS/MS.
2.13. Sample preparation for quantification of free 2-AAA and LysCN
Ice cold methanol (80 μL) containing 10 μM [13C6,15N2]Lys (universally labeled lysine, u-Lys) and 1 μM d3-2AAA were added to 20 μL mouse lavage fluid. The protein in the lavage was pelleted by centrifugation and the free amino acids in the supernatant were analyzed by LC/MS/MS.
2.14. Mass spectrometry analyses
Lysine, 2-AAA, and LysCN were quantified by LC/MS/MS. Calibration curves were prepared using varying concentrations of lysine, 2-AAA, and synthetic LysCN with a fixed amount of stable isotope-labeled internal standards [13C6,15N2]Lys and d3-2-AAA respectively. Internal standards were added to each sample prior to sample preparation, which underwent enzymatic digestion and SAX solid phase extraction. The sample (5 μL) was injected onto a silica column (4.6 × 250 mm, Clipeus silica 5 μm, Higgins Analytical Inc, Mountain view, CA) at a flow rate of 0.8 mL/min. Separation was performed using the following gradient: start with solvent A (0.1% propionic acid aqueous solution); 0–15% solvent B (methanol containing 0.1% acetic acid) for 10 min; 15%–100% solvent B for 1 min; isocratic with 100% solvent B for 3 min; 0–100% solvent A for 1 min; equilibration of the column with solvent A for 15 min. After 3.9 min the HPLC column effluent was introduced into an API 4000 QTRAP mass spectrometer, and after 11 min the HPLC effluent was diverted to waste. The mass spectrometer was set to acquire data from 4 min to 10 min. Analyses were performed using electrospray ionization in positive-ion mode with multiple reaction monitoring of characteristic precursor and product ions specific for the components monitored. The transitions monitored were mass-to-charge ratio (m/z): 147→84 for lysine; 162→98, and 162→55 for 2-AAA; 143→97, and 143→54 for LysCN, and 155→90 for [13C6,15N2]Lys; and finally 165→101 for d3-2AAA.
2.15. Statistical analysis
The statistical significance of differences was determined using the Kruskal–Wallis test by ranks (one-way ANOVA on ranks) [36]. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Conventional acid or base protein hydrolysis methods convert LysCN into 2-AAA
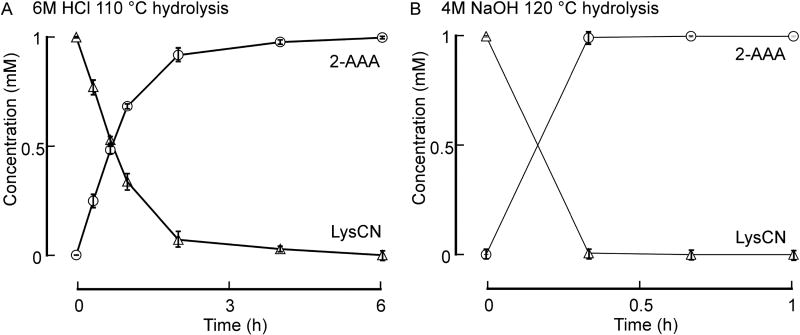
As noted above, debate exists in the field of lysyl residue oxidation chemistry. Recent studies suggest LysCN may be the major stable posttranslational modification of lysine formed in vivo, based upon in vitro studies examining proteins exposed to excess HOCl [18]. We therefore first sought to investigate whether prior studies used protein hydrolysis methods that would accurately measure LysCN and 2-AAA in tissues. LysCN was synthesized as outlined under Materials and Methods and both its stability and the stability of 2-AAA examined during typical protein hydrolysis conditions (either 6N HCl, 110°C; or 4N NaOH, 120°C). Of note, LysCN was virtually quantitatively converted into 2-AAA under both acid and base hydrolysis conditions (Figure 2A,B). Thus, typical protein hydrolysis conditions are not appropriate to accurately quantify either protein-bound LysCN or 2-AAA in biological samples. These results also suggested that ours and others’ previously published studies used conditions during sample preparation (e.g. refs. [3, 11–14]) that may have inadvertently overestimated the 2-AAA content because a portion (or all) of the measured 2-AAA may have come from the hydrolysis of LysCN, if it is present in the sample. Of further note, however, as far as we are aware, no studies to date have reported detection of protein-bound LysCN in vivo.
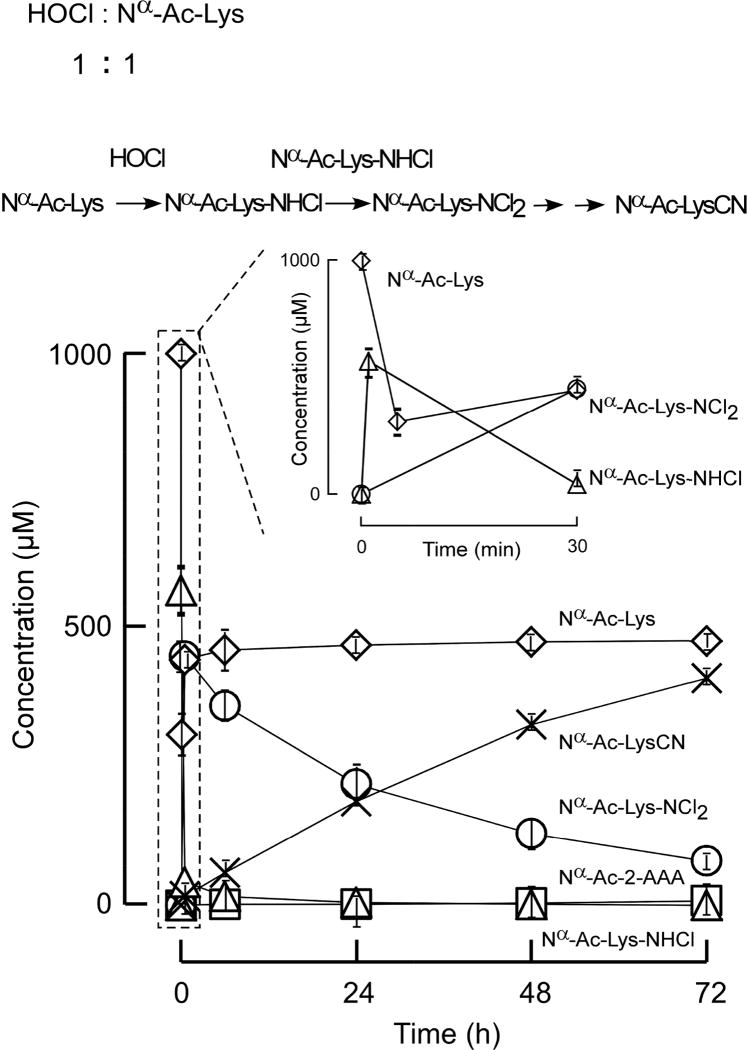
Figure 2. Nα-acetyl-LysCN is the major product in the reaction of Nα-acetyl-lysine with equal moles of HOCl.
HOCl (1 mM) was added to Nα-acetyl-lysine (1 mM) in reaction buffer (see Materials and Methods for details). Initial Nα-acetyl-lys-NHCl (△) production was monitored by UV 254 nm. Nα-acetyl-lys-NCl2 (○) decay was measured by HPLC-UV at 304 nm. All analyte identities (including mono- and di-chloramines) were confirmed by high resolution mass spectrometry analyses. Nα-acetyl-lys (◇), Nα-acetyl-lysCN (×), Nα-acetyl-2-AAA (□) were quantified by LC-Triple-TOF mass spectrometer as described under Experimental Procedures. Data shown represent the mean +/− S.D. of duplicated determinations for a study performed greater than three independent times.
3.2. Quantification of 2-AAA and LysCN in biological samples
To address the issue of LysCN conversion to 2-AAA during sample preparation we used enzymatic proteolytic digestion to break down the protein into its constituent amino acids under conditions where both 2-AAA and LysCN are stable. Subsequently, we developed a mass spectrometry (MS)-based assay to accurately quantify both 2-AAA and LysCN in biological samples. For complete proteolytic digestion of proteins extracted from tissue or plasma we used a series of small amounts (relative to target protein mass) of pronase, a mixture of proteases from Streptomyces griseus, to break the peptide bonds releasing free amino acids (see Materials and Methods). We validated the efficiency of the pronase proteolytic method employed by comparing the digestion yield of various amino acids against conventional acid hydrolysis of the same amount of human serum albumin (HSA) or precipitated plasma protein. We considered HCl hydrolysis as complete, and defined pronase proteolytic efficiency as the ratio of amino acid amounts recovered from pronase digestion to acid hydrolysis. We eliminated contributions from pronase self-digestion by including control studies with every batch to allow subtraction of amino acid amounts recovered in parallel pronase self-digestion reactions (also ensuring amino acids monitored and subtracted were less than 10% total observed in complete reaction samples under the conditions employed). Our control studies revealed that under the methods employed, the overall recovery yield of various amino acids was in excess of 90%: (e.g. Met 91±1%, Lys 95±1%, Phe 94±3%, Tyr 91±2%, Leu 93±3%, Arg 93±3%, Val 97±2%). An accurate recovery estimate for Trp could not be obtained because there is only one Trp residue in HSA, and the self (pronase) enzyme digestion under the conditions employed was observed to contribute >10% to the Trp pool. Additional control studies exposing LysCN and 2-AAA to the same proteolytic conditions showed that both compounds are stable with 99±2% and 102±4% recovery, respectively.
3.3. The mechanism of Nα–protected lysyl residue oxidation by HOCl
To understand the chemistry and clarify the products of posttranslational modification of protein lysyl residues by MPO, we initially investigated lysine oxidation by the major MPO-generated oxidant, HOCl. In these initial mechanistic studies we used Nα-acetyl-lysine (Ac-Lys) as a protein lysyl residue surrogate. The acetyl group was used to protect the α-amine group of lysine from oxidation and to mimic the peptide bond. Following the reaction of Nα-Ac-Lys with HOCl, we discovered that both Nα-acetyl-2-aminoadipic acid (Ac-2AAA) and Nα-acetyl-lysine nitrile (Ac-LysCN) were formed. Moreover, we determined that Ac-2AAA is the major oxidation product in this reaction when a lower molar ratio of HOCl : Ac-Lys is used, whereas at higher oxidant to target exposures, the amount of Ac-LysCN increases and becomes the major product (Figures 2, 3, 4). Previous work has already established that the reaction of HOCl with the backbone α–amino group of free amino acids and side chain amino groups produce chloramine reaction intermediates [24, 25, 37–39]. Our results suggest that Ac-2AAA is produced through a monochloramine reaction intermediate, while Ac-LysCN might be formed through a dichloramine intermediate – hypotheses subsequently confirmed in our studies (see below).
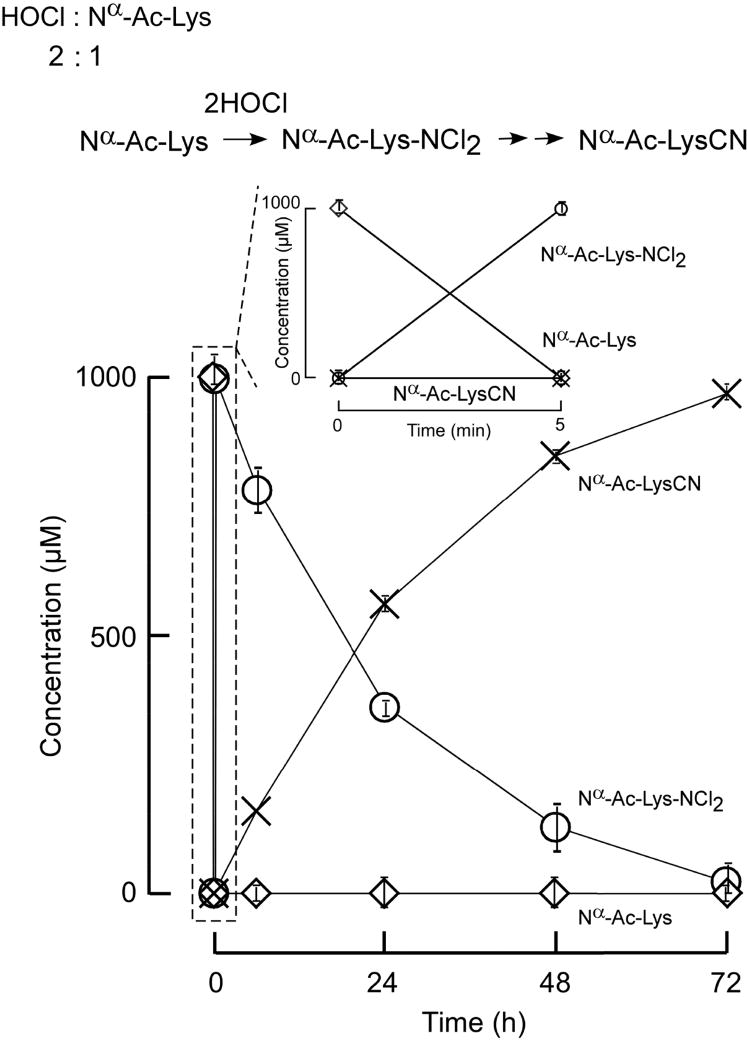
Figure 3. Nα-acetyl-LysCN is the major product in the reaction of Nα-acetyl-lysine with 2 moles of HOCl.
Nα-acetyl-lysine (1 mM) was incubated with HOCl (2 mM) in reaction buffer and reaction products formed monitored as described in Materials and Methods. All analyte identities (including dichloramine) were confirmed by high resolution mass spectrometry analyses. Nα-acetyl-lys-NCl2 (○) decay was measured by UV at 304 nm. Nα-acetyl-lys (◇), Nα-acetyl-lysCN (×), were quantified by LC-Triple-TOF mass spectrometer. Data shown represent the mean +/− S.D. of duplicated determinations for a study performed greater than three independent times.
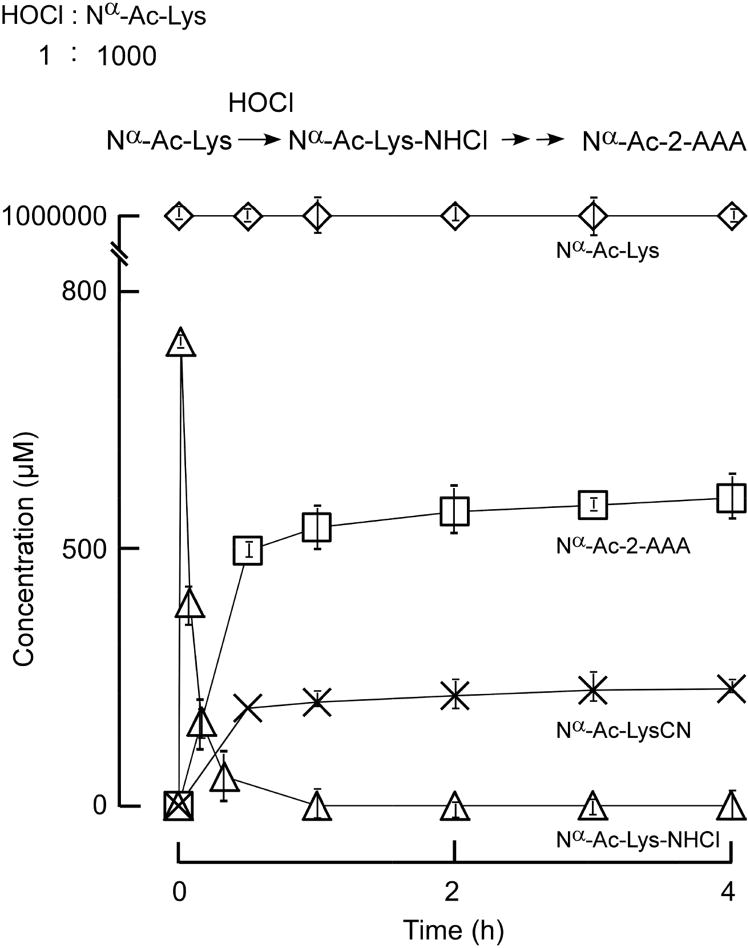
Figure 4. Nα-acetyl-2-AAA is the major product in the reaction of Nα-acetyl-lysine with HOCl (1000:1 molar ratio).
HOCl (1 mM) was reacted with Nα-acetyl-lysine (1 M). The reactions were performed in reaction buffer as described in Materials and Methods. All analyte identities (including mon-chloramine) were confirmed by high resolution mass spectrometry analyses. Nα-acetyl-lys-NHCl (○) decay was measured by UV at 254 nm. Nα-acetyl-lysCN (△), Nα-acetyl-2-AAA (□) were quantified by LC-Triple-TOF mass spectrometer. Data shown represent the mean +/− S.D. of duplicated determinations for a study performed greater than three independent times.
We first examined both the stoichiometry and time course of reaction products formed during interaction of Ac-Lys with HOCl as described under Materials and Methods. Results at 1 mole oxidant (HOCl) to 1 mole target (Nα-Ac-Lys) are shown in Figure 3. Consistent with previous studies showing that the ε-amine group of lysine readily reacts with HOCl forming Nα-Ac-Nε-chloro-lysine (RNHCl) [7, 24, 38–41], we observed that Ac-Lys was rapidly consumed, and an intermediate, RNHCl absorbing at 254 nm, forms and then decays (Figure 3). Monitoring the reaction using high-pressure liquid chromatography (HPLC) coupled with both a photodiode array detector and mass spectrometry analyses revealed that RNHCl decays into a longer-lived intermediate, Nα-Ac-Nε-dichloro-lysine (RNCl2) that absorbs at 304 nm. Furthermore, separate studies starting with RNHCl and mass spectrometry (MS) analyses indicated rapid formation of Ac-Lys and RNCl2, consistent with a disproportionation reaction of RNHCl [42]. Ultimately, RNCl2 decays too as the reaction proceeds [37], leading to Ac-LysCN as the main product and a small amount of Ac-2AAA (Figure 3). When the oxidant to Ac-Lys ratio is increased to 2:1 (HOCl: Nα-Ac-Lys; Figure 4), Ac-Lys is completely consumed shortly after adding HOCl rapidly forming the dichloramine [37], which then further decomposes into Ac-LysCN.
Lysine is one of the most abundant amino acids in proteins (about 1 in 5 residues), but under physiological conditions the amount of oxidant produced by MPO is typically much less than the amount of lysine. At sites of inflammation one would expect monochloramines to be rapidly scavenged by free and protein-bound thiol [43–45]. This is because methionine and cysteine preferentially react with HOCl orders of magnitude faster than lysine [24, 46]. Therefore it is reasonable to assume that under physiological conditions the molar concentration of MPO-generated oxidants will be orders of magnitude less than that of lysine ride chains (especially in the extracellular compartment). Thus, we performed studies where the oxidant is present at only a trace level relative to protein target ratio (e.g. 1:1000, HOCl: Nα-Ac-Lys), as one might predict exist in vivo (Figure 5). Here, the initial RNHCl formed was observed to decompose into 2-AAA as the major product, and LysCN as a minor product (Figure 5). Also observed under these conditions, the monochloramine intermediate formed is at low level relative to unreacted Ac-Lys, and the RNHCl disproportionation reaction (forming RNCl2) does not significantly occur.
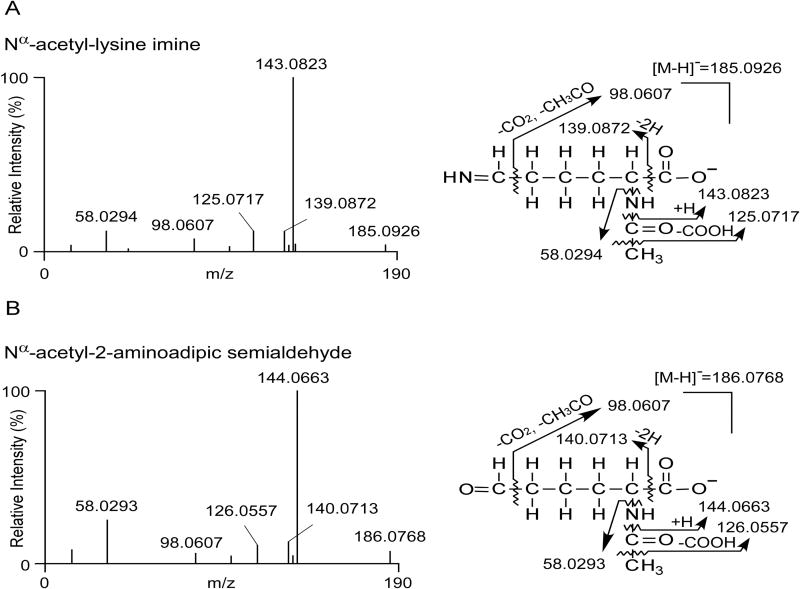
Figure 5. Nα-acetyl-lys-imine (A) and Nα-acetyl-2-aminoadipic semialdehyde (B) are detected in the reaction of Nα-acetyl-lys with HOCl (1000:1).
Generation of Nα-acetyl-lys-imine and Nα-acetyl-2-aminoadipic semialdehyde intermediates were detected by Triple-TOF high resolution mass spectrometry as described under Materials and Methods. Exact mass of both precursor and product ions of the identified molecules match with those of the calculated masses of the proposed reaction intermediates as shown, respectively.
Based on these results we hypothesized that when Ac-Lys is in a large molar excess with respect to the oxidant species, Ac-Lys-NHCl takes a different reaction pathway by eliminating HCl with the formation of an imine intermediate instead of disproportionation. The imine compound would be relatively unstable and further hydrolyze, releasing ammonia to form Nα-acetyl-2-aminoadipic–δ–semialdehyde, which similarly is unstable and reactive in the presence of RNHCl (or HOCl) and could be further oxidized to Ac-2AAA. This reaction pathway has been established for the reaction of HOCl with the α–amino groups of free amino acids, but here we were able to directly test whether this pathway is plausible also for the ε-amino group of lysine by using high resolution mass spectrometry (see Materials and Methods). Both proposed imine and semialdehyde intermediates were identified as sequential products formed in the oxidation reaction.
The MS/MS spectra of the two detected molecules (Figure 6A,B) indicate that these two compounds have identical anticipated elemental composition and fragmentation patterns as the expected imine and semialdehyde intermediates. For example, the measured masses of the parents and their fragment ions (see Fig. 5A,B for proposed fragmentation pattern) are all less than 2 ppm from the theoretical masses, strongly supporting the identity of these proposed labile intermediates. The existence of these two compounds in the oxidation reaction strongly supports our proposed pathway for Ac-2AAA formation.
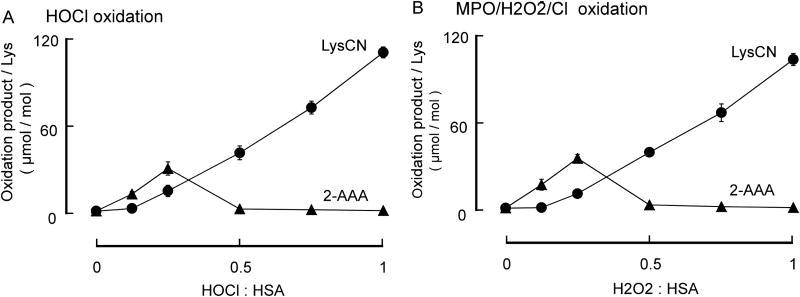
Figure 6. Formation of 2-AAA and LysCN in the reaction of HSA with HOCl and MPO/H2O2/Cl− system.
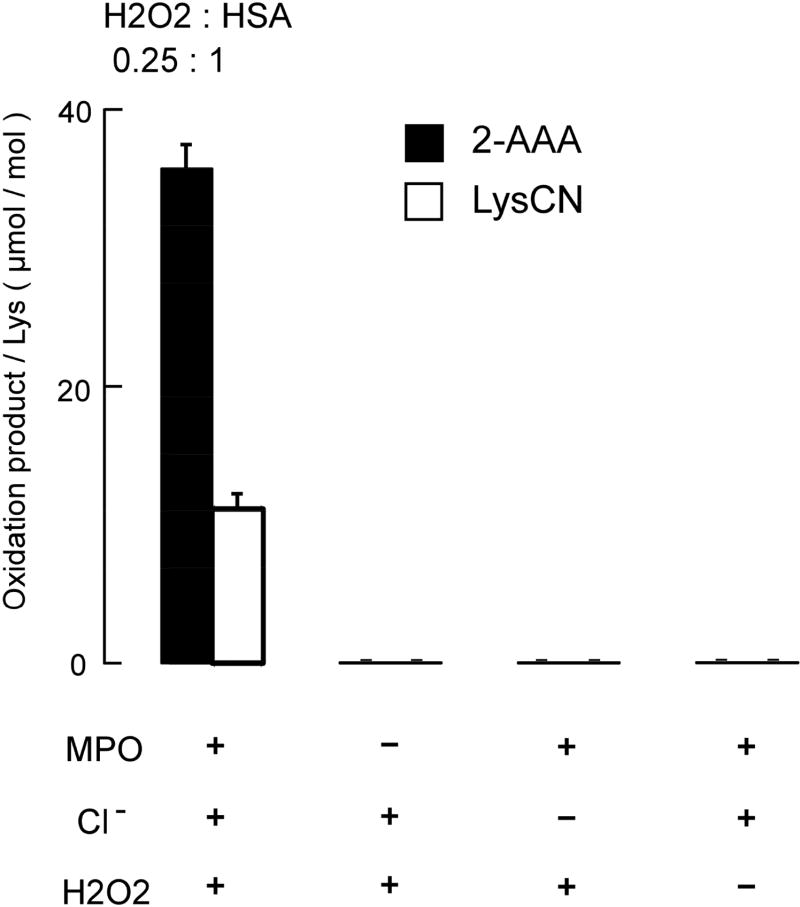
Reactions were carried out in reaction buffer as described in Materials and Methods with HSA (1 mg/mL) and the indicated concentration of oxidant. Oxidation products were subsequently quantified by LC/MS/MS following pronase digestion described under Materials and Methods. A, At lower HOCl to protein ratio, 2-AAA is the major product. At higher HOCl to protein ratio, LysCN is the major product. B, When HSA is oxidized by MPO/H2O2/Cl− system, at lower H2O2 to HSA ratio, 2-AAA is the major product; at higher H2O2 to HSA ratio, LysCN is the major reaction product. Data represent the mean ± S.D. of triplicate determinations for an experiment performed at least 3 separate times. Initially, we used a range of HOCl : HSA molar ratios (0 to 1; HSA 1mg/mL (15 μmol), HOCl 0–15 μmol) and observed results similar to those observed with Ac-Lys oxidation (Figure 7A) – namely, at a low molar ratio of oxidant to target (< 0.3), 2-AAA is the major oxidation product (the yield of 2-AAA is approx. 1% of the HOCl added) of the stable end products of lysine oxidation monitored, while LysCN becomes the major product at a higher molar ratio (the yield of LysCN is approx. 1.3% of the added HOCl). Nearly identical results were obtained with the complete MPO/H2O2/Cl− system (Figure 7B). In additional control studies, we confirmed that each component of the MPO/H2O2/Cl− system is required for HSA lysine oxidation to produce protein-bound 2-AAA and LysCN (H2O2 : HSA = 0.25, Figure 8).
3.4. Human serum albumin lysyl residue oxidation by HOCl and the MPO/H2O2/Cl− system
Next we used human serum albumin (HSA) as a target protein model system to further investigate lysine oxidation by HOCl or mediated via the action of human MPO (Figure 7).
Figure 7. Protein bound 2-AAA and LysCN formation requires protein lysyl residue exposure to the complete MPO/H2O2/Cl− system.
HSA (1 mg/mL, 15 μM) was exposed to the complete MPO/H2O2/Cl− system, or to the indicated components of the MPO system. The production of 2-AAA LysCN and oxidation precursor lysine were quantified by LC/MS/MS as described in Materials and Methods. Only under the complete MPO/H2O2/Cl− system, 2-AAA and LysCN were produced. Data values represent the mean ± S.D. of triplicate samples for an experiment performed at least 3 separate times.
3.5. Human neutrophils oxidize protein lysyl residues forming both 2-AAA (major) and LysCN (minor) as products
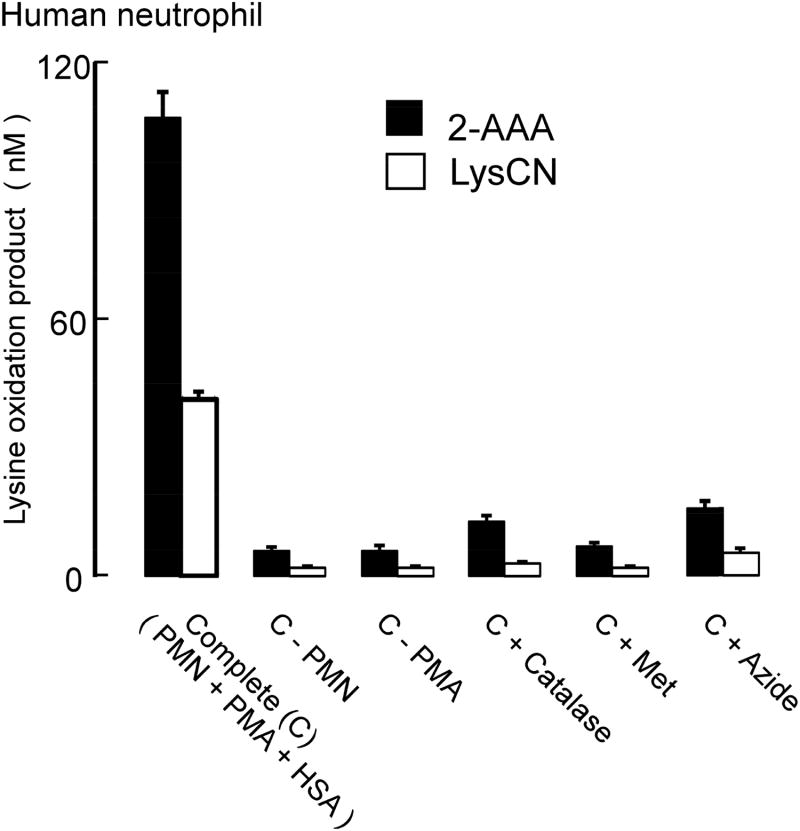
MPO is one of the most abundant proteins in neutrophils [4, 47]. During neutrophil activation at sites of inflammation, MPO is released and uses H2O2 from respiratory bursts and physiological levels of chloride to make HOCl [4, 47]. We therefore next sought to test whether activated isolated human neutrophils can employ the MPO system to oxidize protein lysyl residues and generate 2-AAA and LysCN. HSA was incubated with human neutrophils in the presence of media containing physiological levels of chloride followed by PMA stimulation to activate the neutrophils. DTPA (100 μmol) was also added to the media to inhibit potential free redox active transition metal ion metal-catalyzed oxidation. In the presence of this “Complete System” (i.e. HSA, neutrophils, and PMA), both protein-bound 2-AAA and LysCN were formed with 2-AAA as the major product (Figure 9). On the other hand, in the absence of any one of the components of the oxidation system (neutrophils, or PMA) or in the presence of an MPO inhibitor (e.g. azide), no significant amount of oxidation products were observed. Addition of catalase, a scavenger of H2O2, dramatically reduced the production of both 2-AAA and LysCN, and addition of methionine, a scavenger of HOCl [24, 48, 49], also diminished both 2-AAA and LysCN formation, consistent with neutrophils employing the MPO/H2O2/Cl− system in generation of these post translational modifications of lysine (Figure 9).
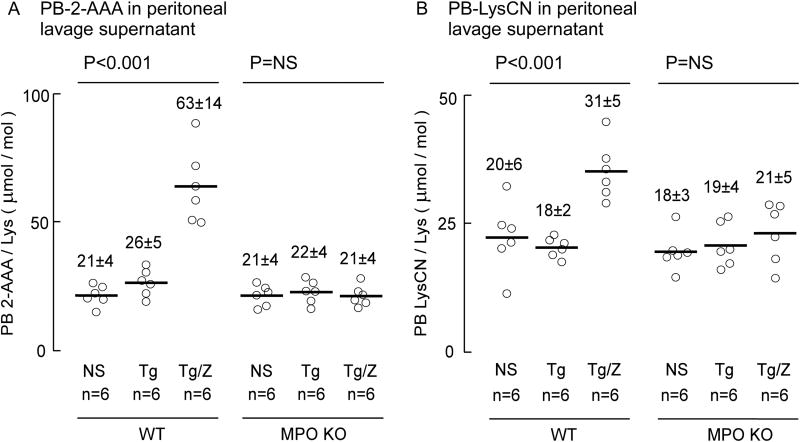
Figure 9. MPO plays a role in 2-AAA and LysCN formation during inflammation.
WT and MPO KO mice were injected intra-peritoneally with thioglycollate (TG) broth or vehicle control (n=6 in each group). Where indicated, zymosan was injected 20 h after injection of TG. Peritoneal lavages were performed 24 h after the initial injection. Protein bound 2-AAA, LysCN and lysine content in soluble proteins from the peritoneal lavages were determined by LC/MS/MS as described under Materials and Methods. Data points shown represent duplicate analyses of a sample from an individual mouse, reported as μmol oxidation product/mol Lys of either 2-AAA or LysCN, as indicated, and the bar represents mean, the number above the data points represents mean ± S.D. P-value shown represents Kruskal–Wallis test within the indicated groups of mice. NS = non-significant.
3.6. MPO forms 2-AAA and LysCN in vivo at sites of inflammation
We further investigated whether MPO is involved in the formation of 2-AAA and LysCN at sites of inflammation in vivo. For these studies we used MPO-KO mice and an acute inflammation model, zymosan-induced peritonitis, that is well characterized and frequently employed to gauge the role of MPO in protein or lipid oxidation in vivo [35]. As shown in Figure 10A, the content of 2-AAA in peritoneal lavage protein from wild type mice significantly (P<0.001) increases following thioglycolate (TG)-induced recruitment of leukocytes and zymosan (ZM) triggered leukocyte activation in WT mice. In sharp contrast, however, the MPO-KO mice showed no significant increase in 2-AAA following TG and ZM injection. Similarly, a significant increase in protein-bound LysCN is formed in WT mice injected with TG and ZM (P<0.001), but not in MPO-KO mice (Figure 10B). Moreover, while at baseline similar protein content of 2-AAA and LysCN in peritoneal lavage proteins were noted, the amount of LysCN produced during inflammation was approximately half that observed for 2-AAA.
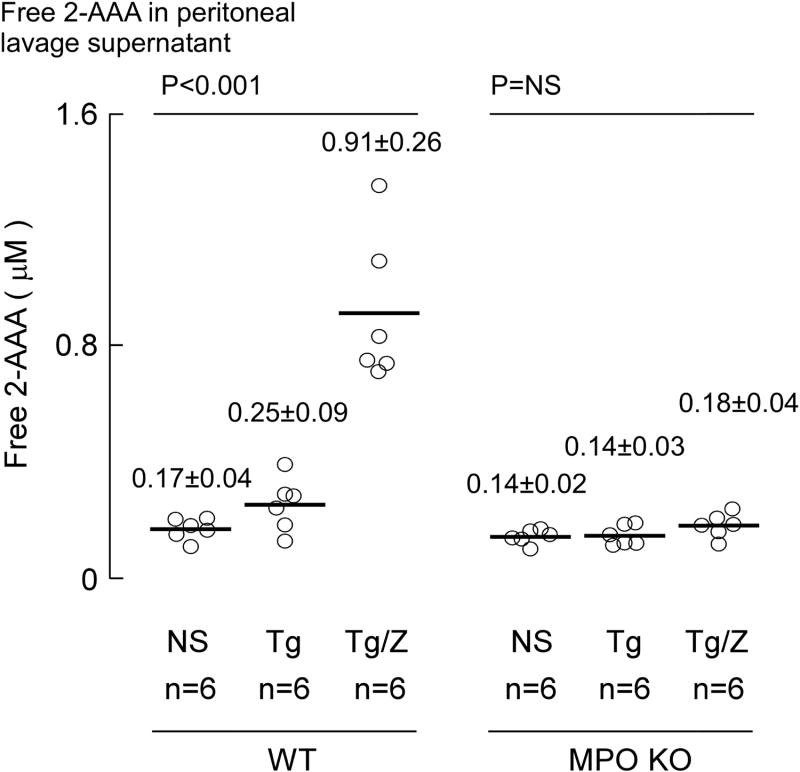
Figure 10. Free 2-AAA level in zymosan induced peritonitis model.
Free 2-AAA in WT and MPO-KO mouse peritoneal lavage under experimental conditions as described in Figure 11 were quantified by stable isotope dilution LC/MS/MS (Materials and Methods). Each data point represents the mean of duplicate analysis of sample from one mouse. Lines indicate mean levels within each group and values reported represent mean ± S.D. P-value shown represents Kruskal–Wallis test within the indicated groups of mice. NS = non-significant.
3.7. MPO oxidation is a source of free 2-AAA production in vivo
We also examined whether MPO can generate free 2-AAA in vivo. Here peritoneal lavage supernatants were analyzed for quantification of free 2-AAA and free LysCN. As shown in Figure 11, after neutrophils were elicited by TG and activated by ZM, there was a significant ~4.5-fold increase (p<0.001) in free 2-AAA noted in WT mice over TG treatment alone. This increase was completely abrogated in MPO-KO mice. Attempts to measure free LysCN in the lavage fluid failed to demonstrate any detectable levels in response to any of the treatments. Under the conditions employed for the assay, the limit of detection (S/N of peak = 3:1) of LysCN was 40 nM.
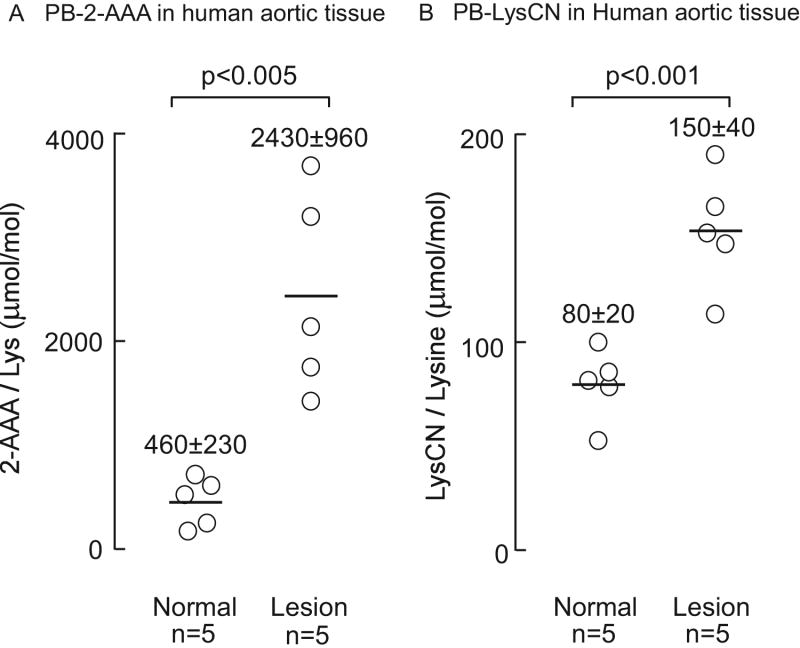
Figure 11. Both 2-aminoadipic acid (2-AAA) and lysine nitrile (LysCN) are enriched in protein recovered from human aortic atherosclerotic tissues compared to normal aorta.
Samples were prepared from normal human aortic tissue (n=5) and atherosclerotic plaque-laden aortic tissue (n=5). Protein bound 2-AAA and LysCN were quantified after protease-mediated hydrolysis by LC/MS/MS mass spectrometric analysis as described under methods. Data points shown represent duplicate analyses of a sample from an individual aorta, reported as μmol oxidation product/mol Lys of either 2-AAA or LysCN, as indicated, and the bar represents mean ± S.D.
3.8. Human aortic lesions are enriched in both 2-AAA and LysCN
In the final series of studies we sought to test whether 2-AAA and LysCN are present in normal human aorta and whether levels of these oxidation products are enriched in aortic atherosclerotic lesions. Using the validated pronase digestion method we readily detected both species and observed the content of both protein-bound 2-AAA and LysCN are elevated in human aortic atherosclerotic plaque compared to healthy aorta. Figure 12A,B shows that protein bound 2-AAA and LysCN content in atherosclerotic lesions are significantly higher (~2-fold, p<0.001) than in normal aortic tissue; moreover, the 2-AAA content in human aortic tissues observed were more than 10-fold higher (p<0.005) than that for LysCN.
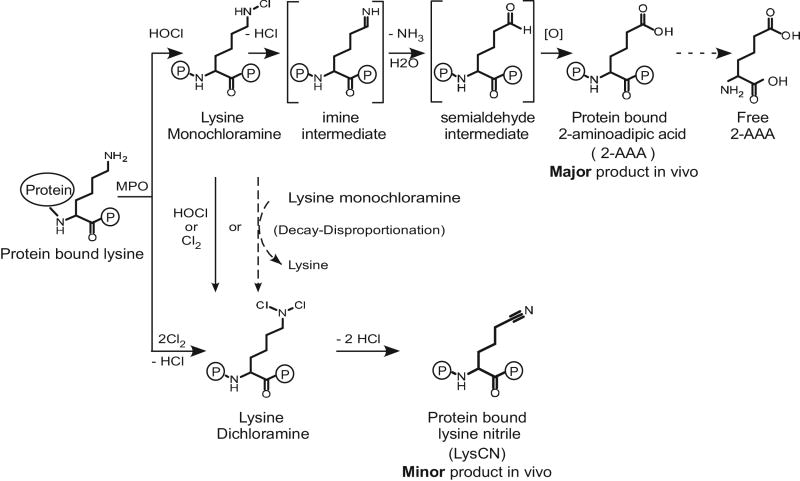
Figure 12.
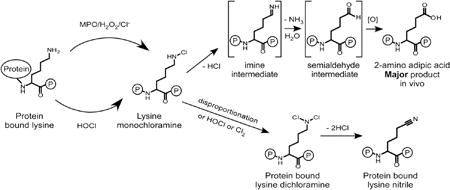
Schematic illustration of reaction pathways for the formation of 2-AAA and LysCN by MPO/H2O2/Cl− (HOCl) oxidation of protein-bound lysine.
4. Discussion
Our present studies explore the chemistry and the stable terminal products of protein lysyl residue oxidation by MPO generated chlorinating oxidants in vitro and in vivo. We provide direct evidence that both 2-AAA and LysCN are posttranslational modifications of protein lysyl residues through action of MPO-generated oxidants in vivo. Moreover, detailed analysis of the reaction mechanism reveals the following scheme summarized in Figure 12. First, activated neutrophils or monocytes generate HOCl, which reacts with the ε–amino group of protein lysine to produce a Nε–monochloramine as a reaction intermediate [37]. The monochloramine is labile, and can further decompose to form 2-AAA. We suggest that because there is still chlorinating oxidant capacity of lysine monochloramine, in the presence of excess protein lysines, as long as it does not get scavenged (e.g. by RSH or RSR groups) it keeps exchanging halogen to other lysine Nε–amine residues reforming Nε–monochloramine until it eliminates HCl, forming an imine intermediate. The latter undergoes subsequent hydrolysis through the elimination of NH3 and addition of H2O to give the 2-aminoadipate–δ–semialdehyde intermediate (Figure 12).
The semialdehyde intermediate is not stable and rapidly forms 2-AAA. Importantly, we were able to directly detect both the indicated imine and semialdehyde intermediates in the reaction mixture by high resolution MS, as well as to show direct precursor → product relationships for most of the proposed transitions indicated including starting from isolated mono-chloramine, further supporting the pathway proposed for the formation of 2-AAA. Our studies further revealed that when the oxidant to lysine target ratio is higher, the RNHCl formed can either disproportionate to produce dichloramine RNCl2 intermediate + lysine, or further react with another HOCl molecule to form the dichloramine RNCl2 intermediate [37]. This latter intermediate was isolated and shown to directly decompose into LysCN. Collectively, our results also help to explain why the prior in vitro study by Sivey et al [18, 50, 51] reported LysCN as the overwhelming product formed without 2-AAA. Our studies show similar results when a comparable vast molar excess of oxidant is used (~100-fold or more relative to target). Further, quantitation of each of the products formed under different molar ratios of oxidant to target (Figures 2–4) is entirely consistent with the stoichiometry of the reactions shown in Figure 12. It is of interest to note that in previous studies we demonstrated that chlorine gas (Cl2) can be produced by the MPO/H2O2/Cl− system of neutrophils [9]. Cl2 is in equilibrium with HOCl in water and was directly detected by mass spectrometry in headspace gas above activated neutrophils [9]. We therefore include the hypothetical pathway involving Cl2 reaction with protein lysyl residues leading directly to lysine monochloramine and dichloramine derivatives as yet another possible pathway envisioned at high ratios of oxidant to target protein Nε–Lys groups for generation of LysCN (Figure 12).
Our studies reveal that prior analyses that reported detection of protein-bound 2-AAA in tissues likely overestimated 2-AAA formation due to acid catalyzed hydrolysis of protein-bound LysCN into 2-AAA. By using MPO-KO mice, our studies for the first time directly demonstrate that 2-AAA is a major product of protein lysyl residues oxidation by MPO at sites of inflammation. It should also be noted that in both WT and MPO-KO mice, protein bound 2-AAA and LysCN were both present at baseline and at approximately equal levels. Thus, these results strongly suggest that pathway(s) alternative to MPO also exist for generation of these post translational modifications to protein lysyl residues. Different pathways have been reported to produce 2-aminoadipic-δ-semialdehyde, including metal catalyzed oxidation [52, 53], and lysyl oxidase [54]. Besides MPO mediated oxidation, it is also possible to form 2-AAA through further oxidation of 2-aminoadipic-δ-semialdehyde. We found MPO catalyzed oxidant of protein lysyl residues is one pathway producing 2-AAA in inflammatory diseases, however further studies of the origins of 2-AAA in different disease states are needed. Moreover, our studies also show an increase in free 2-AAA in mice following MPO catalyzed oxidation during inflammation. We presume the free amino acid is a proteolytic degradation product of an MPO-oxidized protein since levels of free 2-AAA were nominal at baseline, and markedly increased with inflammation in the WT but not MPO-KO mice. It also is noteworthy that in prior reported studies, exposure of free lysine to HOCl oxidizes lysine at the Nα-amine to form Nα-monochloramine, which then decomposes into a distinct aldehyde (and not 2-AAA) via a different pathway [7, 8]. Thus, MPO-catalyzed generation of free 2-AAA observed in our animal model studies presumably arose from oxidation of protein-bound Lys, and not free Lys.
Our findings thus suggest MPO is a likely source of 2-AAA in vivo. While not directly examined in the present studies, we suspect MPO-mediated oxidation is a major pathway responsible for the elevation of protein-bound 2-AAA and LysCN in human atherosclerotic plaque, since MPO and numerous characteristic oxidation products such as 3-chloro-tyrosine are enriched in atherosclerotic plaque [27, 55]. It is also notable that numerous studies have shown elevated levels of circulatory inflammatory factors in obese and prediabetic subjects [56], and a potential role of MPO in diabetes development has been suggested [57]. Inflammation of pancreatic islets is proposed to contribute to the development of type two diabetes [58], and elevated systemic MPO levels have been reported in patients with diabetes or insulin resistance [59]. Interestingly, prior studies with MPO-KO mice have shown protection from high fat diet-induced obesity and insulin resistance [57]. Based on these cumulative findings, more studies exploring the role of MPO, 2-AAA generation and development of insulin resistance seem warranted. Given the ongoing effort for generation of pharmacological agents that inhibit MPO as a potential therapeutic for the treatment of atherosclerosis, it will be interesting to see if such agents also have a potential salutary effect on prevention of prediabetes and diabetes development.
The present studies focused primarily on chemical mechanisms responsible for post translational modification of protein lysyl residues and their conversion into 2-AAA and LysCN. The biological consequences of 2-AAA and LysCN formation on target proteins by MPO-mediated oxidation are unknown. Under physiological conditions, lysine’s ε-amino side chain is positively charged, and often participates in hydrogen bonds and salt bridges, which are crucial to protein structure and function. The modification of lysine to 2-AAA alters its charge from positive to negative, which changes the interaction of this posttranslationally-modified lysine with surrounding molecules. It is thus likely that such oxidative modification to a protein could cause conformational changes at affected local sites, and it can be envisioned to potentially alter protein function. Considering the high content of lysine residues in proteins and the low abundance of MPO modifications, future studies should pay particular emphasis on possible gain of function modifications to protein lysine residues mediated by MPO. It is also noteworthy that the protein bound 2-aminoadipate–δ–semialdehyde intermediate formed has potential to crosslink with a second lysyl residue, which also would of course change protein structure and possibly function. Our studies revealing the end products of MPO-mediated oxidation of lysine in vivo and the reaction mechanisms involved can help to better understand potential functional changes of oxidized proteins found at the sites of inflammation in future investigations.
Figure 1. LysCN stability under acid hydrolysis and base hydrolysis conditions.
LysCN (1 mM) was incubated with either 6N HCl at 110°C or 4N NaOH at 120°C. At the indicated times, 2-AAA and LysCN content in the reaction were analyzed by LC/MS/MS following solid phase extraction (SPE) as described under Materials and Methods. A, Lysine nitrile (△) is not stable under conventional acid hydrolysis condition and quantitatively decomposes to 2-AAA (○). B, Lysine nitrile (△) is not stable under base hydrolysis conditions and quantitatively decomposes to 2-AAA (○). Data shown represent the mean +/− S.D. of duplicated determinations for a study performed greater than three independent times.
Figure 8. Activated human neutrophils employ the MPO/H2O2/Cl− system to oxidize protein bound lysyl residues to 2-AAA and LysCN.
The complete MPO system ( C ) consisted of freshly harvested human neutrophils (PMN) (1×106 cells/mL) activated with 200 nM phorbol myristate acetate (PMA) in the presence HSA (1 mg/mL). Where indicated, either PMN or PMA was omitted; or catalase (20 μg/mL) or methionine (2 mM) was included with the complete MPO system. Results are the mean ± S.D. of triplicate determinations for an experiment performed at least 3 separate times.
Highlights.
Oxidation of protein lysyl residues by myeloperoxidase (MPO) generated chlorinating oxidants produces the stable end products 2-amino adipic acid (major) and lysine nitrile (minor) in vivo.
During MPO-catalyzed oxidation, protein-bound lysine monochlorimine is an intermediate in generation of 2-amino adipic acid.
During MPO-catalyzed oxidation, protein-bound lysine dichlorimine is an intermediate in generation of protein bound lysine nitrile.
MPO serves as one pathway for generating protein-bound and free 2-amino adipic acid in vivo during inflammation.
Acknowledgments
This study was supported by National Institutes of Health grant P01 HL076491, R01 HL128300, R01 HL128268, and R01 DK106000. V.G. acknowledges support from Cleveland State University through the Faculty Scholarship Initiative and Faculty Research Development Awards.
Footnotes
Conflict of interest
Drs. Hazen, Wang and Levison are named as co-inventors on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Hazen reports having been paid as a consultant for the following companies: Esperion and P&G. Dr. Hazen reports receiving research funds from Astra Zeneca, P&G, Pfizer Inc., Roche and Takeda. Drs. Hazen, Wang and Levison report having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab; and Dr Hazen from Siemens, Esperion, Frantz Biomarkers, LLC. All other authors have no relationships to disclose.
Author contributions
HL played a role in the design, performance and analyses of all studies and the initial drafting of the manuscript. BL helped with synthesis, HPLC purifications and high resolution mass spectrometry studies, as well as partial drafting of the manuscript. XF, ZW, VG, and JAD assisted with study design and data analyses. YH with JAB assisted with all in vivo studies. SLH conceived of project idea, assisted in design and analyses of experiments, and the drafting of the manuscript. All authors critically reviewed the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang YF. Lysine metabolism in the human and the monkey: demonstration of pipecolic acid formation in the brain and other organs. Neurochem Res. 1982;7:577–588. doi: 10.1007/BF00965124. [DOI] [PubMed] [Google Scholar]
- 2.Papes F, Kemper EL, Cord-Neto G, Langone F, Arruda P. Lysine degradation through the saccharopine pathway in mammals: involvement of both bifunctional and monofunctional lysine-degrading enzymes in mouse. Biochem J. 1999;344(Pt 2):555–563. [PMC free article] [PubMed] [Google Scholar]
- 3.Wang TJ, Ngo D, Psychogios N, Dejam A, Larson MG, Vasan RS, Ghorbani A, O’Sullivan J, Cheng S, Rhee EP, Sinha S, McCabe E, Fox CS, O’Donnell CJ, Ho JE, Florez JC, Magnusson M, Pierce KA, Souza AL, Yu Y, Carter C, Light PE, Melander O, Clish CB, Gerszten RE. 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest. 2013;123:4309–4317. doi: 10.1172/JCI64801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klebanoff SJ. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980;93:480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 5.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folkes LK, Candeias LP, Wardman P. Kinetics and mechanisms of hypochlorous acid reactions. Arch Biochem Biophys. 1995;323:120–126. doi: 10.1006/abbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- 7.Hazen SL, d’Avignon A, Anderson MM, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to oxidize alpha-amino acids to a family of reactive aldehydes. Mechanistic studies identifying labile intermediates along the reaction pathway. J Biol Chem. 1998;273:4997–5005. doi: 10.1074/jbc.273.9.4997. [DOI] [PubMed] [Google Scholar]
- 8.Hazen SL, Hsu FF, d’Avignon A, Heinecke JW. Human neutrophils employ myeloperoxidase to convert alpha-amino acids to a battery of reactive aldehydes: a pathway for aldehyde generation at sites of inflammation. Biochemistry. 1998;37:6864–6873. doi: 10.1021/bi972449j. [DOI] [PubMed] [Google Scholar]
- 9.Hazen SL, Hsu FF, Mueller DM, Crowley JR, Heinecke JW. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J Clin Invest. 1996;98:1283–1289. doi: 10.1172/JCI118914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng DQ, Wu Z, Brubaker G, Zheng L, Settle M, Gross E, Kinter M, Hazen SL, Smith JD. Tyrosine modification is not required for myeloperoxidase-induced loss of apolipoprotein A-I functional activities. J Biol Chem. 2005;280:33775–33784. doi: 10.1074/jbc.M504092200. [DOI] [PubMed] [Google Scholar]
- 12.Sell DR, Strauch CM, Shen W, Monnier VM. 2-aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: effects of diabetes, renal failure and sepsis. Biochem J. 2007;404:269–277. doi: 10.1042/BJ20061645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sell DR, Strauch CM, Shen W, Monnier VM. Aging, diabetes, and renal failure catalyze the oxidation of lysyl residues to 2-aminoadipic acid in human skin collagen: evidence for metal-catalyzed oxidation mediated by alpha-dicarbonyls. Ann N Y Acad Sci. 2008;1126:205–209. doi: 10.1196/annals.1433.065. [DOI] [PubMed] [Google Scholar]
- 14.Peng DQ, Brubaker G, Wu Z, Zheng L, Willard B, Kinter M, Hazen SL, Smith JD. Apolipoprotein A-I tryptophan substitution leads to resistance to myeloperoxidase-mediated loss of function. Arterioscler Thromb Vasc Biol. 2008;28:2063–2070. doi: 10.1161/ATVBAHA.108.173815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharjee JK. alpha-Aminoadipate pathway for the biosynthesis of lysine in lower eukaryotes. Crit Rev Microbiol. 1985;12:131–151. doi: 10.3109/10408418509104427. [DOI] [PubMed] [Google Scholar]
- 16.Zabriskie TM, Jackson MD. Lysine biosynthesis and metabolism in fungi. Nat Prod Rep. 2000;17:85–97. doi: 10.1039/a801345d. [DOI] [PubMed] [Google Scholar]
- 17.Kosuge T, Hoshino T. The alpha-aminoadipate pathway for lysine biosynthesis is widely distributed among Thermus strains. J Biosci Bioeng. 1999;88:672–675. doi: 10.1016/s1389-1723(00)87099-1. [DOI] [PubMed] [Google Scholar]
- 18.Sivey JD, Howell SC, Bean DJ, McCurry DL, Mitch WA, Wilson CJ. Role of lysine during protein modification by HOCl and HOBr: halogen-transfer agent or sacrificial antioxidant? Biochemistry. 2013;52:1260–1271. doi: 10.1021/bi301523s. [DOI] [PubMed] [Google Scholar]
- 19.Krieble VK, Noll CI. The hydrolysis of nitriles with acids. J Am Chem Soc. 1939;61:560–563. [Google Scholar]
- 20.Zgliczyński JM, Stelmaszyńska T, Domański J, Ostrowski W. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. Biochim Biophys Acta. 1971;235:419–424. doi: 10.1016/0005-2744(71)90281-6. [DOI] [PubMed] [Google Scholar]
- 21.Pereira WE, Hoyano Y, Summons RE, Bacon VA, Duffield AM. Chlorination studies. II. The reaction of aqueous hypochlorous acid with alpha-amino acids and dipeptides. Biochim Biophys Acta. 1973;313:170–180. doi: 10.1016/0304-4165(73)90198-0. [DOI] [PubMed] [Google Scholar]
- 22.Clark RA, Szot S, Williams MA, Kagan HM. Oxidation of lysine side-chains of elastin by the myeloperoxidase system and by stimulated human neutrophils. Biochem Biophys Res Commun. 1986;135:451–457. doi: 10.1016/0006-291x(86)90015-x. [DOI] [PubMed] [Google Scholar]
- 23.Nightingale ZD, Lancha AH, Jr, Handelman SK, Dolnikowski GG, Busse SC, Dratz EA, Blumberg JB, Handelman GJ. Relative reactivity of lysine and other peptide-bound amino acids to oxidation by hypochlorite. Free Radic Biol Med. 2000;29:425–433. doi: 10.1016/s0891-5849(00)00262-8. [DOI] [PubMed] [Google Scholar]
- 24.Pattison DI, Davies MJ. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem Res Toxicol. 2001;14:1453–1464. doi: 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- 25.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 26.Hazen SL, Crowley JR, Mueller DM, Heinecke JW. Mass spectrometric quantification of 3-chlorotyrosine in human tissues with attomole sensitivity: a sensitive and specific marker for myeloperoxidase-catalyzed chlorination at sites of inflammation. Free Radic Biol Med. 1997;23:909–916. doi: 10.1016/s0891-5849(97)00084-1. [DOI] [PubMed] [Google Scholar]
- 27.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Hörkkö S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 29.Zheng L, Settle M, Brubaker G, Schmitt D, Hazen SL, Smith JD, Kinter M. Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative Impairment in ABCA1-dependent cholesterol efflux from macrophages. J Biol Chem. 2005;280:38–47. doi: 10.1074/jbc.M407019200. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC. Acid ionization constant of HOCl from 5 to 35 degrees. J Chem Phys. 1966;70:3798–3805. [Google Scholar]
- 31.Nelson DP, Kiesow LA. Enthalpy of decomposition of hydrogen peroxide by catalase at 25 degrees C (with molar extinction coefficients of H2O2 solutions in the UV) Anal Biochem. 1972;49:474–478. doi: 10.1016/0003-2697(72)90451-4. [DOI] [PubMed] [Google Scholar]
- 32.Hazen SL, Hsu FF, Heinecke JW. p-Hydroxyphenylacetaldehyde is the major product of L-tyrosine oxidation by activated human phagocytes. A chloride-dependent mechanism for the conversion of free amino acids into reactive aldehydes by myeloperoxidase. J Biol Chem. 1996;271:1861–1867. doi: 10.1074/jbc.271.4.1861. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki S. A simple and convenient method for the synthesis of nitriles by oxidation of primary amines with NaOCl in ethanol. Synthetic Commun. 1997;27:3559–3564. [Google Scholar]
- 34.Reddy AV, Ravindranath B. Acetylation under ultrasonic conditions - convenient preparation of N-acetylamino acids. Synthetic Commun. 1992;22:257–264. [Google Scholar]
- 35.Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, Lusis AJ, Lee JJ, Lee NA, Abu-Soud HM, Ischiropoulos H, Hazen SL. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 36.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. [Google Scholar]
- 37.Coker MS, Hu WP, Senthilmohan ST, Kettle AJ. Pathways for the decay of organic dichloramines and liberation of antimicrobial chloramine gases. Chem Res Toxicol. 2008;21:2334–2343. doi: 10.1021/tx800232v. [DOI] [PubMed] [Google Scholar]
- 38.Hawkins CL, Davies MJ. Reaction of HOCl with amino acids and peptides: EPR evidence for rapid rearrangement and fragmentation reactions of nitrogen-centred radicals. J Chem Soc, Perkin Trans. 1998;2:1937–1945. [Google Scholar]
- 39.Hawkins CL, Davies MJ. Hypochlorite-induced damage to proteins: formation of nitrogen-centred radicals from lysine residues and their role in protein fragmentation. Biochem J. 1998;332:617–625. doi: 10.1042/bj3320617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Test ST, Lampert MB, Ossanna PJ, Thoene JG, Weiss SJ. Generation of nitrogen-chlorine oxidants by human phagocytes. J Clin Invest. 1984;74:1341–1349. doi: 10.1172/JCI111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas EL, Grisham MB, Jefferson MM. Preparation and characterization of chloramines. Methods Enzymol. 1986;132:569–585. doi: 10.1016/s0076-6879(86)32042-1. [DOI] [PubMed] [Google Scholar]
- 42.Valentine RL, Jafvert CT. General acid catalysis of monochloramine disproportionation. Environ Sci Technol. 1988;22:691–696. [Google Scholar]
- 43.Thomas EL, Grisham MB, Jefferson MM. Myeloperoxidase-dependent effect of amines on functions of isolated neutrophils. J Clin Invest. 1983;72:441–454. doi: 10.1172/JCI110992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasu VT, de Cruz SJ, Houghton JS, Hayakawa KA, Morrissey BM, Cross CE, Eiserich JP. Evaluation of thiol-based antioxidant therapeutics in cystic fibrosis sputum: Focus on myeloperoxidase. Free Rad Res. 2011;45:165–176. doi: 10.3109/10715762.2010.521154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peskin AV, Winterbourn CC. Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radic Biol Med. 2001;30:572–579. doi: 10.1016/s0891-5849(00)00506-2. [DOI] [PubMed] [Google Scholar]
- 46.Storkey C, Davies MJ, Pattison DI. Reevaluation of the rate constants for the reaction of hypochlorous acid (HOCl) with cysteine, methionine, and peptide derivatives using a new competition kinetic approach. Free Radic Biol Med. 2014;73:60–66. doi: 10.1016/j.freeradbiomed.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 47.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 48.Williams J. The decomposition of hydrogen peroxide by liver catalase. J Gen Physiol. 1928;11:309–337. doi: 10.1085/jgp.11.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winterbourn CC. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985;840:204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 50.Hazell LJ, van den Berg JJ, Stocker R. Oxidation of low-density lipoprotein by hypochlorite causes aggregation that is mediated by modification of lysine residues rather than lipid oxidation. Biochem J. 1994;302:297–304. doi: 10.1042/bj3020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Handelman GJ, Nightingale ZD, Dolnikowski GG, Blumberg JB. Formation of carbonyls during attack on insulin by submolar amounts of hypochlorite. Anal Biochem. 1998;258:339–348. doi: 10.1006/abio.1998.2592. [DOI] [PubMed] [Google Scholar]
- 52.Requena JR, Chao CC, Levine RL, Stadtman ER. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc Natl Acad Sci USA. 2001;98:69–74. doi: 10.1073/pnas.011526698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akagawa M, Sasaki T, Suyama K. Oxidative deamination of lysine residue in plasma protein of diabetic rats. Novel mechanism via the Maillard reaction. Eur J Biochem. 2002;269:5451–5458. doi: 10.1046/j.1432-1033.2002.03243.x. [DOI] [PubMed] [Google Scholar]
- 54.Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16:387–398. doi: 10.1016/s0945-053x(98)90012-9. [DOI] [PubMed] [Google Scholar]
- 55.Heinecke JW, Hsu FF, Crowley JR, Hazen SL, Leeuwenburgh C, Mueller DM, Rasmussen JE, Turk J. Detecting oxidative modification of biomolecules with isotope dilution mass spectrometry: sensitive and quantitative assays for oxidized amino acids in proteins and tissues. Methods Enzymol. 1999;300:124–144. doi: 10.1016/s0076-6879(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 56.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 57.Wang Q, Xie Z, Zhang W, Zhou J, Wu Y, Zhang M, Zhu H, Zou MH. Myeloperoxidase deletion prevents high-fat diet-induced obesity and insulin resistance. Diabetes. 2014;63:4172–4185. doi: 10.2337/db14-0026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 59.Rovira-Llopis S, Rocha M, Falcon R, de Pablo C, Alvarez A, Jover A, Hernandez-Mijares A, Victor VM. Is myeloperoxidase a key component in the ROS-induced vascular damage related to nephropathy in type 2 diabetes? Antioxid Redox Signal. 2013;19:1452–1458. doi: 10.1089/ars.2013.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]