Abstract
Severe vitamin B12 deficiency is well known to cause morphological alterations in bone marrow. In rare instances, these myelodysplastic and megaloblastic changes can coexist with cytogenetic abnormalities. Here, we report a case of a 38-year-old African-American woman with pernicious anaemia, who was found to have an isolated 20q deletion and which resolved after vitamin B12 replacement. We also discuss various mechanisms in which vitamin B12 deficiency can lead to chromosomal abnormalities. A literature review is also performed to evaluate various other chromosomal aberrations associated with B12 deficiency.
Background
Folic acid and vitamin B12 (B12) play an important role in DNA synthesis and gene expression. Although there are numerous in vivo and in vitro studies linking folate to chromosomal abnormalities and mutagenesis, the data on B12 is however surprisingly limited to few case studies. To the best of our knowledge, this is the first reported case of an isolated 20q deletion secondary to vitamin B12 deficiency. Our report adds new findings to the current existing literature of various other cytogenetic abnormalities seen in the bone marrow from severe B12 deficiency. This manuscript also highlights the importance to exclude B12 deficiency in patients with new diagnosis of myelodysplasia with cytogenetic abnormalities.
Case presentation
A 38-year-old African-American woman was initially referred to our haematology clinic for evaluation of macrocytic anaemia. The patient was in a normal state of health until 6 months prior to this presentation. She then experienced progressively worsening fatigue, heart burn, nocturnal cough and weight loss over 20 lbs. Pertinent physical examination and vital signs were grossly unremarkable. Initial laboratory findings were as follows: haemoglobin (Hb): 10 g/dL, mean corpuscular volume: 110 fL, white cell count (WCC): 3.92×103/μL, platelets: 281 000/μL, reticulocyte count: 1.4%, serum B12: 4187 pg/mL, serum folate: 33 ng/mL, iron: 84 μg/dL, total iron binding capacity: 251 μg/dL, ferritin: 119 ng/mL, transferrin saturation: 33%, lactate dehydrogenase: 205 U/L, haptoglobin: 32 mg/dL, thyroid stimulating hormone: 0.650 μIU/mL, copper: 166 μg/dL. Hepatitis and Human Immunodeficiency Virus 1 and 2 serology was negative. Peripheral blood smear revealed macrocytosis, anisocytosis, tear drop cells, adequate platelets and no evidence of dysplastic cells. Secondary to peripheral blood examination revealing macrocytic red blood cells, tear drop cells in the context of normal B12 levels, a bone marrow biopsy was suggested, which was declined by the patient. Six months later after being lost to follow-up, she presented to the emergency room with bilateral leg weakness and her physical examination revealed marked skin hyperpigmentation, glossitis, moderately increased muscular tone, decreased sensation to touch and pressure in the lower extremities, decreased reflexes in the ankle and knee joints and wide based gait.
Investigations
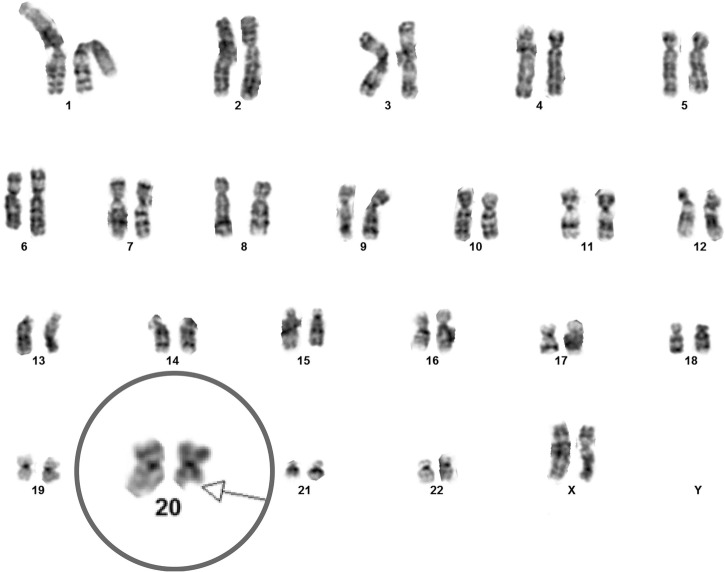
Repeat laboratory findings were: Hb: 8.6 g/dL, WCC: 2.06×103/μL, platelets: 193 000/μL, folate: 19.9 ng/mL. Bone marrow biopsy revealed hypercellular bone marrow with megaloblastic and dysplastic changes, 6% CD34+ blast cells and rare ringed sideroblasts. Metaphase cytogenetic studies on bone marrow aspirate showed del 20 q (figure 1). B12 this time was noted to be at 83 pg/mL, serum homocysteine: 218 μmol/L, serum methyl malonic acid: 23246 nmol/L. Antiparietal antibody and anti-intrinsic factor antibody were strongly positive. The initial finding of a falsely elevated B12 levels can be perceived as an erroneous laboratory finding.
Figure 1.
This figure demonstrates cytogenetic analysis revealing 20 q deletion before vitamin B12 replacement (chromosome 20 magnified (not to scale) to demonstrate the abnormality).
Differential diagnosis
At patient's initial presentation, as all the work up for macrocytic anaemia was negative, myelodysplasia was considered in the differential diagnosis. But, the differential diagnosis was soon narrowed to B12 deficiency when the patient presented later with severe ataxia along with classic neurological manifestations in the presence of macrocytic anaemia. The primary diagnosis of pernicious anemia was confirmed when the serology was positive for both anti-parietal and anti-intrinsic factor antibodies.
Treatment
The patient was subsequently treated with intramuscular B12 supplementation (1000 µg every day for 1 week, every 1 week for 4 weeks and monthly thereafter) with rapid resolution of all haematological parameters in a week and more gradual improvement of neurological symptoms.
Outcome and follow-up
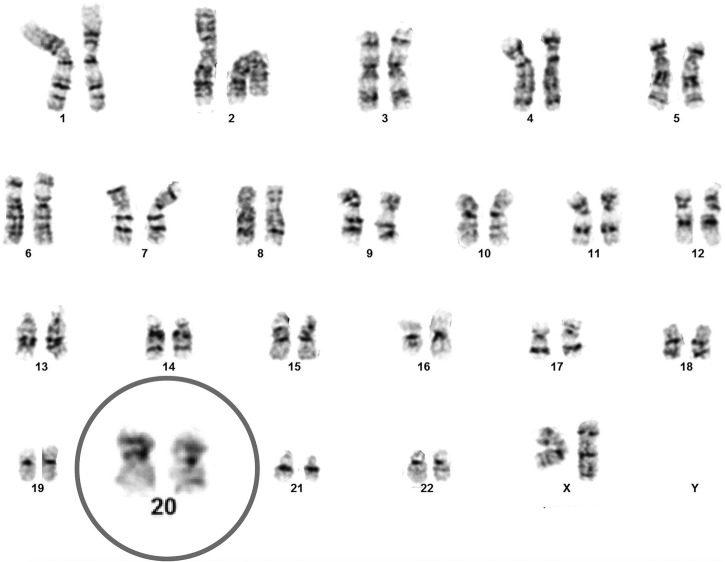
Seventy days after B12 replacement, a repeat bone marrow biopsy was performed and revealed a normocellular bone marrow with no evidence of myelodysplastic features and 3% CD34+ blast cells. Repeat cytogenetic studies on bone marrow aspirate demonstrated normal female karyotype and reversal of prior noted del 20q (figure 2).
Figure 2.
This figure demonstrates normal female karyotype and the reversal of the previously seen 20 q deletion after vitamin B12 replacement.
Discussion
Dietary B12 (cobalamin) is absorbed in the distal ileum after binding to intrinsic factor secreted by the gastric parietal cells.1 Cobalamin aids in the conversion of 5-methyl tetrahydrofolate (THF) to N 5,10 methylene THF, which is necessary for deoxy-thymidine monophosphate (dTMP) synthesis.2 Cobalamin also mediates the conversion of homocysteine to methionine which is a precursor for S-adenosyl methionine, a methyl donor required for the maintenance of methylation patterns in DNA and the synthesis of formyl-THF.2 Deficiency of B12 also leads to accumulation of homocysteine. B12 deficiency ultimately leads to trapping of folate, increased deoxy-uridine monophosphate (dUMP), which prevents incorporation of dTMP into the DNA. High intracellular ratio of dUMP/dTMP and subsequent increase in the deoxy-uridine triphosphate/deoxy-thymidine triphosphate (dUTP/dTTP) ratio results in misincorporation of uracil into the DNA strand.3–5 Uracil DNA glycolase/apyrimidinic nuclease which corrects this mis-incorporation is overwhelmed in the continued absence of B12, and this lack of corrective process leads to accumulation in chromosomal fragile sites, chromosomal breaks and chromosomal deletions without any repair6–7 and chromosomal deletions without any repair. Altered methylation of DNA is also hypothesised to play a role in mutagenesis and carcinogenesis.8–10 Other plausible mechanisms for mutagenesis which have been studied in vitro include increased homocysteine levels,11 and decreased telomere length12 seen in patients with a B12 deficiency that affects genomic integrity. B12 deficiency can also cause micronucleus formation,13–14 and accelerated apoptosis;15 however, their role in mutagenesis is unknown.
Studies attempting to characterise chromosomal changes associated with B12 deficiency date back to as early as 1950s. But, a consistent demonstration of this finding was a challenge secondary to the limitations of laboratory techniques involved.16 As the molecular techniques improved, various groups demonstrated multiple chromosomal abnormalities: increase in nuclear size,17 hypodiploidy18 19 and chromosomal breaks.20–23 Some of these reports also demonstrate reversal of these abnormalities on replacement of B12.20 21 24 25 For example, B12 deficient patients with isolated 7q and 3p chromosomal deletions had reversal of these chromosomal findings on replacement therapy.26 27 Table 1 summarises the various reports of chromosomal abnormalities which were associated with B12 deficiency.
Table 1.
Other reports of B12 deficiency associated with chromosomal abnormalities
| Reports | N* | Deficiency | Comments | |
|---|---|---|---|---|
| 1 | Ford, 195916 | 1 | B12 | Hypodiploidy; subsequently reported as an error |
| 3 | Astaldi et al, 196218 | 1 | B12 | Aneuploidy and changes in chromosome morphology Less pronounced after B12 replacement |
| 4 | Forteza and Baguena, 196328 | 1 | B12 | Aneuploidy |
| 5 | Kiossoglou et al, 196519 | 3 | B12 | Aneuploidy, chromosomal and chromatid breaks Changes persisted in one after B12 replacement |
| 6 | Jensen et al, 196722 | 4 | B12 | Increased chromosomal breaks |
| 7 | Heath, 196621 | 14 | B12 and folate | Chromosome breakage along with severe megaloblastic changes |
| 8 | Bottura et al, 196824 | 1 | B12 | Chromosomal breaks in metaphase; corrected after B12 replacement |
| 9 | Keller et al, 197020 | 3 | B12 | Chromosomal breaks resolved after B12 replacement in one patient |
| 10 | Lawler et al, 197123 | 15 | B12 and folate | Chromosomal breaks. Reversal seen in 48 hours with B12 replacement |
| 11 | Goh, 198129 | 1 | B12 | Del (18p), persisted with replacement. Unlikely from B12 deficiency |
| 12 | Wollman et al, 199626 | 1 | B12 and folate | Del (7q) with reversal after B12 replacement |
| 13 | Chintagumpala et al, 199625 | 3 | B12 | Spontaneous chromosomal fragility. Reversal with B12 replacement in one patient |
| 14 | Parmentier et al 201227 | 1 | B12 | Del (3p), with reversal of cytogenetic abnormalities after B12 replacement |
*N, number of patients described in the case report.
B12, vitamin B12.
Our case involved an isolated 20q deletion, a non-specific, yet a recurrent chromosomal abnormality seen in myelodysplastic syndrome (MDS).30 Del (20q) preferentially involves erythroid and megakaryocytic precursors and this isolated abnormality when associated with MDS portends a favourable prognosis in terms of its overt progression into acute myeloid leukemia (AML).31 32 It is to be noted that other conflicting reports about poor prognosis of del (20q) and higher conversion to AML have also been reported.33 Del 20q is also known to be associated with myeloproliferative disorders, acute myeloid leukaemia, pure red blood cell aplasia and angioimmunoblastic lymphadenopathy with dysproteinaemia.34 Morphologically bone marrow examination in a B12-deficient individual can mimic those with frank myelodysplasia/leukaemia.35–37 Cases have been reported where B12 deficiency and MDS/AML coexist.38–39 Das et al40 demonstrated that reversal of chromosomal breaks and despiralisation can take up to 12 weeks to normalise after B12 and folate supplementation.
Learning points.
This case demonstrates reversal of haematological parameters and cytogenetic abnormality after vitamin B12 replacement in a patient with pernicious anaemia.
Reversible clonal cytogenetic abnormalities and bone marrow changes mimicking myelodysplastic syndrome may be seen in patients with severe B12 deficiency. It is prudent to identify and treat the B12 deficiency before making a misdiagnosis of myelodysplastic syndrome.
Although B12 deficiency can cause chromosomal and hematopoetic abnormalities, its role in leukemogenesis is not clear. Further studies are needed to confirm if B12 deficiency can lead to mutagenesis and carcinogenesis.
Footnotes
Contributors: SRC, DV and PP contributed to data collection. SRC and PP are responsible for drafting the article. PP, SRC and NK are responsible for critical revision of the article. All authors are responsible for final approval of the version to be published.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Festen HP. Intrinsic factor secretion and cobalamin absorption: physiology and pathophysiology in the gastrointestinal tract. Scand J Gastroenterol 1991;26(Suppl 188):1–7. 10.3109/00365529109111222 [DOI] [PubMed] [Google Scholar]
- 2.Solomon LR. Disorders of cobalamin (vitamin B12) metabolism: emerging concepts in pathophysiology, diagnosis and treatment. Blood Rev 2007;21:113–30. 10.1016/j.blre.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 3.Blount BC, Ames BN. 2 DNA damage in folate deficiency. Baillières Clin Haematol 1995;8:461–78. 10.1016/S0950-3536(05)80216-1 [DOI] [PubMed] [Google Scholar]
- 4.Blount BC, Mack MM, Wehr CM et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci USA 1997;94:3290–5. 10.1073/pnas.94.7.3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wickramasinghe SN, Fida S. Bone marrow cells from vitamin B12-and folate-deficient patients misincorporate uracil into DNA. Blood 1994;83: 1656–61. [PubMed] [Google Scholar]
- 6.Dianov GL, Timchenko TV, Sinitsina OI et al. Repair of uracil residues closely spaced on the opposite strands of plasmid DNA results in double-strand break and deletion formation. Mol Gen Genet 1991;225:448–52. 10.1007/BF00261686 [DOI] [PubMed] [Google Scholar]
- 7.Reidy JA, Zhou X, Chen AT. Folic acid and chromosome breakage I. Implications for genotoxicity studies. Mutat Res Lett 1983;122:217–21. 10.1016/0165-7992(83)90062-3 [DOI] [PubMed] [Google Scholar]
- 8.Wainfan E, Poirier LA. Methyl groups in carcinogenesis: effects on DNA methylation and gene expression. Cancer Res 1992;52(7 Suppl):2071s–7s. [PubMed] [Google Scholar]
- 9.Chen RZ, Pettersson U, Beard C et al. DNA hypomethylation leads to elevated mutation rates. Nature 1998;395:89–93. 10.1038/25779 [DOI] [PubMed] [Google Scholar]
- 10.Ehrlich M. DNA methylation in cancer: too much, but also too little. Oncogene 2002;21:5400–13. 10.1038/sj.onc.1205651 [DOI] [PubMed] [Google Scholar]
- 11.Huang RF, Huang SM, Lin BS et al. Homocysteine thiolactone induces apoptotic DNA damage mediated by increased intracellular hydrogen peroxide and caspase 3 activation in HL-60 cells. Life Sci 2001;68:2799–811. 10.1016/S0024-3205(01)01066-9 [DOI] [PubMed] [Google Scholar]
- 12.Pusceddu I, Herrmann M, Kirsch SH et al. One-carbon metabolites and telomere length in a prospective and randomized study of B-and/or D-vitamin supplementation. Eur J Nutr 2016:1–2. DOI: 10.1007/s00394-016-1231-z [DOI] [PubMed] [Google Scholar]
- 13.Jensen MK. Cytogenetic findings in pernicious anaemia. Comparison between results obtained with chromosome studies and the micronucleus test. Mutat Res 1977;45:249–52. [DOI] [PubMed] [Google Scholar]
- 14.Fenech M. Micronucleus frequency in human lymphocytes is related to plasma vitamin B12 and homocysteine. Mutat Res 1999;428:299–304. [DOI] [PubMed] [Google Scholar]
- 15.Koury MJ, Price JO, Hicks GG. Apoptosis in megaloblastic anemia occurs during DNA synthesis by a p53-independent, nucleoside-reversible mechanism. Blood 2000;96:3249–55. [PubMed] [Google Scholar]
- 16.Ford CE. The interpretation of chromosome counts. Lancet 1959;274:567 10.1016/S0140-6736(59)91808-2 [DOI] [Google Scholar]
- 17.Powsner ER, Berman L, MacLeod AI. Human bone marrow chromosomes in megaloblastic anemia. Blood 1965;26:784–9. [PubMed] [Google Scholar]
- 18.Astaldi G, Strosselli E, Airo R et al. [Cytogenetic research in pernicious anemia after treatment.]. Schweiz Med Wochenschr 1962;92:1332–3. [PubMed] [Google Scholar]
- 19.Kiossoglou KA, Mitus WJ, Dameshek W. Chromosomal aberrations in pernicious anemia. Study of three cases before and after therapy. Blood 1965;25:662–82. [PubMed] [Google Scholar]
- 20.Keller R, Lindstrand K, Nordén A. Disappearance of chromosomal abnormalities in megaloblastic anaemia after treatment. Scand J Haematol 1970;7:478–85. 10.1111/j.1600-0609.1970.tb01935.x [DOI] [PubMed] [Google Scholar]
- 21.Heath CW, Fliegelman L. Cytogenetic observations in vitamin B12 and folate deficiency. Blood 1966;27:800–15. [PubMed] [Google Scholar]
- 22.Jensen MK, Friis-Møller A. Chromosome studies in pernicious anaemia. Acta Med Scand 1967;181:571–6. 10.1111/j.0954-6820.1967.tb07277.x [DOI] [PubMed] [Google Scholar]
- 23.Lawler SD, Roberts PD, Hoffbrand AV. Chromosome studies in megaloblastic anaemia before and after treatment. Scand J Haematol 1971;8:309–20. 10.1111/j.1600-0609.1971.tb00880.x [DOI] [PubMed] [Google Scholar]
- 24.Bottura C, Coutinho V. The chromosome anomalies of the megaloblastic anaemias. Blut 1968;16:193–9. 10.1007/BF01631668 [DOI] [PubMed] [Google Scholar]
- 25.Chintagumpala MM, Dreyer ZA, Steuber CP et al. Pancytopenia with chromosomal fragility: vitamin B12 deficiency. J Pediatr Hematol Oncol 1996;18:166–70. 10.1097/00043426-199605000-00014 [DOI] [PubMed] [Google Scholar]
- 26.Wollman MR, Penchansky L, Shekhter-Levin S. Transient 7q-in association with megaloblastic anemia due to dietary folate and vitamin Bl2 deficiency. J Pediatr Hematol Oncol 1996;18:162–5. 10.1097/00043426-199605000-00013 [DOI] [PubMed] [Google Scholar]
- 27.Parmentier S, Meinel J, Oelschlaegel U et al. Severe pernicious anemia with distinct cytogenetic and flow cytometric aberrations mimicking myelodysplastic syndrome. Ann Hematol 2012;91:1979–81. 10.1007/s00277-012-1488-0 [DOI] [PubMed] [Google Scholar]
- 28.Forteza BG, Baguena CR. [Cytogenetic analysis of a case of pernicious anemia before and after treatment.]. Rev Clin Esp 1963;88:251–4. [PubMed] [Google Scholar]
- 29.Goh K. Vitamin B12 deficiency in an 18p-patient. Arch Pathol Lab Med 1981;105:164. [PubMed] [Google Scholar]
- 30.Haase D. Cytogenetic features in myelodysplastic syndromes. Ann Hematol 2008;87:515–26. 10.1007/s00277-008-0483-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braun T, de Botton S, Taksin AL et al. Characteristics and outcome of myelodysplastic syndromes (MDS) with isolated 20q deletion: a report on 62 cases. Leuk Res 2011;35:863–7. 10.1016/j.leukres.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 32.Wattel E, Laï JL, Hebbar M et al. De novo myelodysplastic syndrome (MDS) with deletion of the long arm of chromosome 20: a subtype of MDS with distinct hematological and prognostic features? Leuk Res 1993;17:921–6. 10.1016/0145-2126(93)90038-M [DOI] [PubMed] [Google Scholar]
- 33.Campbell LJ, Garson OM. The prognostic significance of deletion of the long arm of chromosome 20 in myeloid disorders. Leukemia 1994;8:67–71. [PubMed] [Google Scholar]
- 34.Kurtin PJ, Dewald GW, Shields DJ et al. Hematologic disorders associated with deletions of chromosome 20q: a clinicopathologic study of 107 patients. Am J Clin Pathol 1996;106:680–8. 10.1093/ajcp/106.5.680 [DOI] [PubMed] [Google Scholar]
- 35.Kim M, Lee SE, Park J et al. Vitamin B(12)-responsive pancytopenia mimicking myelodysplastic syndrome. Acta Haematol 2011;125:198–201. 10.1159/000322941 [DOI] [PubMed] [Google Scholar]
- 36.Bharath V, Hsia CC. Vitamin B12 deficiency presenting as pancytopenia with ring sideroblasts. Blood 2015;126:2255 10.1182/blood-2015-08-664862 [DOI] [PubMed] [Google Scholar]
- 37.Aitelli C, Wasson L, Page R. Pernicious anemia: presentations mimicking acute leukemia. South Med J 2004;97:295–8. 10.1097/01.SMJ.0000082003.98003.88 [DOI] [PubMed] [Google Scholar]
- 38.Drabick JJ, Davis BJ, Byrd JC. Concurrent pernicious anemia and myelodysplastic syndrome. Ann Hematol 2001;80:243–5. 10.1007/s002770000272 [DOI] [PubMed] [Google Scholar]
- 39.Mufti GJ, Figes A, Hamblin TJ et al. Immunological abnormalities in myelodysplastic syndromes I. Serum immunoglobulins and autoantibodies. Br J Haematol 1986;63:143–7. 10.1111/j.1365-2141.1986.tb07504.x [DOI] [PubMed] [Google Scholar]
- 40.Das KC, Herbert V. The lymphocyte as a marker of past nutritional status: persistence of abnormal lymphocyte deoxyuridine (dU) suppression test and chromosomes in patients with past deficiency of folate and vitamin B12. Br J Haematol 1978;38:219–33. 10.1111/j.1365-2141.1978.tb01038.x [DOI] [PubMed] [Google Scholar]