Abstract
Several studies have described the adaptive remodeling of the heart during exercise. In some more practiced endurance athletes, there is a disproportionate load on the right ventricle (RV), at least during exercise, and this might be the basis for a chronic pro-arrhythmic RV remodeling. Especially, in these kinds of athletes the recovery after detraining might be incomplete, in particular for RV changes. The observation of acute myocardial injury based on transient elevation of biomarkers and chronic myocardial scar, not completely reversible changes of the RV and an increased prevalence of some arrhythmias support the existence of an “exercise-induced cardiomyopathy.” The aim of this paper is to review current knowledge about changes in the right heart in highly trained athletes and how these change influence cardiac function.
Keywords: Athletes heart, Doppler, exercise-induced cardiomyopathy, right heart, sport training, strain
INTRODUCTION
During last decades, most studies have described this adaptive remodeling also referred to as “athletes heart.” Exercise causes structural, functional, and electrical changes of the heart which are physiological responses to the hemodynamic demands of increased cardiac output during exercise.
The main changes are represented by cardiac enlargement with preserved compliance and contractility such that, according to the law Laplace, “the larger heart can fill and empty larger volumes more efficiently.”
The type of training and age, sex, ethnicity, genetic factors, and body size can influence cardiac remodeling.
Actually, in literature, there are many evidences that suggest that the majority of sports induce a variable combination of both endurance and strength exercise, rather than only one of them.
While strength training seems to impact minimally on the right ventricle (RV), endurance exercise seems to be associated with the greatest cardiac remodeling.[1,2,3]
Other reports described a disproportionate load on RV, at least during exercise, and this might be the basis for a chronic pro-arrhythmic RV remodeling.[4]
Indeed, while the reversibility of the changes induced by sport after detraining was considered a typical feature of athletes heart, several studies showed that recovery might be incomplete, in particular for RV changes and this is particularly true in more practiced athletes.
Moreover, there is some evolving evidence which suggests that some of the exercise-induced changes may be associated with acute and chronic cardiac damage and that in a small number of athletes this may predispose to atrial and ventricular arrhythmias.
The observation of acute myocardial injury and chronic myocardial scar in some athletes [Figures 1 and 2], the not completely reversible changes of the RV and an increased prevalence of some arrhythmias among endurance athletes, support the existence of an “exercise-induced cardiomyopathy”.
Figure 1.
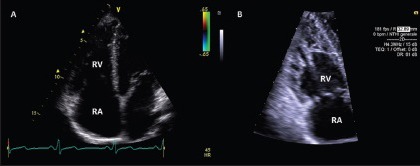
Standard Doppler echocardiography (apical 4-chamber view) showing two possible right ventricle morphologic adaptation to training, even in presence of normal function: Dilatation (a) and hypertrabeculation (b)
Figure 2.
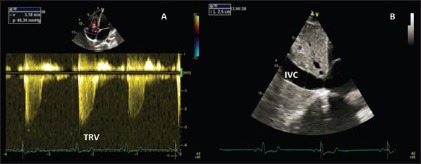
Standard Doppler echocardiography outlining increased tricuspidation velocity and inferior vena cava diameter in an endurance athlete, as a result of physiologic volume overload
The aim of this paper is to review current knowledge about physiologic and pathophysiologic changes in the right heart (RH) in highly trained athletes.
THE PHYSIOLOGY
RH clearly participates in the process of enlargement of the athlete's heart, with an increase in internal diameters and thickness of its free walls, as demonstrated by studies with standard echocardiography.
RV shows greater inflow and outflow dimensions comparing with sedentary controls, with no significant difference in the systolic function.
In particular, with respect to the type of long-term training, D’Andrea et al. documented that RH measures were all significantly greater in endurance highly trained athletes, compared to age and sex matched strength-trained athletes.[4] Left ventricular (LV) stroke volume and pulmonary artery systolic pressure (PASP) were found to be powerful independent predictors of both RV and right atrium dimensions.
Right sided-valves (pulmonary and tricuspid) present a higher prevalence of regurgitation in athletes, possibly as a result of chambers enlargement due to the long-term overload caused by training.[2,5] These regurgitation are generally considered “physiological,” because of the absence of structural abnormalities of the valve apparatus and the absence of aliasing at the color-Doppler analysis.
In close relationship with RH adaptation to long-term training, interesting changes in pulmonary vascular hemodynamics of highly trained athletes can be detected at rest. Range of resting PASP values, as estimated by tricuspid regurgitant velocity, have been described by D’Andrea et al. in both endurance- and strength-trained athletes.[6] The upper limit of normal was 40 mmHg, in the presence of normal pulmonary vascular resistance. Endurance-trained athletes showed the highest values, compared with strength-trained athletes, and LV stroke volume was an independent predictor of PASP.
Resting RV global systolic function as measured by fractional area change and tricuspid annular plain systolic excursion seems to be lower in endurance athletes comparing with nonathletic controls, as reported in recent studies. This finding is in conflict with previous small studies, which reported augmented resting RV systolic function in athletes cohort.[10,7] A subsequent large cardiovascular magnetic resonance (CMR) study has confirmed the reduction in RV systolic function of the endurance athletes.[8] The reduction was more pronounced in the presence of higher RV dilation. A recent study by D’Andrea et al., found comparable two-dimensional (2D) and 3D RV systolic indexes between endurance athletes and controls. In this setting, a mild reduction in global RV function could be considered a physiological consequence of RV dilation, since an efficient stroke volume will be reached with higher end-diastolic volumes and then at lower ejection fraction. On the other hand, a severe reduction in RV global systolic function should be considered an abnormal finding even among athletes.[9]
Novel echocardiographic techniques provide data about regional RV systolic function modification among highly trained athletes. In particular, both tissue Doppler and 2D-strain derived deformation indexes are reduced at rest in endurance athletes at the RV inlet and mid free wall level [Figures 3 and 4].
Figure 3.
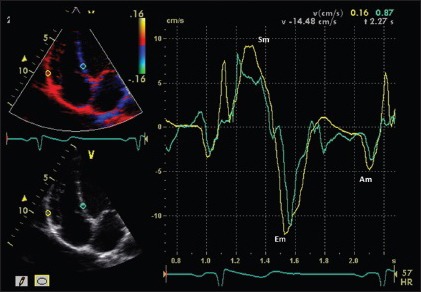
Right ventricle tissue Doppler of endurance athlete, showing normal systolic (Sm) and diastolic (Em and Am) disatolic velocities at a myocardial level
Figure 4.
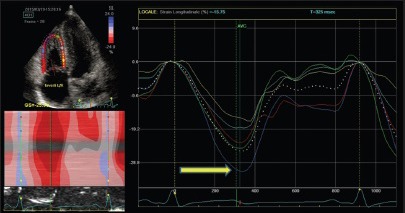
Right ventricle two-dimensional strain of endurance athlete, showing normal myocardial deformation (arrow)
Inconsistent data have been reported with respect to RV diastolic function in athletes, since several studies reported enhanced RV diastolic function among athletes, while others found no significant differences in comparison with controls.[10,11] As demonstrated by tissue Doppler velocity measurements, the early-diastolic phase of ventricular filling was increased along with a prolonged isometric relaxation time. LV stroke volume was an independent predictor of the early diastolic velocity (Em) and the time of regional isovolumic release (RTm) of the free right ventricular walls.[10] These findings are consistent with the known left and right ventricular interdependence.
These findings have important implications for the physician involved in the preparticipation screening and follow-up of elite athletes. In particular, further explanation of the resting changes of the right ventricular morphology and function might come from the assessment of the right ventricular performance during exercise, which is an important area of ongoing study.
Right heart morphological changes and chronic functional adaptation during exercise
As noted above, changes in dimensions and hemodynamics of RV and pulmonary circulation can be detected. These make athletes evaluation challenging for the overlap of RV parameters with those detected in pathological conditions. In this context, the evaluation of RV and pulmonary circulation during exercise is of fundamental importance to elucidate the physiological basis of such changes.
With respect to pulmonary circulation hemodynamics, a number of studies have reported substantial increases in pulmonary artery pressures during exercise using echocardiographic estimates[8,10,11,12] and direct invasive measures.[13,14] Lewis et al.[13] observed an increase of 1.5 mmHg in mean pulmonary artery pressure for each liter increase in CO. Thus, an increase in CO of 30 L/min would equate to a mean pulmonary artery pressure exceeding 50 mmHg representing an increase of 3-fold or more from the rest. La Gerche et al. sought to assess this seemingly disproportionate load using a combination of magnetic resonance and echocardiographic imaging at rest and during exercise to quantify RV systolic wall stress, as compared with that of the LV.[12] Using the simple construct of the LaPlace relationship, we found that during exercise increases in both pressures and volumes were greater for the RV, while increases in wall thickness were relatively less than for the LV. As a result, RV wall stress estimates increased 125% during exercise as compared with a modest 14% increase in LV wall stress.[20] Thus, it may be contended that the stress, work, and metabolic demands placed on the RV during exercise are relatively far greater than that of the LV.[23] Remembering that the RV is approximately one-quarter the mass of the LV, it would seem better matched to its arterial load at rest than during exercise. The substantial increases in afterload would seem a significant burden for the contractile reserve of the RV and raises the possibility that in the extremes of exercise the RV/pulmonary vascular unit may limit output. However, echocardiographic estimates of contractility seem to increase proportional to increases in pulmonary artery pressures during intense exercise of short duration[15] suggesting that the RV has the contractile reserve to meet exercise demands, at least for a while.
While the healthy RV seems capable of meeting the work requirements of short bouts of intense exercise, the aforementioned disparity in ventricular load would suggest that if prolonged exercise can induce cardiac “fatigue” then the RV would be most susceptible. Numerous studies have demonstrated the evidence of cardiac fatigue or injury following intense exercise sustained over many hours, such as following marathon running and ultra-endurance triathlons. Biochemical evidence of cardiac injury is common, usually minor and mostly associated with little if any LV dysfunction.[16]
In contrast, virtually all studies in which the RV has been assessed following intense endurance exercise have reported RV dysfunction.[17,18,19,20,21,22,23] A recent meta-analysis of multiple postendurance exercise studies confirmed that intense prolonged exercise is associated with a measurable reduction in RV function while LV function is relatively unaffected.[24] The magnitude of the effect appears related to both exercise intensity and duration.
The main arguments which are used to support the existence of an “exercise-induced cardiomyopathy” are:
The observation of acute myocardial injury and chronic myocardial scar in some athletes;
That exercise-induced remodeling results in structural myocardial changes which are not completely reversible, thereby implying some extracellular expansion, as well as muscular hypertrophy, and
An increased prevalence of some arrhythmias among endurance athletes.
Studies of endurance athletes following competitive events in different disciplines and of different durations have shown evidence of acute myocardial injury based on the transient elevation of biomarkers (Troponin and B-type natriuretic peptide) following these events.[25]
A meta-analysis suggested that elevated cardiac troponin levels occur in approximately 50% of endurance exercise event participants.[26] In all studies, cardiac enzyme levels returned to normal within a few days of the exercise prompting a debate regarding the origin, mechanisms, and significance of these biomarkers. However, it would seem that the changes in RV function are both more profound and may provide a better explanation for biomarker elevations. As discussed above, postendurance exercise studies have consistently reported decrements in RV function which are far more substantive than those observed for the LV.[33] Also, while multiple studies have documented that there is no relationship between biomarkers of cardiac injury and changes in LV function,[34] two recent studies have documented moderately strong inverse correlations between the decrease in measures of RV function and the increase in release of troponin and B-type natriuretic peptide.[5,32] Thus, it would seem that the RV is potentially the “Achilles’ heel” of the endurance athlete which is disproportionately fatigued or injured following endurance exercise.
The question is whether the transient changes in cardiac function and elevations in biomarkers result in any long-term cardiac damage with clinical consequences.
A potential deleterious consequence of any cardiac damage is chronic scar or fibrosis that has the potential to affect cardiac function and/or cause arrhythmias. Histology provides the only direct evidence of fibrosis and in the case of the heart, this involves an invasive procedure with significant risks. Thus, biopsies are performed in those athletes in whom there is a high pretest probability of identifying pathology and hence, it is unsurprising that inflammation and/or fibrosis is identified in a considerable proportion.[27,28,29] A multimodality imaging approach to the athlete's heart aims to differentiate physiological changes due to intensive training in the athlete's heart from serious cardiac diseases with similar morphological features.
Perhaps the most promising tool for identifying fibrosis noninvasively is by combining cardiac magnetic resonance imaging following gadolinium contrast. This delayed gadolinium enhancement (DGE) technique has been investigated in small cohorts of athletes and while it appears that DGE tends to be absent in athletes with modest training histories,[26,28,30] four recent studies have each reported DGE in 12-50% of extensively trained veteran athletes[5,31,32,33] La Gerche et al. identified DGE in 5 of 39 well-trained endurance athletes, and found that those with DGE had a more extensive history of training and had greater cardiac dimensions, particularly of the RV.[12]
As noted above, increasing interest has been placed in the study of the RV dysfunction noted in high endurance athletes and its relationship with a possible pro-arrhythmic remodeling and an exercise-induced cardiomyopathy. In particular, in some more practiced athletes features of possible chronic RV alteration were seen, that is, DGE of the interventricular septum at the site of RV attachment.[5,42,34] There is some limitation in the evaluation of RV fibrosis with CMR, such as the difficult visualization of the RV free wall myocardium. Then, it is possible that cardiac fibrosis may be underappreciated with current techniques that assess relative enhancement.
All the changes in RH morphology and function have important correlates in resting electrocardiography patterns. Among these, both complete and incomplete right bundle branch block are common among trained athletes and are important overlap findings with primary conduction disturbance and cardiomyopathies that involve RV, most notably arrhythmogenic right ventricular cardiomyopathy (ARVC).
There is now reasonably compelling evidence that some cardiac arrhythmias are associated with long-standing endurance training. Almost every study conducted in endurance athletes of middle-age or greater has observed an increased prevalence of atrial fibrillation as compared with nonathletic referents.[35,36,37,38,39,40,41,42]
Biffi et al. reported that ventricular ectopic beats were common among athletes but was a benign and potentially reversible phenomenon so long as underlying cardiac disease was excluded.[43,44]
In contrast, Heidbuchel et al. observed a high incidence of major arrhythmic events (39%) including sudden cardiac death (20%) among 46 athletes who presented with frequent ventricular ectopy or non/sustained ventricular tachycardia that were followed for 5 years.[45] Somewhat surprisingly, in 89% of cases the arrhythmias arose from the RV and were frequently associated with functional and/or structural changes of the RV. The degree to which many years of intense endurance exercise promotes ventricular arrhythmias is yet to be determined. Furthermore, it is unknown to what extent these issues are caused by sports practice in its own right as opposed to being an expression of an underlying familial predisposition such as ARVC.[46]
CONCLUSIONS
Vigorous exercise practice, both for recreational and competitive purpose, is spreading worldwide because of a number of factors, such as documented health benefits, growing numbers of sport events (i.e., community-based road running races). Then, an increase in the number of subjects with features of exercise-induced cardiac remodeling could be expected. It is increasingly important for the cardiologist and sports medicine practitioners to possess at least a basic knowledge of this subject. With respect to the contemplated potential existence of an “exercise-induced cardiomyopathy” or of any potential excess of arrhythmias in athletes, it is critical to maintain some balance. There are a multitude of health benefits from exercise and these are very unlikely to be outweighed by any small risk of arrhythmias. However, there is much work still to be done on the relative risks and benefits of “extreme” exercise, but present evidence should not alarm exercise enthusiasts.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.D’Andrea A, Galderisi M, Sciomer S, Nistri S, Agricola E, Ballo P, et al. Echocardiographic evaluation of the athlete's heart: From morphological adaptations to myocardial function. G Ital Cardiol (Rome) 2009;10:533–44. [PubMed] [Google Scholar]
- 2.Luijkx T, Cramer MJ, Prakken NH, Buckens CF, Mosterd A, Rienks R, et al. Sport category is an important determinant of cardiac adaptation: An MRI study. Br J Sports Med. 2012;46:1119–24. doi: 10.1136/bjsports-2011-090520. [DOI] [PubMed] [Google Scholar]
- 3.Wernstedt P, Sjöstedt C, Ekman I, Du H, Thuomas KA, Areskog NH, et al. Adaptation of cardiac morphology and function to endurance and strength training. A comparative study using MR imaging and echocardiography in males and females. Scand J Med Sci Sports. 2002;12:17–25. doi: 10.1034/j.1600-0838.2002.120104.x. [DOI] [PubMed] [Google Scholar]
- 4.D’Andrea A, Caso P, Sarubbi B, Limongelli G, Liccardo B, Cice G, et al. Right ventricular myocardial adaptation to different training protocols in top-level athletes. Echocardiography. 2003;20:329–36. doi: 10.1046/j.1540-8175.2003.03038.x. [DOI] [PubMed] [Google Scholar]
- 5.Pollak SJ, McMillan SA, Knopff WD, Wharff R, Yoganathan AP, Felner JM. Cardiac evaluation of women distance runners by echocardiographic color Doppler flow mapping. J Am Coll Cardiol. 1988;11:89–93. doi: 10.1016/0735-1097(88)90171-4. [DOI] [PubMed] [Google Scholar]
- 6.D’Andrea A, Naeije R, D’Alto M, Argiento P, Golia E, Cocchia R, et al. Range in pulmonary artery systolic pressure among highly trained athletes. Chest. 2011;139:788–94. doi: 10.1378/chest.10-1260. [DOI] [PubMed] [Google Scholar]
- 7.Kasikcioglu E, Oflaz H, Akhan H, Kayserilioglu A. Right ventricular myocardial performance index and exercise capacity in athletes. Heart Vessels. 2005;20:147–52. doi: 10.1007/s00380-005-0824-x. [DOI] [PubMed] [Google Scholar]
- 8.Prakken NH, Velthuis BK, Teske AJ, Mosterd A, Mali WP, Cramer MJ. Cardiac MRI reference values for athletes and nonathletes corrected for body surface area, training hours/week and sex. Eur J Cardiovasc Prev Rehabil. 2010;17:198–203. doi: 10.1097/HJR.0b013e3283347fdb. [DOI] [PubMed] [Google Scholar]
- 9.D’Andrea A, Riegler L, Morra S, Scarafile R, Salerno G, Cocchia R, et al. Right ventricular morphology and function in top-level athletes: A three-dimensional echocardiographic study. J Am Soc Echocardiogr. 2012;25:1268–76. doi: 10.1016/j.echo.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Bidart CM, Abbas AE, Parish JM, Chaliki HP, Moreno CA, Lester SJ. The noninvasive evaluation of exercise-induced changes in pulmonary artery pressure and pulmonary vascular resistance. J Am Soc Echocardiogr. 2007;20:270–5. doi: 10.1016/j.echo.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 11.La Gerche A, Heidbüchel H, Burns AT, Mooney DJ, Taylor AJ, Pfluger HB, et al. Disproportionate exercise load and remodeling of the athlete's right ventricle. Med Sci Sports Exerc. 2011;43:974–81. doi: 10.1249/MSS.0b013e31820607a3. [DOI] [PubMed] [Google Scholar]
- 12.La Gerche A, MacIsaac AI, Burns AT, Mooney DJ, Inder WJ, Voigt JU, et al. Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol (1985) 2010;109:1307–17. doi: 10.1152/japplphysiol.00457.2010. [DOI] [PubMed] [Google Scholar]
- 13.Lewis GD, Murphy RM, Shah RV, Pappagianopoulos PP, Malhotra R, Bloch KD, et al. Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ Heart Fail. 2011;4:276–85. doi: 10.1161/CIRCHEARTFAILURE.110.959437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: A systematic review. Eur Respir J. 2009;34:888–94. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 15.La Gerche A, Burns AT, D’Hooge J, Macisaac AI, Heidbüchel H, Prior DL. Exercise strain rate imaging demonstrates normal right ventricular contractile reserve and clarifies ambiguous resting measures in endurance athletes. J Am Soc Echocardiogr. 2012;25:253–262.e1. doi: 10.1016/j.echo.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Shave R, George K, Whyte G, Hart E, Middleton N. Postexercise changes in left ventricular function: The evidence so far. Med Sci Sports Exerc. 2008;40:1393–9. doi: 10.1249/MSS.0b013e318172cf36. [DOI] [PubMed] [Google Scholar]
- 17.Trivax JE, Franklin BA, Goldstein JA, Chinnaiyan KM, Gallagher MJ, deJong AT, et al. Acute cardiac effects of marathon running. J Appl Physiol (1985) 2010;108:1148–53. doi: 10.1152/japplphysiol.01151.2009. [DOI] [PubMed] [Google Scholar]
- 18.Neilan TG, Yoerger DM, Douglas PS, Marshall JE, Halpern EF, Lawlor D, et al. Persistent and reversible cardiac dysfunction among amateur marathon runners. Eur Heart J. 2006;27:1079–84. doi: 10.1093/eurheartj/ehi813. [DOI] [PubMed] [Google Scholar]
- 19.Mousavi N, Czarnecki A, Kumar K, Fallah-Rad N, Lytwyn M, Han SY, et al. Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am J Cardiol. 2009;103:1467–72. doi: 10.1016/j.amjcard.2009.01.294. [DOI] [PubMed] [Google Scholar]
- 20.Douglas PS, O’Toole ML, Hiller WD, Reichek N. Different effects of prolonged exercise on the right and left ventricles. J Am Coll Cardiol. 1990;15:64–9. doi: 10.1016/0735-1097(90)90176-p. [DOI] [PubMed] [Google Scholar]
- 21.La Gerche A, Connelly KA, Mooney DJ, MacIsaac AI, Prior DL. Biochemical and functional abnormalities of left and right ventricular function after ultra-endurance exercise. Heart. 2008;94:860–6. doi: 10.1136/hrt.2006.101063. [DOI] [PubMed] [Google Scholar]
- 22.Oxborough D, Shave R, Warburton D, Williams K, Oxborough A, Charlesworth S, et al. Dilatation and dysfunction of the right ventricle immediately after ultraendurance exercise: Exploratory insights from conventional two-dimensional and speckle tracking echocardiography. Circ Cardiovasc Imaging. 2011;4:253–63. doi: 10.1161/CIRCIMAGING.110.961938. [DOI] [PubMed] [Google Scholar]
- 23.Neilan TG, Januzzi JL, Lee-Lewandrowski E, Ton-Nu TT, Yoerger DM, Jassal DS, et al. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation. 2006;114:2325–33. doi: 10.1161/CIRCULATIONAHA.106.647461. [DOI] [PubMed] [Google Scholar]
- 24.Elliott AD, La Gerche A. The right ventricle following prolonged endurance exercise: Are we overlooking the more important side of the heart. A meta-analysis? Br J Sports Med. 2015;49:724–9. doi: 10.1136/bjsports-2014-093895. [DOI] [PubMed] [Google Scholar]
- 25.Shave R, Baggish A, George K, Wood M, Scharhag J, Whyte G, et al. Exercise-induced cardiac troponin elevation: Evidence, mechanisms, and implications. J Am Coll Cardiol. 2010;56:169–76. doi: 10.1016/j.jacc.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 26.Shave R, George KP, Atkinson G, Hart E, Middleton N, Whyte G, et al. Exercise-induced cardiac troponin T release: A meta-analysis. Med Sci Sports Exerc. 2007;39:2099–106. doi: 10.1249/mss.0b013e318153ff78. [DOI] [PubMed] [Google Scholar]
- 27.La Gerche A, Robberecht C, Kuiperi C, Nuyens D, Willems R, de Ravel T, et al. Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin. Heart. 2010;96:1268–74. doi: 10.1136/hrt.2009.189621. [DOI] [PubMed] [Google Scholar]
- 28.Dello Russo A, Pieroni M, Santangeli P, Bartoletti S, Casella M, Pelargonio G, et al. Concealed cardiomyopathies in competitive athletes with ventricular arrhythmias and an apparently normal heart: Role of cardiac electroanatomical mapping and biopsy. Heart Rhythm. 2011;8:1915–22. doi: 10.1016/j.hrthm.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Zeppilli P, Santini C, Palmieri V, Vannicelli R, Giordano A, Frustaci A. Role of myocarditis in athletes with minor arrhythmias and/or echocardiographic abnormalities. Chest. 1994;106:373–80. doi: 10.1378/chest.106.2.373. [DOI] [PubMed] [Google Scholar]
- 30.Galderisi M, Cardim N, D’Andrea A, Bruder O, Cosyns B, Davin L, et al. The multi-modality cardiac imaging approach to the Athlete's heart: An expert consensus of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:353. doi: 10.1093/ehjci/jeu323. [DOI] [PubMed] [Google Scholar]
- 31.Breuckmann F, Möhlenkamp S, Nassenstein K, Lehmann N, Ladd S, Schmermund A, et al. Myocardial late gadolinium enhancement: Prevalence, pattern, and prognostic relevance in marathon runners. Radiology. 2009;251:50–7. doi: 10.1148/radiol.2511081118. [DOI] [PubMed] [Google Scholar]
- 32.Möhlenkamp S, Lehmann N, Breuckmann F, Bröcker-Preuss M, Nassenstein K, Halle M, et al. Running: The risk of coronary events: Prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J. 2008;29:1903–10. doi: 10.1093/eurheartj/ehn163. [DOI] [PubMed] [Google Scholar]
- 33.Wilson M, O’Hanlon R, Prasad S, Deighan A, Macmillan P, Oxborough D, et al. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol (1985) 2011;110:1622–6. doi: 10.1152/japplphysiol.01280.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlstedt E, Chelvanathan A, Da Silva M, Cleverley K, Kumar K, Bhullar N, et al. The impact of repeated marathon running on cardiovascular function in the aging population. J Cardiovasc Magn Reson. 2012;14:58. doi: 10.1186/1532-429X-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karjalainen J, Kujala UM, Kaprio J, Sarna S, Viitasalo M. Lone atrial fibrillation in vigorously exercising middle aged men: Case-control study. BMJ. 1998;316:1784–5. doi: 10.1136/bmj.316.7147.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimsmo J, Grundvold I, Maehlum S, Arnesen H. High prevalence of atrial fibrillation in long-term endurance cross-country skiers: Echocardiographic findings and possible predictors — A 28-30 years follow-up study. Eur J Cardiovasc Prev Rehabil. 2010;17:100–5. doi: 10.1097/HJR.0b013e32833226be. [DOI] [PubMed] [Google Scholar]
- 37.Molina L, Mont L, Marrugat J, Berruezo A, Brugada J, Bruguera J, et al. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: A follow-up study. Europace. 2008;10:618–23. doi: 10.1093/europace/eun071. [DOI] [PubMed] [Google Scholar]
- 38.Mont L, Sambola A, Brugada J, Vacca M, Marrugat J, Elosua R, et al. Long-lasting sport practice and lone atrial fibrillation. Eur Heart J. 2002;23:477–82. doi: 10.1053/euhj.2001.2802. [DOI] [PubMed] [Google Scholar]
- 39.Elosua R, Arquer A, Mont L, Sambola A, Molina L, García-Morán E, et al. Sport practice and the risk of lone atrial fibrillation: A case-control study. Int J Cardiol. 2006;108:332–7. doi: 10.1016/j.ijcard.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Baldesberger S, Bauersfeld U, Candinas R, Seifert B, Zuber M, Ritter M, et al. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J. 2008;29:71–8. doi: 10.1093/eurheartj/ehm555. [DOI] [PubMed] [Google Scholar]
- 41.Heidbüchel H, Anné W, Willems R, Adriaenssens B, Van de Werf F, Ector H. Endurance sports is a risk factor for atrial fibrillation after ablation for atrial flutter. Int J Cardiol. 2006;107:67–72. doi: 10.1016/j.ijcard.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 42.La Gerche A, Schmied CM. Atrial fibrillation in athletes and the interplay between exercise and health. Eur Heart J. 2013;34:3599–602. doi: 10.1093/eurheartj/eht265. [DOI] [PubMed] [Google Scholar]
- 43.Biffi A, Maron BJ, Verdile L, Fernando F, Spataro A, Marcello G, et al. Impact of physical deconditioning on ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2004;44:1053–8. doi: 10.1016/j.jacc.2004.05.065. [DOI] [PubMed] [Google Scholar]
- 44.Biffi A, Pelliccia A, Verdile L, Fernando F, Spataro A, Caselli S, et al. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2002;40:446–52. doi: 10.1016/s0735-1097(02)01977-0. [DOI] [PubMed] [Google Scholar]
- 45.Heidbüchel H, Hoogsteen J, Fagard R, Vanhees L, Ector H, Willems R, et al. High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias. Role of an electrophysiologic study in risk stratification. Eur Heart J. 2003;24:1473–80. doi: 10.1016/s0195-668x(03)00282-3. [DOI] [PubMed] [Google Scholar]
- 46.La Gerche A. Defining the interaction between exercise and arrhythmogenic right ventricular cardiomyopathy. Eur J Heart Fail. 2015;17:128–31. doi: 10.1002/ejhf.224. [DOI] [PubMed] [Google Scholar]


