Abstract
The aim of this review is to summarize the recent developments in strain imaging, an evolving technique – from tissue Doppler to 3D echocardiography – for resolving the complex left ventricular mechanics. Following a brief overview of the different used technique to extract myocardial deformation data, the authors summarize the role of the technique in the assessment of cardiac mechanics and its role in the clinical arena.
Keywords: Cardiac mechanics, clinical application, strain echocardiography, speckle tracking
INTRODUCTION
The assessment of left ventricular (LV) systolic function is the main goal of the cardiological examination, and most of the clinical management and the prognosis are based on that.[1]
In the clinical practice, ejection fraction (EF) is the most used parameter to judge LV systolic function. Unfortunately, EF is limited by several technical and hemodynamic factors. Usually LV EF is estimated by “eyeballing,” which is influenced by reader experience and thus highly variable. Quantitative approaches are dependent on geometrical assumptions and on endocardial border definition. EF only reflects global LV function and does not take into consideration that a hyperkinetic segment may compensate a hypokinetic one leading to a false “normal” result.[2] Even if we overcome all the technical limitations to assess EF (by using 3D echo or cardiac MRI), still this is a load-dependent parameter.[1] In addition, cardiac mechanics is a complex process and EF can only roughly describe it. For all these reasons in the last 15 years, a significant effort has been done to develop strain imaging technique in order to objectively quantify regional and global myocardial function without geometric assumptions, limiting (as much as technically possible) the influence of loading conditions and allowing a comprehensive approach to study myocardial mechanics.
CARDIAC MECHANICS
Cardiac mechanics is the net result of three major components:[3,4,5]
The cardiac base moves toward the relatively fixed apex.
Inward motion of the ventricular walls.
The base and the apex rotate in opposite direction (ventricular twist).
This complex mechanics is due to the interaction among the 3 different muscle layers constituting the heart. According to Torrent Guasp model,[6] the heart is a single myocardial muscle band consisting of helical and circumferential fibers. The myocardial band folds in 1 basal loop and 2 segments, one ascending and the other one descending, these two segments cross each other at the apical level, forming the apical loop. The basal loop is formed by transverse-oriented circumferential fibers wrapping around the ascending and descending segments sparing the apex and the septum. The contraction of the basal loop is responsible for circumferential shortening. The inner part of the ventricular walls had subendocardial right-handed fibers (the descending segment according to Torrent Gasp's theory) with an average angle of about 60-degree counterclockwise rotated.[3]
The outer part of the wall had subepicardial left-handed fibers (the ascending segment of the Torrent Gasp's model) with an average angle of 50-degree clockwise rotated. The remaining 55% of mid wall muscle fibers had a circumferential orientation.
The subendocardial fibers (descending segment] are responsible for longitudinal shortening and of the clockwise rotation of the apex and counterclockwise rotation of the base. The epicardial (ascending segment) fibers are responsible for longitudinal lengthening (during isovolumic relaxation] of the apical counterclockwise rotation and of the base clockwise rotation.
The interventricular septum has a bi-layer structure: The right ventricular side made of subepicardial fibers and the LV side of subendocardial fibers with a dominant longitudinal motion. The interaction between longitudinal and circumferential fibers contracting according with different timings creates the mechanical reasons for the LV twist motion.
Basically during systole and diastole, the apex and the base rotate in opposite directions, while during the isovolumic periods both rotate in the same direction.
Systole
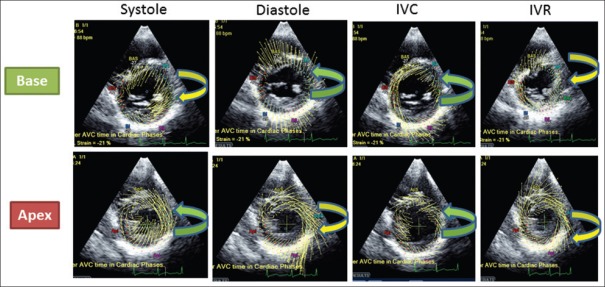
The apical mechanics is the result of the interaction between the subepicardial (ascendant) and the subendocardial (descendant) segments. Because of its larger radius, the ascendant segment overpowers the descent segment leading to a counterclockwise apical rotation and the smaller clockwise basal rotation as net result of the interaction among all the 3 muscle layers at basal level [Figure 1].
Figure 1.
Rotation of LV base and apex during the different phases of the cardiac cycle. Systole: Counterclockwise apical rotation and the smaller clockwise basal rotation. Diastole: The apex moves clockwise and the base counterclockwise. IVC (Isovolumic contraction period): Both the base and apex rotate in a counterclockwise direction. IVR (Isovolumic relaxation period): Apex and base rotate in a clockwise direction
Diastole
The apex moves clockwise and the base counterclockwise [Figure 1].
Isovolumic contraction period
Both the base and apex rotate in a counterclockwise direction. This is due to contraction of the circumferential circular fibers. The ascending segment does not contract during this phase. The ventricle also narrows (because of circumferential fiber shortening) and elongates (because the contraction of the circumferential fibers shortens the basal loop and push toward the apex the two segments) [Figure 1].
Isovolumic relaxation period
Apex and base rotate in a clockwise direction. During this phase, there is no contraction of the circumferential circular muscle layer or of the descending segment as both recoil. However, the ascending segment continues to contract, but at a reduced force so there is no ejection. The ventricle widens and lengthens during this period [Figure 1].
The normal range of time between the termination of the contraction of the descending and ascending segments is about 80-90 ms. If this interval is shortened due to the persisting contraction of the descending segment during the diastolic period (post-systolic shortening), this will adversely affect diastolic filling, inhibiting the suction since about 50% of the untwisting occurs at this time.
Strain Imaging
Strain is a dimensionless parameter that represents the change in length of a segment relative to its baseline length and is expressed as a percentage (%). Strain rate is a quantification of the rate at which myocardial deformation takes place and is expressed as 1/s.[1] While strain correlates well with stroke volume[7] and fibrosis[8], strain rate relates more preponderance to parameters reflecting myocardial contractility like end-systolic elastance.[7] Loading conditions influence more strain, while strain rate seems less affected by preload and after load changes.[1] The advantages of myocardial deformation parameters are their independence from tethering, and global cardiac motion, which impact on all the wall motion-based parameters (as myocardial velocity, displacement etc.), reflecting the true regional function allowing an early detection of subtle myocardial abnormalities.[1]
The left ventricle has a 3D structure and thus its deformation is defined by the three normal strains (longitudinal, circumferential, and radial) [Figures 2 and 3] and three shear strains (circumferential-longitudinal, circumferential-radial, and longitudinal-radial). Shear strains amplify about 15% shortening of myocytes into 40% radial LV wall thickening. Left ventricular shearing increases from subepicardium to subendocardium. Twist or torsional deformation is the result of counterclockwise apical rotation and clockwise basal rotation [Figure 4]. During ejection, LV torsion allows the storage of potential energy into the deformed myofibers. This stored energy is released at the onset of relaxation and results in suction.[9]
Figure 2.
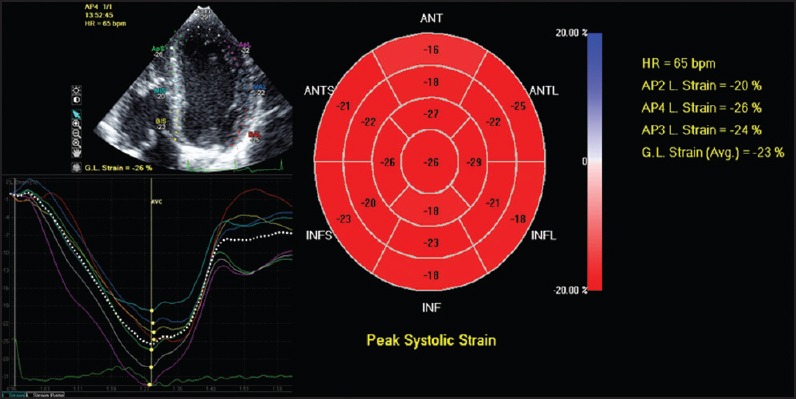
From apical views, longitudinal myocardial strain can be calculated. In most of the commercially available systems, a bull's eye showing the peak systolic longitudinal strain is displayed
Figure 3.
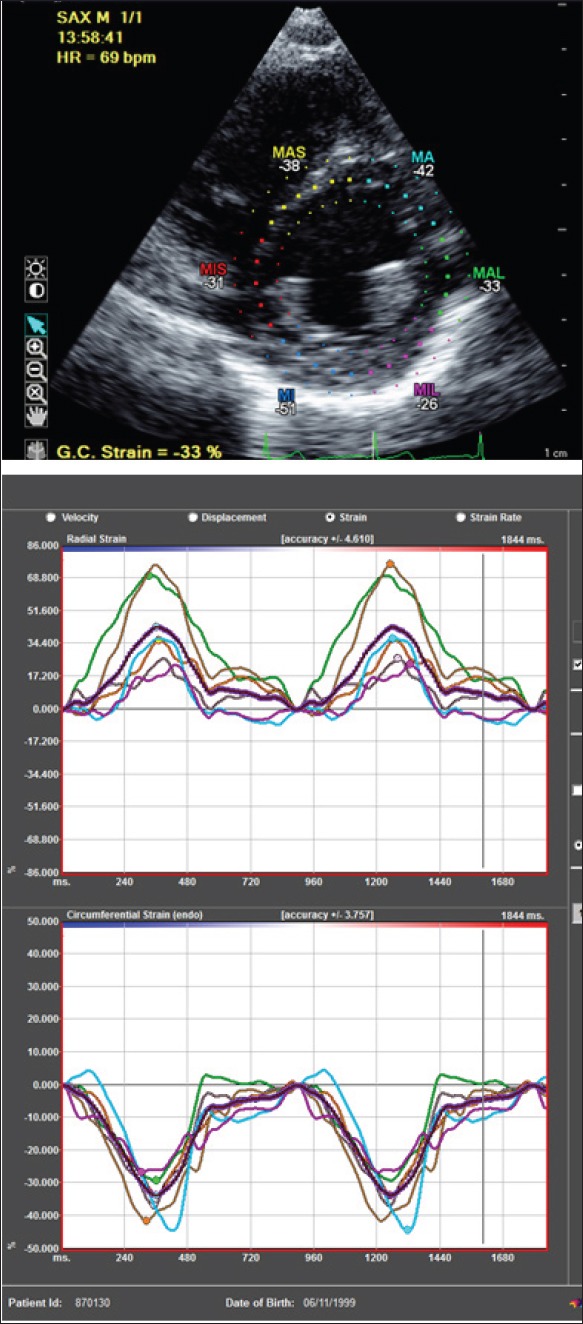
From short axis view, radial strain (upper panel) and circumferential strain (lower panel) can be calculated
Figure 4.
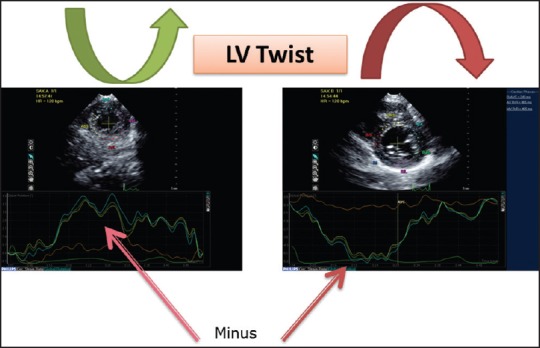
The apical mechanics is the result of the interaction between the subepicardial (ascendant) and the subendocardial (descendant) segments. Because of its larger radius, the ascendant segment overpowers the descent segment leading to a counterclockwise apical rotation and the smaller clockwise basal rotation. Practically, LV twist is calculated as the difference between the maximal rotation (positive) during systole of the apex and the maximal (negative) systolic rotation of the base
Speckle tracking
Originally strain imaging was derived by tissue Doppler myocardial velocities[1], but in recent years speckle tracking echocardiography (STE) has gained popularity and has now been widely accepted as the technique of choice.[10]
This modality is based on 2D echocardiographic technology, thus is relatively angle independent obviating the need for parallel alignment between the ultrasound beam and myocardial wall being studied.[11] The software enables to automatically perform the tracking demanding less post-processing.
After having been considered for many years as an undesirable characteristic of the image, speckles started to be employed as myocardial natural markers, capable of evaluation and quantification of the cardiac function in a reproducible, accurate and simple way [Figure 5].
Figure 5.
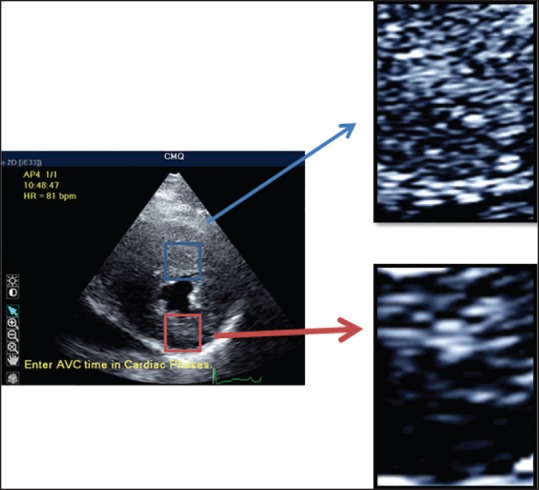
STE is based on the identification and on the tracking of speckles (spots generated by the interaction between the ultrasound beam and myocardial fibers) on routine 2-dimensional ultrasonic derived images. These spots, temporally stable, are used as natural acoustic markers, able to identify, as fingerprint, that specific myocardial segment and moving together with the tissue. Thus, the analysis of the spatial dislocation (tracking) of speckle represents the movement of that segment of tissue. From this, natural strain can be obtained and strain rate can be calculated as temporal derivative
STE is based on B-mode gray scale tracking of 2D “speckles.” Speckles are small dots or groups of myocardial pixels that are routinely created by the interaction of ultrasonic beams and the myocardium.[12] “Kernel” is a number of adjacent speckles clumped together with unique arrangements. This unique signature kernel is easy to track, just like a fingerprint that identifies the unique myocardial segment. Using the end-diastolic dimensions as a surrogate for original length and reference point, these speckles can be tracked as they move through the cardiac cycle to determine direction, speed and the distance of such movement, and thus the amount of deformation (i.e., strain). The derivative of strain will give the strain rate.
2D STE limitations
The bi-dimensional STE has several limitations:
frame rates are lower than tissue Doppler-derived strain (50-90 fps vs. 180-220 fps), which specifically hamper strain rate measurements.
Different post-processing algorithms and frequent software upgrades for 2D STE are used by different vendors giving rise to different values. Despite the name, some vendors rather than tracking the speckles, track user-defined points along the manually defined interface between endocardium and blood pool (edge tracking).
STE is critically dependent on 2D image quality.
STE provides the average value of deformation for each LV segment ignoring differences in myocardial deformation within the segment. In this regard, tissue Doppler derived myocardial deformation parameters are more sensitive to early myocardial abnormalities.
Rotation measurements are highly dependent on image acquisition. Transducer angulation can result in oblique short-axis views and the real apical short axis view can be difficult to obtain.
Out of plane motion of speckles throughout the cardiac cycle.
3D STE
The out of plane motion hampering 2D STE and the natural 3D deformation of the heart has raised interest for 3D STE.
Particularly, the new software requiring just one beat to acquire a full volume dataset for the analysis of all the chambers and all the segments are particularly promising.[2,13]
First-generation volume imaging system requires multiple beats to build the full volume data making it susceptible to stitching artifacts.
Advantages of 3D STE over 2D STE are:
The avoidance of LV foreshortening.
Simultaneous assessment of all strain and rotation parameters from a single 3D dataset.
No out of plane motion.
Data about reproducibility of 3D STE are rather conflictual with some claiming a better reproducibility than 2D STE and other showing less good results.[14,15] The variability of 3D STE measurements rely on the post-processing need for manual adjustment of endocardial and epicardial borders, which are less well-defined than in 2D.
3D STE limitations
A major disadvantage of 3D STE is its critical dependency on the quality of 2D images used for acquisition, even more than 2D STE because of its relatively low spatial resolution.
The tracking accuracy is limited by the relatively low temporal (volume rate) and spatial resolution. Especially in presence of higher heart rate (children, arrhythmias, etc.) the tracking accuracy significantly deteriorates, particularly for strain rate, twist, and torsion. Radial strain is estimated by tracking both the endocardial and epicardial borders and therefore is critically dependent on image quality and less reliable.
Discrepancies between different vendor platforms apply for 3D-STE-derived measurements too.[14] As for 2D STE, the obtained deformation parameters are an average of myocardial segment function overlooking local differences within the segment.
Clinical applications
The assessment of myocardial deformation properties has emerged as clinically useful in several cardiac diseases and especially for early detecting of subclinical cardiac abnormalities in conditions where standard echocardiography or myocardial velocity failed to identify early abnormalities, such as heart failure with preserved ejection fraction or cardiac abnormalities after chemotherapy.[16,17] However, global longitudinal strain is probably the only parameter that can be used in the routine clinical practice.
Atrial function
Several clinical studies demonstrated that the left atrial volume and size are both predictors of cardiovascular events.[18,19] Initial studies by using tissue Doppler-derived strain were very promising on the clinical application of this technique. The most interesting results have been obtained in the field of atrial fibrillation where atrial strain has emerged as a strong prognosticator for atrial fibrillation recurrence for its strong correlation with atrial fibrosis[20,21] But atrial strain demonstrated an important prognostic value also in patients with mitral valve diseases[22] or hypertrophic cardiomyopathy.[23]
DILATED CARDIOMYOPATHY
Longitudinal strain has demonstrated an additional predictive value in patients with dilated cardiomyopathy.[24,25] The application of this technique also provides new insight on cardiac mechanics of DCM patients showing opposite apical rotation in those with more severe dilatation.[26]
Hypertrophic cardiomyopathy
Myocardial deformation parameters are significantly impaired in hypertrophic cardiomyopathy patients[27] even in segment without hypertrophy[28] [Figure 6] and in presence of normal LV EF. The amount of myocardial deformation impairment is correlated with the amount of fibrosis[29] and some authors suggest a predictive value of longitudinal strain in hypertrophic cardiomyopathy patients to detect ventricular tachycardia.[30] These findings if confirmed in larger and multicenter study may help to better stratify hypertrophic cardiomyopathy patients for the risk of sudden death.
Figure 6.

Hypertrophic cardiomyopathy. During systole, the normal myocardial shortening is color-coded in yellow (as shown in the top panel corresponding to the apical segment). Conversely, the mid panel shows during systole abnormal myocardial deformation, suggestive of fibrosis and myocardial disarray, as demonstrated by the abnormal red and blue lines. Minor abnormalities showed in the basal panel (corresponding to the basal septum) suggesting some minor degree of fibrosis
Myocardial deformation analysis could be also of help to differentiate subgroup of cardiomyopathies exhibiting a peculiar myocardial deformation pattern[31]: Fabry's disease shows the double peak sign [consisting of an early systolic peak followed by a rapid fall and a second peak during the isovolumic relaxation phase indicative of post-systolic shortening on the strain rate curves[32]). - Friederich's ataxia: Marked and homogeneously reduced systolic and diastolic myocardial deformation properties even in non-hypertrophied segments. — Amyloidosis: Early and severe impairment in longitudinal deformation sparing the apex with normal radial and circumferential deformation.[31]
CORONARY ARTERY DISEASE
Myocardial deformation parameters are able to provide an objective and quantitative way of assessing regional myocardial function both at rest or during exercise test differentiating all the ischemic substrates.[1] Although speckle tracking analysis of regional myocardial deformation is best performed during dipyridamole challenge rather than other type of stress echocardiography because frame rate limitation, several reports demonstrated an additional value also during dobutamine challenge.[33]
Global longitudinal strain may predict infarct size better than LV EF as demonstrated by cardiac MRI.[34,35,36]
Valvular heart disease
The surgical timing is often difficult to define in asymptomatic patients with moderate to severe aortic regurgitation and normal ejection fraction. Several studies both in adults and in pediatrics demonstrated that peak systolic strain is able to detect early cardiac abnormalities and the prognosis of such patients is strongly correlated to the amount of myocardial deformation impairment.[37,38,39,40]
Similar results have been obtained in patients with mitral regurgitation by using LV strain and atrial strain.[40,41,42,43,44,45]
Patients with severe aortic stenosis (AS), normal LVEF still asymptomatic showed impairment in the longitudinal strain.[40,41,42,43,44,45,46,47,48]
All these findings clearly demonstrate that LVEF is not enough to identify subtle changes in myocardial function in the presence of significant changes in loading conditions.
Right ventricular evaluation
The right ventricular function is the Achilles’ heel in the echocardiographic evaluation. This is because of right ventricular complex geometry, heterogeneous morphology and function. In addition, the evaluation of right ventricular function is more clinically important in presence of abnormal loading conditions, which further challenge the application of traditional echo indices. Speckle tracking echocardiography overcomes all these limitations[49,50], as it is not dependent on geometrical assumptions, it can describe regional myocardial deformation patterns and is less load dependent (especially strain rate).
Congenital heart disease
Myocardial deformation assessment is particularly helpful in the evaluation of ventricular function in children with congenital heart disease[51], including assessment of the systemic right ventricle[52], single ventricles[53], and morphologically abnormal left ventricles[54] even in the presence of normal or supranormal ejection fraction. The evaluation of myocardial deformation properties has provided new insights into the pathophysiology of congenital heart disease and diagnostic and prognostic information otherwise not available by conventional echocardiography.[51]
CONCLUSIONS
Cardiac mechanics assessment by STE is a promising tool, considering its property of early diagnosis and prediction of events. We hypothesize that this semi-automated, noninvasive and low-cost methodology may shed light on the comprehension of the sophisticated cardiomyocyte physiology and also on the physiopathology of cardiac diseases.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sutherland GR, Di Salvo G, Claus P, D’hooge J, Bijnens B. Strain and strain rate imaging: A new clinical approach to quantifying regional myocardial function. J Am Soc Echocardiogr. 2004;17:788–802. doi: 10.1016/j.echo.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Seo Y, Ishizu T, Aonuma K. Current status of 3-dimensional speckle tracking echocardiography: A review from our experiences. J Cardiovasc Ultrasound. 2014;22:49–57. doi: 10.4250/jcu.2014.22.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckberg G, Hoffman JI, Nanda NC, Coghlan C, Saleh S, Athanasuleas C. Ventricular torsion and untwisting: Further insights into mechanics and timing interdependence: A viewpoint. Echocardiography. 2011;28:782–804. doi: 10.1111/j.1540-8175.2011.01448.x. [DOI] [PubMed] [Google Scholar]
- 4.Buckberg G, Hoffman JI, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: The relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–87. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- 5.Biswas M, Sudhakar S, Nanda NC, Buckberg G, Pradhan M, Roomi AU, et al. Two- and three-dimensional speckle tracking echocardiography: Clinical applications and future directions. Echocardiography. 2013;30:88–105. doi: 10.1111/echo.12079. [DOI] [PubMed] [Google Scholar]
- 6.Carreras F, Ballester M, Pujadas S, Leta R, Pons-Llado G. Morphological and functional evidences of the helical heart from non-invasive cardiac imaging. Eur J Cardiothorac Surg. 2006;29:S50–5. doi: 10.1016/j.ejcts.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 7.Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation. 2000;102:1158–64. doi: 10.1161/01.cir.102.10.1158. [DOI] [PubMed] [Google Scholar]
- 8.Saito M, Okayama H, Yoshii T, Higashi H, Morioka H, Hiasa G, et al. Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2012;13:617–23. doi: 10.1093/ejechocard/jer318. [DOI] [PubMed] [Google Scholar]
- 9.D’Hooge J, Heimdal A, Jamal F, Kukulski T, Bijnens B, Rademakers F, et al. Regional strain and strain rate measurements by cardiac ultrasound: Principles, implementation and limitations. Eur J Echocardiogr. 2000;1:154–70. doi: 10.1053/euje.2000.0031. [DOI] [PubMed] [Google Scholar]
- 10.Feigenbaum H, Mastouri R, Sawada S. A Practical approach to using strain echocardiography to evaluate the left ventricle. [Last cited 2014 Aug 3];Circ J. 2012 76:1550–5. doi: 10.1253/circj.cj-12-0665. Available from: http://japanlinkcenter.org/DN/JST.JSTAGE/circj/CJ-12-0665?lang=en&from=CrossRef&type=abstract . [DOI] [PubMed] [Google Scholar]
- 11.Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: Definition of normal range. JACC Cardiovasc Imaging. 2009;2:80–4. doi: 10.1016/j.jcmg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J Am Soc Echocardiogr. 2010:351–69. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Urbano-Moral JA, Patel AR, Maron MS, Arias-Godinez JA, Pandian NG. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography. 2012:997–1010. doi: 10.1111/j.1540-8175.2012.01773.x. [DOI] [PubMed] [Google Scholar]
- 14.Gayat E, Ahmad H, Weinert L, Lang RM, Mor-Avi V. Reproducibility and inter-vendor variability of left ventricular deformation measurements by three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2011;24:878–85. doi: 10.1016/j.echo.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Xu TY, Sun JP, Lee AP, Yang XS, Qiao Z, Luo X, et al. Three-dimensional speckle strain echocardiography is more accurate and efficient than 2D strain in the evaluation of left ventricular function. Int J Cardiol. 2014;176:360–6. doi: 10.1016/j.ijcard.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Iarussi D, Di Salvo G, Pergola V, Coppolino P, Tedesco MA, Ratti G, et al. Pulsed Doppler tissue imaging and myocardial function in thalassemia major. Heart Vessels. 2003;18:1–6. doi: 10.1007/s003800300000. [DOI] [PubMed] [Google Scholar]
- 17.Imbalzano E, Zito C, Carerj S, Oreto G, Mandraffino G, Cusmà-Piccione M, et al. Left ventricular function in hypertension: New insight by speckle tracking echocardiography. Echocardiography. 2011;28:649–57. doi: 10.1111/j.1540-8175.2011.01410.x. [DOI] [PubMed] [Google Scholar]
- 18.Tedesco MA, Di Salvo G, Ratti G, Natale F, Iarussi D, Iacono A. Left atrial size in 164 hypertensive patients: An echocardiographic and ambulatory blood pressure study. Clin Cardiol. 2001;24:603–7. doi: 10.1002/clc.4960240907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ancona R, Comenale Pinto S, Caso P, D’Andrea A, Di Salvo G, Arenga F, et al. Left atrium by echocardiography in clinical practice: From conventional methods to new echocardiographic techniques. ScientificWorldJournal 2014. 2014 doi: 10.1155/2014/451042. 451042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Salvo G, Caso P, Lo Piccolo R, Fusco A, Martiniello AR, Russo MG, et al. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: A color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation. 2005;112:387–95. doi: 10.1161/CIRCULATIONAHA.104.463125. [DOI] [PubMed] [Google Scholar]
- 21.Longobardo L, Todaro MC, Zito C, Piccione MC, Di Bella G, Oreto L, et al. Role of imaging in assessment of atrial fibrosis in patients with atrial fibrillation: State-of-the-art review. Eur Hear J Cardiovasc Imaging. 2013;15:1–5. doi: 10.1093/ehjci/jet116. [DOI] [PubMed] [Google Scholar]
- 22.Ancona R, Comenale Pinto S, Caso P, Di Salvo G, Severino S, D’Andrea A, et al. Two-dimensional atrial systolic strain imaging predicts atrial fibrillation at 4-year follow-up in asymptomatic rheumatic mitral stenosis. J Am Soc Echocardiogr. 2013;26:270–7. doi: 10.1016/j.echo.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Roca M, Popescu BA, Beladan CC, Clin A, Muraru D, Popa EC, et al. Left atrial dysfunction as a correlate of heart failure symptoms in hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2010;23:1090–8. doi: 10.1016/j.echo.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Haugaa KH, Goebel B, Dahlslett T, Meyer K, Jung C, Lauten A, et al. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J Am Soc Echocardiogr. 2012;25:667–73. doi: 10.1016/j.echo.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Jasaityte R, Dandel M, Lehmkuhl H, Hetzer R. Prediction of short-term outcomes in patients with idiopathic dilated cardiomyopathy referred for transplantation using standard echocardiography and strain imaging. Transplant Proc. 2009;41:277–80. doi: 10.1016/j.transproceed.2008.10.083. [DOI] [PubMed] [Google Scholar]
- 26.Popescu BA, Beladan CC, Caˇlin A, Muraru D, Deleanu D, Roşca M, et al. Left ventricular remodelling and torsional dynamics in dilated cardiomyopathy: Reversed apical rotation as a marker of disease severity. Eur J Heart Fail. 2009;11:945–51. doi: 10.1093/eurjhf/hfp124. [DOI] [PubMed] [Google Scholar]
- 27.Weidemann F, Mertens L, Gewillig M, Sutherland GR. Quantitation of localized abnormal deformation in asymmetric nonobstructive hypertrophic cardiomyopathy: A velocity, strain rate, and strain Doppler myocardial imaging study. Pediatr Cardiol. 2001;22:534–7. doi: 10.1007/s002460010293. [DOI] [PubMed] [Google Scholar]
- 28.Carasso S, Yang H, Woo A, Vannan MA, Jamorski M, Wigle ED, et al. Systolic myocardial mechanics in hypertrophic cardiomyopathy: Novel concepts and implications for clinical status. J Am Soc Echocardiogr. 2008;21:675–83. doi: 10.1016/j.echo.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Popović ZB, Kwon DH, Mishra M, Buakhamsri A, Greenberg NL, Thamilarasan M, et al. Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhancement magnetic resonance imaging. J Am Soc Echocardiogr. 2008;21:1299–305. doi: 10.1016/j.echo.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Di Salvo G, Pacileo G, Limongelli G, Baldini L, Rea A, Verrengia M, et al. Non sustained ventricular tachycardia in hypertrophic cardiomyopathy and new ultrasonic derived parameters. J Am Soc Echocardiogr. 2010;23:581–90. doi: 10.1016/j.echo.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Fadel B, Baldini L, Pergola V, Al Bulbul Z, Di Salvo G. Myocardial deformation imaging and rare cardiomyopathies with hypertrophic phenotype: A review focused on Fabry disease, Friedreich ataxia and amyloidosis. Cardiogenetics. 2013. [Last cited on 2014 Sep 16]. p. 3. Available from: http://www.pagepressjournals.org/index.php/cardiogen/article/view/528 .
- 32.Weidemann F, Störk S, Herrmann S, Ertl G, Niemann M. The various forms of left ventricular hypertrophy: Diagnostic value of echocardiography. Herz. 2011;36:713–23. doi: 10.1007/s00059-010-3416-1. [DOI] [PubMed] [Google Scholar]
- 33.Hanekom L, Cho GY, Leano R, Jeffriess L, Marwick TH. Comparison of two-dimensional speckle and tissue Doppler strain measurement during dobutamine stress echocardiography: An angiographic correlation. Eur Heart J. 2007;28:1765–72. doi: 10.1093/eurheartj/ehm188. [DOI] [PubMed] [Google Scholar]
- 34.Munk K, Andersen NH, Nielsen SS, Bibby BM, Bøtker HE, Nielsen TT, et al. Global longitudinal strain by speckle tracking for infarct size estimation. Eur J Echocardiogr. 2011;12:156–65. doi: 10.1093/ejechocard/jeq168. [DOI] [PubMed] [Google Scholar]
- 35.Zahid W, Eek CH, Remme EW, Skulstad H, Fosse E, Edvardsen T. Early systolic lengthening may identify minimal myocardial damage in patients with non-ST-elevation acute coronary syndrome. Eur Heart J Cardiovasc Imaging. 2014 doi: 10.1093/ehjci/jeu101. [DOI] [PubMed] [Google Scholar]
- 36.Bière L, Donal E, Terrien G, Kervio G, Willoteaux S, Furber A, et al. Longitudinal strain is a marker of microvascular obstruction and infarct size in patients with acute ST-segment elevation myocardial infarction. PLoS One. 2014;9:e86959. doi: 10.1371/journal.pone.0086959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C, Li C, Bai W, Zhang X, Tang H, Qing Z, et al. Value of three-dimensional speckle-tracking in detecting left ventricular dysfunction in patients with aortic valvular diseases. J Am Soc Echocardiogr. 2013;26:1245–52. doi: 10.1016/j.echo.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 38.Kusunose K, Agarwal S, Marwick TH, Griffin BP, Popović ZB. Decision making in asymptomatic aortic regurgitation in the era of guidelines: Incremental values of resting and exercise cardiac dysfunction. Circ Cardiovasc Imaging. 2014;7:352–62. doi: 10.1161/CIRCIMAGING.113.001177. [DOI] [PubMed] [Google Scholar]
- 39.Kaneko A, Tanaka H, Onishi T, Ryo K, Matsumoto K, Okita Y, et al. Subendocardial dysfunction in patients with chronic severe aortic regurgitation and preserved ejection fraction detected with speckle-tracking strain imaging and transmural myocardial strain profile. Eur Heart J Cardiovasc Imaging. 2013;14:339–46. doi: 10.1093/ehjci/jes160. [DOI] [PubMed] [Google Scholar]
- 40.Di Salvo G, D’Aiello AF, Castaldi B, Fadel B, Limongelli G, D’Andrea A, et al. Early left ventricular abnormalities in children with heterozygous familial hypercholesterolemia. J Am Soc Echocardiogr. 2012;25:1075–82. doi: 10.1016/j.echo.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Candan O, Ozdemir N, Aung SM, Hatipoglu S, Karabay CY, Guler A, et al. Atrial longitudinal strain parameters predict left atrial reverse remodeling after mitral valve surgery: A speckle tracking echocardiography study. Int J Cardiovasc Imaging. 2014;30:1049–56. doi: 10.1007/s10554-014-0433-9. [DOI] [PubMed] [Google Scholar]
- 42.Pandis D, Sengupta PP, Castillo JG, Caracciolo G, Fischer GW, Narula J, et al. Assessment of longitudinal myocardial mechanics in patients with degenerative mitral valve regurgitation predicts postoperative worsening of left ventricular systolic function. J Am Soc Echocardiogr. 2014;27:627–38. doi: 10.1016/j.echo.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Ring L, Rana BS, Wells FC, Kydd AC, Dutka DP. Atrial function as a guide to timing of intervention in mitral valve prolapse with mitral regurgitation. JACC Cardiovasc Imaging. 2014;7:225–32. doi: 10.1016/j.jcmg.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Yang LT, Shih JY, Liu YW, Li YH, Tsai LM, Luo CY, et al. Effects of left atrial strain on functional capacity in chronic severe mitral regurgitation. Int J Cardiol. 2013;168:e151–3. doi: 10.1016/j.ijcard.2013.08.070. [DOI] [PubMed] [Google Scholar]
- 45.Zito C, Carerj S, Todaro MC, Cusmà-Piccione M, Caprino A, Di Bella G, et al. Myocardial deformation and rotational profiles in mitral valve prolapse. Am J Cardiol. 2013;112:984–90. doi: 10.1016/j.amjcard.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann R, Altiok E, Friedman Z, Becker M, Frick M. Myocardial deformation imaging by two-dimensional speckle-tracking echocardiography in comparison to late gadolinium enhancement cardiac magnetic resonance for analysis of myocardial fibrosis in severe aortic stenosis. Am J Cardiol. 2014;114:1083–8. doi: 10.1016/j.amjcard.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Bartko PE, Heinze G, Graf S, Clavel MA, Khorsand A, Bergler-Klein J, et al. Two-dimensional strain for the assessment of left ventricular function in low flow-low gradient aortic stenosis, relationship to hemodynamics, and outcome: A substudy of the multicenter TOPAS study. Circ Cardiovasc Imaging. 2013;6:268–76. doi: 10.1161/CIRCIMAGING.112.980201. [DOI] [PubMed] [Google Scholar]
- 48.Zito C, Salvia J, Cusmà-Piccione M, Antonini-Canterin F, Lentini S, Oreto G, et al. Prognostic significance of valvuloarterial impedance and left ventricular longitudinal function in asymptomatic severe aortic stenosis involving three-cuspid valves. Am J Cardiol. 2011;108:1463–9. doi: 10.1016/j.amjcard.2011.06.070. [DOI] [PubMed] [Google Scholar]
- 49.Bussadori C, Salvo GD, Pluchinotta FR, Piazza L, Gaio G, Russo MG, et al. Evaluation of right ventricular function in adults with congenital heart defects. Echocardiography. 2014 doi: 10.1111/echo.12566. [DOI] [PubMed] [Google Scholar]
- 50.D’Andrea A, Caso P, Bossone E, Scarafile R, Riegler L, Di Salvo G, et al. Right ventricular myocardial involvement in either physiological or pathological left ventricular hypertrophy: An ultrasound speckle-tracking two-dimensional strain analysis. [Last cited on 2014 Sep 18];Eur J Echocardiogr. 2010 11:492–500. doi: 10.1093/ejechocard/jeq007. Available from: http://ehjcimaging.oxfordjournals.org/cgi/content/long/jeq007v1 . [DOI] [PubMed] [Google Scholar]
- 51.Friedberg MK, Mertens L. Deformation imaging in selected congenital heart disease: Is it evolving to clinical use? J Am Soc Echocardiogr. 2012;25:919–31. doi: 10.1016/j.echo.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Di Salvo G, Pacileo G, Rea A, Limongelli G, Baldini L, D’Andrea A, et al. Transverse strain predicts exercise capacity in systemic right ventricle patients. Int J Cardiol. 2010;145:193–6. doi: 10.1016/j.ijcard.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 53.Pacileo G, Di Salvo G, Rea A, Calabrò R. Findings from new echocardiographic techniques concerning ventricular remodelling and function in the functionally univentricular heart. Cardiol Young. 2005;15(Suppl 3):45–50. doi: 10.1017/S1047951105001630. [DOI] [PubMed] [Google Scholar]
- 54.Di Salvo G, Pacileo G, Limongelli G, Verrengia M, Rea A, Santoro G, et al. Abnormal regional myocardial deformation properties and increased aortic stiffness in normotensive patients with aortic coarctation despite successful correction: An ABPM, standard echocardiography and strain rate imaging study. Clin Sci (Lond) 2007;113:259–66. doi: 10.1042/CS20070085. [DOI] [PubMed] [Google Scholar]