Abstract
Bicuspid aortic valve (BAV) cannot be considered an innocent finding, but it is not necessarily a life-threatening condition. Athletes with BAV should undergo a thorough staging of the valve anatomy, taking into consideration hemodynamic factors, as well as aortic diameters and looking for other associated significant cardiovascular anomalies by use of a multimodality cardiac imaging approach. Furthermore an accurate follow-up is mandatory with serial cardiological controls in those allowed to continue sports.
Keywords: Athlete's heart, bicuspid aortic valve, cardiac magnetic resonance, computed tomography, echocardiography, multimodality imaging, power, sports training, endurance
ANATOMY AND CLASSIFICATION
The bicuspid aortic valve (BAV) is the most common congenital cardiac malformation, affecting 0.5-2% of the general population.[1,2,3,4,5] Compared to the normally formed aortic valve, it features two unequally sized cusps (leaflets) with or without a raphe, generally located in the largest leaflet. The raphe is believed to correspond to the area of congenital fusion between two rudimentary cusps, may vary in extension along the leaflet, and it does not contain valvular tissue. Several anatomical classifications of the BAV have been proposed, based on the pattern of cusp fusion (s), presence of the raphe, and commissural orientation.[6,7] The most common form is characterized by fusion of the left coronary and right coronary cusps (type 1 BAV or RL-BAV), also referred to as anteroposterior at transthoracic echocardiography (TTE); the fusion of the right coronary and noncoronary cusps is called type 2 or atypical or RN-BAV (right-left orientation at TTE); and the rarest form is fusion of the left and noncoronary cusps (type 3 or LN-BAV). According to another nomenclature, the “type” indicates the number of fusions, so that type 0 is the “naturally bicuspid” valve with only two equally sized cusps and no raphe, type 1 is the more common BAV (subdivided into RL, RN, and LN), and type 2 corresponds to the unicuspid aortic valve, with two fusions/raphes [Figure 1].[1,2,3,4,5,6,7]
Figure 1.
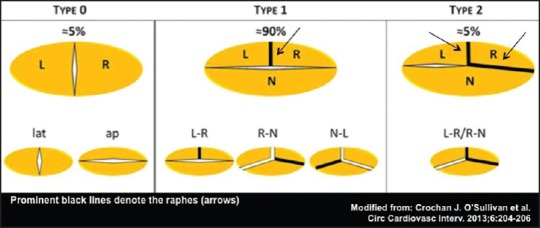
Anatomic classification of bicuspid aortic valve (BAV). L = Left, R = Right, N = Noncoronary, lat = Lateral, ap = Anteroposterior
MORPHOGENESIS AND HEREDITY
The events that lead to bicuspid malformation during human valvulogenesis are not yet fully understood: Animal model studies have suggested that aberrations in different signaling pathways involving endothelial-to-mesenchymal transition, neural crest cell migration, and septation of the conotruncus may subtend the abovementioned different anatomical forms.[8] BAV is inherited as an autosomal dominant trait with incomplete penetrance and variable expressivity: Many clinical studies have reported an increased prevalence (about 9%) in the first degree relatives (FDRs) of subjects with BAV, with an inheritability of about 89%.[9,10,11,12] Therefore, the current American College of Cardiology (ACC)/American Heart Association (AHA) guidelines recommend echocardiographic screening in all FDRs of patients with BAV.[13]
COMPLICATIONS AND TREATMENT
BAV can be either isolated or associated with other congenital anomalies: Among patients with aortic coarctation, a BAV is found in 50-75% cases: The diagnosis of BAV in a patient with known coarctation is crucial, as it implies a relevant increase of the risk for aortic complications.[14,15,16] BAV can be also associated with hypoplastic left heart syndrome, Shone syndrome, Williams’ syndrome, and Turner syndrome with coarctation.[17,18,19,20,21] A wide spectrum of cardiovascular complications, occurring either in pediatric age or in the adult life, is associated with congenital BAV, including valvular (stenosis, regurgitation, and endocarditis) and vascular (aortic aneurysm, annuloaortic ectasia, and acute aortic dissection) complications; so that the BAV represents a socially important source of morbidity for the healthcare system. An estimation of the actual incidence of such cardiovascular complications has been object of a number of studies, often yielding different figures; depending on referral bias, study period, mean age of the patients, diagnostic accuracy, etc. By screening about 21,000 Italian military conscripts (mean age 18 ± 2 years), Nistri and coworkers identified 167 BAVs (0.8%), of which 48 (29%) were normofunctional; whereas, 110 (66%) were regurgitant (moderate-severe degree of regurgitation in 20 patients) and only nine (5%) were stenotic.[22] This is remarkably different from the high prevalence of aortic stenosis (AS) that is reported in surgical series (up to 75% of BAVs), in which the mean age of the patients is generally older. The BAV indeed accounts for more than 50% of the cases of AS requiring surgery in adult age, a percentage that increases when the younger ages are considered selectively.[23] In fact, AS reaches the severity criteria for surgical indication 5–10 years earlier than in subjects with tricuspid aortic valve (TAV). Studies of natural history have been issued in recent years, ultimately depicting the clinical course of BAV-associated disease in the general population, reducing the referral bias to a minimum: Both in a study from the Olmsted County (Minnesota)[24] and in another series from Toronto (Ontario);[25] a relatively high rate of surgical operation was found, but lethal events were rare and life expectancy was not different from the general age-matched population. The presence of a BAV is also associated with an aortopathy that places the patients at increased risk for aneurysm formation (generally at the level of the ascending aorta) and dissection (generally type A). A dilatation of the ascending aorta has been reported in percentages between 33 and 80% of BAV patients, again depending on patient age and type of referral: Given the high prevalence of BAV in the cardiac surgical patient population, the clinician is quite frequently faced with the dilemma whether to treat a concomitant nonsevere aortic dilatation in a patient with surgical indications for bicuspid valve disease.[26] In such a situation it has been recently demonstrated that different surgeons use to adopt different policies, often even deviating from the current guidelines, some with a more aggressive attitude than officially recommended, some more conservatively. The question of how to manage a moderately dilated ascending aorta in patients with BAV, with or without valve dysfunction, remains debated mainly because of the unresolved questions and persisting gaps in knowledge about the pathogenesis of the BAV aortopathy.[27] Aortic wall alterations observed with BAV, commonly included among those forms of aortic pathology defined as “medial degeneration”, have led several authors to theorize that a common genetic defect may affect both valve and aorta development. Nevertheless a responsible gene has never been identified and only negative studies have been issued when possible linkages have been investigated with mutations in supposedly involved loci. Opposed to the genetic theory, the “hemodynamic” theory has been supported by others, whereby the peculiar aortic wall fragility could be caused by undue mechanical burden on the aortic wall persisting since first moments in life even in the absence of (and before the development of) significant stenosis or regurgitation. It has been demonstrated that BAV anatomy yields flow derangements that are more than enough to cause abnormal aortic wall shear stress even in the absence of transvalvular gradients.[28] The dichotomy of the pathogenetic theories translates into the abovementioned inconsistencies between official guidelines and the policies of individual cardiac surgery centers. At present, indications are fundamentally based on dimensional criteria (aortic diameter, either in absolute terms or normalized to anthropometrics). However, the most recent literature has focused on the heterogeneity of BAV condition, especially in terms of risk of valve dysfunction and of aortic aneurysm or dissection: Some patients remain free from complications for their entire lifetime, others experience much worse natural histories.[29,30] In the face of this prognostic complexity, the diameter alone appears to be a limited criterion and the research on possible additional clinical, imaging-based risk markers, and biomarkers of BAV aortopathy severity is currently notably active.[27,31] A recently proposed theory suggests that the heterogeneity of anatomical forms of aortic dilatation that can be encountered in association with BAV may reflect an heterogeneity in terms of pathogenesis and prognosis, that is, the two main different anatomical forms of dilatation (or “aortic phenotypes”) might be subtended by different combinations of genetic defects and hemodynamic derangements. The “root phenotype”, accounting for 20% of aortic dilatations with BAV, mainly observed in young male patients with pure aortic regurgitation (AR) or normally functioning valve, is characterized by greater dilatation of the sinuses of Valsalva compared to the tubular ascending tract: This form has been found associated with faster growth of the aortic diameter over time, higher rates of postoperative aortic complications following simple aortic valve replacement, greater aortic diameters in the FDRs with TAV, and is supposed to be linked to a greater risk of aortic dissection.[32,33,34,35] The more common “ascending phenotype” has a more variable natural history, but generally is characterized by more indolent progression and lower risk of complications.[33]
EXERCISE PERFORMANCE IN PATIENTS WITH BAV
Athlete's heart: Cardiovascular adaptations and diagnostic findings
Long-term intensive physical training determines progressive cardiac changes, characterized by modifications in cavity diameters, wall thickness, and functional parameters; which represent the so-called athlete's heart.[36,37,38]
Standard color-Doppler echocardiography and, in selected cases, cardiac magnetic resonance (CMR) have been widely used in the analysis of the characteristics of the athlete's heart, becoming sometimes irreplaceable in the evaluation of top-level athletes. Furthermore, novel echocardiographic technologies, like Doppler myocardial imaging (DMI) and strain imaging have shown their usefulness also in athlete's heart description and understanding, mostly because of their ability to earlier detection of myocardial systolic and diastolic dysfunction.
Athlete's left heart
The echocardiographic analysis of the athlete's left ventricle has shown different forms of adaptation, according to genetic factors, age, and mostly to the type and the intensity of chronic training. Isotonic exercise such as in endurance sports is the most responsible for a predominant volume overload, inducing an increase in left ventricular (LV) mass and end-diastolic diameter (eccentric LV hypertrophy); while isometric exercise, such as in strength disciplines, is instead associated with an increase of LV mass and wall thickness (concentric LV hypertrophy).[36,37,38] However, the hypertrophy patterns often show mixed characteristics of eccentric or concentric hypertrophy depending to the type and level of the sports activity. Some reports and different studies in fact showed that 55% of the athletes had an increased LV end-diastolic diameter on echocardiography. Interestingly, about 15% of the endurance athletes demonstrated cavity diameters >60 mm, in presence of normal cardiac function, in contrast with the impairment of both diastolic and systolic indexes typical of dilated cardiomyopathy (DCM).[39] Similarly, the maximum thickness of the interventricular septum (IVS) was lower than 12 mm in most of the athletes, and only 2% of them had a wall thickness between 13 and 16 mm, obviously depending onage, gender, and race.[40] Interestingly, most of the adaptations induced by physical training seem to regress after temporary suspension of training of only few weeks. In only 20% of the cases, however, a persistence of the dilation in the LV end-diastolic diameter was noted.[41] Of note, in athletes abusing of anabolic steroids, the development of LV myocardial thickening does not seem to be reversible, even after discontinuation of the drug.[42,43] By standard Doppler analysis, one of the peculiar characteristics of the athlete's heart is the normality of both systolic and diastolic LV functional indexes. In particular, athlete's transmitral flow pattern demonstrates a “supernormal” aspect with an increased contribution of early-diastolic phase in baseline conditions (early wave/atrial wave (E/A) ratio >2). This is a difference of pathological from physiological hypertrophy. By DMI, the “supernormal” diastolic function of the athlete has been confirmed, with high early-diastolic myocardial velocity (Em), and increased Em/Am ratio of the basal IVS as well as of the lateral walls.[44,45] In recent years, some studies have analyzed athlete's LV hypertrophy also by strain imaging, finding no significant differences in both systolic and diastolic strain parameters compared with control subjects.[46,47,48] CMR can provide detailed anatomical and functional information in the assessment of athlete's heart and its differential diagnosis. From these images highly accurate and reproducible measurements of LV volumes, mass, ejection fraction, and stroke volumes can be derived.[49,50,51,52,53,54,55,56] In addition, the high resolution and image contrast allow the detection of localized areas of pathology and fibrosis, including the apex or the basal anteroseptal wall. Sport activities are also responsible of left atrial (LA) size remodeling.[57,58] LA enlargement is common in a lot of athletes, and this modification does not regress completely after cessation of athletic activity and may predispose athletes to an increased risk of subsequent atrial fibrillation.[59] Pelliccia et al., for the first time studied the prevalence and the clinical significance of LA enlargement in competitive athletes.[57] They reported a mild increase of LA diameter (≥40 mm) in 18% of athletes and a marked dilatation (≥45 mm) in 2%. Our group performed a longitudinal study that provided reference values for LA volume index, confirming a higher prevalence of LA volume index increase in athletes: Mild enlargement in 24.3% and moderate enlargement in 3.2%. The most powerful independent determinants of LA volume index were type and duration of training and LV end-diastolic volume.[58]
Athlete's right heart
Like all four chambers of the athlete's heart, the right atrium (RA) and right ventricle (RV) undergoes functional, structural, and electrical remodeling as a result of the hemodynamic challenges of intense exercise training.[60,61] Some research suggests that the hemodynamic stress of intense exercise is greater for the right heart and, as a result, remodeling is slightly more profound than the left heart. The clinical consequence of this is that the right heart of the athlete can become quite profoundly dilated which may present issues in differentiating healthy remodeling from pathologies such as left-to-right shunts, arrhythmogenic right ventricular cardiomyopathy (ARVC), and DCM. During exercise, however, pulmonary artery pressures and right ventricular after load increase disproportionately relative to the systemic circulation.[62,63] Instead, normally, the right ventricular mass is approximately one-fifth that of the LV, reflecting the fact that the load of the pulmonary circulation is markedly less at rest. As a result, right ventricular wall stress exceeds that of the LV making it more susceptible to acute injury during prolonged endurance exercise.[64,65,66,67,68] Endurance training, more than strength training, due enlargement of cavity dimensions and increases of wall thickness of the size of right atrium (RA) and RV.[69,70,71,72,73] The degree of remodeling is similar to that of the left heart, leading to the concept of ‘balanced remodeling’.[74,75] However, some recent work has suggested that regular exercise may be associated with greater relative remodeling of the RV as has been shown in large studies of nonathletes and in smaller studies of endurance athletes, possibly reflecting the slight excess in wall stress to which the RV is exposed.[76,77] In routine clinical practice, the slight differences in the relative remodeling of the RV and LV are minimal. Clinicians will be familiar with the appearance in athletes of a volume loaded RV with some degree of septal flattening, “pouching” of the RV free wall below the lateral tricuspid annulus, and a pseudoaneurysmal appearance of RV free wall either side of where the moderator band attaches. All these features are commonly seen in healthy endurance athletes and do not represent pathology. One of the challenges in clinical practice has been to accurately measure RV size due to the complex three-dimensional (3D) structure of this chamber and the use of CMR and 3D echocardiography may make this more practicable in the future. D’Andrea et al., have provided some important initial experience in what constitutes the range of RV volumes in healthy athletes using 3D echocardiography.[72]
BAV AND PHYSICAL EXERCISE
Physical activity is a well-known protective factor for a multitude of pathological conditions. For this reason, national and international health organizations promote regular physical training among younger as well as older people. In this way, young athletes are examined by sports physicians and, an increasing number of them who have BAV, which is often asymptomatic, are diagnosed. However, as BAV can be associated with AS and/or AR, as well as with primitive or secondary aortic dilation, sports physicians are strongly involved in the decision about their eligibility to participate in sports. There is growing awareness that many patients with BAVs have disorders of vascular connective tissue, involving loss of elastic tissue, that may result in aortic root dilatation even in the absence of hemodynamically significant AS or AR.[78,79] Indeed, patients are prone to develop dilatation of the aortic root (right–left fusion) or of the ascending aorta/aortic arch (right-noncoronary cusp fusion).[80,81] Actually, a well-documented link exists between hypertension and aortic dilation in subjects with a normal TAV.[82,83] On the other hand, a greater prevalence of hypertension than expected for age-matched populations has been reported among young subjects suffering from aortic dissection.[84] Therefore, a facilitating role of high blood pressure (BP) values on aortic enlargement, and possibly dissection, can be hypothesized in subjects with BAV. Longitudinal studies in subjects with BAV are limited to competitive athletes and they show slow progression in LV and ascending aortic diameters with regular training compared to those athletes with TAV.[85] However, no data is available on strict endurance training programs. Several studies carried out in patients without aortic valve malfunction have shown that various kinds of endurance training are able to improve the elastic properties of the aorta.[86,87,88,89,90] We performed a study about aortic root dimensions in elite athletes which demonstrated that diameters at all levels are significantly greater in strength-trained athletes.[91] Even though there is no definitive proof in patients with BAV, it could be argued that moderate endurance training should be promoted in patients with BAV after AS, AR, aortic aneurysm, and coarctation are ruled out.
ELIGIBILITY IN COMPETITIVE SPORTS
In order to take any decision about sports eligibility, sports physicians should perform an initial accurate staging of the BAV; taking into account hemodynamic factors, aortic complications, and associated significant cardiovascular anomalies. A strict follow-up, with serial cardiological controls, is mandatory. Since rapid valve deterioration and progressive severe aortic dilation have been documented; complete cardiological examinations, comprising at least electrocardiogram (ECG), bi-dimensional echocardiography (2DE) with color-Doppler analysis, ECG stress test, and, in selected cases, BP monitoring and 24-h Holter ECG monitoring, should be required every year in athletes allowed to continue sports, even in subjects with a ‘near normal’ or uncomplicated BAV.[92,93] Until the late 1970s, in the majority of cases, BAV was an occasional finding at surgical inspection or necropsy. With the advent of 2DE, an early in vivo diagnosis has become feasible and the current modern imaging techniques (i. e., magnetic resonance, computed tomography, etc.) allow the collection of novel useful data on thoracic aorta in subjects with BAV. Clinically, suspicion of BAV may arise due to the presence of an ejection click at the base of the heart that can be linked to a systolic and/or diastolic murmur. Familial history may be helpful since in an increasing number of cases this condition appears heritable.[9,10,12] ECG abnormalities, such as LV hypertrophy, atrial enlargement, and arrhythmias can be associated with a BAV, but are a specific.
Arterial pulse examination and BP measurement at rest and during exercise may be of use, in order to recognize hypertensive or hypotensive conditions due to associated cardiovascular anomalies, such as severe AS and/or insufficiency, or aortic coarctation. Nevertheless, unequivocal diagnosis of BAV can be made by the presence of only two cusps, clearly identified in systole and diastole on 2DE in the parasternal short-axis view, following precise criteria.[94,95] Once a BAV condition has been diagnosed, it is of paramount importance to assess the presence or absence and the entity of valvular obstruction/incompetence by means of color-Doppler analysis. Moreover, as patients with BAV, independently from associated cardiovascular abnormalities and/or complications, may exhibit dilation of the aorta, aortic diameters should be measured at different levels [Figures 2–6].[92,93]
Figure 2.
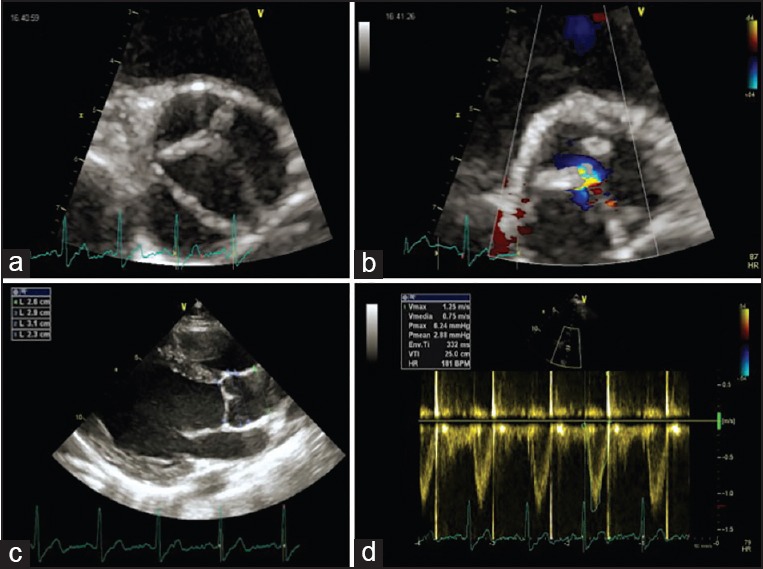
Transthoracic echocardiography in uncomplicated type 1 BAV. Aortic short-axis view (a), mild aortic regurgitation (b), normal ascending aorta diameters (c), and absence of significant aortic stenosis (d)
Figure 6.
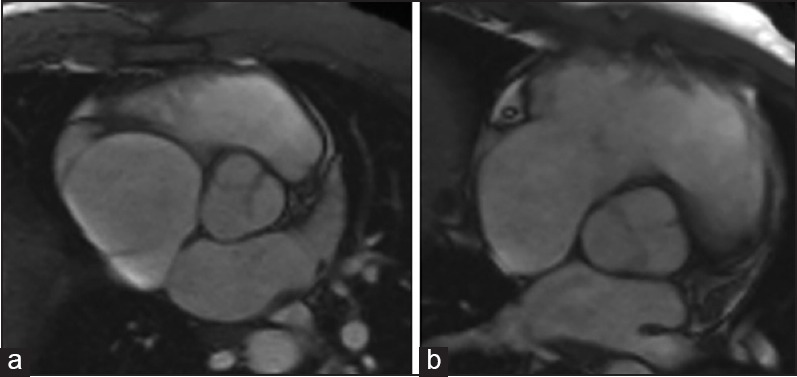
Aortic magnetic resonance in type 1 BAV with complete (a) and uncomplete (b) raphe
Figure 3.
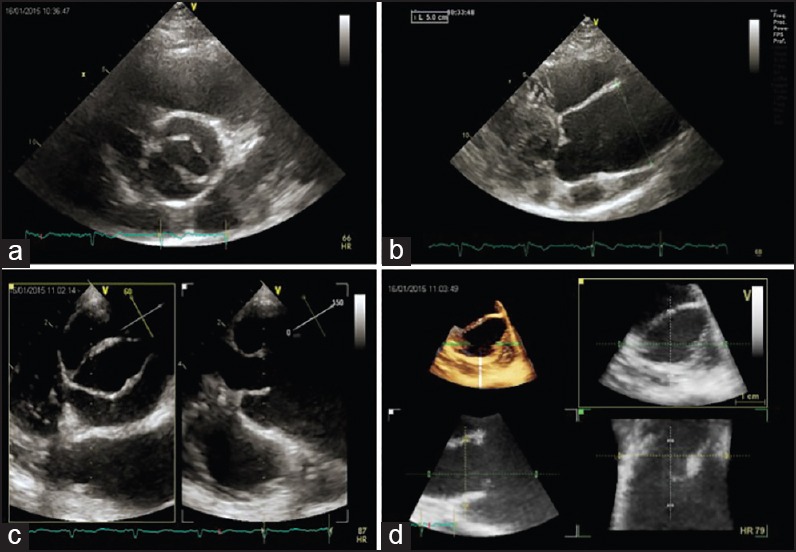
Transthoracic and transesophageal echocardiography in complicated type 1 BAV. Aortic short-axis view (a), parasternal long-axis view with ascending aorta aneurism (b), and three-dimensional reconstruction of aortic valve (c-d)
Figure 4.
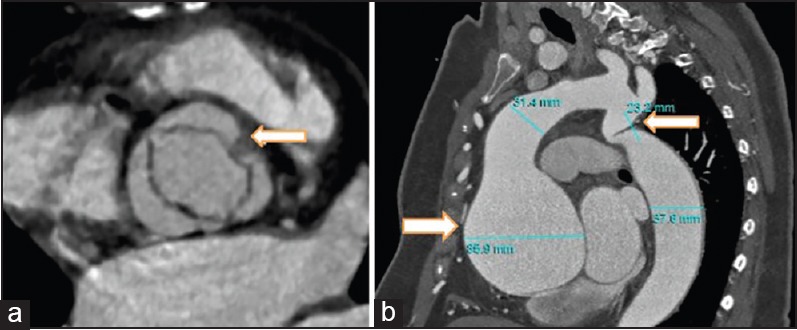
Aortic computed tomography in complicated type 1 BAV. Aortic short-axis view (a), and long-axis (b) with ascending aorta aneurism
Figure 5.
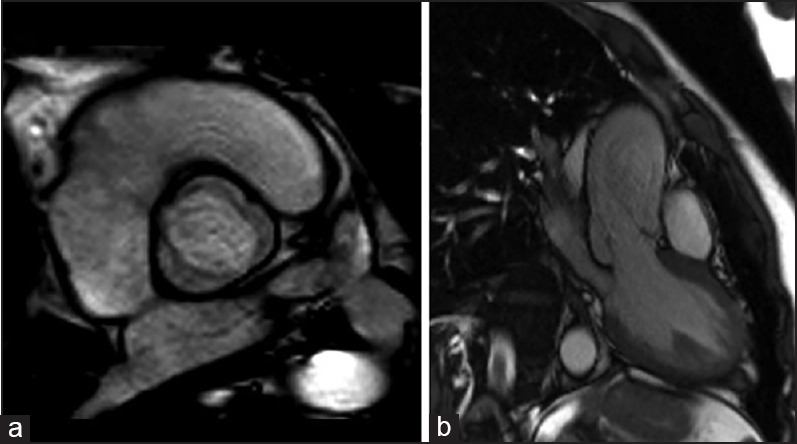
Aortic magnetic resonance in uncomplicated type 1 BAV. Aortic short-axis view (a), and long-axis (b) with mild eccentric regurgitation jet
A good correlation, actually, exists between aortic calibers by 2 DE and those obtained by magnetic resonance imaging of the thoracic aorta.[96] Echocardiography is also useful to detect an anomalous origin of the coronary arteries origin, a condition which account for up to 20% of cases of sudden cardiac death (SCD) in young athletes.[97,98] Anatomical coronary abnormalities can imply a potential risk of coronary artery compression, especially following exercise. These consequences predispose to myocardial ischemia, syncope, or decompensated heart failure, and likely to account for most cases of SCD.
There are recommendations about sport eligibility for patients with BAV from the ACC Foundation (36th Bethesda Conference-2004), European Association for Cardiovascular Prevention and Rehabilitation (Recommendations for physical activity, recreation sport, and exercise training in pediatric patients with congenital heart disease: A report from the Exercise, Basic & Translational Research Section of the European Association of Cardiovascular Prevention and Rehabilitation, the European Congenital Heart and Lung Exercise Group, and the Association for European Paediatric Cardiology-2011), and from the Italian Cardiological Guidelines for Sports Eligibility in Athletes with Heart Disease (COCIS 2009). They have some common aspects, but also some differences.
In order to formulate eligibility criteria; the assessment of a classification of sports, based on the cardiovascular engagement, appears to be crucial.
In the 36th Bethesda Conference report, there is a classification of sports according to the type and intensity of exercise performed and also with regard to the danger of bodily injury from collision, as well as the consequences of syncope.[99] Exercise can be divided into two broad types: Dynamic (isotonic) and static (isometric). Dynamic exercise involves changes in muscle length and joint movement with rhythmic contractions that develop a relatively small intramuscular force; and static exercise involves development of a relatively large intramuscular force with little or no change in muscle length or joint movement. These two types of exercise should be thought of as the two opposite poles of a continuum, with most physical activities involving both static and dynamic components. A classification of sports relates individual competitive sports to the two general types of exercise: Dynamic and static.[100] Each sport is categorized by the level of intensity (low, medium, and high) of dynamic or static exercise generally required to perform that sport during competition. It also recognizes those sports that pose significant risk due to bodily collision, either because of the probability of hard impact between competitors or between a competitor and an object, projectile, or the ground; as well as the degree of risk to the athlete or others if a sudden syncopal event occurs. Thus, in terms of their dynamic and static demands, sports can be classified as IIIC (high static, high dynamic), IIB (moderate static, moderate dynamic), IA (low static, low dynamic), and so forth.
The recommendations of the ACC that follow are for patients with bicuspid valves and associated aortic root enlargement:
Patients with BAVs with no aortic root dilatation (less than 40 mm or the equivalent according to body surface area in children and adolescents) and no significant AS or AR may participate in all competitive sports.
Patients with BAVs and dilated aortic roots between 40 and 45 mm may participate in low and moderate static or low and moderate dynamic competitive sports (classes IA, IB, IIA, and IIB), but should avoid any sports in these categories that involve the potential for bodily collision or trauma.
Patients with BAVs and dilated aortic root greater than 45 mm can participate only in low-intensity competitive sports (class IA).
If such patients also have significant AS, AR, or Marfan syndrome; these recommendations should be considered in concert with those discussed in the same document for patients with these valvular disease or disorder of connective tissue.[100,101]
European Association for Cardiovascular Prevention and Rehabilitation document focuses on eligibility criteria for competitive sports and extends the recommendations to leisure sports, physical activity, and even exercise training programs; in order to promote physical activity and exercise, identifying circumstances for precautions and specific guidance, and counseling.[102]
The level of evidence and the strength of recommendation are weighed and grading according to predefined scale. All physical activities can be characterized according to their static (need for strength) and dynamic (need for endurance) components, as described in the American classification.
European guidelines provide these recommendations:
Patients with BAV can participate in all sport activities if AS, AR, aortic aneurysm, and coarctation are ruled out.
Strength training with a very high static component (i. e., weight lifting) enhances aortic stiffness and dilatation and should be avoided (Class IIa, level of evidence C).[103,104]
Patients with mild aortic dilatation must stay at close surveillance with at least yearly echocardiography as dilatation may become progressive with any kind of training.[85]
Patients with stable moderate aortic aneurysm (40-45 mm in adults and their equivalents in children) may participate in low static and low or moderate dynamic competitive sports, additionally excluding sports with body collision and trauma (Class IIa, level of evidence C).[105]
Patients with progressive or large aortic aneurysm (>45 mm in adults or equivalent in children) must be consulted individually. They must limit their sports activities to low static/low dynamic activities or in more severe cases referred to surgery (Class I, level of evidence C).
Interval training should be omitted and resistance training should be limited to low or moderate intensities involving small muscle groups separately (Class IIa, level of evidence C).
If AS or regurgitation is present, physical activities must be curtailed as suggested by the guidelines for the management of valvular heart disease.[106]
According to Italian Cardiological Guidelines for Sports Eligibility in Athletes with Heart Disease (COCIS 2009), the classification of sports activities, based on the engagement of the cardiovascular system, is based primarily on the behavior of some important parameters, such as heart rate (HR) and BP, and their integration with physiological parameters, in order to take into consideration three basic indicators: Peripheral resistance, cardiac output, and level of adrenergic stimulation [Table 1].[107]
Table 1.
Modified from Italian cardiological guidelines for sports eligibility in athletes with heart disease 2013 [107]
| Group A: Neurogenic | |
| Characterized mainly by increases in heart rate from minimum to moderate, due to emotional component | Bowls (Raffa and petanque), bowling, curling, skittles |
| Golf | |
| Fishing (marine and inland waters) | |
| Shooting sports (archery, flying, archery, etc). | |
| Sport hunting | |
| Biliards sports | |
| Bridge, checkers, chess | |
| Group B: Neurogenic | |
| Characterized mainly by increases in heart rate from moderate to high (and minor cardic output and peripheral resistance) | aMotor recing (speed, rally, autocross, regularity, Slalom National Karting) |
| aAviation | |
| Horse riding | |
| aParachuting | |
| bApnea diving, scuba diving, underwater photos, video diving, underwater shooting Sailing | |
| Group C: Pressure | |
| Characterized by heart rate increased to maximum, medium to high peripheral resistance, submaximal cardiac output | aAlpinism |
| aFree climbing | |
| Athletics (sprint, throwing, jumping, heptathlonb, decathlonb) | |
| aBob, Luge, Steleton | |
| Body building | |
| Cycling (speed, keinin, downhill mountain biking, BMX) | |
| Artistic gymnastics | |
| aMotor racing (motocross, endura, trial) | |
| Synchronized swrnming | |
| Weightlifting | |
| aWater skiing | |
| aAlpine and cross-country skiing (slalom, giant Skiing, Super G, Downhill, Alpine Skiing, Cross-country speed, carving skis, grass skiing, snawboard, jump) | |
| Surfing | |
| Tug of war | |
| Group D: High cardiovascular engagement | |
| Dl: characterized by variable trend of HR, peripheral resistance, and cardiac output | Badminton |
| Baseball | |
| Bawls (flight) | |
| Football, Futsal | |
| Canaeing polo | |
| bAmerican Football | |
| Rhythmic Gymnastics, Twirling | |
| aHockey on ice, on track, on field | |
| aWrestling, Judo, Karate, Taekwondo, Kendo, Wushu kung iu | |
| Basketball | |
| Handball | |
| aWater polo | |
| Handball | |
| Volleyball, Beach Volleyball | |
| Polo | |
| aBoxing, Kick boxing | |
| aRugby, aUnderwater Rugby | |
| Fencing | |
| Softball | |
| Squash | |
| Tambourine | |
| Tennis | |
| Table tennis | |
| D2: characterized by regular increases in submaximal or maximum frequency and cardiac output, and reduced peripheral resistance | Track and fields (middle distance running, duing, running, marathon, ultramarathon, mountain running, cross-country) |
| Biathlon | |
| aRawing, Olympic Canoeing, Rafting Canoeing | |
| aCycling (individual and team pursuit, points race, the American line, individual time trial, mountain bike cross-country, cross-cycling) | |
| Nordic Combined | |
| Sport Dancing | |
| Swimming | |
| Fin swimming | |
| Orienteering | |
| Ice skating, roller skating, figure skating and other specialty shapes | |
| Modem Pentathlon | |
| Cross-country sking | |
| Triathlon | |
| aWind surf |
aIntrinsic risk is considered, bTraumatic risk is considered.
The Italian document recommends that athletes without hemodynamically significant AS or with uncomplicated BAV are eligible for all competitive sports in the absence of the following:
LV hypertrophy and LV systolic or diastolic dysfunction.
Abnormally elevated systolic BP or ST-segment changes during maximal stress test electrocardiogram.
Significant arrhythmias either at rest or during exercise and/or 24-h Holter ECG monitoring, including one training session.
Athletes with hemodynamically significant AS (mean gradient >20 mmHg) should avoid competitive sports. Six months after successful balloon valvuloplasty or surgical correction (residual mean gradient <20 mmHg — no significant AR), competitive sports eligibility may be allowed in selected cases. However, the same exclusion criteria listed above for athletes without hemodynamically significant AS or with uncomplicated BAV should be applied.[108] The occurrence of residual defects after the Ross procedure (pulmonary autograft replacement of the aortic valve, with or without reimplantation of the coronary arteries, followed by pulmonary homograft insertion) is relatively common. A 6-month eligibility period for competitive sports activities of Groups A and B (horse riding and sailing) may be considered in athletes with normal cardiac chamber size and function, and RV to pulmonary artery peak systolic gradient less than 30 mmHg. Conversely, athletes with more than mild AR, ECG alterations, and/or arrhythmias during maximal stress test electrocardiogram or 24-h Holter ECG monitoring, including one training session, should be restricted from competitive sports.
CONCLUSIONS
BAV cannot be considered an innocent finding, but it is not necessarily a life-threatening condition. In Italy, where sports physicians are directly involved in deciding the eligibility for competitive sports, athletes with BAV should undergo a thorough staging of the valve anatomy, taking into consideration hemodynamic factors, as well as aortic diameters and looking for other associated significant cardiovascular anomalies. Furthermore an accurate follow-up is mandatory with serial cardiological controls in those allowed to continue sports. Antibiotic prophylaxis for endocarditis is also recommended, particularly in subjects engaged in contact sports which can create infected wounds, although European Society of Cardiology (ESC) guidelines on infective endocarditis donot recommend this preventive measure in patients with BAV.[108,109]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Osler W. The bicuspid condition of the aortic valve. Trans Assoc Am Physicians. 1886;2:185–92. [Google Scholar]
- 2.Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol. 1970;26:72–83. doi: 10.1016/0002-9149(70)90761-7. [DOI] [PubMed] [Google Scholar]
- 3.Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83:81–5. doi: 10.1136/heart.83.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson EW, Edwards WD. Risk factors for aortic dissection: A necropsy study of 161 cases. Am J Cardiol. 1984;53:849–55. doi: 10.1016/0002-9149(84)90418-1. [DOI] [PubMed] [Google Scholar]
- 5.Basso C, Boschello M, Perrone C, Mecenero A, Cera A, Bicego D, et al. An echocardiographic survey of primary school children for bicuspid aortic valve. Am J Cardiol. 2004;93:661–3. doi: 10.1016/j.amjcard.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–33. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: A surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2715 additional cases. Mayo Clin Proc. 1999;74:14–26. doi: 10.4065/74.1.14. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez B, Duran AC, Fernadez-Gallego T, Fernández MC, Such M, Arqué JM, et al. Bicuspid aortic valves with different spatial orientations of the leaflets are distinct etiological entities. J Am Coll Cardiol. 2009;54:2312–8. doi: 10.1016/j.jacc.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 9.Clementi M, Notari L, Borghi A, Tenconi R. Familial congenital bicuspid aortic valve: A disorder of uncertain inheritance. Am J Med Genet. 1996;62:336–8. doi: 10.1002/(SICI)1096-8628(19960424)62:4<336::AID-AJMG2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44:138–43. doi: 10.1016/j.jacc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 11.Loscalzo ML, Goh DL, Loeys B, Kent KC, Spevak PJ, Dietz HC. Familial thoracic aortic dilation and bicommissural aortic valve: A prospective analysis of natural history and inheritance. Am J Med Genet A. 2007;143A:1960–7. doi: 10.1002/ajmg.a.31872. [DOI] [PubMed] [Google Scholar]
- 12.Huntington K, Hunter AG, Chan KL. A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J Am Coll Cardiol. 1997;30:1809–12. doi: 10.1016/s0735-1097(97)00372-0. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, et al. ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–92. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 14.Roos-Hesselink JW, Scholzel BE, Heijdra RJ, Spitaels SE, Meijboom FJ, Boersma E, et al. E. Aortic valve and aortic arch pathology after coarctation repair. Heart. 2003;89:1074–7. doi: 10.1136/heart.89.9.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warnes CA. Bicuspid aortic valve and coarctation: Two villains part of a diffuse problem. Heart. 2003;89:965–6. doi: 10.1136/heart.89.9.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliver JM, Gallego P, Gonzalez A, Aroca A, Bret M, Mesa JM. Risk factors for aortic complications in adults with coarctation of the aorta. J Am Coll Cardiol. 2004;44:1641–7. doi: 10.1016/j.jacc.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 17.Brenner JI, Berg KA, Schneider DS, Clark EB, Boughman JA. Cardiac malformations in relatives of infants with hypoplastic left-heart syndrome. Am J Dis Child. 1989;143:1492–4. doi: 10.1001/archpedi.1989.02150240114030. [DOI] [PubMed] [Google Scholar]
- 18.Roberts WC, Morrow AG, Braunwald E. Complete interruption of the aortic arch. Circulation. 1962;26:39–59. doi: 10.1161/01.cir.26.1.39. [DOI] [PubMed] [Google Scholar]
- 19.Hinton RB, Jr, Martin LJ, Tabangin ME, Mazwi ML, Cripe LH, Benson DW. Hypoplastic left heart syndrome is heritable. J Am Coll Cardiol. 2007;50:1590–5. doi: 10.1016/j.jacc.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Bolling SF, Iannettoni MD, Dick M, 2nd, Rosenthal A, Bove EL. Shone's anomaly: Operative results and late outcome. Ann Thorac Surg. 1990;49:887–93. doi: 10.1016/0003-4975(90)90861-y. [DOI] [PubMed] [Google Scholar]
- 21.Sybert VP. Cardiovascular malformations and complications in Turner syndrome. Pediatrics. 1998;101:E11. doi: 10.1542/peds.101.1.e11. [DOI] [PubMed] [Google Scholar]
- 22.Nistri S, Basso C, Marzari C, Mormino P, Thiene G. Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. Am J Cardiol. 2005;96:718–21. doi: 10.1016/j.amjcard.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 23.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–5. doi: 10.1161/01.CIR.0000155623.48408.C5. [DOI] [PubMed] [Google Scholar]
- 24.Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nkomo VT, Sundt TM, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117:2776–84. doi: 10.1161/CIRCULATIONAHA.107.740878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, et al. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300:1317–25. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- 26.Della Corte A, Bancone C, Quarto C, Dialetto G, Covino FE, Scardone M, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: A wide spectrum of disease expression. Eur J Cardiothorac Surg. 2007;31:397–404. doi: 10.1016/j.ejcts.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Della Corte A, Body SC, Booher AM, Schaefers HJ, Milewski RK, Michelena HI, et al. International Bicuspid Aortic Valve Consortium [BAVCon] Investigators. Surgical treatment of bicuspid aortic valve disease: Knowledge gaps and research perspectives. J Thorac Cardiovasc Surg. 2014;147:1749–57. doi: 10.1016/j.jtcvs.2014.01.021. 1757 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bissell MM, Hess AT, Biasiolli L, Glaze SJ, Loudon M, Pitcher A, et al. Aortic dilation in bicuspid aortic valve disease: Flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging. 2013;6:499–507. doi: 10.1161/CIRCIMAGING.113.000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michelena HI, Prakash SK, Della Corte A, Bissell MM, Anavekar N, Mathieu P, et al. BAVCon Investigators. Bicuspid aortic valve: Identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium [BAVCon] Circulation. 2014;129:2691–704. doi: 10.1161/CIRCULATIONAHA.113.007851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Della Corte A. Phenotypic heterogeneity of bicuspid aortopathy: A potential key to decode the prognosis? Heart. 2014;100:96–7. doi: 10.1136/heartjnl-2013-305004. [DOI] [PubMed] [Google Scholar]
- 31.Fedak PW, Verma S. The molecular fingerprint of bicuspid aortopathy. J Thorac Cardiovasc Surg. 2013;145:1334. doi: 10.1016/j.jtcvs.2013.02.067. [DOI] [PubMed] [Google Scholar]
- 32.Della Corte A, Bancone C, Buonocore M, Dialetto G, Covino FE, Manduca S, et al. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovasc Imaging. 2013;6:1301–10. doi: 10.1016/j.jcmg.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg. 2012;42:832–7. doi: 10.1093/ejcts/ezs137. [DOI] [PubMed] [Google Scholar]
- 34.Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol. 2009;53:2288–95. doi: 10.1016/j.jacc.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Della Corte A. The conundrum of aortic dissection in patients with bicuspid aortic valve: The tissue, the mechanics and the mathematics. Eur J Cardiothorac Surg. 2014 doi: 10.1093/ejcts/ezu418. [DOI] [PubMed] [Google Scholar]
- 36.Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Intern Med. 1975;82:521–4. doi: 10.7326/0003-4819-82-4-521. [DOI] [PubMed] [Google Scholar]
- 37.D’Andrea A, Limongelli G, Caso P, Sarubbi B, Della Pietra A, Brancaccio P, et al. Association between left ventricular structure and cardiac performance during effort in two morphological forms of athlete's heart. Int J Cardiol. 2002;86:177–84. doi: 10.1016/s0167-5273(02)00194-8. [DOI] [PubMed] [Google Scholar]
- 38.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete's heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101:336–44. doi: 10.1161/01.cir.101.3.336. [DOI] [PubMed] [Google Scholar]
- 39.Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991;324:295–301. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- 40.Spirito P, Pelliccia A, Proschan MA, Granata M, Spataro A, Bellone P, et al. Morphology of the “athlete's heart” assessed by echocardiography in 947 elite athletes representing 27 sports. Am J Cardiol. 1994;74:802–6. doi: 10.1016/0002-9149(94)90439-1. [DOI] [PubMed] [Google Scholar]
- 41.Pelliccia A, Maron BJ, De Luca R, Di Paolo FM, Spataro A, Culasso F. Remodeling of left ventricular hypertrophy in elite athletes after long-term deconditioning. Circulation. 2002;105:944–9. doi: 10.1161/hc0802.104534. [DOI] [PubMed] [Google Scholar]
- 42.Urhausen A, Albers T, Kindermann W. Are the cardiac effects of anabolic steroid abuse in strength athletes reversible? Heart. 2004;90:496–501. doi: 10.1136/hrt.2003.015719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Bello V, Giorgi D, Bianchi M, Bertini A, Caputo MT, Valenti G, et al. Effects of anabolic-androgenic steroids on weight-lifters’ myocardium: An ultrasonic videodensitometric study. Med Sci Sports Exerc. 1999;31:514–21. doi: 10.1097/00005768-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Caso P, D’Andrea A, Galderisi M, Liccardo B, Severino S, De Simone, et al. Pulsed Doppler tissue imaging in endurance athletes: Relation between left ventricular preload and myocardial regional diastolic function. Am J Cardiol. 2000;85:1131–6. doi: 10.1016/s0002-9149(00)00709-8. [DOI] [PubMed] [Google Scholar]
- 45.D’Andrea A, Caso P, Severino S, Galderisi M, Sarubbi B, Limongelli G, et al. Effects of different training protocols on left ventricular myocardial function in competitive athletes: A Doppler tissue imaging study. Ital Heart J. 2002;3:34–40. [PubMed] [Google Scholar]
- 46.Richand V, Lafitte S, Reant P, Serri K, Lafitte M, Brette S, et al. An ultrasound speckle tracking (two-dimensional strain) analysis of myocardial deformation in professional soccer players compared with healthy subjects and hypertrophic cardiomyopathy. Am J Cardiol. 2007;100:128–32. doi: 10.1016/j.amjcard.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 47.Saghir M, Areces M, Makan M. Strain rate imaging differentiates hypertensive cardiac hypertrophy from physiologic cardiac hypertrophy (athlete's heart) J Am Soc Echocardiogr. 2007;20:151–7. doi: 10.1016/j.echo.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Poh KK, Ton-Nu TT, Neilan TG, Tournoux FB, Picard MH, Wood MJ. Myocardial adaptation and efficiency in response to intensive physical training in elite speedskaters. Int J Cardiol. 2008;126:346–51. doi: 10.1016/j.ijcard.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 49.D’Andrea A, Cocchia R, Riegler L, Scarafile R, Salerno G, Gravino R, et al. Left ventricular myocardial velocities and deformation indexes in top-level athletes. J Am Soc Echocardiogr. 2010;23:1281–8. doi: 10.1016/j.echo.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Pattynama PM, Lamb HJ, van der Velde EA, van der Wall EE, de Roos A. Left ventricular measurements with cine and spin-echo MR imaging: A study of reproducibility with variance component analysis. Radiology. 1993;187:261–8. doi: 10.1148/radiology.187.1.8451425. [DOI] [PubMed] [Google Scholar]
- 51.Semelka RC, Tomei E, Wagner S, Mayo J, Caputo G, O’Sullivan M, et al. Interstudy reproducibility of dimensional and functional measurements between cine magnetic resonance studies in the morphologically abnormal left ventricle. Am Heart J. 1990;119:1367–73. doi: 10.1016/s0002-8703(05)80187-5. [DOI] [PubMed] [Google Scholar]
- 52.Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, et al. Society for Cardiovascular Magnetic Resonance Imaging; Cardiovascular Magnetic Resonance Working Group of the European Society of Cardiology. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen SE, Selvanayagam JB, Francis JM, Myerson SG, Wiesmann F, Robson MD, et al. Differentiation of athlete's heart from pathological forms of cardiac hypertrophy by means of geometric indices derived from cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2005;7:551–8. doi: 10.1081/jcmr-200060631. [DOI] [PubMed] [Google Scholar]
- 54.Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112:855–61. doi: 10.1161/CIRCULATIONAHA.104.507723. [DOI] [PubMed] [Google Scholar]
- 55.Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart. 2004;90:645–9. doi: 10.1136/hrt.2003.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875–87. doi: 10.1016/j.jacc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Pelliccia A, Maron BJ, Di Paolo FM, Biffi A, Quattrini FM, Pisicchio C, et al. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol. 2005;46:690–6. doi: 10.1016/j.jacc.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 58.D’Andrea A, Riegler L, Cocchia R, Scarafile R, Salerno G, Gravino R, et al. Left atrial volume index in highly trained athletes. Am Heart J. 2010;159:1155–61. doi: 10.1016/j.ahj.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 59.Abdulla J, Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. 2009;11:1156–9. doi: 10.1093/europace/eup197. [DOI] [PubMed] [Google Scholar]
- 60.La Gerche A, Taylor AJ, Prior DL. Athlete's heart: The potential for multimodality imaging to address the critical remaining questions. JACC Cardiovasc Imaging. 2009;2:350–63. doi: 10.1016/j.jcmg.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 61.Prior DL, La Gerche A. The athlete's heart. Heart. 2012;98:947–55. doi: 10.1136/heartjnl-2011-301329. [DOI] [PubMed] [Google Scholar]
- 62.La Gerche A, Heidbuchel H, Burns AT, Mooney DJ, Taylor AJ, Pfluger HB, et al. Disproportionate exercise load and remodeling of the athlete's right ventricle. Med Sci Sports Exerc. 2011;43:974–81. doi: 10.1249/MSS.0b013e31820607a3. [DOI] [PubMed] [Google Scholar]
- 63.Lewis GD, Bossone E, Naeije R, Grünig E, Saggar R, Lancellotti P, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–9. doi: 10.1161/CIRCULATIONAHA.112.000667. [DOI] [PubMed] [Google Scholar]
- 64.La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, et al. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 65.Claessen G, Claus P, Ghysels S, Vermeersch P, Dymarkowski S, LA Gerche A, et al. Right ventricular fatigue developing during endurance exercise: An exercise cardiac magnetic resonance study. Med Sci Sports Exerc. 2014;46:1717–26. doi: 10.1249/MSS.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 66.Douglas PS, O’Toole ML, Hiller WD, Reichek N. Different effects of prolonged exercise on the right and left ventricles. J Am Coll Cardiol. 1990;15:64–9. doi: 10.1016/0735-1097(90)90176-p. [DOI] [PubMed] [Google Scholar]
- 67.Oxborough D, Shave R, Warburton D, Williams K, Oxborough A, Charlesworth S, et al. Dilatation and dysfunction of the right ventricle immediately after ultraendurance exercise: Exploratory insights from conventional two-dimensional and speckle tracking echocardiography. Circ Cardiovasc Imaging. 2011;4:253–63. doi: 10.1161/CIRCIMAGING.110.961938. [DOI] [PubMed] [Google Scholar]
- 68.Trivax JE, Franklin BA, Goldstein JA, Chinnaiyan KM, Gallagher MJ, deJong AT, et al. Acute cardiac effects of marathon running. J Appl Physiol (1985) 2010;108:1148–53. doi: 10.1152/japplphysiol.01151.2009. [DOI] [PubMed] [Google Scholar]
- 69.Henriksen E, Kangro T, Jonason T, Landelius J, Hedberg P, Ekstrand P, et al. An echocardiographic study of right ventricular adaptation to physical exercise in elite male orienteers. Clin Physiol. 1998;18:498–503. doi: 10.1046/j.1365-2281.1998.00130.x. [DOI] [PubMed] [Google Scholar]
- 70.Henriksen E, Landelius J, Kangro T, Jonason T, Hedberg P, Wesslén L, et al. An echocardiographic study of right and left ventricular adaptation to physical exercise in elite female orienteers. Eur Heart J. 1999;20:309–16. doi: 10.1053/euhj.1998.1197. [DOI] [PubMed] [Google Scholar]
- 71.D’Andrea A, Riegler L, Golia E, Cocchia R, Scarafile R, Salerno G, et al. Range of right heart measurements in top-level athletes: The training impact. Int J Cardiol. 2013;164:48–57. doi: 10.1016/j.ijcard.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 72.D’Andrea A, Riegler L, Morra S, Scarafile R, Salerno G, Cocchia R, et al. Right ventricular morphology and function in top-level athletes: A three-dimensional echocardiographic study. J Am Soc Echocardiogr. 2012;25:1268–76. doi: 10.1016/j.echo.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 73.Utomi V, Oxborough D, Whyte GP, Somauroo J, Sharma S, Shave R, et al. Systematic review and meta-analysis of training mode, imaging modality and body size influences on the morphology and function of the male athlete's heart. Heart. 2013;99:1727–33. doi: 10.1136/heartjnl-2012-303465. [DOI] [PubMed] [Google Scholar]
- 74.Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W. Athlete's heart: Right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol. 2002;40:1856–63. doi: 10.1016/s0735-1097(02)02478-6. [DOI] [PubMed] [Google Scholar]
- 75.Luijkx T, Velthuis BK, Prakken NH, Cox MG, Bots ML, Mali WP, et al. Impact of revised Task Force Criteria: Distinguishing the athlete's heart from ARVC/D using cardiac magnetic resonance imaging. Eur J Prev Cardiol. 2012;19:885–91. doi: 10.1177/1741826711414215. [DOI] [PubMed] [Google Scholar]
- 76.Aaron CP, Tandri H, Barr RG, Johnson WC, Bagiella E, Chahal H, et al. Physical activity and right ventricular structure and function. The MESA-Right Ventricle Study. Am J Respir Crit Care Med. 2011;183:396–404. doi: 10.1164/rccm.201003-0469OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perseghin G, De Cobelli F, Esposito A, Lattuada G, Terruzzi I, La Torre A, et al. Effect of the sporting discipline on the right and left ventricular morphology and function of elite male track runners: A magnetic resonance imaging and phosphorus 31 spectroscopy study. Am Heart J. 2007;154:937–42. doi: 10.1016/j.ahj.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 78.Nistri S, Sorbo MD, Marin M, Palisi M, Scognamiglio R, Thiene G. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart. 1999;82:19–22. doi: 10.1136/hrt.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg. 2003;126:892–3. doi: 10.1016/s0022-5223(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 80.Schaefer BM, Lewin MB, Stout KK, Gill E, Prueitt A, Byers PH, et al. The bicuspid aortic valve: An integrated phenotypic classification of leaflet morphology and aortic root shape. Heart. 2008;94:1634–8. doi: 10.1136/hrt.2007.132092. [DOI] [PubMed] [Google Scholar]
- 81.Schaefer BM, Lewin MB, Stout KK, Byers PH, Otto CM. Usefulness of bicuspid aortic valve phenotype to predict elastic properties of the ascending aorta. Am J Cardiol. 2007;99:686–90. doi: 10.1016/j.amjcard.2006.09.118. [DOI] [PubMed] [Google Scholar]
- 82.Kim M, Roman MJ, Cavallini MC, Schwartz JE, Pickering TG, Devereux RB. Effect of hypertension on aortic root size and prevalence of aortic regurgitation. Hypertension. 1996;28:47–52. doi: 10.1161/01.hyp.28.1.47. [DOI] [PubMed] [Google Scholar]
- 83.Palmieri V, Bella JN, Arnett DK, Roman MJ, Oberman A, Kitzman DW, et al. Aortic root dilatation at sinuses of valsalva and aortic regurgitation in hypertensive and normotensive subjects: The Hypertension Genetic Epidemiology Network Study. Hypertension. 2001;37:1229–35. doi: 10.1161/01.hyp.37.5.1229. [DOI] [PubMed] [Google Scholar]
- 84.Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE, Fang J, et al. International Registry of Aortic Dissection (IRAD). Characterizing the young patient with aortic dissection: Results from the International Registry of Aortic Dissection (IRAD) J Am Coll Cardiol. 2004;43:665–9. doi: 10.1016/j.jacc.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 85.Galanti G, Stefani L, Toncelli L, Vono MC, Mercuri R, Maffulli N. Effects of sports activity in athletes with bicuspid aortic valve and mild aortic regurgitation. Br J Sports Med. 2010;44:275–9. doi: 10.1136/bjsm.2008.047407. [DOI] [PubMed] [Google Scholar]
- 86.Cameron JD, Dart AM. Exercise training increases total systemic arterial compliance in humans. Am J Physiol. 1994;266:H693–701. doi: 10.1152/ajpheart.1994.266.2.H693. [DOI] [PubMed] [Google Scholar]
- 87.Kakiyama T, Sugawara J, Murakami H, Maeda S, Kuno S, Matsuda M. Effects of short-term endurance training on aortic distensibility in young males. Med Sci Sports Exerc. 2005;37:267–71. doi: 10.1249/01.mss.0000152733.12578.5a. [DOI] [PubMed] [Google Scholar]
- 88.Kakiyama T, Matsuda M, Koseki S. Effect of physical activity on the distensibility of the aortic wall in healthy males. Angiology. 1998;49:749–57. doi: 10.1177/000331979804901007. [DOI] [PubMed] [Google Scholar]
- 89.Sugawara J, Otsuki T, Tanabe T, Maeda S, Kuno S, Ajisaka R, et al. The effects of low-intensity single-leg exercise on regional arterial stiffness. Jpn J Physiol. 2003;53:239–41. doi: 10.2170/jjphysiol.53.239. [DOI] [PubMed] [Google Scholar]
- 90.Sugawara J, Otsuki T, Tanabe T, Hayashi K, Maeda S, Matsuda M. Physical activity duration, intensity, and arterial stiffening in postmenopausal women. Am J Hypertens. 2006;19:1032–6. doi: 10.1016/j.amjhyper.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 91.D’Andrea A, Cocchia R, Riegler L, Scarafile R, Salerno G, Gravino R, et al. Aortic root dimensions in elite athletes. Am J Cardiol. 2010;105:1629–34. doi: 10.1016/j.amjcard.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 92.Keane M, Wiegers S, Plappert T, Pochettino A, Bavaria JE, Sutton MG. Bicuspid aortic valves are associated with aortic valve dilatation out of proportion to coexistent valvular lesions. Circulation. 2000;102:III-35-9. doi: 10.1161/01.cir.102.suppl_3.iii-35. [DOI] [PubMed] [Google Scholar]
- 93.Cecconi M, Manfrin M, Moraca A, Zanoli R, Colonna PL, Bettuzzi MG, et al. Aortic dimensions in patients with bicuspid aortic valve without significant valve dysfunction. Am J Cardiol. 2005;95:292–4. doi: 10.1016/j.amjcard.2004.08.098. [DOI] [PubMed] [Google Scholar]
- 94.Branderburg RO, Jr, Tajik AJ, Edwards WD, Reeder GS, Shub C, Seward JB. Accuracy of 2-dimensional echocardiography diagnosis of congenitally bicuspid aortic valve: Echocardiographic — anatomic correlations in 115 patients. Am J Cardiol. 1983;51:1469–73. doi: 10.1016/0002-9149(83)90659-8. [DOI] [PubMed] [Google Scholar]
- 95.Zema MJ, Caccavano M. Two-dimensional echocardiographic assessment of aortic valve morphology: Feasibility of bicuspid aortic valve detection. Prospective study of 100 adult patients. Br Heart J. 1982;48:428–33. doi: 10.1136/hrt.48.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loschiavo A, Fabbricatore C, Gentili F, Bianco M, Bria S, Ramadori A, et al. Confronto ecocardiografia-risonanza magnetica nello studio delle patologie aortiche associate a valvola aortica bicuspide. J Sports Cardiol. 2005;2:92. [Google Scholar]
- 97.Maron BJ, Gohman TE, Aeppli D. Prevalence of sudden cardiac death during competitive sports activities in Minnesota high school athletes. J Am Coll Cardiol. 1998;32:1881–4. doi: 10.1016/s0735-1097(98)00491-4. [DOI] [PubMed] [Google Scholar]
- 98.Popovic D, Ostojic MC, Popovic N, Stojiljković S, Sćepanović L. Causes of sudden cardiac death in athletes. Med Pregl. 2007;60:61–5. doi: 10.2298/mpns0702061p. [DOI] [PubMed] [Google Scholar]
- 99.Bonow RO, Cheitlin MD, Crawford MH, Douglas PS. Task Force 3: Valvular heart disease. J Am Coll Cardiol. 2005;45:1334–40. doi: 10.1016/j.jacc.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 100.Maron BJ, Ackerman MJ, Nishimura RA, Pyeritz RE, Towbin JA, Udelson JE. Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and marfan syndrome. J Am Coll Cardiol. 2005;45:1340–5. doi: 10.1016/j.jacc.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 101.Asmussen E. Similarities and dissimilarities between static and dynamic exercise. Circ Res. 1981;48:I3–10. [PubMed] [Google Scholar]
- 102.Takken T, Giardini A, Reybrouck T, Gewillig M, Hövels-Gürich HH, Longmuir PE, et al. Recommendations for physical activity, recreation sport, and exercise training in paediatric patients with congenital heart disease: A report from the Exercise, Basic and Translational Research Section of the European Association of Cardiovascular Prevention and Rehabilitation, the European Congenital Heart and Lung Exercise Group, and the Association for European Paediatric Cardiology. Eur J Prev Cardiol. 2012;19:1034–65. doi: 10.1177/1741826711420000. [DOI] [PubMed] [Google Scholar]
- 103.Bertovic DA, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA. Muscular strength training is associated with low arterial compliance and high pulse pressure. Hypertension. 1999;33:1385–91. doi: 10.1161/01.hyp.33.6.1385. [DOI] [PubMed] [Google Scholar]
- 104.Babaee Bigi MA, Aslani A. Aortic root size and prevalence of aortic regurgitation in elite strength trained athletes. Am J Cardiol. 2007;100:528–30. doi: 10.1016/j.amjcard.2007.02.108. [DOI] [PubMed] [Google Scholar]
- 105.Hatzaras I, Tranquilli M, Coady M, Barrett PM, Bible J, Elefteriades JA. Weight lifting and aortic dissection: More evidence for a connection. Cardiology. 2007;107:103–6. doi: 10.1159/000094530. [DOI] [PubMed] [Google Scholar]
- 106.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, et al. Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease [version 2012] Eur Heart J. 2012;33:2451–96. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 107.Biffi A, Delise P, Zeppilli P, Giada F, Pelliccia A, Penco M, et al. Italian Society of Sports Cardiology and Italian Sports Medicine Federation. Italian cardiological guidelines for sports eligibility in athletes with heart disease: Part 1. J Cardiovasc Med (Hagerstown) 2013;14:477–99. doi: 10.2459/JCM.0b013e32835f6a21. [DOI] [PubMed] [Google Scholar]
- 108.Zeppilli P, Bianco M, Bria S, Palmieri V. Bicuspid aortic valve: An innocent finding or a potentially life-threatening anomaly whose complications may be elicited by sports activity. J Cardiovasc Med (Hagerstown) 2006;7:282–7. doi: 10.2459/01.JCM.0000219322.04881.9e. [DOI] [PubMed] [Google Scholar]
- 109.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, et al. ESC Committee for Practice Guidelines. Guidelines on the prevention, diagnosis and treatment of infective endocarditis (new version 2009): The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30:2369–413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]


