Abstract
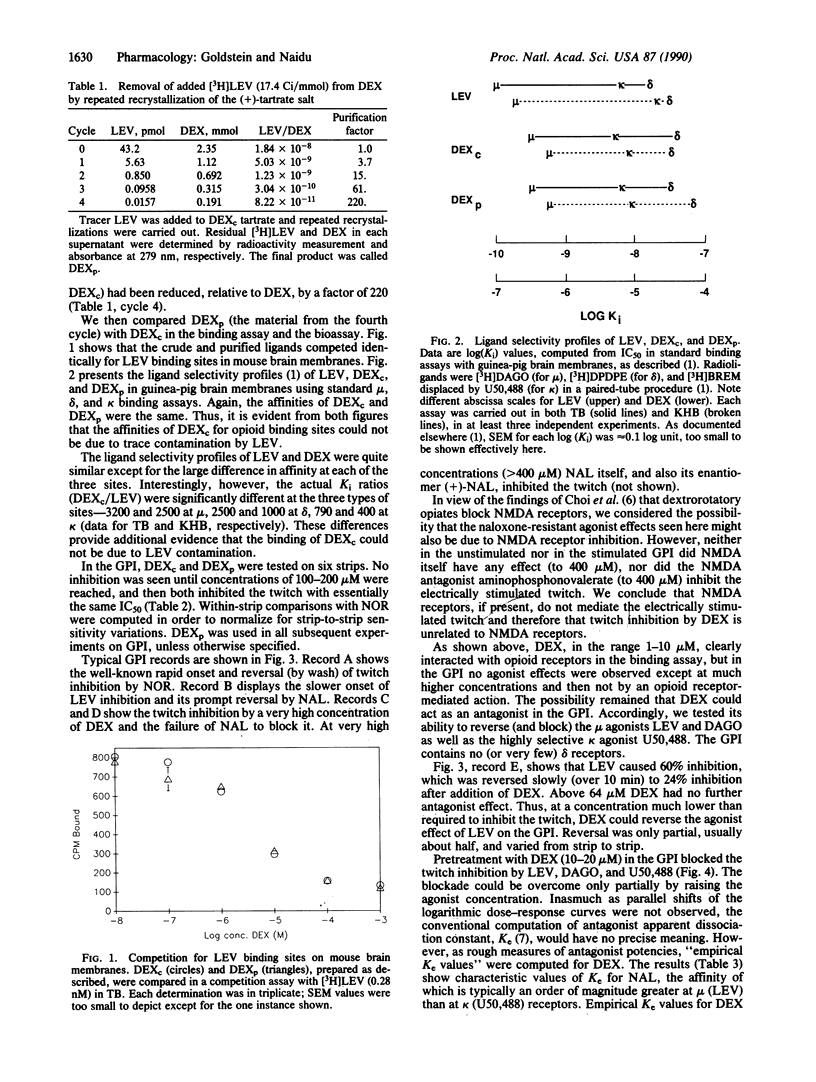
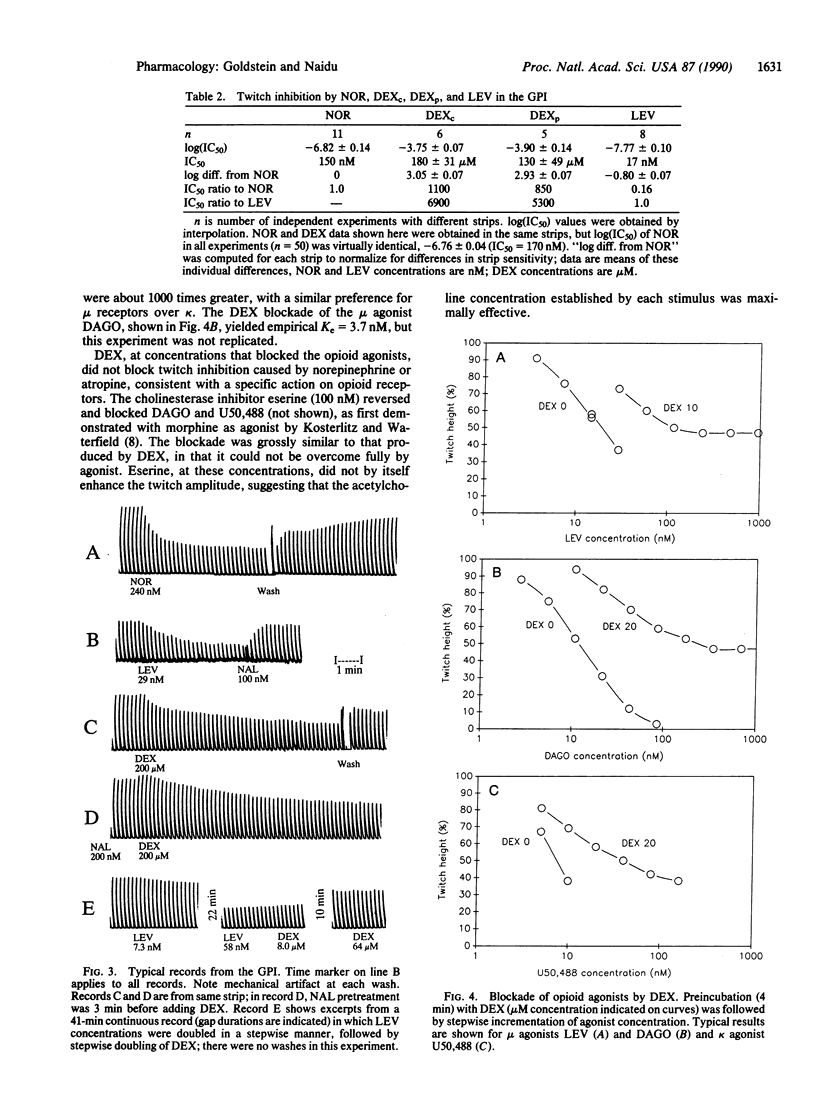
Dextrorphan (+)-tartrate, purified by repeated crystallization to remove all traces of the enantiomer levorphanol, binds to mu, delta, and kappa sites on guinea-pig brain membranes with lower affinities (by a factor of 400-3200) than levorphanol. In the guinea-pig ileum myenteric plexus longitudinal muscle preparation (GPI), dextrorphan, at 100-200 microM, inhibits the electrically stimulated twitch, but this action is not blocked or reversed by naloxone; both (+)- and (-)-naloxone produce similar non-opioid twitch inhibition at comparable concentrations. At 10-20 microM, dextrorphan blocks and reverses the twitch inhibition due to mu and kappa agonists, but the blockade can be overcome only partially by increasing the agonist concentration. We conclude that dextrorphan is an opioid ligand with low affinity and with antagonist effect on opioid receptors in the GPI.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choi D. W., Peters S., Viseskul V. Dextrorphan and levorphanol selectively block N-methyl-D-aspartate receptor-mediated neurotoxicity on cortical neurons. J Pharmacol Exp Ther. 1987 Aug;242(2):713–720. [PubMed] [Google Scholar]
- Gillan M. G., Kosterlitz H. W. Spectrum of the mu, delta- and kappa-binding sites in homogenates of rat brain. Br J Pharmacol. 1982 Nov;77(3):461–469. doi: 10.1111/j.1476-5381.1982.tb09319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A., Naidu A. Multiple opioid receptors: ligand selectivity profiles and binding site signatures. Mol Pharmacol. 1989 Aug;36(2):265–272. [PubMed] [Google Scholar]
- Goldstein A., Schulz R. Morphine-tolerant longitudinal muscle strip from guinea-pig ileum. Br J Pharmacol. 1973 Aug;48(4):655–666. doi: 10.1111/j.1476-5381.1973.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachur J. F., Morgan D. W., Gaginella T. S. Effect of dextromethorphan on guinea pig ileal contractility in vitro: comparison with levomethorphan, loperamide and codeine. J Pharmacol Exp Ther. 1986 Dec;239(3):661–667. [PubMed] [Google Scholar]
- Kosterlitz H. W., Waterfield A. A. An analysis of the phenomenon of acute tolerance to morphine in the guinea-pig isolated ileum. Br J Pharmacol. 1975 Jan;53(1):131–138. doi: 10.1111/j.1476-5381.1975.tb07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Waterfield A. A. In vitro models in the study of structure-activity relationships of narcotic analgesics. Annu Rev Pharmacol. 1975;15:29–47. doi: 10.1146/annurev.pa.15.040175.000333. [DOI] [PubMed] [Google Scholar]
- Vonvoigtlander P. F., Lahti R. A., Ludens J. H. U-50,488: a selective and structurally novel non-Mu (kappa) opioid agonist. J Pharmacol Exp Ther. 1983 Jan;224(1):7–12. [PubMed] [Google Scholar]



