Abstract
Since 2013, highly pathogenic (HP) H5N6 influenza A viruses (IAVs) have emerged in poultry in Asia, especially Southeast Asia. These viruses have also caused sporadic infections in humans within the same geographic areas. Active IAV surveillance in wild birds sampled in Guangdong province, China from August 2014 through February 2015 resulted in the recovery of three H5N6 IAVs. These H5N6 IAV isolates possess the basic amino acid motif at the HA1-HA2 cleavage site that is associated with highly pathogenic IAVs infecting chickens. Noteworthy findings include: (1) the HP H5N6 IAV isolates were recovered from three species of apparently healthy wild birds (most other isolates of HP H5N6 IAV in Asia are recovered from dead wild birds or fecal samples in the environment) and (2) these isolates were apparently the first recoveries of HP H5N6 IAV for two of the three species thus expanding the demonstrated natural host range for these lineages of virus. This investigation provides additional insight into the natural history of HP H5N6 IAVs and identifies the occurrence of non-lethal, HP H5N6 IAV infections in wild birds thereby demonstrating the value of active IAV surveillance in wild birds.
Avian influenza A viruses (AIVs) pose significant threats to animal and human health. Among the 18 influenza haemagglutinin (HA) subtypes, the H5 subtype, in combination with a variety of individual neuraminidase (NA) subtypes (Nx), cause the most frequent and widespread epizootics resulting in severe economic losses to the poultry industry1,2. In addition several H5Nx influenza A virus (IAV) combinations have also been proven to be zoonotic, thereby raising global concern for human health. The most recent emergent lineages of concern are the highly pathogenic (HP) and/or zoonotic lineages of H5N6 AIVs in China3,4,5,6 and Southeast Asia7, and more recently in Korea8. These viruses have caused human infections in China since 20149. Human infections of H5N6 viruses reappeared in December 2015, in Guangdong province, China10. This has raised concerns about the threat of these viruses to public health.
Poultry are usually considered to be the source of the human infections of AIVs, and a lot of work has focused on live poultry markets10. Wild birds are considered to be natural reservoirs for lowly pathogenic AIVs as well as being an important source of low pathogenic AIVs infecting poultry11. The Qinghai-like highly pathogenic avian influenza A (HPAI) H5N1 viruses caused epizootic outbreaks in wild birds in 2005, and were rapidly disseminated throughout Africa, Europe and the Middle East along flyways by migratory birds resulting in hundreds of cases of human infection and the death of tens of millions of poultry12. Also, H5N8 HPAIVs appeared to be spread by long-distance migratory birds13 which add additional evidence supporting the role of wild birds in the long-distance transmission and spread of HPAIVs during their seasonal migrations along their flyways14. In this investigation we examined wild birds in Guangdong Province for active IAV infections from August 2014 through February 2015.
Result
Influenza virus surveillance in wild birds
From August 2014 to February 2015, a total of 1092 oropharyngeal and cloacal samples were collected from apparently healthy wild birds in Guangdong Province, China (Table 1). 11 HA-positive specimens were recovered. Three H5N6 subtype AIVs were isolated from an oriental magpie-robin (oropharyngeal swab), a common moorhen (oropharyngeal swab), and a Pallas’s sandgrouse (both oropharyngeal and cloacal swabs). These H5N6 AIVs were designated as A/oriental magpie-robin/Guangdong/SW8/2014 (H5N6) (SW8), A/common moorhen/Guangdong/GZ174/2014 (H5N6) (GZ174) and A/Pallas’s sandgrouse/Guangdong/ZH283/2015 (H5N6) (ZH283) respectively.
Table 1. Surveillance statistics for Influenza A virus in Guangdong during 2014–2015.
| City | Sampling time | Site | Common name | Scientific name | No. of sample | No. of positive | Positive rate (%) | Subtype (n)a |
|---|---|---|---|---|---|---|---|---|
| Guangzhou | 08-9-2014 | wetland | common moorhen | Gallinula chloropus | 18 | 1 | 5.56 | H5N6 (1) |
| great white egret | Ardea alba | 6 | 0 | 0 | ||||
| little egret | Egretta garzetta | 8 | 0 | 0 | ||||
| night heron | Nycticorax nycticorax | 8 | 0 | 0 | ||||
| gray heron | Ardea cinerea | 14 | 0 | 0 | ||||
| cattle egret | Bubulcus ibis | 10 | 0 | 0 | ||||
| Chinese pond heron | Ardeola bacchus | 11 | 0 | 0 | ||||
| rose-ringed parrakeet | Psittacula krameri | 13 | 0 | 0 | ||||
| Subtotal | 88 | 1 | 1.14 | |||||
| 16-9-2014 | lake | night heron | Nycticorax nycticorax | 18 | 0 | 0 | ||
| common kingfisher | Alcedo atthis | 16 | 0 | 0 | ||||
| white-cheeked starling | Spodiopsar cineraceus | 24 | 3 | 12.5 | H5N1 (3) | |||
| sooty-headed bulbul | Pycnonotus aurigaster | 4 | 0 | 0 | ||||
| little egret | Egretta garzetta | 35 | 0 | 0 | ||||
| cormorant | Phalacrocorax carbo | 3 | 0 | 0 | ||||
| heuglin’s gull | Larus heuglini | 8 | 0 | 0 | ||||
| purple heron | Ardea purpurea | 14 | 0 | 0 | ||||
| gray heron | Ardea cinerea | 20 | 0 | 0 | ||||
| Subtotal | 142 | 3 | 2.11 | |||||
| Zhuhai | 2- 9-2014 | wetland | common moorhen | Gallinula chloropus | 20 | 0 | 0 | |
| common gull | Larus canus | 46 | 0 | 0 | ||||
| little egret | Egretta garzetta | 14 | 0 | 0 | ||||
| night heron | Nycticorax nycticorax | 28 | 0 | 0 | ||||
| Chinese hwamei | Garrulax canorus | 6 | 1 | 16.7 | H5N1 (1) | |||
| Eurasian Tree sparrow | Passer montanus | 4 | 0 | 0 | ||||
| white-breasted waterhen | Amaurornis phoenicurus | 24 | 0 | 0 | ||||
| vega gull | Larus vegae | 36 | 0 | 0 | ||||
| Subtotal | 178 | 1 | 0.56 | |||||
| 20-2-2015 | wetland | night heron | Nycticorax nycticorax | 36 | 0 | 0 | ||
| common moorhen | Gallinula chloropus | 120 | 0 | 0 | ||||
| common gull | Larus canus | 58 | 0 | 0 | ||||
| Pallas's sandgrouse | Syrrhaptes paradoxus | 42 | 1 | 2.38 | H5N6 (1) | |||
| purple heron | Ardea purpurea | 42 | 0 | 0 | ||||
| little egret | Egretta garzetta | 36 | 0 | 0 | ||||
| whooper swan | Cygnus cygnus | 6 | 0 | 0 | ||||
| daurian redstart | Phoenicurus auroreus | 4 | 0 | 0 | ||||
| plain prinia | Prinia inornata | 12 | 0 | 0 | ||||
| dusky warbler | Phylloscopus fuscatus | 11 | 0 | 0 | ||||
| Japanese white-eye | Zosterops japonicus | 4 | 0 | 0 | ||||
| green-winged teal | Anas crecca | 8 | 0 | 0 | ||||
| spot-billed duck | Anas poecilorhyncha | 12 | 2 | 16.67 | H6N6 (2) | |||
| common kingfisher | Alcedo atthis | 14 | 0 | 0 | ||||
| Subtotal | 405 | 3 | 0.74 | |||||
| Shanwei | 02-8-2014 | wetland | rose-ringed parrakeet | Psittacula krameri | 10 | 0 | 0 | |
| night heron | Nycticorax nycticorax | 20 | 0 | 0 | ||||
| oriental magpie-robin | Copsychus saularis | 14 | 1 | 7.14 | H5N6 (1) | |||
| common moorhen | Gallinula chloropus | 12 | 0 | 0 | ||||
| great white egret | Ardea alba | 6 | 0 | 0 | ||||
| little egret | Egretta garzetta | 8 | 0 | 0 | ||||
| purple heron | Ardeapurpurea | 9 | 0 | 0 | ||||
| gray heron | Ardeacinerea | 5 | 0 | 0 | ||||
| Subtotal | 84 | 1 | 1.19 | |||||
| 25-12-2014 | lake | common moorhen | Gallinula chloropus | 14 | 0 | 0 | ||
| spotted dove | Streptopeliachinensis | 18 | 0 | 0 | ||||
| night heron | Nycticorax nycticorax | 20 | 0 | 0 | ||||
| purple heron | Ardea purpurea | 8 | 0 | 0 | ||||
| great white egret | Ardea alba | 13 | 0 | 0 | ||||
| swan goose | Anser cygnoides | 31 | 2 | 6.45 | H5N1 (2) | |||
| daurian redstart | Phoenicurus auroreus | 4 | 0 | 0 | ||||
| dusky warbler | Phylloscopus fuscatus | 12 | 0 | 0 | ||||
| grebe | Podicipediformes | 6 | 0 | 0 | ||||
| black-throated stone-chat | Saxicola torquata | 4 | 0 | 0 | ||||
| spot-billed duck | Anas poecilorhyncha | 22 | 0 | 0 | ||||
| turtle dove | Streptopelia orientalis | 43 | 0 | 0 | ||||
| Subtotal | 195 | 2 | 1.03 | |||||
| Total | 1092 | 11 | 1.01 |
Note: a“n” represents the number of positive samples.
Phylogenetic analyses of the H5N6 viruses
We constructed maximum likelihood trees for each of the eight viral genomic segments from our new sequences combined with data from public sources (listed in Supplementary Table 1). The HA gene of our three new isolates grouped with clade 2.3.4.4 H5 of influenza viruses (Fig. 1A). Phylogenetic analysis of the N6-NA gene showed that it could be delineated into two major lineages (marked as lineages A and B, in Supplementary Figure 1A).
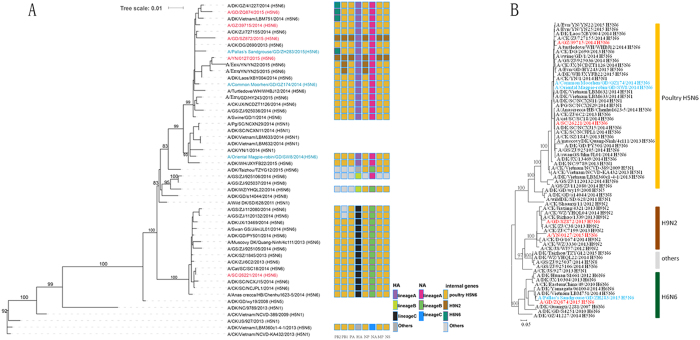
Figure 1.
Phylogenetic analyses of complete hemagglutinin (A) and PB2 (B) genes of indicated H5N6 viruses. Viruses highlighted in blue were characterized in this study. Human-isolated H5N6 are marked in red. Genotypes of the influenza viruses are shown on the right (A) as eight coloured blocks representing each gene segment (from left to right: PB2, PB1, polymerase acidic, haemagglutinin, nucleoprotein, neuraminidase, matrix and non-structural) with the colour indicating the lineage of that segment. Host species are: CK (chicken), DK (duck), GS (goose), PG (pigeon). Geographic locations are: ZJ (Zhejiang), GD (Guangdong), JX (Jiangxi), YN (Yunnan), DG (Dongguan), JS (Jiangsu), GZ (Guangzhou), Env (environment), SC (Sichuan), WZ (Wenzhou), SD (Shandong), SZ (Shenzhen), NC (Nanchang), WH (Wuhan), and HB (Hubei).
Except for PB2, the other genomic segments (HA, NA, PB1, PA, NP, MP and NS) of these three IAV isolates cluster with poultry H5N6 viruses (Supplementary Figure 1B–F). The gene sequences of ZH283 (HA, 99.2%; NA, 98.9%; PB1, 99.3%; PA, 99.3%; NP, 99.7%; MP, 99.3%; and NS, 99.2%), SW8 (HA, 98.4%; NA, 99.7%; PB1, 99.1%; PA, 99.3%; NP, 99.3%; MP, 99.3%; and NS, 99.1%), and GZ174 (HA, 99.7%; NA, 99.9%; PB1, 99.2%; PA, 99.1%; NP, 99.5%; MP, 98.9%; and NS, 99.0%) have high identity with those of A/chicken/Dongguan/2690/2013 (H5N6). For PB2, GZ174 and SW8 cluster with poultry H5N6 viruses, and have high identify with that of A/chicken/Dongguan/2690/2013 (H5N6) (99.4%, and 99.4%, respectively). While the PB2 gene of ZH283 is derived from poultry H6N6 (Fig. 1B), and has the highest identify (98.3%) with that of A/duck/Yamagata/061004/2014 (H6N6).
Molecular characterization
The SW8, GZ174 and ZH283 IAV isolates have a string of basic amino acids at the HA cleavage site (-RRRKR↓G-), which is characteristic of HPAIVs15,16. The HA1 receptor-binding pocket of all three isolates retained the amino acid residues Q226 and G228 (H3 numbering), which preferentially recognize the avian influenza virus receptor (α-2,3 galactose sialic acids) (Supplementary Table 2)17. However, positions 138 A and 155 T of these three isolates (except position I155 of the ZH283 virus, which was not mutated) indicate that these isolates should also have affinity for human-type receptors (α-2,6 galactose sialic acids)18,19 (Supplementary Table 2).
The three isolates have an eleven amino acid residue deletion (-TIINNHPQNNF-) in the stalk of the NA protein, which contributes to increased virulence in mice20. The H274Y mutation was not found in the NA protein, indicating that these isolates might be sensitive to neuraminidase inhibitor drugs such as Oseltamivir (Tamiflu)21,22.
The PB2 genes of these three isolates do not have the mutations E627K or D701N, which are considered to be the predominant factor for cross-species transmission from avian to mammals23,24. The amino-acid substitution S31N in the M2 protein was not observed, indicating that the three isolates should be sensitive to influenza A M2 protein inhibitors such as amantadine25. Studies have shown that the virulence of the influenza virus in humans is associated with viral resistance to the antiviral effects of cytokines, such as interferons, and that the mutation D92E in the NS1 protein promotes greater resistance to these cytokines26, and all three isolates characterized in this study had the D92E mutation in the NS1 protein (Supplementary Table 2).
Discussion
From August 2014 through February 2015, our active surveillance efforts resulted in the recovery of three H5N6 (SW8, GZ174 and ZH283) IAV isolates from wild birds (Table 1). The sequences of these isolates have a string of basic amino acids at the HA cleavage site (-RRRKR↓G-), which is characteristic of HPAIVs15,16. Our study supports that Palla’s sandgrouse (ZH283) and the common moorhen (GZ174) are within the host range of HP H5N6 AIVs currently circulating in poultry and sporadically infecting humans in Asia. These wild birds do not migrate very long-distance. However, H5N6 IAVs can infect wild birds, such as swan geese27 and teal ducks28, which are long-distance migrators. The common moorhen, swan geese and teal ducks have overlapping habitats across the Eurasia (data from Avibase database: https://avibase.bsc-eoc.org). Wild birds have a proven role in the long-distance spread of HPAIVs, such as H5N1 and H5N811,12,13 along their migratory flyways. The detection of H5N6 AIVs in apparently healthy wild birds in this study raises the possibility that wild birds might carry H5N6 to other areas.
The gene sequences of these three H5N6 isolates shared high identity for all genes except PB2. The PB2 genes of SW8 and GZ174 have high identify with those of poultry H5N6 viruses (such as A/chicken/Dongguan/2690/2013 (H5N6)). While the PB2 of ZH283 clustered with A/duck/GZ/41227/2014 (H5N6), A/Guangdong/ZQ874/2015 (H5N6), and A/duck/Vietnam/LBM751/2014 (H5N6), and is derived from duck H6N6 (Fig. 1B). The H5N6 AIVs have different reassortments. Although sequence analysis suggest that these viruses preferentially recognize the avian influenza virus receptor (α-2,3 galactose sialic acids), they have some amino acids that are associated with affinity for human-type receptors (α-2,6 galactose sialic acids). Also these three isolates all have an eleven amino acid residue deletion in the stalk of the NA protein, which is associated with increased virulence in mice20. Thus, it’s possible that they could pose a threat to public health.
The H5N6 viruses have circulated within poultry in China and have caused several human infection cases since 20133,4,5,10. In this study, we systematically analyzed the genetics and phylogeny of three HPAV H5N6 isolates obtained from wild birds in southern China. We found that the H5N6 HPAIVs had different reassortments and affected several species of wild birds in southern China. Future large-scale surveillance efforts of wild birds and poultry are needed to reveal the circulation of these HPAIVs.
Materials and Methods
Ethics Statement
We conducted all animal experiments under the guidance of both the Guangdong Provincial Center for Disease Control and Prevention’s Institutional Animal Care and Use Committee and the Association for Assessment and Accreditation of Laboratory Animal Care International. South China Agricultural University’s Committee on the Ethics of Animal Experiments of Animal Biosafety Level 3 reviewed and approved the study protocols (permit no. 2015–10). The methods were carried out in accordance with these approved guidelines.
Sample Collection
Oropharyngeal and cloacal swab samples were collected from wild birds from August 2014 through February 2015 in Guangdong province, southern China (Table 1). Wetlands or lakes of three cities (Guangzhou, Zhuhai and Shanwei) in Guangdong province were chosen for this surveillance. These three cities represent the three regions of the Pearl River Delta Region. Mist nets and traps were used to capture wild birds. The Forest Police Administration provided some of the samples (e.g. Chinese hwamei and Pallas’s sandgrouse). Oropharyngeal and cloacal swab samples were taken and placed separately in 1.0 ml of transport medium (medium 199) containing antibiotics, and kept in cool boxes until they arrived in the laboratory.
Virus isolation, identification and genomic sequencing
The protocols for virus isolation, and identification were described in our previous studies29. Briefly, samples were inoculated into 9–11 day old embryonated chicken eggs for 48 to 96 hours at 37 °C. Three eggs were inoculated per sample. Allantoic fluid was harvested and tested for the presence of active hemagglutinin. Haemagglutinin-positive isolates were further tested by real-time RT-PCR for the influenza matrix gene, and subtyped by the haemagglutination and neuraminidase inhibition (HAI and NAI) assays.
All three H5N6 positive samples of this surveillance (Table 1) were chosen for viral genome sequencing. Firstly, viral RNA was extracted from the first passage allantoic fluid from the eggs using the QIAamp Viral RNA Minikit (Qiagen, Germany). PCR amplification was performed using segment-specific primers as previously described30. PCR products were purified with the QIAquick PCR purification kit (QIAGEN) and sequenced using an automatic ABI Prism 3730 genetic analyzer (Applied Biosystems). The full length genomic sequences of the three H5N6 IAV isolates, A/Oriental Magpie-robin/Guangdong/SW8/2014 (H5N6), A/Common Moorhen/Guangdong/GZ174/2014 (H5N6) and A/Pallas’s Sandgrouse/Guangdong/ZH283/2015 (H5N6), were deposited into GenBank (accession numbers: KT454936-KT454959).
Phylogenetic analyses
On 20 January 2016, all available H5, H6N6 and H9N2 AIV subtype sequences were collated from GenBank and the Global Initiative on Sharing Avian Influenza Data (GISAID). These sequences were combined with the genome sequences obtained in this study, and the sequences of each gene were separately aligned using the European Bioinformatics Institute’s MUSCLE31. Phylogenetic trees were constructed using RAxML v8.2.432, and 66 sequences that appeared to be the most closely related to the H5N6 outbreak isolates were selected for further analysis (listed in Supplementary Table 1). Maximum likelihood phylogenies of these sequences were constructed using PhyML with bootstrap analysis (1,000 replicates)33. The phylogenies deduced for all segments are shown in Fig. 1 and Supplementary Figure 1.
Additional Information
How to cite this article: Kang, Y. et al. Highly pathogenic H5N6 influenza A viruses recovered from wild birds in Guangdong, southern China, 2014–2015. Sci. Rep. 7, 44410; doi: 10.1038/srep44410 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was supported by grants from the Science and Technology Projects of Guangdong Province (No. 20140224), and Guangdong Natural Science Funds for Distinguished Young Scholar (2014A030306046).
Footnotes
The authors declare no competing financial interests.
Author Contributions T.R. and Y.S. conceived and designed the experiments. Y.K., L.L., M.F., R.Y., C.H., Y.T., P.G., D.X., X.Z., and Y.L. performed the experiments. Y.K. and Y.S. analyzed the data. Y.K., Y.S. and D.M.I. wrote the paper. All authors reviewed the manuscript.
References
- Guan Y. & Smith G. J. D. The emergence and diversification of panzootic H5N1 influenza viruses. Virus Res 178, 35–43 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J. S., de Jong M. D. & Guan Y. Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev 20, 243–267 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Wu B., Chen Y., Bi Y. & Xie Q. Influenza A(H5N6) Virus Reassortant, Southern China, 2014. Emerg Infect Dis 21, 1261–1262 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y. et al. Two novel reassortants of avian influenza A (H5N6) virus in China. J Gen Virol. 96, 975–981 (2015). [DOI] [PubMed] [Google Scholar]
- Qi X., Cui L., Yu H., Ge Y. & Tang F. Whole-Genome Sequence of a Reassortant H5N6 Avian Influenza Virus Isolated from a Live Poultry Market in China, 2013. Genome Announc 2, e00706–00714 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y. et al. Genesis, Evolution and Prevalence of H5N6 Avian Influenza Viruses in China. Cell Host Microbe 25, 30448–30446 (2016). [DOI] [PubMed] [Google Scholar]
- Wong F. Y. K. et al. Reassortant Highly Pathogenic Influenza A(H5N6) Virus in Laos. Emerging infectious diseases 21, 511–516 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y. J. et al. Genetic characterisation of novel, highly pathogenic avian influenza (HPAI) H5N6 viruses isolated in birds, South Korea, November 2016. Euro Surveill 22, 1560–7917 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok C. K. P. et al. Genetic Characterization of Highly Pathogenic Avian Influenza A(H5N6) Virus, Guangdong, China. Emerg Infect Dis 21, 2268–2271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y. Y. et al. Novel Reassortant Avian Influenza A(H5N6) Viruses in Humans, Guangdong, China, 2015. Emerg Infect Dis 22, 1507–1509 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. et al. Global Patterns of Influenza A Virus in Wild Birds. Science 312, 384–388 (2006). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science 309, 1206 (2005). [DOI] [PubMed] [Google Scholar]
- Global Consortium for H5N8 and Related Influenza Viruses. Role for migratory wild birds in the global spread of avian influenza H5N8. Science 354, 213–217 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. et al. Global patterns of influenza a virus in wild birds. Science 312, 384–388 (2006). [DOI] [PubMed] [Google Scholar]
- Belser J. A., Szretter K. J., Katz J. M. & Tumpey T. M. Use of animal models to understand the pandemic potential of highly pathogenic avian influenza viruses. Adv Virus Res 73, 55–97 (2009). [DOI] [PubMed] [Google Scholar]
- Nobusawa E. et al. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology 182, 475–485 (1991). [DOI] [PubMed] [Google Scholar]
- Ha Y., Stevens D. J., Skehel J. J. & Wiley D. C. X-ray structures of H5 avian and H9 swine influenza virus hemagglutinins bound to avian and human receptor analogs. Proc Natl Acad Sci USA 98, 11181–11186 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamuri A. U., Dos Reis M., Hay A. J. & Goldstein R. A. Identifying changes in selective constraints: host shifts in influenza. PLoS Comput Biol 5, e1000564 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. et al. Genetics, receptor binding property, and transmissibility in mammals of naturally isolated H9N2 Avian Influenza viruses. PLoS Pathog 10, e1004508 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y. et al. Neuraminidase stalk length and additional glycosylation of the hemagglutinin influence the virulence of influenza H5N1 viruses for mice. J Virol 83, 4704–4708 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Quack G., Klenk H. D. & Webster R. G. How to overcome resistance of influenza A viruses against adamantane derivatives. Antiviral Res 37, 83–95 (1998). [DOI] [PubMed] [Google Scholar]
- Suzuki H. et al. Emergence of amantadine-resistant influenza A viruses: epidemiological study. J Infect Chemother 9, 195–200 (2003). [DOI] [PubMed] [Google Scholar]
- Massin P., van der Werf S. & Naffakh N. Residue 627 of PB2 is a determinant of cold sensitivity in RNA replication of avian influenza viruses. J Virol 75, 5398–5404 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. et al. Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model. J Virol 79, 12058–12064 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell J. R. & Chou J. J. Structure and mechanism of the M2 proton channel of influenza A virus. Nature 451, 591–595 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X. et al. Molecular characterization of highly pathogenic H5N1 avian influenza A viruses isolated from raccoon dogs in China. PLoS One 4, e4682 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z. et al. Fatal H5N6 Avian Influenza Virus Infection in a Domestic Cat and Wild Birds in China. Sci Rep-Uk 5, 10704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S. et al. Epidemiology, Evolution, and Recent Outbreaks of Avian Influenza Virus in China. J Virol 89, 8671–8676 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T. T. et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 502, 241–244 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E., Stech J., Guan Y., Webster R. G. & Perez D. R. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol 146, 2275–2289 (2001). [DOI] [PubMed] [Google Scholar]
- Edgar R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 (2006). [DOI] [PubMed] [Google Scholar]
- Guindon S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59, 307–321 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.