Abstract
Patent foramen ovale (PFO) is a remnant of fetal circulation commonly found in healthy population. However, a large number of clinical conditions have been linked to PFO, the most important being ischemic strokes of undetermined cause (cryptogenic strokes) and migraine, especially migraine with aura. Coexistent atrial septal aneurysm, size of PFO, degree of the shunt, shunt at rest, pelvic deep vein thrombosis, and prothrombotic states (G20210A prothrombin gene mutation, Factor V Leiden mutation, MTHFR: C677T, basal homocystine, recent surgery, trauma, or use of contraceptives) could enhance stroke risk in subjects with PFO. Owing to the complexity of this issue, for any individual presenting with a PFO, particularly in the setting of cryptogenic stroke, it is not clear whether the PFO is pathogenically related to the neurological event or an incidental finding. Thus, a heart-brain team, which individually plans the best strategy, in accordance with neuroimaging findings and anatomical characteristics of PFO, is strongly recommended. In the first part of this review, we discuss the embryologic and anatomic features of PFO, the diagnostic techniques for its identification and evaluation, and the relationship between PFO and neurological syndromes. A special attention is made to provide some key points, useful in a daily clinical practice, which summarize how better we understand PFO clinical significance
Keywords: Cryptogenic stroke, echocardiography, patent foramen ovale
INTRODUCTION
The role of patent foramen ovale (PFO) as underlying mechanism of neurological syndromes is still debated and patients’ management is debated as well. Compared to previous years, recent advances in echocardiography and neuroimaging allow today an accurate individual diagnosis. Moreover, medical and interventional strategy may benefit from information provided by echocardiography, neuroimaging, and genetics, leading to a more tailored therapeutic approach. In this review, we aim to provide an overview on PFO anatomical features and diagnostic techniques for its detection by focusing our attention on management of patients with PFO and cryptogenic stroke (CS) or migraine.
Embriology
PFO is a remnant of fetal circulation, commonly found in the healthy population, with an overall prevalence of 27% in autopsy studies.[1] It presents as a slit or tunnel-like passage in the atrial septum formed by failure of postnatal fusion of the septum primum and septum secundum at level of the fossa ovalis.[2] This “flap valve,” often presenting as a tunnel, permits the right to left shunting (RLS) of blood that is necessary for normal fetal circulation. At birth, the increase in pulmonary blood flow causes the left atrial pressure to exceed the right atrial pressure, leading to closure of the foramen ovale.[3] By the age of 2 years, 75% of people have closure of the foramen ovale while it remains unclosed in the remaining population.[4] Therefore, PFO was considered as an anatomical variant rather than a pathological condition for a long time.
Anatomy: Are all pfo the same?
Since the overlap between the flap and septum secundum is variable, PFO anatomy is also variable, resulting in a tunnel of different width and length, ranging from 5-13 mm and 1-6 mm, respectively.[5] Usually the opening to the right atrium lies between the fixed antero-superior border of fossa ovalis and the thin flap valve posteriorly. On the left side, the entrance usually has a crescent shape and is bounded by the free edge of the flap valve. Under normal physiological conditions, the higher left atrial pressure gently pushes the thin septum primum against the septum secundum and, except for very brief periods in each cardiac cycle, seals the potential opening of the PFO. Actions, such as the release of a Valsalva maneuver (VM) or severe pulmonary hypertension, can transiently reverse the normal left-to-right pressure gradient and cause an exaggerated transient leftward shift of the free edge of the septum primum with enlargement of the PFO. The PFO anatomy can shift from simple form to complex structural conformation: The septum primum may be aneurysmal (atrial septum aneurysm, ASA) and may have single or multiple openings.[6] Furthermore, PFO may be associated withother defects: The Eustachian ridge, the Eustachian valve, the Chiari network, and it constitutes a hybrid lesion when associated with an atrial septal defect (ASD). In particular cases, the distinction between ASD and PFO is not simple. Usually, these borderline defects present a rest left-to-right shunt, which is instead less common in a simple PFO.
Key point
With the name “PFO” we actually include a spectrum of different defects that may show various anatomical complexity. Clinical significance of a single PFO depends on its anatomical features, thus a complex PFO can be considered at higher risk for neurological events rather than a simple defect. Furthermore, the assessment of anatomical PFO complexity is of paramount importance to guide patient management (PFO closure or medical therapy) and in the choice of closure device.
Diagnosis: Which is the best technique?
Several echocardiographic techniques can be used for PFO detection, including echocardiography -transthoracic (TTE) and transoesophageal (TEE), and transcranial Doppler ultrasonography (TCD).[7] All these imaging modalities require the use of a contrast, typically saline solution, and rarely commercially available contrast agents. Because of its superior image resolution and ability to identify the origin of RLS, contrast-enhanced TEE (c-TEE) is considered the gold standard for PFO diagnosis [Figure 1]. However, in the context of a cryptogenic stroke (CS) work-up, c-TEE is usually preceded by a standard TTE.[8,9,10] If this examination reveals a structurally normal heart and any intra-cardiac sources of emboli, the next step should be to assess for the presence of a PFO through a contrast-enhanced TTE (c-TTE).
Figure 1.
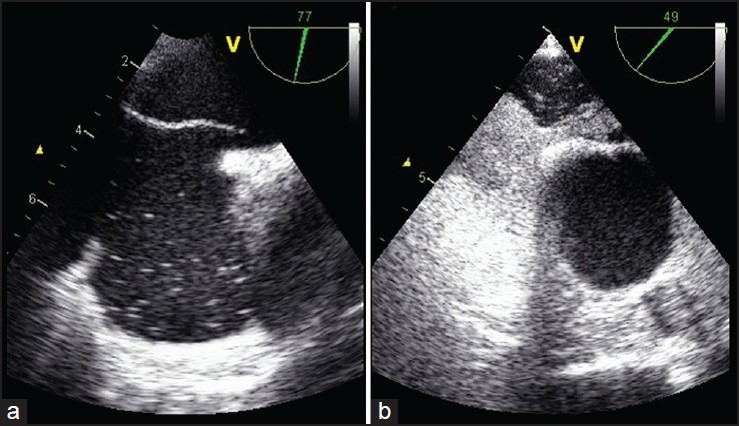
Multiplanare two-dimensional transoesophageal (2D-TEE). a) Bicaval view (77°) identifies a large patent foramen ovale (PFO); b) Short-axis section with aorta (49°): Contrast injection of agitated saline solution shows a severe paradoxical shunt through the PFO, during Valsalva Maneuver
Contrast-enhanced TTE and TEE: Complementary not alternative
c-TTE is a reliable, feasible, cheap, non-invasive technique that enables PFO detection with high specificity, therefore useful as first-line test for screening a PFO.[11] In a recent meta-analysis of 15 studies, c-TTE with harmonic imaging obtained mean sensitivity and specificity of 91% and 93%, respectively, compared to c-TEE for PFO detection.[12] Practice recommendations have been suggested for c-TTE and c-TEEstudies including:
Obtaining a righatrium dense opacification,
Using a temporal cut-off of three cardiac cycles for bubbles appearance in the left atrium[1,11,13,14,15,16] (although some authors prefer to take into account five cardiac cycles, defining early RLS as a shunt occurring within the third cardiac cycle, and late RLS as a shunt manifesting at the fourth or fifth cardiac cycle);[17,18,19]
Ruling-out false positive results (bubbles appearing in the left atrium beyond five cycles are suggestive of a transpulmonary shunting and account for some false positive results);
Demonstrating the effectiveness of VM, which needs to be proved through the visualization of an atrial septal shift to the left.[17]
Nonetheless, c-TTE is affected by some bias, the most important being a low sensitivity in small shunts and the lack of information about aortic sources of emboli. Therefore, if c-TTE study is negative or images are inadequate but the index of suspicion is high, a c-TEE will be useful to assess the presence of thrombus in left atrial appendage, aortic atheroma, cardiac masses, and vegetations that may have been missed by TTE. On the other hand, if c-TTE study is positive for a RLS, a c-TEE will be also required to define the anatomy of the atrial septum, to assess its suitability for device closure, and to confirm that the shunt is due to a PFO rather than a pulmonary shunt or other atrial septum defects.[20]
It is important to recognize, however, that c-TEE is a semi-invasive technique, poorly tolerated by the patient, which often requires sedation. Moreover, an increasing number of false negative results (the most of them due to inaccurate VM) have been demonstrated even with this modality, for a long time considered the gold standard technique for PFO detection.[21] Nowadays, TEE plays instead a pivotal role in monitoring the percutaneous PFO closure. When the device is deployed but still connected to the delivery cable, a fast TEE inspection is indicated in checking for proper device position, performing the “wiggle-maneuver,” and observing the device while it is being released from the cable. Moreover, through a continuous TEE monitoring, a thrombosis of the device can be immediately revealed, even this is a rare complication occurring in about 2% of interventional device closure procedures, mainly related to hypercoagulable states.[22]
Contrast-enhanced transcranial doppler (c-TCD): A very sensitive tool
An attractive alternative to c-TEE in the recognition of a PFO is c-TCD. The literature data show a good concordance between c-TCD and c-TEE, with sensitivity ranging from 70% to 100% and specificity > 95%.[23,24] A recent meta-analysis demonstrated mean sensitivity of 97% and specificity of 93% for c-TCD in comparison with c-TEE.[25] Consequently, this technique is considered as a reliable, non-invasive test with excellent diagnostic accuracy, very useful during CS work-up, with a position in class IIA for RLS identification. The effectiveness of VM can be easily verified throughout the test [Figure 2]. A detailed quantification of paradoxical shunt by c-TCD is based on the number of microembolic signals (MES) in the Doppler flow velocity spectrum of medium cerebral artery (MCA): 0 MES, no shunt; 1-10 MES small shunt; > 10 MES medium shunt; > 10 MES with “curtain effect,” large shunt.[23] The test is deemed positive if at least one MES is recorded on the spectrum of MCA within the first three cardiac cycles from contrast injection [Figure 3].
Figure 2.
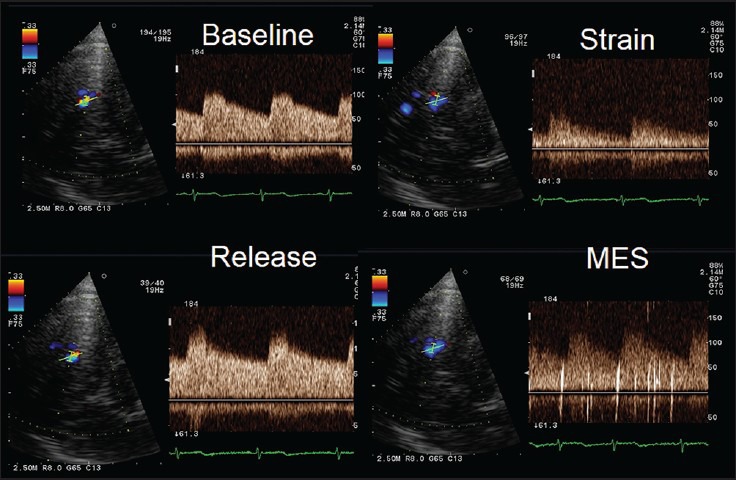
Changes in the middle cerebral artery (MCA) spectrum during contrast-transcranial Doppler (c-TCD) demonstrating the effectiveness of performed Valsalva maneuver. During the strain phase of the maneuver, MCA flow velocity decreases of about 50% compared to baseline evaluation. After realising the maneuver, some microembolic signals (MES) appear suggesting; in this case, a mild paradoxical shunt
Figure 3.
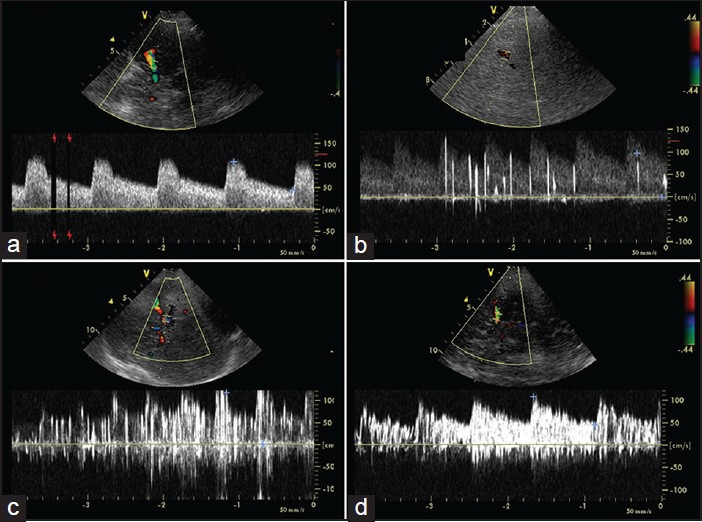
Paradoxical shunt quantification by contrast transcranial Doppler (TCD) based on the number of microembolic signals (MES): a) 0 MES, no shunt; b) 0-10 MES, small shunt; c) 10-20 MES, medium shunt; d: large shunt (> 20 MES) with “curtain effect”
The principal limitation of c-TCD is the difficulty in discriminating between atrial and pulmonary shunts due to the overlap in time when microbubbles are detected in the MCA.[26]
In summary, after a positive c-TTE and/or c-TCD, whereas knowledge of precise PFO anatomy is required, c-TEE can be used, particularly before scheduling a patient for percutaneous closure. On the contrary, after a negative c-TTE and/or c-TCD the search for a PFO should be ended.
The emerging role of three-dimensional echocardiography
A detailed assessment of the PFO is feasible with real-time three-dimensional echocardiography (RT-3DE).[27] This technique enables a clearer understanding of PFO morphology and is particularly helpful in delineating the relationship of the PFO with surrounding structures within the left and right atria and assistance during the closure procedure.[28] Three-dimensional echo is reproducible and accurate in comparison to 2D c-TEE except in small PFO (less than 2 mm). Moreover, by RT-3DE, the types of possible defects can be broadly grouped into simple and complex lesions. A simple PFO is a standard PFO with tunnel length < 8 mm, without ASA, large Eustachian ridge or valve, thickened septum secundum (10 mm), and without other defects of the fossa ovalis. Essentially a PFO falls into this group by excluding specific characteristics that, if present, are considered “complex”[29] that are the following:
PFO with a long tunnel length, usually > 8mm [Figure 4a].
Multiple openings of PFO on left atrial side [Figure 4d].
Hybrid defect (the concomitant occurrence of a PFO with additional defects of the fossa ovalis) [Figures 4b and 5D].
Excessive thickening of septum secundum (due to the amount of extra-cardiac tissue, usually adipose tissue) [Figure 5a].
Presence of Eustachian ridge.
Presence of Eustachian valve (or Chiari network) [Figure 5a].
Figure 4.
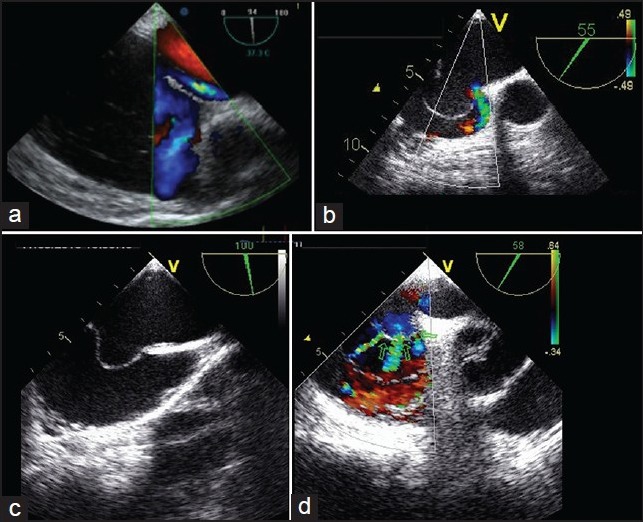
Examples of complex patent foramen ovale (PFO) by transoesophageal (TEE). A) Short-axis view with aorta (94°): A “tunnel-like” PFO (tunnel length > 8 mm), identified by color doppler. b) Short-axis view with aorta (55°): A PFO associated with an ostium secundum defect, identified by color doppler; c) Bicaval view (100°): An atrial septal aneurysm with, d) (58°) multiple septal openings (green arrows)
Figure 5.
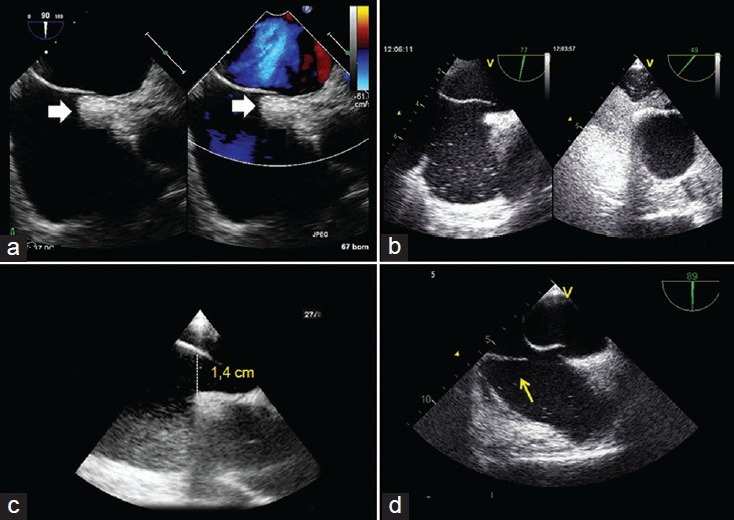
Examples of complex patent foramen ovale (PFO) by transoesophageal (TEE): a) Bicaval view (90°): The white arrows show the excessive thickening of septum secundum; b) (77° and 49°) a PFO with a large shunt; c) a large (1.4 cm) opening of a PFO toward the right atrium; d) bicaval view (89°), the yellow arrow shows a redundant Eustachian valve in the right atrium
Key point
Contrast echocardiography has become the method of choice to discover a PFO, since it permits direct shunt visualization. c-TEE appears superior to other ultrasonographic techniques because it provides high-quality images, but it is poorly tolerated and time-consuming. c-TTE is accurate and easy to be performed, but it shows low sensitivity particularly in small shunts. c-TCD is a very sensitive, feasible, and accurate tool for RLS detection; accordingly, c-TCD and c-TTE can be considered complementary and both included as standard examinations during CS work-up. Thus, an empiric approach could take into account an integration of c-TTE and c-TCD, as first-line tools while c-TEE and, if available, 3D-TEE, should be limited to the following selected categories of subjects:
Patients scheduled for transcatheter PFO closure;
Patients in whom either the PFO diagnosis is uncertain or an alternative embolic cardiac source must be considered;
Patients with high-risk PFO, particularly those experiencing recurrent CE and
Patients with ASA and/or large RLS or curtain effect on TCD [Table 1].
Table 1.
Ultrasonographic tools for evaluating PFO morphology, shunt severity, and for patient management: Advantages and limitations
| Requirements | C-TEE | C-TTE | C-TCD |
|---|---|---|---|
| Sensitivity | +++ | ++ | +++ |
| Specificity | +++ | +++ | ++ |
| Patient tolerance | + | +++ | +++ |
| Feasibility | ++ | +++ | ++++ |
| Safety | ++ | +++ | +++ |
| Low-cost | + | +++ | +++ |
| Shunt quantification | ++ | ++ | +++ |
| Anatomic details | +++ | ++ | — |
| ASA detection | +++ | +++ | — |
| Guide to PFO closure | +++ | + | — |
| Follow up after PFO closure | + | ++ | +++ |
– = Not fulfilled, + = Poorly fulfilled, ++ = Sufficiently fulfilled, +++ = Widely fulfilled, PFO = Patent foramen ovale, C-TEE = Contrast-enhanced transoesophageal echocardiography, C-TTE = Contrast-enhanced transthoracic echocardiography, C-TCD = Contrast-enhanced transcranial Doppler ultrasonography
Why searching for a PFO? the challenging of guilty or not guilty PFO in neurological syndromes
Several pathological conditions are associated with PFO, including CS, migraine, systemic arterial embolism, decompression illness, and platypnea-orthodeoxia syndrome, etc. However, the most frequently encountered clinical situations are CS and migraine, especially migraine with aura (MA). Since several years, a consistent pool of studies aiming to demonstrate a pathophysiological relationship between PFO and neurological syndromes has been published with discordant results. Furthermore, there is, to date, inconsistency with regards clinical management of patients with PFO and neurological syndromes.
Cryptogenic stroke (CS) and PFO: Tips and tricks
A CS is generally defined as a stroke of unknown cause, despite extensive investigation to exclude other causes, such as aortic and carotid atheroma, carotid dissection, and atrial fibrillation (AF), as well as intracerebral haemorrhage and space-occupying lesions.[10,32,33]
The association between CS and PFO is one of the most controversial issues of the literature, owing to the instance that paradoxical embolism (the systemic passage of thrombi of venous origin through an interatrial conduit) remains frequently a diagnosis of suspicion.[34]
Since 1988, with the first case-control study by Lechat et al.,[35] several further retrospective studies were published,[33,36,37,38,39,40,41] showing how PFO prevalence was 4- and 2-fold increased in young (less than 55 years) and old (more than 55 years) stroke patients, respectively, compared to stroke free control subjects of the same sex and age.[42] [Table 2]. Nonetheless, in the following years, these data were denied by larger prospective studies with a long-term follow-up. In fact, in 2006, Meissner et al., demonstrated that in a population of 585 subjects, during a follow-up of 5.1 years, PFO was not a significant independent predictor of stroke, and that the risk of a cerebrovascular event among subjects with ASA was nearly four times higher than that in those without ASA, introducing the role of ASA as risk factor for stroke [Table 3].[43] Later, in 2007 Di Tullio et al.,[44] sought to assess the risk of ischemic stroke from a PFO in a multiethnic prospective cohort; their results showed that PFO was not found to be significantly associated with stroke, and that the coexistence of PFO and ASA did not increase the stroke risk, but isolated ASA was associated with elevated stroke incidence [Table 3]. These data were recently confirmed from the same group that evaluated the risk of stroke associated with PFO after adjusting for established stroke risk factors, as well the odds of having silent brain infarcts among patients with and without PFO. Authors concluded that PFO was not associated with an increased risk of clinical stroke or subclinical cerebrovascular disease[45] [Table 3].
Table 2.
Relationship between cryptogenic stroke and PFO in young and old patients (case-controls studies from 1988 to 2006)
| Study | Number of patients (n) | Age of patients (years) | PFO (cryptogenic) (%) | PFO (controls) (%) | P |
|---|---|---|---|---|---|
| Prevalence of PFO in young patients | |||||
| Lechat[35] | 26 | <55 | 54 (14/26) | 10 (10/100) | <0.001 |
| Webster[36] | 34 | <40 | 56 (19/34) | 15 (6/40) | <0.001 |
| Cabanes[37] | 64 | <55 | 56 (36/64) | 18 (9/50) | <0.001 |
| De Belder[38] | 39 | <55 | 13 (5/39) | 3 (1/39) | — |
| Di Tullio[39] | 21 | <55 | 47 (10/21) | 4 (1/24) | <0.001 |
| Hausmann[40] | 18 | <40 | 50 (9/18) | 11 (2/18) | <0.05 |
| Handke[33] | 82 | <55 | 44 (36/82) | 14 (7/49) | <0.001 |
| Total | 284 | 45 (129/284) | 11 (36/320) | <0.001 | |
| Prevalence of PFO in old patients | |||||
| De Belder[38] | 64 | >55 | 20 (13/64) | 5 (3/56) | <0.001 |
| Di Tullio[39] | 24 | >55 | 38 (9/24) | 8 (6/77) | <0.001 |
| Hausmann[40] | 20 | >40 | 15 (3/20) | 23 (23/98) | ns |
| Jones[41] | 57 | >50 | 18 (10/57) | 16 (29/183) | ns |
| Handke[33] | 145 | >55 | 28 (41/145) | 12 (28/232) | <0.001 |
| Total | 310 | 25 (76/310) | 14 (89/646) | <0.001 | |
PFO = Patent foramen ovale
Table 3.
Relationship of cryptogenic stroke and PFO in prospective studies from 2006 to 2013
| Variable | Meissner 2006[43] | Di Tullio 2007[44] | Di Tullio 2013[45] |
|---|---|---|---|
| Patients, n | 585 | 1100 | 1100 |
| Age, years (mean age) | >45 (66.9±13.3) | >39 (68.7±10.0) | >39 |
| PFO, n (%) | 140 (24.3) | 164 (14.9) | 164 (14.9) |
| PFO + ASA, n (%) | 6 (4.3) | 19 (1.7) | 19 (11.6) |
| Follow-up | 5.1 years | 79.7±28 months | 11±4.5 years |
| CVE during F. U., n (%) | 41 (7) | 68 (6.2) | 111 (10.1) |
| Cryptogenic stroke in PFO (+) % | 12 | 18.2 | 15 (9.2) |
| Cryptogenic stroke in PFO (−) % | — | 21.2 | 96 (10.3) |
| Adjusted PFO alone HR (CI) for ischemic stroke | 1.46 (0.74-2.88) P=ns* | 1.79 (0.93-3.45) P=ns† | 1.23 (0.70-2.16) P=ns† |
| Adjusted PFO + ASA HR (CI) for ischemic stroke | 3.72 (0.88-15.71) P=0.07# | 1.04 (0.14-7.74) P=ns† | 0.48 (0.07-3.50) P=ns† |
*Prior myocardial infarction and atrial fibrillation, †Adjusted for age, sex, atrial fibrillation, diabetes mellitus, hypertension, hypercholesterolemia, and current smoking, #for ASA alone, CI=confidence interval, CVE=cerebro-vascular events, FU=follow-up, HR=hazard ratio, PFO = Patent foramen ovale, ASA = Atrial septum aneurysm
Key point
The discrepancy regarding PFO pathogencity can be explained taking into consideration two main concerns, the first is that, owing to its large prevalence in the general population, PFO may be often an incidental finding in CS patients; the second includes various features (event-, patient-, and PFO-related) that must be deeply known in order to distinguish a guilty from a non-guilty PFO, in a case by case evaluation.
An integrated approach for a complex problem
The first aim of any diagnostic workflow in the context of a CS is to identify a link between the index cryptogenic cerebral ischemic event and any given PFO. However, we must recognize that, in the majority of cases, the association between PFO and CS is not causal but probabilistic and that the degree of probability of a causal association will guide the next steps of treatment decisions. For this purpose, the search for a causal relationship needs to be centered on the clinical and anatomical factors that can estimate the probability that a given and specific PFO may be a critical element of a larger clinical syndrome. Considering the complexity of these assessments, which include cardiac, neurological, hematological, and imaging aspects, it is necessary the formal establishment of a working group that fulfills the need for a multidisciplinary estimate. It is therefore recommended that patients with CS and PFO would refer to a brain-heart team for a joint evaluation of individual cases in order to get to personalized choices which take into account neurological and cardiological aspects (clinical, imaging, and interventional).[7]
Firstly, it is important to remind that subclinical atrial fibrillation (AF) and PFO are the major causes of CS. Before any invasive testing, an accurate clinical history must be taken, focusing on symptoms potentially related to cardiac embolism and symptoms suggestive of arrhythmias. Moreover, neuroimaging lesion patterns may help to distinguish AF from PFO-related strokes. Indeed, it has been recently demonstrated that PFO-stroke is more frequently observed as a single cortical or multiple small-scattered lesions and is more often found in the vertebro-basilar circulation, without any visible occlusion by magnetic resonance angiography; moreover, the recanalization rate seems to be lower than that observed in stroke due to AF. These differences in imaging characteristics may be useful to identify the mechanisms of CS and choose the best treatment for secondary prevention.[46] The essential information provided by neuroimaging techniques in CS and PFO has been recently confirmed by two studies that tried to detect, through T2-weighted magnetic resonance imaging (T2WI) and fluid-attenuated inversion recovery (FLAIR) sequences, the lesion patterns, and the stroke mechanisms in CS patients with PFO compared to those of patients with CS but no PFO. They found that multiple small ischemic lesions and subcortical frontal and parietal small lesions were significantly associated with CS patients with PFO, confirming that paradoxical embolism may be the mechanism of PFO-associated CS patients.[47,48]
Another concern, in this clinical setting, regards the role of cofactors. Actually, coexistent ASA,[49] large PFO (defined as at least 2 mm separation of septa or at least 10 microbubbles in the left atrium),[50] the presence of spontaneous RLS at rest and its magnitude,[51,52] pelvic deep vein thrombosis[53] and prothrombotic states (G20210A prothrombin gene mutation, Factor V Leiden mutation, MTHFR: C677T, basal homocystine, recent surgery, trauma, or use of contraceptives)[54,55] are recognized factors that may potentiate stroke risk in patients with PFO.
Key point
Ruling out AF, looking at the neuroimaging lesion patterns, screening for thrombophilia, searching for anatomical (ASA, large PFO, RLS at rest, Eustachian valve >10 mm, Chiari network, and long PFO tunnel) and clinical (multiple ischemic lesions on CT or MRI, recurrent clinical events, history of deep vein thrombosis, pulmonary embolism, event related to travel or prolonged immobilization) data, all constitute pivotal elements in the insidious path of understanding PFO clinical significance.
Migraine and PFO: More doubts than certainties
Migraine has an important impact on the quality of life and seems to be one of the most disabling medical illnesses. There is an association between PFO and migraine, particularly MA, but it is unclear if there is a causal relationship or if it is only comorbidity.[56,57]
One possible mechanism of explaining how RLS may play a role in MA is related to the occurrence of subclinical emboli and/or higher concentrations of serotonin and other metabolites that avoid the lungs and directly enter the systemic circulation. This causes irritation of the trigeminal nerve and brain vasculature, triggering migraine.[58]
An important role of ASA, beyond PFO, in the genesis of aura has been previously demonstrated in a study showing that the prevalence of isolated ASA is higher in patients with MA than in patients with migraine without aura or in control subjects.[59]
Recently, the relationship between migraine and PFO has been investigated in a review of 9 studies, revealing that approximately 57% of patients with MA had PFO compared with 19% of healthy subjects. On the contrary, the prevalence of PFO in patients with migraine without aura is similar to that of healthy population.[60] However, there are data rejecting the correlation between PFO and migraine. In 2012, a systematic review and stratified meta-analysis concluded that there is not a strong causal role for PFO in migraine.[61] However, this conclusion about PFO and migraine was taken from the only population-based study assessing the relation of PFO and migraine, the NOMAS study[62] that is an epidemiologic study evaluating patients who had a stroke and reported history of migraine. Fifteen percent of patients had PFO and 16% of patients self-reported migraine. The mean age of the cohort group was 69 ± 10 years, and 58% were women. Prevalence of PFO was similar in patients with and without migraine. But, several limitations affect this study: TTE was the only technique used for PFO diagnosis, the age-group was older for migraine patients, a self-reporting of disease may not be reliable. Moreover, Schwerzmann et al., in a TEE study found that small shunts were equally prevalent in migraineurs and controls, while larger shunts were more prevalent in patients with MA, conferring a role of RLS severity in determining the aura.[63]
Key point
Observational studies suggest that there is a higher incidence of migraine, especially MA, in patients with PFO and vice versa, but the only population-based study, does not support this evidence.
Conclusion part I
As pieces of a puzzle, several elements need to be accurately researched prior to consider a PFO responsible for a neurological event. Echocardiography, together with other hematological and instrumental investigations, play an essential role to treat this challenging condition with a logical approach. Certainly, we cannot oversimplify with a standard common management for each subject wo has a PFO.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gupta V, Yesilbursa D, Huang WY, Aggarwal K, Gupta V, Gomez C, et al. Patent foramen ovale in a large population of ischemic stroke patients: Diagnosis, age distribution, gender and race. Echocardiography. 2008;25:217–27. doi: 10.1111/j.1540-8175.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 2.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- 3.Calvert PA, Rana BS, Kydd AC, Shapiro LM. Patent foramen ovale: Anatomy, outcomes, and closure. Nat Rev Cardiol. 2011;8:148–60. doi: 10.1038/nrcardio.2010.224. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed S, Sadiq A, Siddiqui AK, Borgen E, Mattana J. Paradoxical arterial emboli causing acute limb ischemia in a patient with essential thrombocytosis. Am J Med Sci. 2003;326:156–8. doi: 10.1097/00000441-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Ho SY, McCarthy KP, Rigby ML. Morphological features pertinent to interventional closure of patent oval foramen. J Interv Cardiol. 2003;16:33–8. doi: 10.1046/j.1540-8183.2003.08000.x. [DOI] [PubMed] [Google Scholar]
- 6.Kutty S, Sengupta P, Khandheria BK. Patent foramen ovale: The known and to be known. J Am Coll Cardiol. 2012;59:1665–71. doi: 10.1016/j.jacc.2011.09.085. [DOI] [PubMed] [Google Scholar]
- 7.Pristipino C, Anzola GP, Ballerini L, Bartorelli A, Cecconi M, Chessa M, et al. Italian Society of Invasive Cardiology (SICI-GISE); Italian Stroke Association (ISA-AIS); Italian Association of Hospital Neurologists, Neuroradiologists, Neurosurgeons (SNO); Congenital Heart Disease Study Group of Italian Society of Cardiology; Italian Association of Hospital Cardiologists (ANMCO); Italian Society of Pediatric Cardiology (SICP), et al. Management of patients with patent foramen ovale and cryptogenic stroke: A collaborative, multidisciplinary, position paper: Executive summary. Catheter Cardiovasc Interv. 2013;82:122–9. doi: 10.1002/ccd.24693. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, et al. American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council: Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873–923. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:483S–512S. doi: 10.1378/chest.126.3_suppl.483S. [DOI] [PubMed] [Google Scholar]
- 10.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicentre clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 11.Ha JW, Shin MS, Kang S, Pyun WB, Jang KJ, Byun KH, et al. Enhanced detection of right-to-left shunt through patent foramen ovale by transthoracic contrast echocardiography using harmonic imaging. Am J Cardiol. 2001;87:669–71. doi: 10.1016/s0002-9149(00)01455-7. [DOI] [PubMed] [Google Scholar]
- 12.Mojadidi MK, Winoker JS, Roberts SC, Msaouel P, Gevorgyan R, Zolty R. Two-dimensional echocardiography using second harmonic imaging for the diagnosis of intracardiac right-to-left shunt: A meta-analysis of prospective studies. Int J Cardiovasc Imaging. 2014;30:911–23. doi: 10.1007/s10554-014-0426-8. [DOI] [PubMed] [Google Scholar]
- 13.Van Camp G, Franken P, Melis P, Cosyns B, Schoors D, Vanoverschelde JL. Comparison of transthoracic echocardiography with second harmonic imaging with transesophageal echocardiography in the detection of right to left shunts. Am J Cardiol. 2000;86:1284–7, A9. doi: 10.1016/s0002-9149(00)01224-8. [DOI] [PubMed] [Google Scholar]
- 14.Clarke NR, Timperley J, Kelion AD, Banning AP. Transthoracic echocardiography using second harmonic imaging with Valsalva manoeuvre for the detection of right to left shunts. Eur J Echocardiogr. 2004;5:176–81. doi: 10.1016/S1525-2167(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 15.Souteyrand G, Motreff P, Lusson JR, Rodriguez R, Geoffroy E, Dauphin C, et al. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr. 2006;7:147–54. doi: 10.1016/j.euje.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Kerut EK, Lee S, Fox E. Diagnosis of an anatomically and physiologically significant patent foramen ovale. Echocardiography. 2006;23:810–5. doi: 10.1111/j.1540-8175.2006.00318.x. [DOI] [PubMed] [Google Scholar]
- 17.Attaran RR, Ata I, Kudithipudi V, Foster L, Sorrell VL. Protocol for optimal detection and exclusion of a patent foramen ovale using transthoracic echocardiography with agitated saline microbubbles. Echocardiography. 2006;23:616–22. doi: 10.1111/j.1540-8175.2006.00272.x. [DOI] [PubMed] [Google Scholar]
- 18.Trevelyan J, Steeds RP. Comparison of transthoracic echocardiography with harmonic imaging with transoesophageal echocardiography for the diagnosis of patent foramen ovale. Postgrad Med J. 2006;82:613–4. doi: 10.1136/pgmj.2006.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thanigaraj S, Valika A, Zajarias A, Lasala JM, Perez JE. Comparison of transthoracic versus transesophageal echocardiography for detection of right-to-left atrial shunting using agitated saline contrast. Am J Cardiol. 2005;96:1007–10. doi: 10.1016/j.amjcard.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 20.Hildick-Smith D, Behan M, Haworth P, Rana B, Thomas M. Patent foramen ovale closure without echocardiographic control: Use of “standby” intracardiac ultrasound. JACC Cardiovasc Interv. 2008;1:387–91. doi: 10.1016/j.jcin.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues AC, Picard MH, Carbone A, Arruda AL, Flores T, Kloh J, et al. Importance of adequately performed valsalvamaneuver to detect patent foramen ovale during transesophageal echocardiography. J Am Soc Echocardiogr. 2013;26:1337–43. doi: 10.1016/j.echo.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Bartel T, Müller S. Contemporary echocardiographic guiding tools for device closure of interatrial communications. Cardiovasc Diagn Ther. 2013;3:38–46. doi: 10.3978/j.issn.2223-3652.2013.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sloan MA, Alexandrov AV, Tegeler CH, Spencer MP, Caplan LR, Feldmann E, et al. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: Transcranial Doppler ultrasonography: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2004;62:1468–81. doi: 10.1212/wnl.62.9.1468. [DOI] [PubMed] [Google Scholar]
- 24.Zito C, Dattilo G, Oreto G, Di Bella G, Lamari A, Iudicello R, et al. Patent Foramen Ovale: Comparison among diagnostic strategies in cryptogenic stroke and migraine. Echocardiography. 2009;26:495–503. doi: 10.1111/j.1540-8175.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- 25.Mojadidi MK, Roberts SC, Winoker JS, Romero J, Goodman-Meza D, Gevorgyan R, et al. Accuracy of transcranial Doppler for the diagnosis of intracardiac right-to-left shunt: A bivariate meta-analysis of prospective studies. JACC Cardiovasc Imaging. 2014;7:236–50. doi: 10.1016/j.jcmg.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Jauss M, Zanette E. Detection of right-to-left shunt with ultrasound contrast agent and transcranial Doppler sonography. Cerebrovasc Dis. 2000;10:490–6. doi: 10.1159/000016119. [DOI] [PubMed] [Google Scholar]
- 27.Rana BS, Thomas MR, Calvert PA, Monaghan MJ, Hildick-Smith D. Echocardiographic evaluation of patent foramen ovale prior to device closure. JACC Cardiovasc Imaging. 2010;3:749–60. doi: 10.1016/j.jcmg.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Balzer J, Kelm M, Kuhl HP. Real time three-dimensional transoesophageal echocardiography for guidance of non-coronary interventions in the catheter laboratory. Eur J Echocardiogr. 2009;10:341–9. doi: 10.1093/ejechocard/jep006. [DOI] [PubMed] [Google Scholar]
- 29.Rana BS, Shapiro LM, McCarthy KP, Ho SY. Three-dimensional imaging of the atrial septum and patent foramen ovale anatomy: Defining the morphological phenotypes of patent foramen ovale. Eur J Echocardiogr. 2010;11:i19–25. doi: 10.1093/ejechocard/jeq122. [DOI] [PubMed] [Google Scholar]
- 30.Hanley PC, Tajik AJ, Hynes JK, Edwards WD, Reeder GS, Hagler DJ, et al. Diagnosis and classification of atrial septal aneurysm by two-dimensional echocardiography: Report of 80 consecutive cases. J Am Coll Cardiol. 1985;6:1370–82. doi: 10.1016/s0735-1097(85)80228-x. [DOI] [PubMed] [Google Scholar]
- 31.Pearson AC, Nagelhout D, Castello R, Gomez CR, Labovitz AJ. Atrial septal aneurysm and stroke: A transesophageal echocardiographic study. J Am Coll Cardiol. 1991;18:1223–9. doi: 10.1016/0735-1097(91)90539-l. [DOI] [PubMed] [Google Scholar]
- 32.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: A meta-analysis of case-control studies. Neurology. 2000;55:1172–9. doi: 10.1212/wnl.55.8.1172. [DOI] [PubMed] [Google Scholar]
- 33.Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262–8. doi: 10.1056/NEJMoa071422. [DOI] [PubMed] [Google Scholar]
- 34.Stolfo D, Piazza R, Cassin M, Grandis U, Antonini-Canterin F, Pavan D, et al. Patent foramen ovale and cardiovascular stroke: The eternal dilemma. G Ital Cardiol (Rome) 2012;13:474–89. doi: 10.1714/1114.12243. [DOI] [PubMed] [Google Scholar]
- 35.Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–52. doi: 10.1056/NEJM198805053181802. [DOI] [PubMed] [Google Scholar]
- 36.Webster MW, Chancellor AM, Smith HJ, Swift DL, Sharpe DN, Bass NM, et al. Patent foramen ovale in young stroke patients. Lancet. 1988;2:11–2. doi: 10.1016/s0140-6736(88)92944-3. [DOI] [PubMed] [Google Scholar]
- 37.Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, Oubary P, et al. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke. 1993;24:1865–73. doi: 10.1161/01.str.24.12.1865. [DOI] [PubMed] [Google Scholar]
- 38.de Belder MA, Tourikis L, Leech G, Camm AJ. Risk of patent foramen ovale for thromboembolic events in all age groups. Am J Cardiol. 1992;69:1316–20. doi: 10.1016/0002-9149(92)91228-v. [DOI] [PubMed] [Google Scholar]
- 39.Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. 1992;117:461–5. doi: 10.7326/0003-4819-117-6-461. [DOI] [PubMed] [Google Scholar]
- 40.Hausmann D, Mugge A, Becht I, Daniel WG. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol. 1992;70:668–72. doi: 10.1016/0002-9149(92)90210-p. [DOI] [PubMed] [Google Scholar]
- 41.Jones EF, Calafiore P, Donnan GA, Tonkin AM. Evidence that patent foramen ovale is not a risk factor for cerebral ischemia in the elderly. Am J Cardiol. 1994;74:596–9. doi: 10.1016/0002-9149(94)90750-1. [DOI] [PubMed] [Google Scholar]
- 42.Homma S, Di Tullio MR. Patent foramen ovale and stroke. J Cardiol. 2010;56:134–41. doi: 10.1016/j.jjcc.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meissner I, Khandheria BK, Heit JA, Petty GW, Sheps SG, Schwartz GL, et al. Patent foramen ovale: Innocent or guilty. Evidence from a prospective population-based study? J Am Coll Cardiol. 2006;47:440–5. doi: 10.1016/j.jacc.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 44.Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol. 2007;49:797–802. doi: 10.1016/j.jacc.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 45.Di Tullio MR, Jin Z, Russo C, Elkind MS, Rundek T, Yoshita M, et al. Patent foramen ovale, subclinical cerebrovascular disease and ischemic stroke in a population-based cohort. J Am Coll Cardiol. 2013;62:35–41. doi: 10.1016/j.jacc.2013.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim BJ, Sohn H, Sun BJ, Song JK, Kang DW, Kim JS, et al. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke. 2013;44:3350–6. doi: 10.1161/STROKEAHA.113.002459. [DOI] [PubMed] [Google Scholar]
- 47.Huang YY, Shao B, Ni XD. Differential lesion patterns on T2-weighted magnetic resonance imaging and fluid-attenuated inversion recovery sequences in cryptogenic stroke patients with patent foramen ovale. J Stroke Cerebrovasc Dis. 2014;23:1690–5. doi: 10.1016/j.jstrokecerebrovasdis.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 48.Boutet C, Rouffiange-Leclair L, Garnier P, Quenet S, Delsart D, Varvat J, et al. Brain magnetic resonance imaging findings in cryptogenic stroke patients under 60 years with patent foramen ovale. Eur J Radiol. 2014;83:824–8. doi: 10.1016/j.ejrad.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 49.Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, et al. Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740–6. doi: 10.1056/NEJMoa011503. [DOI] [PubMed] [Google Scholar]
- 50.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP PFO in Cryptogenic Stroke Study (PICSS) Investigators. Effect of medical treatment in stroke patients with patent foramen ovale: Patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002;105:2625–31. doi: 10.1161/01.cir.0000017498.88393.44. [DOI] [PubMed] [Google Scholar]
- 51.De Castro S, Cartoni D, Fiorelli M, Rasura M, Anzini A, Zanette EM, et al. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke. 2000;31:2407–13. doi: 10.1161/01.str.31.10.2407. [DOI] [PubMed] [Google Scholar]
- 52.Anzola GP, Zavarize P, Morandi E, Rozzini L, Parrinello G. Transcranial Doppler and risk of recurrence in patients with stroke and patent foramen ovale. Eur J Neurol. 2003;10:129–35. doi: 10.1046/j.1468-1331.2003.00561.x. [DOI] [PubMed] [Google Scholar]
- 53.Ranoux D, Cohen A, Cabanes L, Amarenco P, Bousser MG, Mas JL. Patent foramen ovale: Is stroke due to paradoxical embolism? Stroke. 1993;24:31–4. doi: 10.1161/01.str.24.1.31. [DOI] [PubMed] [Google Scholar]
- 54.Pezzini A, Del Zotto E, Magoni M, Costa A, Archetti S, Grassi M, et al. Inherited thrombophilic disorders in young adults with ischemic stroke and patent foramen ovale. Stroke. 2003;34:28–33. doi: 10.1161/01.str.0000046457.54037.cc. [DOI] [PubMed] [Google Scholar]
- 55.Karttunen V, Hiltunen L, Rasi V, Vahtera E, Hillbom M. Factor V Leiden and prothrombin gene mutation may predispose to paradoxical embolism in subjects with patent foramen ovale. Blood Coagul Fibrinolysis. 2003;14:261–8. doi: 10.1097/01.mbc.0000061288.28953.c8. [DOI] [PubMed] [Google Scholar]
- 56.Schwedt TJ, Dodick DW. Patent foramen ovale and migraine - bringing closure to the subject. Headache. 2006;46:663–71. doi: 10.1111/j.1526-4610.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 57.Schwedt TJ, Demaerschalk BM, Dodick DW. Patent foramen ovale and migraine: A quantitative systematic review. Cephalalgia. 2008;28:531–40. doi: 10.1111/j.1468-2982.2008.01554.x. [DOI] [PubMed] [Google Scholar]
- 58.Sharma A, Gheewala N, Silver P. Role of patent foramen ovale in migraine etiology and treatment: A review. Echocardiography. 2011;28:913–7. doi: 10.1111/j.1540-8175.2011.01460.x. [DOI] [PubMed] [Google Scholar]
- 59.Carerj S, Narbone MC, Zito C, Serra S, Coglitore S, Pugliatti P, et al. Prevalence of atrial septal aneurysm in patients with migraine: An echocardiographic study. Headache. 2003;43:725–8. doi: 10.1046/j.1526-4610.2003.03129.x. [DOI] [PubMed] [Google Scholar]
- 60.Schwerzmann M, Meirer B. Patent foramen ovale and stroke risk: The devil is in the detail. J Am Coll Cardiol. 2007;50:80. doi: 10.1016/j.jacc.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 61.Davis D, Gregson J, Willeit P, Stephan B, Al-Shahi Salman R, Brayne C. Patent foramen ovale, ischemic stroke and migraine: Systematic review and stratified meta-analysis of association studies. Neuroepidemiology. 2013;40:56–67. doi: 10.1159/000341924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rundek T, Elkin MS, Di Tullio MR, Carrera E, Jin Z, Sacco RL, et al. Patent foramen ovale and migraine: A cross-sectional study from the Northern Manhattan Study (NOMAS) Circulation. 2008;118:1419–24. doi: 10.1161/CIRCULATIONAHA.108.771303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwerzmann M, Nedeltchev K, Lagger F, Mattle HP, Windecker S, Meier B, et al. Prevalence and size of directly detected patent foramen ovale in migraine with aura. Neurology. 2005;65:1415–8. doi: 10.1212/01.wnl.0000179800.73706.20. [DOI] [PubMed] [Google Scholar]


